Abstract
FK506 binding protein 51 (FKBP5) alters stress response system functioning, and childhood maltreatment is associated with methylation of the FKBP5 gene. Yet it is unknown if maltreatment contributes to change in FKBP5 methylation over time. The current study draws upon a sample of 231 preschoolers, including 123 with child welfare documentation of moderate-severe maltreatment in the past 6 months, to understand if maltreatment contributes to change in FKBP5 methylation over a 6-month period. Review of child protection records and semi-structured interviews in the home were used to assess maltreatment and exposure to other contextual stressors, as well as service utilization. Methylation of FKBP5 at two CpG sites in intron 7 was measured from saliva DNA at the time of initial study enrollment, and 6 months following enrollment. Child maltreatment was associated with change in FKBP5 methylation over time, but only when children were exposed to high levels of other contextual stressors. Service utilization was associated with increases in methylation over time, but only among children with the FKBP5 rs1360780 protective CC genotype. Methylation of FKBP5 is sensitive to stress exposure and may be a mechanism linking early adversity to long term health and developmental outcomes.
Keywords: FKBP5, methylation, maltreatment, service utilization, children
Each year nearly 700,000 American children are identified by child protection services as victims of maltreatment (U.S. Department of Health & Human Services, 2017) and evidence suggests that as few as 5% of abuse cases are reported (Gilbert et al., 2009). It is well established that children who experience major adversity and trauma are at high risk for the development of psychiatric disorders (Gilbert et al., 2009; Burns et al., 2004), as well as major medical conditions (Cohen, Janicki-Deverts, & Miller, 2007; Grippo & Johnson, 2009; Schneiderman, Ironson, & Siegel, 2005). Emerging evidence suggests that epigenetic processes represent key mechanisms underlying the biobehavioral encoding of early adversity, and that childhood maltreatment is associated with epigenetic alteration in the genes that regulate stress responses. Yet, the importance of these changes likely unfolds over time across development, and little is known about the stability or responsiveness of epigenetic changes to adversity in stress-sensitive genes over time.
Childhood maltreatment and exposure to other adversities activate biological stress response systems including the hypothalamic-pituitary-adrenal (HPA) axis (Evans, 2003; Shonkoff, Boyce, & McEwen, 2009). The glucocorticoid receptor (GR), encoded by the gene NR3C1, is a key nuclear hormone receptor that is a major regulator of the stress response. GRs are widely distributed throughout the body and brain and are activated by binding of cortisol. GRs regulate the basal activity of a variety of physiologic systems as well as the physiological response to acute stress (de Kloet, Joels, & Holsboer, 2005; Kadmiel & Cidlowski, 2013). The acute cortisol response to stress allows for short-term cognitive and physical coping through activation of the GR, but excessive or prolonged glucocorticoid activation in response to chronic or severe stress, such as childhood maltreatment, can be toxic to the brain and other organ systems (McEwen, 2008). Cortisol activation of GRs in the hypothalamus and pituitary initiates a negative feedback loop that helps to prevent excessive glucocorticoid activation (Laryea et al., 2015).
FK506 binding protein 51 (FKBP5) represents an additional negative feedback loop within the HPA axis. Activation of the GR by cortisol results in rapid induction of FKBP5 which binds to the GR, and consequently reduces GR sensitivity to cortisol and impairs negative feedback of the HPA axis (Binder, 2009; Cioffi, Hubler & Scammell, 2011; Schmidt et al., 2015; Tatro, Everall, Kaul, & Achim, 2009). This process is modulated by genetic variation in FKBP5, which alters GR function and the neuroendocrine response to stress (Zannas & Binder, 2014). There is now evidence that a single nucleotide polymorphism (SNP) in FKBP5 (C to T SNP in intron 2, rs1360780) alters sensitivity of the GR to cortisol, such that the “risk” T allele is associated with reduced GR sensitivity (Hohne et al., 2015; Ising et al., 2008; Klengel et al., 2013; Menke et al., 2013). The T allele has also been linked to Major Depressive Disorder, PTSD, and mood and anxiety symptoms (Leszczynska-Rodziewicz et al., 2014; Suzuki et al., 2014; Szczepankiewicz et al., 2014; VanZomeren-Dohm, Pitula, Koss, Thomas, & Gunnar, 2015; Zannas & Binder, 2014). More recent work has demonstrated that epigenetic regulation of FKBP5 may also play a role in these processes.
Epigenetics is a means by which the body can respond to the environment by changing levels of gene expression to allow for adaptations to environmental conditions, with positive and/or negative long-term consequences. Epigenetic modulation of DNA does not change the DNA sequence, but renders it more or less likely to be expressed (Moore, Le, & Fan, 2013; Szyf, 2007). DNA methylation, which is among the most commonly studied epigenetic processes, generally occurs when a methyl group is added at sites in the DNA where a cytosine nucleotide occurs next to a guanine nucleotide (CpG dinucleotides). Methylation at CpG sites can result in transcriptional silencing of the gene due to blocking of transcription factor binding (Moore et al., 2013).
There is now evidence that childhood maltreatment and other adversities are associated with altered methylation of FKBP5. In the current sample of preschoolers, we demonstrated that childhood maltreatment is associated with demethylation of two CpG sites in intron 7 of FKBP5 in saliva DNA (Tyrka et al., 2015). Lower levels of methylation of FKBP5 in intron 7 have also been demonstrated in adults with a history of childhood maltreatment compared to adults with no child maltreatment history (Klengel et al., 2013). Whereas Klengel and colleagues demonstrated demethylation with childhood maltreatment only among those adults with the rs1360780 T “risk” allele, we found demethylation in association with maltreatment regardless of rs1360780 genotype. Related to this work, time spent in institutional care was also negatively associated with FKBP5 methylation in intron 7 at age 12 among children in the Bucharest Early Intervention Project (Non et al., 2016). Critically, to our knowledge no prior work has examined the possibility that childhood maltreatment is associated with change in FKBP5 methylation over time.
DNA methylation is thought to be among the most stable epigenetic processes, yet the stability of methylation, and the extent to which it can be altered over time in relation to adversity has not been adequately studied. Prior work utilizing epigenome-wide approaches suggests that whole genome methylation longitudinally changes over time (Alisch et al., 2012; Martino et al., 2013), and alterations in FKBP5 methylation from pre to post treatment have been observed with treatment response to cognitive behavior therapy in youth with anxiety disorders (Roberts et al., 2015). Thus, childhood maltreatment may contribute to change in methylation of stress sensitive genes over time. The current study draws upon two repeated assessments of methylation of FKPB5 at two CpG sites in intron 7 to understand if childhood maltreatment contributes to change in methylation over a six-month period. We also examined the possibility that contextual stress exposure, service utilization (i.e. engagement in therapeutic interventions, child behavioral supports), and FKBP5 genotype independently predict change in methylation over time, and moderate effects of maltreatment on change in methylation over time.
Method
Sample
Data were available from 231 families for the current report. One child from each family was included. Children ranged in age from 3 to 5 years (M = 51.2 months; SD = 9 months), 121 were female and 110 were male. The sample was racially and ethnically diverse. Ninety-three children were white, 37 black, 49 biracial, and 52 other races. One hundred and two children were Hispanic. Most caregivers (n = 217) were biological mothers. Forty-six caregivers had less than a high school degree, 93 completed high school, 68 had some post-secondary education, 23 had a bachelor’s degree, and one did not provide education information. One-hundred and twenty-five caregivers were single parents, and 49 were under 20 at the time of the child’s birth. One-hundred and twenty-six caregivers were unemployed, 207 of the families qualified for public assistance, and 18 families experienced homeless within the past year. One hundred and twenty-three children (53%) had substantiated cases of moderate to severe maltreatment within the past 6 months and 108 had no lifetime substantiated case of maltreatment, as described below.
Procedure
Families with a maltreated child were identified via record review from the local child welfare agency or an emergency maltreatment assessment service. Families of children with no indicated case of maltreatment within the past 6 months were recruited from a pediatric medical clinic during a well-child visit as well as at childcare centers. Based on review of available medical records and parent report, children with a chronic illness, medication use, obesity, and failure-to-thrive were excluded. Those with acute illness or medication use were included no less than 2 weeks following resolution of illness and discontinuation of medication. Families completed a baseline set of assessments at the time of initial study enrollment and a follow-up set of assessments 6 months following enrollment (M = 6.43 months, SD = .67 months). At each wave of assessment, families completed two home visits and questionnaires between the visits. The current report focuses on data from the first home visit during the baseline assessment during which caregivers completed interviews on child stress exposure and a baseline saliva sample for DNA isolation was collected from the children, as well as the first home visit during the follow-up assessment during which caregivers completed an interview on service utilization and a follow-up saliva sample for DNA isolation was collected from children.
Measures
Child maltreatment status
All families consented to examination of child welfare records to determine maltreatment status. Trained research staff coded the records using the System for Coding Subtype and Severity of Maltreatment in Child Protective Records (Barnett, Manly, & Cicchetti, 1993). Five maltreatment subtypes and severity scores ranging from 1 (least severe) to 5 (most severe) were derived. Children with an episode that met the criteria for moderate to severe maltreatment (score of 3–5) within the last 6 months were included in the maltreated group (n = 123). Twenty-one children had substantiated cases of physical abuse, 29 sexual abuse, 13 physical neglect/failure to provide, 35 physical neglect/lack of supervision, and 78 emotional maltreatment (including witnessing domestic violence). The comparison group (n = 108) included children who had never had a substantiated case of maltreatment regardless of severity type.
Contextual Stress
Caregivers completed a semi-structured interview developed in our laboratory to assess contextual stressors experienced in the child’s lifetime. Categories were: death of a caregiver, separation from a caregiver, housing instability, inadequate food or clothing, and other stressful events which included witnessing neighborhood violence or parental arrest. Interviews were conducted and scored by trained clinical social workers and Ph.D. level psychologists. The project coordinator reviewed each interview to ensure compliance with the scoring protocol. Each domain was scored positive if at least one episode occurred, and domains were summed to determine the number of contextual stressor categories the child experienced in their lifetime. Possible scores ranged from 0 (no stressors) to 5 (stressors in all five domains). In the current sample the number of stressor categories ranged from 0 to 5 (M = 1.50, SD = 1.20).
Service Utilization
Caregivers completed a semi-structured interview developed in our laboratory to assess service utilization. Caregivers were first queried broadly if their child or family ever received any services to support their child’s development or behavior, or services to support family wellbeing. Following the initial broad inquiry, caregivers were queried about engagement in specific services available in the community including outpatient mental health treatment, home based services, and services provided by the local school department. Interviews were conducted by trained research assistants, then scored by a single research assistant with supervision by the lead author. The number of unique episodes of services ever received by the family were summed for data analysis. In the current sample, the number of unique episodes of service utilization ranged from 0 to 9 (M = 1.68, SD = 1.96).
FKBP5 genotype
Saliva samples were obtained using the Oragene DISCOVER kits (OGR-575) for Assisted Collections (DNA Genotek, Kanata, Ontario, Canada), and DNA was isolated following the manufacturer’s instructions. DNA samples were genotyped for the FKBP5 SNP rs1360780 through an allelic discrimination assay using predesigned Taqman primers (part #C_8852038_10, Life Technologies) and Taqman universal master mix (Life Technologies) via established protocols as directed by the manufacturer on a Bio-Rad CFX connect. One hundred and twelve children were homozygotes for the C allele, 100 children were CT heterozygotes, and 19 children were homozygotes for the T allele. Longitudinal models for hypothesis testing utilized a dichotomous variable that included children with the CC genotype versus children with the T risk allele (heterozygous or homozygous).
FKBP5 methylation
Two CpGs in intron 7 (Chr 6: 35558488, CpG 1 and 35558514, CpG2) were studied based on findings of Klengel and colleagues (2013) using methods as previously described (Paquette et al., 2014; Tyrka et al., 2015). Briefly, sodium bisulfite modification was performed with 500 ng of DNA using the EZ DNA methylation Kit (Zymo Research, Irvine, CA, USA), the region of interest was amplified by PCR, sequenced using a PyroMark MD system (Qiagen), and percent DNA methylation at each CpG locus quantified with the PyroMark CpG software, version 1.0.11 (Qiagen).
Modeling Ancestry Differences Using Principal Component Analysis
Allele frequency differences due to systematic ancestry differences could cause spurious associations. We used principal components analysis (PCA) to model ancestry differences in the current study using genome-wide SNP markers from saliva DNA genotyped using the Illumina Infinium PsychArray-24 beadchip (over 588,000 autosomal SNPs). Genotypes were cleaned using standard quality control procedures. We conducted the linkage disequilibrium (LD)-based pruning first, and followed by PCA using PLINK (Purcell et al., 2007). LD-based pruning reduces correlation among SNPs such that the principal components (PCs) of the genetic variation in the sample would not be over-weighted by the contribution of correlated SNPs. The first two PCs obtained using PLINK were used for controlling the potential population stratification (Price et al., 2006).
Statistical analysis
Mean differences in demographic characteristics, stress exposure, and methylation based on genotype were examined using ANOVA and χ2. Simple correlations between demographic characteristics and methylation were conducted to determine inclusion of covariates. Mplus 6.11 software (Muthén & Muthén, 1998–2011) was used to conduct all analyses. Latent change score modeling was used to assess child maltreatment status, contextual stress exposure, service utilization, and FKBP5 genotype as predictors of baseline methylation and within-child change in methylation over time. Latent change score modeling is similar to latent growth curve modeling and allows for simultaneous estimation of individual differences in initial level and change over time, but utilizes two waves of assessment (McArdle, 2009). Two PCs used to adjust for genetic ancestry and the length of time between the baseline and follow-up assessments were included in the latent change score models as a-priori covariates. Outliers, defined as values more than three standard deviations from the mean, were Winsorized by setting them to the next highest value within three standard deviations.
Full information maximum likelihood estimation techniques with robust standard errors (MLR) were used to account for missing data to allow for inclusion of all available data. Eighty-six percent of children (n = 199) had FKBP5 methylation data at the follow-up assessment, and less than 4% of data was missing overall. Little’s Missing Completely at Random test (Little, 1988) demonstrated that the data were missing completely at random. Chi-square (χ2: p > .05 excellent), Comparative Fit Index (CFI; > .95 excellent), Root Mean Square Error of Approximation (RMSEA; < .05 excellent) and the Standardized Root Mean Square Residual (SRMR; < .05 excellent) were used to assess fit of the unconditional latent change score model prior to hypothesis testing (Hu & Bentler, 1999). Significant interaction terms were probed using procedures outlined by Aiken and West (1991). Simple slopes within the latent change score modeling framework were calculated at low (< 1 SD) and high (> 1 SD) levels of continuous moderators.
Results
Sample characteristics
The minor allele frequency (MAF) of the FKBP5 allelic variant in the sample was .30 and the distribution conformed to the Hardy-Weinberg equilibrium (X2 = .26, p = .61). Table 1 shows sample characteristics in relation to FKBP5 genotype. None of the variables differed according to genotype. Child age and sex were not associated with baseline or follow-up methylation at CpG 1 or CpG 2. Children who were maltreated had more contextual stressors (p < .001) and had families who engaged in more services (p = .002) than children with no maltreatment history. Contextual stress and service utilization were positively associated with each other (p = .001).
Table 1.
Descriptive statistics and mean differences by FKBP5 genotype
| CC (n = 112) |
CT (n = 100) |
TT (n = 19) |
p | |
|---|---|---|---|---|
| Sex, N (%) female | 65 (58.0) | 48 (48.0) | 8 (42.1) | .22 |
| Age, M (SD) | 4.2 (0.7) | 4.3 (0.8) | 4.1 (0.6) | .28 |
| PC for Genetic Ancestry 1, M (SD) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.0) | .64 |
| PC for Genetic Ancestry 2, M (SD) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.0) | .68 |
| Follow-Up Length (Months), M (SD) | 6.5 (0.7) | 6.4 (0.7) | 6.2 (0.3) | .40 |
| Maltreated, N (%) | 57 (50.1) | 58 (58.0) | 8 (42.1) | .35 |
| Contextual Stress, M (SD) | 1.5 (1.2) | 1.5 (1.2) | 1.7 (1.2) | .76 |
| Service Utilization, M (SD) | 1.6 (1.8) | 1.6 (2.0) | 2.4 (2.3) | .33 |
| CpG 1 Baseline Methylation, M (SD) | 87.6 (3.3) | 87.8 (3.1) | 87.8 (2.8) | .86 |
| CpG 2 Baseline Methylation, M (SD) | 87.9 (3.7) | 88.4 (3.8) | 89.6 (2.7) | .17 |
| CpG 1 Follow-Up Methylation, M (SD) | 87.1 (2.8) | 87.3 (3.0) | 87.4 (1.8) | .89 |
| CpG 2 Follow-Up Methylation, M (SD) | 88.9 (4.1) | 89.2 (3.9) | 89.5 (3.5) | .81 |
Note: p values indicate F-test or χ2 significance level.
Unconditional model
The unconditional latent change score model with the two PCs used to adjust for genetic ancestry differences and length of time between the baseline and follow-up assessments included as covariates demonstrated excellent fit to the data at CpG 1 (Chi-square (1) = .14, p = .705, RMSEA = .00, CFI = 1.00, SRMR = .005) and CpG 2 (Chi-square (1) = .13, p = .719, RMSEA = .00, CFI = 1.00, SRMR = .004). Across the entire sample, average FKBP5 methylation at CpG 1 and CpG 2 did not change over time. The variance component, however, demonstrated that there was significant variability in change over time at both CpG 1 (B = 13.94, SE = 1.54, p < .001) and CpG 2 (B = 18.86, SE = 2.02, p < .001), suggesting that contextual factors may contribute to change in methylation over time. The following sections therefore test effects of potential contextual influences on change in methylation over time.
Maltreatment
Child maltreatment was associated with baseline methylation at CpG 1 and CpG 2 (B = -1.24, SE = .43, p = .004, and B = -1.09, SE = .52, p = .037, respectively). Consistent with our prior work with the current sample, maltreated children had lower levels of baseline methylation at CpG 1 (M = 87.03, SD = 3.03) and CpG 2 (M = 87.62 SD = 3.64) than children with no maltreatment history (M = 88.49, SD = 3.11 and M = 88.97, SD = 3.63 at CpG 1 and 2, respectively). In contrast, child maltreatment did not predict change in methylation at CpG 1 or CpG 2 over time.
Contextual stress
Also consistent with our prior work, contextual stress did not predict baseline methylation, and contextual stress also did not predict change in methylation over time at CpG 1 or CpG 2. However, the interaction of maltreatment status and contextual stress was a significant predictor of change in methylation over time at CpG 1 (B = .83, SE = .42 p = .049). Examination of simple slopes revealed that child maltreatment was a significant predictor of change in methylation over time when contextual stress was high (B = 1.80, SE = .77, p = .019) but not when contextual stress was low (B = -.21, SE = .79, p = .794). To better understand change in methylation when contextual stress was high, we plotted change in methylation over time for the group of children above the median contextual stress score (Median = 1 contextual stressor). As illustrated in Figure 1, when contextual stress was high, children who were maltreated demonstrated consistently low methylation over time whereas children who did not experience maltreatment demonstrated declines in methylation from the baseline assessment to the follow-up assessment. Contextual stress did not moderate the effect of maltreatment on baseline methylation at CpG 1 or CpG 2, or change in CpG 2 methylation over time.
Figure 1.
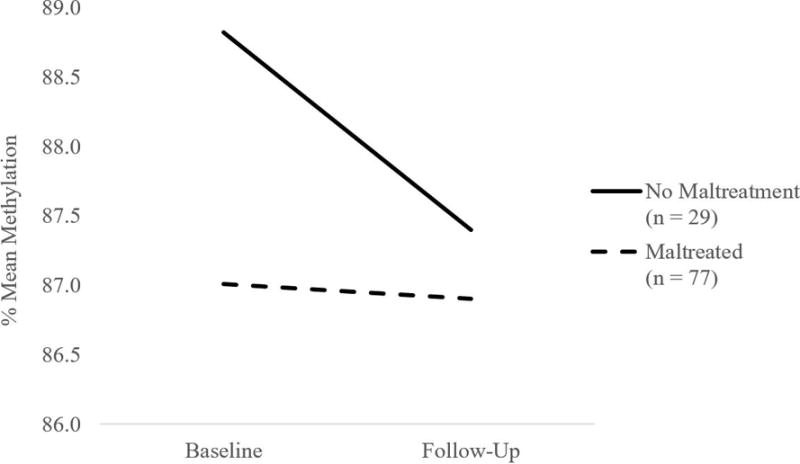
Maltreatment status is associated with change in FKBP5 methylation at CpG 1 when contextual stress is high.
Service utilization and methylation
Service utilization was positively associated with baseline methylation at CpG 1 (B = .25, SE = .11, p = .029), and this effect remained significant when maltreatment status and contextual stress were included in the model (B = .35, SE = .12, p = .003). Service utilization was not associated with change in methylation at CpG 1 over time. In contrast, service utilization was positively associated with change in methylation over time at CpG 2 (B = .40, SE = .14, p = .003). To better understand the effect of service utilization, we plotted change in methylation over time for children below the median service utilization score (0 services), at the median service utilization score (1 service), and above the median service utilization score (≥ 2 services). As illustrated in Figure 2, children whose families who received two or more services demonstrated increases in methylation over time, children whose families who received 1 service demonstrated consistent levels of methylation over time, and children whose families who did not receive any services demonstrated declines in methylation over time. The effect of service utilization on change in methylation over time remained significant when maltreatment status and contextual stress were included in the model (B = .45, SE = .15, p = .002). Service utilization was not associated with baseline methylation at CpG 2. Interactions of service utilization with maltreatment status and contextual stress did not predict baseline methylation or change in methylation over time.
Figure 2.
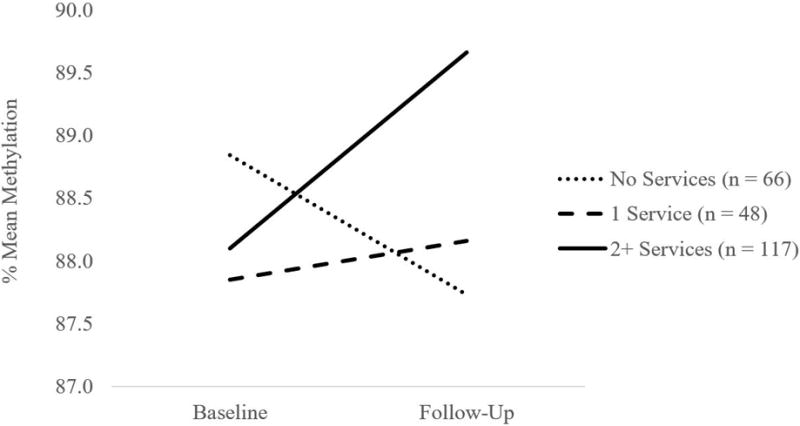
Service utilization is associated with change in FKBP5 CpG 2 methylation over time.
FKBP5 genotype and methylation
FKBP5 genotype was not a significant predictor of baseline methylation or change in methylation over time at CpG 1 or CpG 2. Likewise, FKBP5 genotype did not moderate effects of maltreatment status or contextual stress on baseline methylation or change in methylation over time at CpG 1 or CpG 2. In contrast, the interaction of FKBP5 genotype and service utilization predicted change in methylation at CpG 2 over time (B = -.64, SE = .25, p = .009), and the interaction effect remained significant when maltreatment status and contextual stress were included in the model (B = -.67, SE = .25 p = .007). Simple slopes revealed that service utilization was positively associated with change in methylation over time among those with the protective genotype, C homozygotes (B = .76, SE = .18, < .001), but was not associated with change in methylation over time among children with the T risk allele (B = .12, SE = .17, p = .49). To better understand change in methylation among C homozygotes, we plotted change in methylation over time for children below the median service utilization score (0 services), at the median service utilization score (1 service), and above the median service utilization score (≥ 2 services). As illustrated in Figure 3, among children with the C homozygote protective genotype, those whose families received two or more services demonstrated increases in methylation over time, whereas children whose families received 1 service or no services demonstrated consistent levels of methylation over time. The interaction of FKBP5 genotype and service utilization was not a predictor of baseline methylation at CpG 1 or CpG 2, or change in methylation over time at CpG 1.
Figure 3.
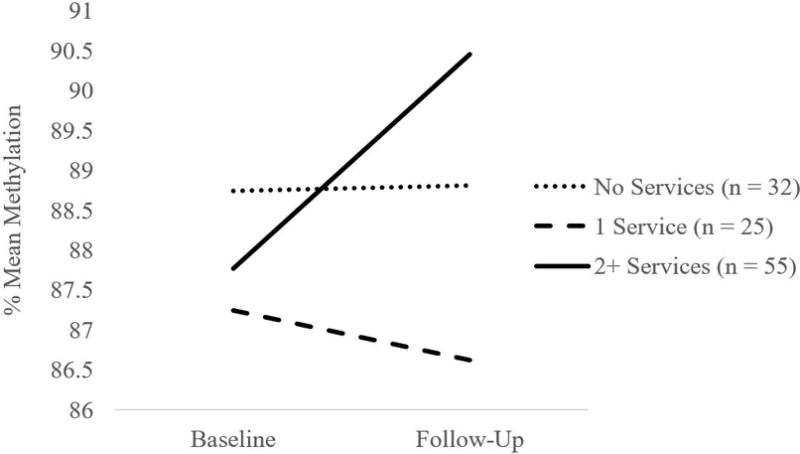
Service utilization is associated with change in FKBP5 CpG 2 methylation over time among children with CC genotype.
Discussion
The current study is the first to examine links between maltreatment and other stressors and change in FKBP5 methylation over time. As we showed previously, childhood maltreatment was linked with lower levels of baseline methylation at CpG 1 and CpG 2, and this demethylation or lower methylation is consistent with results of other work on adults and children with early stress. In this longitudinal analysis we found that although methylation did not change over time on average in the sample, individual variability in change over time at both CpG sites was important. Child maltreatment and contextual stress did not independently contribute to change in FKBP5 methylation over time, but rather interacted to predict change. Maltreatment was associated with change in CpG 1 methylation over time, but only when contextual stress was high, wherein children who were not maltreated demonstrated declines in methylation over time whereas children who experienced maltreatment demonstrated consistently low methylation over time. These findings suggest that contextual stress can have similar effects on FKBP5 demethylation to those of maltreatment, and that there may be a “floor effect” such that effects of maltreatment plus contextual stressors are not necessarily additive in this context.
Turning to effects of service utilization, there was a linear relationship between service utilization and increases in methylation over time at CpG 2, even after controlling for effects of maltreatment and contextual stress. Those children whose families did not have any services had further declines in methylation over time, but those who had two or more services showed increases in methylation over time, and those with one service showed no change. This suggests the possibility that mental health and support services might improve the methylation of a key regular of the biological stress response. Our study extends findings of Roberts and colleagues (2015) who showed that pre- to post-treatment change in FKBP5 methylation is associated with treatment response to cognitive behavior therapy in youth with anxiety disorders (although their effect was no longer significant when correcting for multiple comparisons).
Two important aspects of the findings on service utilization bear further consideration when interpreting these results. First, it is possible that the increases seen among families with two episodes of service utilization might reflect an unmeasured resilience factor that predisposes to both engagement in services and increases in methylation. That baseline methylation of CpG 1 was also associated with service utilization suggests this possible explanation, however note that it was at CpG 2 where the effect of services on change in methylation was seen. Second, analyses of FKBP5 genotype showed that effects of service utilization on CpG 2 methylation over time were only present among children with the protective CC genotype. This indicates that variation of this gene may confer sensitivity to effects of the environment on methylation, consistent with some previous work (Klengel et al., 2013; Han et al., 2017; Van Zomeren-Dohm et al., 2015). Interestingly, a recent study of adults found that DNA methylation was positively associated with cortical thickness at the right transverse frontopolar gyrus, but only among CC homozygotes (Han et al., 2017). Likewise, placenta FKPB5 methylation was negatively associated with FKBP5 gene expression, but only among CC homozygotes (Paquette et al., 2014), suggesting that the effects of methylation may also differ depending on variation in this gene.
Our study is characterized by several strengths including a diverse sample of preschoolers exposed to a range of adversities including maltreatment, our focus on receipt of services in addition to stress exposure, and our longitudinal approach to understanding change in methylation over time. Despite these strengths, there are limitations of this work. Our assessment of service utilization is limited in that we are unable to verify family engagement and adherence to services with providers. Likewise, although child protection records were reviewed for all children in the study, it is possible that some children in our comparison group had undocumented maltreatment which may have reduced the strength of associations between maltreatment and methylation. Related, consideration of additional lifetime stressors such as food insecurity and parental loss is a strength, however there may be other exposures we did not assess including prenatal exposures. Finally, our use of PCs to adjust for genetic ancestry addresses potential genetic population stratification, but may not fully adjust for methylation variation among racial and ethnic groups. Despite these limitations, the current study is among the first to examine change in methylation over time using a candidate gene approach, and the first to examine child maltreatment as a predictor of change in FKBP5 methylation over time.
Taken together, the current study supports the view that childhood stress exposure contributes to epigenetic changes in stress sensitive genes over time, and that engagement in services to support child and family functioning also play an important role in these longitudinal processes. Interventions to support the healthy development of children exposed to maltreatment and other adversities may enhance child biopsychosocial functioning through effects on the epigenome. Future work should further examine these longitudinal epigenetic processes and draw upon experimental designs to better understand the role of interventions and other support services.
Acknowledgments
This research was supported by grant R01 MH083704 awarded to the last author from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIMH. We are grateful to the children and families who participated in this study, and we thank Hasbro Children’s Hospital, Rhode Island Head Start, and the Rhode Island Department of Children, Youth, and Families for assisting in recruitment of study participants. We also thank Brittney Josefson and the numerous other research assistants who contributed to this project, and Asi Polly Gobin for data management. Isolation of DNA and the genotyping array were done in the laboratory of Joel Gelernter, M.D., and we are grateful to Dr. Gelernter and his staff for their contribution.
References
- Aiken LS, West SG. Testing and interpreting interactions in multiple regression. Sage Publications; 1991. [Google Scholar]
- Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST. Age-associated DNA methylation in pediatric populations. Genome Research. 2012;22:623–632. doi: 10.1101/gr.125187.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Burns BJ, Phillips SD, Wagner HR, Barth RP, Kolko DJ, Campbell Y, Landsverk J. Mental health need and access to mental health services by youths involved with child welfare: A national survey. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:960–70. doi: 10.1097/01.chi.0000127590.95585.65. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA: The Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Hubler TR, Scammell JG. Organization and function of the FKBP52 and FKBP51 genes. Current Opinion in Pharmacology. 2011;11:308–313. doi: 10.1016/j.coph.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews: Neuroscience. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Wisdom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Won E, Sim Y, Kang J, Han C, Kim Y, Ham B. Influence of FKBP5 polymorphism and DNA methylation on structural changes of the brain in major depressive disorder. Scientific Reports. 2017;7:42621. doi: 10.1038/srep42621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohne N, Poidinger M, Merz F, Pfister H, Bruckl T, Zimmermann P, Ising M. FKBP5 genotype-dependent DNA methylation and mRNA regulation after psychosocial stress in remitted depression and healthy controls. International Journal of Neuropsychopharmacology. 2015;18:pyu087. doi: 10.1093/ijnp/pyu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. European Journal Neuroscience. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences. 2013;34:518–30. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryea G, Muglia L, Arnett M, Muglia LJ. Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic–pituitary– adrenal axis by gene targeting in mice. Frontiers in Neuroendocrinology. 2015;36:150–164. doi: 10.1016/j.yfrne.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynska-Rodziewicz A, Szczepankiewicz A, Narozna B, Skibinska M, Pawlak J, Dmitrzak-Weglarz M, Hauser J. Possible association between haplotypes of the FKBP5 gene and suicidal disorder, but not with melancholic depression and psychotic features, in the course of bipolar disorder. Neuropsychiatric Disease and Treatment. 2014;10:243–248. doi: 10.2147/NDT.S54538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ. A Test of Missing Completely at Random for Multivariate Date with Missing Values. Journal of the American Statistical Association. 1988;83:1198–1202. doi: 10.2307/2290157. [DOI] [Google Scholar]
- Martino D, Loke YJ, Gordon L, Ollikainen M, Cruickshank MN, Saffery R, Craig JM. Longitudinal, genome-scale analysis of DNA methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biology. 2013;14:R42. doi: 10.1186/gb-2013-14-5-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Latent Variable Modeling of Differences and Changes with Longitudinal Data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Klengel T, Rubel J, Brückl T, Pfister H, Lucae S, Binder EB. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes, Brain and Behavior. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non AL, Hollister BM, Humphreys KL, Childebayeva A, Esteves K, Zeanah C, Drury S. DNA methylation at stress-related genes is associated with exposure to early life institutionalization. American Journal of Physical Anthropology. 2016;61:84–93. doi: 10.1002/ajpa.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ. Placental FKBP5 genetic and epigenetic variationis associated with infant neurobehavioral outcomes in the RICHS cohort. PLOS ONE. 2014;9:e104913. doi: 10.1371/journal.pone.0104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Ferreira MA, Bender D, Maller J, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Keers R, Lester KJ, Coleman JR, Breen G, Arendt K, Wong CC. HPA axis related genes and response to psychological therapies: Genetics and epigenetics. Depression and Anxiety. 2015;32:861–70. doi: 10.1002/da.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA: The Journal of the American Medical Association. 2009;21:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Buell DR, Ionescu IA, Gassen NC, Holsboer F, Cox MB, Touma C. A role for synapsin in in FKBP51 modulation of stress responsiveness: Convergent evidence from animal and human studies. Psychoneuroendocrinology. 2015;52:43–58. doi: 10.1016/j.psyneuen.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annual Review Clinical Psychology. 2005;1:607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Matsumoto Y, Sadahiro R, Enokido M, Goto K, Otani K. Relationship of the FKBP5 C/T polymorphism with dysfunctional attitudes predisposing to depression. Comprehensive Psychiatry. 2014;55:1422–5. doi: 10.1016/j.comppsych.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Szyf M. The dynamic epigenome and its implications in toxicology. Toxicological Sciences. 2007;100:7–23. doi: 10.1093/toxsci/kfm177. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz A, Leszczynska-Rodziewicz A, Pawlak J, Narozna B, Rajewska-Rager A, Wilkosc M, Twarowska-Hauser J. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. Journal of Affective Disorders. 2014;164:33–7. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Kaul M, Achim CL. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Research. 2009;1286:1–12. doi: 10.1016/j.brainres.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Eslinger NM, Seifer R, Marsit CJ, Lesseur C, Josefson B. Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor gene promoter and exposure to adversity in pre-school aged children. Development and Psychopathology. 2015;27:577–585. doi: 10.1017/S0954579415000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. Child Maltreatment 2015. 2017 Available from http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment.
- VanZomeren-Dohm AA, Pitula CE, Koss KJ, Thomas K, Gunnar MR. FKBP5 moderation of depressive symptoms in peer victimized, post-institutionalized children. Psychoneuroendocrinology. 2015;51:426–30. doi: 10.1016/j.psyneuen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism. Genes, Brain and Behavior. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]


