Abstract
Background/aims
The mortality of hospitalized patients for complications of cirrhosis is very high. We examined the independent predictors of mortality, particularly the impact of increments in creatinine, in 339 consecutive patients (636 admissions) who were admitted for complications of cirrhosis.
Methods
Clinical characteristics, biochemical parameters including serum creatinine levels at various time intervals, and mortality data were recorded for all admissions. Data were analyzed for initial as well for all repeated admissions to identify independent predictors of mortality.
Results
The in-hospital mortality, 30-day, 90-day, 180 days, and 365 days mortality were 6%, 15%, 23%, 30%, and 41% respectively. Those admitted with spontaneous bacterial peritonitis had the worst survival. Increase in creatinine was noted in 29% of patients and they had lower 30-day (78% vs.91%) and 90-day (73% vs. 82%) survival than those without increase in creatinine. Any increment in serum creatinine (≥0.1 mg/dL) within 48 h after admission (peak 48 h – admission) was associated with a step-wise increase in mortality, but only if peak creatinine reached above 1.2 mg/dL. If peak creatinine levels were below 1.2 mg/dL, increases in serum creatinine had no impact on survival. Cox regression analysis showed that increments in serum creatinine of 0.3 mg/dL or higher had the worst outcome (HR 2.51, CI 1.65–3.81). Etiology of cirrhosis or the use of PPI, beta blockers or rifaxamin did not predict mortality. Other independent predictors of mortality were age, reason for admission, hyponatremia, and INR.
Conclusion
In patients with cirrhosis, any increment in serum creatinine within 48 h from hospitalization is associated with a higher mortality provided the peak serum creatinine within 48 h is above 1.2 mg/dL.
Abbreviations: ADQI, Acute Dialysis Quality Initiative (ADQI); AKI, acute kidney injury; AKIN, acute kidney injury network; HRS, hepatorenal syndrome; INR, international normalized ratio; MELD, model for end-stage liver disease; PPI, proton pump inhibitor; SBP, spontaneous bacterial peritonitis
Keywords: acute kidney injury, hospitalized patients, cirrhosis, liver failure, complications of cirrhosis
Cirrhosis is a major cause of morbidity and mortality worldwide, and as per Healthcare Cost and Utilization Project (HCUP), the number of people hospitalized with cirrhosis in the US hospitals increased steadily (6.2% increase per year) from 403,665 in 2004 to 578,573 in 2011. It has been estimated that 25.7 per 100,000 people died from liver related disease in the US in 2008.1 The above estimates suggest that the management of people with end-stage liver disease will continue to cause significant financial burden on the entire health care system.2
The transition from a compensated cirrhosis to a decompensated (ascites, encephalopathy, variceal bleeding) state occurs at an annual rate of five to seven percent, and this reduces the median survival time from twelve to two years.3 It has been estimated that about 58% of patients with cirrhosis will decompensate within 10 years from the time of diagnosis of cirrhosis and the 10-year survival of people with cirrhosis is only 47%.4 To reduce short-term and long-term mortality of people with cirrhosis, it is important to identify important negative risk factors and optimize their care. Previous studies showed that people hospitalized with complications of cirrhosis have a very high mortality5, 6 but reliable prognostic indicators and risk factors for worse outcomes are currently unavailable. Although the MELD or Child–Pugh score scores could predict short term mortality, it is important to identify modifiable risk factors in hospitalized cirrhotic patients to improve the outcomes.7, 8, 9, 10, 11, 12, 13, 14, 15
Recently, development of acute kidney injury (AKI) has shown to be an independent negative predictor of mortality in people with cirrhosis, and it is possible that this is a modifiable risk factor. The Acute Kidney Injury Network (AKIN)16 defined AKI as an abrupt (within 48 h) increase in the serum creatinine level by at least 0.3 mg/dL or the equivalent to a percentage increase of 50% (1.5 fold) from baseline, irrespective of final serum creatinine. The International Ascites Club (IAC) and the Acute Dialysis Quality Initiative (ADQI) group17 proposed a modified definition for AKI in people with cirrhosis by removing the staging aspect of AKI (Stage 1–3) proposed by AKIN. People with cirrhosis are susceptible to AKI because of a progressive vasodilatory state, reduced effective circulating blood volume, and subsequent compensatory stimulation of vasoconstrictor hormones.18, 19 It has been estimated that approximately 20% of people admitted to hospitals with a complication of cirrhosis will have AKI and the mortality is higher in those with AKI when compared to those who do not develop AKI.20, 21, 22 However, the reported studies of mortality using the AKI criteria in patients with cirrhosis have mostly focused on specific groups (i.e. intensive care, outpatients and decompensated patients with ascites or infections), which may limit the generalization of these results.23, 24, 25, 26 In this study, our objective was to identify the risk factors, including AKI, of mortality in unselected and consecutive patients admitted to a hospital for complications of cirrhosis, and to critically evaluate the utility of existing diagnostic criteria for AKI in cirrhotic patients.
Subjects and Methods
After obtaining institutional review board approval, all adult patients (age 18 and above) admitted to the Mercy Medical Centre in Baltimore, MD from January 2009 to December 2013 were screened for inclusion in the study. To be eligible for inclusion, patients must have been admitted for complications resulting from underlying cirrhosis. Patients admitted for elective procedures (i.e. transarterial chemoembolization or percutaneous tumor ablation, transjugular portosystemic shunt, large-volume paracentesis or surgical procedures) and patients admitted for observations were excluded. For eligible patients admitted to our hospital between January 2009 and December 2012, data were collected in a retrospective fashion using a comprehensive, electronic patient record database. For eligible patients admitted between January 2013 and December 2013, data were prospectively collected after obtaining informed consent.
During the study period, there were no changes in the management of complications of cirrhosis at the hospital. All patients were managed by experienced hepatologists. All patients with ascites had diagnostic paracentesis on admission, received albumin infusion if there was presence of spontaneous bacterial peritonitis (SBP), received prophylactic antibiotics for 5 days if admitted with gastrointestinal bleeding, and had appropriate secondary prophylaxis after discharge. Prophylactic antibiotics (primary prophylaxis) were not given for patients with ascites without a history of SBP. Patients with MELD score above 14 were considered for liver transplant evaluation unless there were obvious contraindications. Terlipressin was not given to any patients during the study period.
Cirrhosis was diagnosed with a combination of clinical, biochemical, radiological, and endoscopic findings in the absence of a liver biopsy confirmation. The collected data included age, race, sex, employment status, etiology of cirrhosis, medication use prior to admission (particularly rifaximin, beta-blockers and acid suppressive agents), admission vital signs, complete blood counts, basic metabolic panel, liver function tests, coagulation panel, severity of liver dysfunction (as determined by Child–Pugh and MELD calculations), and reason for admission. Data regarding the overall length of hospital stay in days and number of days in ICU, data regarding clinical outcomes (i.e. liver transplantation, discharge to home/nursing home/rehab/hospice) and readmission rates at 3, 6, 12 and 24 months were also collected. Mortality data of patients who were discharged alive from the hospital were obtained from the social security database.
For this study, AKI is defined according to the clinically validated AKIN criteria.16 To analyze the impact of AKI on outcomes, pre-admission serum creatinine values were obtained from a combination of outpatient and emergency department visit records. Pre-admission creatinine is defined as the average of all available serum creatinine measurements within 90 days prior to admission. Serum creatinine on admission, peak creatinine within 48 h, peak creatinine values during the entire length of stay, and creatinine at discharge were recorded. The duration of AKI was also recorded. The differences between peak serum creatinine during the first 48 h admission and admission serum creatinine (peak serum creatinine within 48 h—admission serum creatinine) were calculated in all patients. On admission, peak within 48 h—pre-admission creatinine was also obtained whenever reliable pre-admission creatinine was available. The impact of serum creatinine changes on outcomes when serum creatinine increase was within normal ranges (normal ranges for our laboratory—serum creatinine 0.7 mg/dL to 1.2 mg/dL) and outside the normal ranges (peak serum creatinine more than 1.2 mg/dL) was assessed. We also sought to find the optimal cut-off point at which sharp increases in creatinine related to a meaningful difference in survival. Additionally, we examined the survival based on conventional definition of type 2 hepatorenal syndrome (HRS, peak serum creatinine 1.5 mg/dL or higher). The effect of diuretic use (either before or during admission) or beta blockers, and the mean arterial pressure on the development of AKI were assessed.
Statistical Methods
The primary outcomes of the analysis were 30-day survival, 90-day survival, and survival time. The 30-day and 90-day survival outcomes were dichotomous, and characteristics of patients who did and did not have these outcomes were compared using Pearson's chi-square tests (categorical outcomes), Fisher's exact tests (categorical outcomes for which at least one cell size was fewer than five patients), and Student's t tests (continuous outcomes). Patient-level analysis, which examined baseline patient characteristics, used only data from each patient's first admission in the dataset, and the survival outcomes were based on time to death from the first admission.
Visit-level analysis examined the effect of incremental increases in creatinine (from the admission creatinine to the peak creatinine within 48 h of admission) on 30-day and 90-day survival, using data from each visit in the database. Generalized estimating equations, with logarithmic link functions, were used to uncover significant differences in survival between groups, accounting for correlated data within patients. In instances with few data points, binomial exact tests were used instead.
Cox proportional hazards regression produced adjusted hazard ratios for time-to-death, comparing patients along several baseline factors, as well as time-dependent laboratory values. The main effect of interest in this model involved a four-level creatinine variable, which divided patients among those who did and did not have a peak creatinine of at least 1.20 mg/dL and those who did and did not have an incremental increase of at least +0.3 mg/dL. Survival times were relative to the date of each patient's first admission in the dataset, and times were censored at the end of the data collection period: November 10, 2013.
Survival times were also compared between three groups of patients, based on their maximum creatinine value ever recorded during the study period, with the following cut-offs: 0.7–1.20 mg/dL, 1.21–1.50 mg/dL, and >1.50 mg/dL. This analysis first used the Kaplan–Meier method, which also produced graphical curves that allowed for a visual representation of survival times between groups. An unadjusted Cox proportion hazards regression model then produced crude hazard ratios, comparing these three maximum-creatinine groups.
Results
During the study period, 339 patients fulfilled the predefined criteria for inclusion in the study, and many were readmitted during the study period (total admissions 636). Patient characteristics at their first admission are shown in Table 1. Majorities of patients were male (63%) and white (60%), and the mean age at first visit was 57.0 (standard deviation [SD] 10.1) years old. We had a substantial proportion of black patients (37%) reflecting an inner city location of the hospital. Sixty-one percent of the patients reported being unemployed at their first visit, and 27% reported being retired. While 212 patients (63%) had only one admission during the study period, 127 had two or more admissions. Proton pump inhibitors were used by 136 patients (40%) at first admission, beta blockers by 137 patients (40%), and rifaxamin by 46 (14%).
Table 1.
Sample Characteristics (n = 339), Using Data Collected at First Admission.
| Characteristic | % of sample (n) |
|---|---|
| Sex | |
| Female | 37% (126) |
| Male | 63% (213) |
| Race | |
| White | 60% (204) |
| Black | 37% (127) |
| Other | 2% (8) |
| Age, mean (SD) | 57.0 (10.1) |
| ≤49 years old | 21% (71) |
| 50–59 | 41% (140) |
| 60–69 | 27% (92) |
| 70+ | 11% (36) |
| Employment status | |
| Employed | 12% (41) |
| Unemployed | 61% (208) |
| Retired | 27% (90) |
| Number of visits, total n | 636 |
| Mean (SD) | 1.9 (1.8) |
| 1 visit | 63% (212) |
| 2 visits | 18% (62) |
| 3 visits | 8% (28) |
| 4 visits | 5%(16) |
| 5+ visits | 6% (21) |
| Ever transplanteda | 1% (5/339) |
| Pharmaceutical use at admission | |
| Proton pump inhibitors | 40% (136/339) |
| Beta blockers | 40% (137/339) |
| Rifaxamin | 14% (46/339) |
| AKI | 29% (96/330) |
| AKIN stage | |
| No AKI | 71% (234) |
| 1a | 18% (60) |
| 1b | 7% (23) |
| 2 | 2% (7) |
| 3 | 2% (2) |
| Survival | |
| In-hospital | 94% (317/339) |
| 30 days | 85% (286/338) |
| 90 days | 77% (255/331) |
| 180 days | 70% (222/319) |
| 365 days | 59% (172/293) |
Discharge status listed as “Transfer”.
We could not obtain preadmission (‘baseline’) creatinine in majority of patients (n = 216/339) despite our rigorous attempts. Acute kidney injury (AKI) was noted in 96 patients (29%) using admission creatinine as baseline creatinine.
The in-hospital mortality was 6% (22/339), and 30-day and 90-day mortality were 15% and 23% respectively; 30% of patients died within 180 days after the initial hospitalization and 41% died within 1 year irrespective of the reason for admission.
30-Day and 90-Day Survival
As shown in Table 2, many factors predicted survival. The two unexpected findings were higher survival (89%) in unemployed patients when compared to employed (80%) or retired (75%), and a relatively better 30-day (90% vs. 81%, P = 0.04) and 90-day survival among blacks (85% vs. 72%, P = 0.01) when compared to white patients. However, employment status and race were not independent predictors when adjusted for other covariates.
Table 2.
Factors Associated with 30-Day and 90-Day Survival, Using Independent Variables Collected During the Patients’ First Admission Only (n = 339 Patients).
| Categorical variables | 30-Day survival % | px | 90-Day survival % | px |
|---|---|---|---|---|
| Sex | 0.29 | 0.33 | ||
| Female | 87% (110/126) | 80% (96/120) | ||
| Male | 83% (176/212) | 75% (159/211) | ||
| Race | 0.04 | 0.01 | ||
| White | 81% (166/204) | 72% (143/199) | ||
| Black or other | 90% (120/134) | 85% (112/132) | ||
| Employment | 0.01 | <0.01 | ||
| Employed | 80% (33/41) | 73% (29/40) | ||
| Unemployed | 89% (186/208) | 84% (173/206) | ||
| Retired | 75% (67/89) | 62% (53/85) | ||
| Reason for admission | 0.03 | <0.01 | ||
| Enceph. | 76% (68/89) | 67% (56/84) | ||
| Ref. Asc. | 91% (63/69) | 83% (57/69) | ||
| SBP | 74% (17/23) | 52% (12/23) | ||
| Sepsis | 85% (52/61) | 82% (50/61) | ||
| GI bleed | 90% (86/96) | 85% (80/94) | ||
| AKI and 48 h peak Cr | 0.01 | <0.01 | ||
| No AKI | 91% (213/233) | 82% (186/227) | ||
| AKI, peak Cr ≤1.5 mg/dL | 78% (35/45) | 73% (33/45) | ||
| AKI, peak Cr >1.5 mg/dL | 61% (31/51) | 58% (29/50) | ||
| AKIN stage | 0.46f | 0.32f | ||
| 1a | 68% (41/60) | 65% (39/60) | ||
| 1b | 78% (18/23) | 77% (17/22) | ||
| 2 | 57% (4/7) | 43% (3/7) | ||
| 3 | 50% (3/6) | 50% (3/6) | ||
| MELD | <0.01 | <0.01 | ||
| 0–9 | 97% (35/36) | 89% (31/35) | ||
| 10–19 | 89% (157/176) | 83% (145/174) | ||
| 20–29 | 80% (75/94) | 68% (62/91) | ||
| 30–40 | 45% (9/20) | 37% (7/19) | ||
| MELD-Na | <0.01 | <0.01 | ||
| 0–9 | 97% (30/31) | 90% (27/30) | ||
| 10–19 | 92% (145/158) | 86% (134/156) | ||
| 20–29 | 84% (71/85) | 73% (60/82) | ||
| 30+ | 58% (30/52) | 47% (24/51) |
| Continuous variables | 30-day survival, mean (SD) |
90-day survival, mean (SD) |
||||
|---|---|---|---|---|---|---|
| Died | Survived | pt | Died | Survived | pt | |
| n | 52 | 286 | 76 | 255 | ||
| Age, years | 59.7 (9.5) | 56.5 (10.1) | 0.03 | 60.4 (10.4) | 55.8 (9.7) | <0.01 |
| Length of stay, days | 8.9 (6.0) | 6.4 (6.3) | <0.01 | 8.7 (8.0) | 6.2 (5.6) | <0.01 |
| Cr, Admission,mg/dL | 1.91 (1.70) | 1.29 (0.84) | <0.01 | 1.67 (1.45) | 1.30 (0.87) | 0.01 |
| Peak 48 h, mg/dL | 2.21 (1.75) | 1.40 (0.90) | <0.01 | 1.90 (1.54) | 1.42 (0.93) | <0.01 |
| Peak 48 h—Adm, mg/dL | 0.29 (0.35) | 0.12 (0.22) | <0.01 | 0.23 (0.33) | 0.12 (0.22) | <0.01 |
| INR | 1.9 (0.7) | 1.7 (0.6) | 0.01 | 1.9 (0.7) | 1.6 (0.6) | <0.01 |
| Na, mEq/L | 133.2 (6.2) | 137.2 (5.4) | <0.01 | 133.8 (6.2) | 137.4 (5.3) | <0.01 |
| Alb, mg/dL | 2.5 (0.5) | 2.8 (0.7) | 0.01 | 2.6 (0.6) | 2.8 (0.7) | <0.01 |
| Tbili, mg/dL | 8.0 (8.7) | 3.4 (4.3) | <0.01 | 6.7 (7.9) | 3.3 (4.3) | <0.01 |
| PT, sec | 21.5 (6.4) | 17.6 (5.9) | <0.01 | 21.1 (6.9) | 17.6 (5.5) | <0.01 |
| MAP, mmHg | 79.7 (13.9) | 90.2 (18.2) | <0.01 | 82.8 (15.6) | 90.5 (18.5) | <0.01 |
xPearson's chi-square test.
tStudent's t-test.
fFisher's exact test.
AKI: acute kidney injury at admission; MELD: Model for End-State Liver Disease (CITE); MELD-Na: Model for End-State Liver Disease, With Sodium (CITE); INR: international normalized ratio; Na: sodium; Alb: albumin; Tbili: total bilirubin; PT: prothrombin time; MAP: mean arterial pressure.
Peak 48 h: peak within 48 hours of admission.
Peak 48 h—Adm: peak within 48 hours of admission, minus admission level.
Patients who died within 30 days or 90 days were older, had longer hospitalization, had higher serum creatinine, and longer duration of AKI. Those who died had comparatively worse liver disease as shown by higher INR, higher total bilirubin, lower sodium and lower albumin. Mean arterial pressure was also lower in patients who died reflecting more advanced portal hypertension. Patients who died had higher MELD and MELD-Na scores. Only 45% of patients with MELD score 30 or above survived 30 days and 37% survived 90 days.
Those admitted with spontaneous bacterial peritonitis (SBP) had the worst survival, with only 52% (12/23) surviving at least 90 days. In addition, 24% of patients who were admitted for hepatic encephalopathy died within 30 days and 33% died within 90 days.
Those with AKI had worse 30-day (71%) and 90-day survival (67%) than those without AKI (91% and 82% respectively). More importantly, in the sub-group who fulfilled the criteria for AKI, those with peak serum creatinine 1.5 mg/dL or higher had a higher mortality at 30 days and 90-days (39% and 42% respectively) than those who had peak serum creatinine less than 1.5 mg/dL (22% and 27% respectively).
Although it has been suggested that AKIN staging is removed for people with cirrhosis, we looked at AKIN stages in our group. Of the 96 patients with AKI, 60 had stage 1a, 23 stage 1b, 7 stage 2 and 6 stage 3, and in-hospital mortality in these groups were 0%, 13.9%, 16.7 and 27.3%, respectively (P = 0.003).
Serum Creatinine and Survival
As shown in Table 3, 32 patients who fulfilled the criteria for AKI (serum creatinine increase by ≥0.3 mg/dL) had peak serum creatinine within the normal range (1.2 mg/dL or less). In this group of 32 patients, AKI did not have a significant impact on 30-day or 90-day survival. Similarly, increments of serum creatinine of 0.1 mg/dL or more (n = 80) or 0.2 mg/dL or more (n = 45) did not have an impact on survival if the peak is within the normal ranges (less than 1.2 mg/dL). However, any increase in serum creatinine levels after admission (peak 48 h—admission creatinine) had an impact on 30-day and 90-day survival provided the peak serum creatinine level was above the normal range (1.2 mg/dL or higher). With a cut-off of +0.3 mg/dL, 30-day and 90-day survival was 69% and 63% for those with +0.3 mg/dL or above compared to 89% and 79%, respectively, for those less than 0.3 mg/dL.
Table 3.
Effect of Creatinine Spikes on Survival, Using Data From All Admissions (n = 636 Admissions).
| 30-Day survival % |
90-Day survival % |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cut-off, Peak 48 h—Adm | Peak <1.2 mg/dL | P | Peak 1.2+ mg/dL | P | Peak <1.2 mg/dL | P | Peak 1.2+ mg/dL | P |
| +0.1 mg/dL | 0.52 | <0.01 | 0.71 | 0.01 | ||||
| Less than +0.1 | 95% (193/204) | 90% (168/187) | 87% (173/200) | 80% (142/178) | ||||
| At least +0.1 | 93% (74/80) | 72% (106/148) | 85% (66/78) | 66% (96/146) | ||||
| +0.2 mg/dL | 0.12 | <0.01 | 0.33 | <0.01 | ||||
| Less than +0.2 | 95% (227/239) | 89% (185/208) | 87% (203/234) | 79% (158/199) | ||||
| At least +0.2 | 89% (40/45) | 70% (89/127) | 82% (36/44) | 64% (80/125) | ||||
| +0.3 mg/dL | 0.11 | <0.01 | 0.71 | <0.01 | ||||
| Less than +0.3 | 95% (239/252) | 89% (194/219) | 86% (213/247) | 79% (166/209) | ||||
| At least +0.3 | 88% (28/32) | 69% (80/116) | 84% (26/31) | 63% (72/115) | ||||
| +0.4 mg/dL | 1a | 0.02 | 1a | 0.06 | ||||
| Less than +0.4 | 94% (263/280) | 84% (234/279) | 86% (235/274) | 75% (202/269) | ||||
| At least +0.4 | 100% (4/4) | 71% (40/56) | 100% (4/4) | 65% (36/55) | ||||
| +0.5 mg/dL | 1a | 0.01 | 1a | 0.02 | ||||
| Less than +0.5 | 94% (266/283) | 84% (255/305) | 86% (238/277) | 75% (222/295) | ||||
| At least +0.5 | 100% (1/1) | 63% (19/30) | 100% (1/1) | 55% (16/29) | ||||
Peak 48 h—Adm: peak creatinine within 48 hours of admission, minus admission level.
P-values from GEE, except abinomial exact test.
Of 636 admission, 619 admissions had adequate data and occurred within prescribed time frame for analysis.
Those with AKI had a longer hospital stay than those without AKI (10.2 ± 7.6 vs. 5.4 ± 5.2, P < 0.01). In those with AKI, duration of AKI was higher in those who died when compared to those who survived at every time point (in-hospital duration of AKI 6.1 vs. 1.8 days).
Cox Regression Analysis
The Cox regression analysis combined all survival outcomes and data from all visits into a single, continuous summary measure, and allowed adjustment for covariates (Table 4). In this analysis, race and sex were not significant predictors of survival. Age at baseline (2% hazard-of-death per year older), INR (3% increase per +0.1increase above 1) and hyponatremia (5% for −1 mEq/L below 135 mEq/L) were independent predictors.
Table 4.
Survival Analysis—Cox Proportional Hazards Model, Showing Adjusted Hazard Ratios of Death (n = 322 Patients).
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Cr Peak 48 h | ||
| <1.2 mg/dL, increment +0 to 0.29 | Ref. | |
| <1.2 mg/dL, increment +0.3+ | 0.79 (0.24–2.60) | 0.70 |
| 1.2+ mg/dL, increment +0 to 0.29 | 1.37 (0.91–2.08) | 0.13 |
| 1.2+ mg/dL, increment +0.3+ | 2.51 (1.65–3.81) | <0.01a |
| INR (per +0.1 above 1) | 1.03 (1.00–1.05) | 0.04 |
| TBILI (per +1 mg/dL above 1) | 1.02 (0.99–1.05) | 0.14 |
| Na (per −1 mEq/L below 135) | 1.05 (1.01–1.09) | 0.02 |
| MAP | ||
| Normal (70+ mmHg) | Ref. | |
| Abnormal (<70 mmHg) | 1.37 (0.89–2.11) | 0.16 |
| Reason for admission | ||
| 6-Ref. Asc. | Ref. | |
| 1-Enceph. | 1.98 (1.20–3.27) | 0.01 |
| 7-SBP | 2.61 (1.36–5.03) | <0.01 |
| 8-Sepsis | 1.45 (0.82–2.58) | 0.20 |
| 9-GI bleed | 0.90 (0.50–1.62) | 0.73 |
| Age (per +1 year) | 1.02 (1.00–1.04) | 0.02 |
| Race | ||
| Black or other | Ref. | |
| White | 1.31 (0.93–1.85) | 0.13 |
| Sex | ||
| Female | Ref. | |
| Male | 1.29 (0.93–1.82) | 0.13 |
Hazard ratio 1.83 (95% CI 1.24–2.70) (P < 0.01) compared to “1.2+ mg/dL, increment +0 to 0.29″. Also, Hazard ratio 3.16 (95% CI 0.96–10.48) (P = 0.06) compared to “<1.2 mg/dL, increment +0.3+”.
Interpretations:
Those with a high peak (1.2+) and high increment (+0.3+) had significantly worse survival (151% worse) than those with a low peak (<1.2) and low increment (<+0.3).
Those with a high peak and high increment also had significantly worse survival (83% worse) than those with a high peak (1.2+) and low increment (<+0.3).
These hazard ratios take into account all other variables in the table.
INR: international normalized ratio; TBILI: total bilirubin; Na: sodium; MAP: mean arterial pressure.
Single Cox regression model, adjusted for all variables in the table.
Data from 607 admissions were used. Deaths were recorded for 158 patients in this sample.
Patients with a 48-h peak creatinine of at least 1.2 mg/dL and an incremental increase of at least +0.3 mg/dL had a 2.51 times higher hazard of death (95% confidence interval 1.65–3.81) compared to those with a lower peak and increment. If the peak 48-h creatinine was less than 1.2 mg/dL, increments of serum creatinine of 0.3 mg/dL or more did not have a significant independent effect on survival. However, the spike in creatinine of 0.3 mg/dL or more was an important predictor of survival if 48-h peak was 1.2 mg/dL and moreover, in that group, a difference of at least 0.3 mg/dL from their admission creatinine level to their 48-hour peak had 1.83 times worse survival (95% CI 1.24–2.70) compared to those with a smaller spikes (0–0.29 mg/dL) (see footnotes of Table 4).
Reason for admission persisted as a predictor of survival in the Cox models. Patients admitted for encephalopathy, SBP, or sepsis all had worse survival outcomes than did patients admitted with refractory ascites or a gastrointestinal bleeding.
The etiology of cirrhosis, use of proton pump inhibitors, beta blockers or rifaximin had no impact on survival. Similarly, use of proton pump inhibitors was not associated with an increased risk of sepsis or SBP.
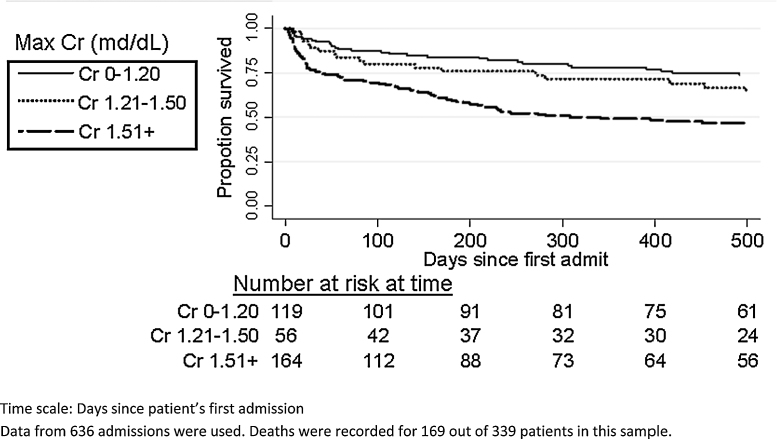
Figure 1 shows Kaplan–Meier survival stratified by maximum serum creatinine ever recorded during the entire study period. The conventional definition of serum creatinine for the diagnosis of type 2 hepatorenal syndrome is still relevant as those who had peak creatinine above 1.5 mg had the worst outcomes. The worst outcomes (Table 5) were in the group with serum creatinine above 1.50 mg/dL (hazard ratio 2.27, 95% CI 1.58–3.25, compared to those with a maximum creatinine of 1.20 mg/dL or lower).
Figure 1.
Kaplan–Meier survival showing differences in survival based on maximum serum creatinine recorded during the entire study period.
Table 5.
Hazard of Death, by Maximum Creatinine (n = 339 Patients).
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Max Cr during admission | ||
| 0.7–1.20 mg/dL | Ref. | |
| 1.21–1.50 mg/dL | 1.57 (0.96–2.54) | 0.07 |
| 1.51+ mg/dL | 2.27 (1.58–3.25) | <0.01 |
Note: The 1.21–1.50 and 1.51+ groups are not significantly different from each other (P = 0.09).
Data from 636 admissions were used. Deaths were recorded for 169 out of 339 patients in this sample.
Discussion
In this study, we have shown that hospitalized patients with complications of cirrhosis have a very high mortality, and the mortality is dependent on the age, the severity of liver disease, reason for hospitalization, hyponatremia and AKI. Our study showed that any increment above 1.2 mg/dL is associated with a higher mortality, but increments within the normal ranges of serum creatinine (less than 1.2 mg/dL) had no impact on survival. The International Ascites Club and the Acute Dialysis Quality Initiative (ADQI) group had proposed that acute kidney injury (AKI) in cirrhosis should be redefined as an increase in serum creatinine level of 0.3 mg/dL in less than 48 h or a 50% increase in serum creatinine level from a stable baseline (pre-admission) reading within the previous 6 months, irrespective of the final serum creatinine level. Our study suggests an increase in serum creatinine from admission creatinine is significant only if the peak is above 1.2 mg/dL (or upper limit of normal range).
One major limitation of the AKI definition proposed by the International Ascites Club and ADQI is the need for establishing the change in serum creatinine from the baseline (pre-admission). For patients being hospitalized with complications of cirrhosis, the pre-admission creatinine may not always be easily determined (as we found in our analysis) leading to inaccurate classification of AKI. An important aspect of the original definition of AKI by AKIN is the ‘abrupt’ increase in creatinine within 48 h, and therefore, a model that predicts mortality based on changes in serum creatinine from the admission creatinine may have more practical and clinical utility.
To date, there has been no consensus on the definition of ‘baseline’ creatinine. Although AKI is defined as abrupt increase of serum creatinine within 48 h, previous studies had used pre-admission serum creatinine ranging from 90 to 365 days prior to hospitalization as ‘baseline creatinine.16, 17, 27, 28, 29 The International Ascites Club and ADQI defined ‘baseline’ as stable serum creatinine within the previous 6 months.16 Another study used the most recent stable creatinine measurement within 12 months prior to the index hospitalization as ‘baseline’ creatinine, but if the patient had at least 5 days of stable values within the normal creatinine range following admission, admission creatinine was used as ‘baseline’.19 In a recent study, ‘baseline’ creatinine was the admission creatinine value except for those who had an elevated creatinine level on admission when serum creatinine within the 3 months prior to hospitalization was used as ‘baseline’.25 Another large study used the average of serum creatinine measurements between 7 and 90 days before admission as ‘baseline’, but if serum creatinine levels were not available during that period (7–90 days), any measurement within 12 months prior to hospitalization was used as ‘baseline.28 It is conceivable that some of the patients diagnosed as AKI in previous studies had pre-existing type 2 hepatorenal syndrome, and do not truly represent AKI. However, the disadvantage of using admission creatinine as ‘baseline’ is that it does not take into consideration the possibility the renal changes may have preceded the hospitalization.27 For the sake of consistency and reproducibility, we believe that it is prudent to use admission serum creatinine as ‘baseline’ for future studies.
Our study found that a difference between admission creatinine and creatinine at 48 h of ≥0.1 had significantly higher mortality at all time points compared to those with less than 0.1 mg/dL provided the peak is above 1·2 mg/dL (Table 3). Highest mortality was seen when change in creatinine level was 0·3 mg/dL or more from the admission creatinine level. Although smaller increments in serum creatinine may fall well within the laboratory variations, it is perhaps reasonable to optimize the fluid management and supportive care to all patients with cirrhosis who show a serum increase of 0.1 mg/dL or higher provided the peak within 48 h is above the normal range. Our findings differ from that of Fagundes et al., who found similar 90-day survival in patients with AKI when serum creatinine ≤1.5 and those without AKI.28 We found that mortality is higher in patients with AKI if peak creatinine values within 48 h after admission is ≥1.2 mg/dL, but not if peak creatinine values are <1.2 mg/dL. However, mortality was higher when peak serum creatinine was higher than 1.5 mg/dL when compared to those with peak creatinine less than 1.5 mg/dL confirming step-wise increase in mortality with higher creatinine levels.
As in previous studies, we showed that there are many independent predictors beside serum creatinine increases that determine mortality in hospitalized patients with cirrhosis. In our study, hyponatremia, defined as serum sodium less than 135 mg Eq/L, was an independent predictor of mortality.30, 31, 32, 33, 34 Every 1 mEq/L decrease in serum sodium was associated with a 5% increase in mortality. Similarly, mean arterial pressure was lower in those who died (Table 2). These associations, along with serum creatinine changes, suggest that early alteration of systemic hemodynamics may be predisposing patients with portal hypertension to higher complications and mortality. Early and effective interventions during the immediate hospitalization period may improve survival, and clinical studies need to be conducted to determine whether it is possible using currently available treatments (such as albumin infusion, terlipressin etc.) or newer modalities. It is also equally possible that the ‘irreversible’ damage occurred prior to hospitalization, and we may not be able to change the natural history despite the optimal care. In our institution, we had standardized the treatment based on current guidelines and management of cirrhosis, and during the study period, there has been no change in the treatment strategy. Since terlipressin is not approved in the USA, our patients were not treated with terlipressin. We believe that more aggressive hemodynamic interventions for minimal increase in serum creatinine from admission creatinine should be tested in controlled trials.
In univariate analysis, we found that Whites had a higher mortality than African Americans. Although this disappeared when adjusted for other co-variates, it is reassuring to find that African Americans, very well represented in our study (37%), had similar survival as compared with White patients. Contrary to prior studies,13 we found that pre-hospital gastric acid suppressant use did not have a statistically significant association between mortality and those hospitalized with infectious (SBP and sepsis) and non-infectious complications of cirrhosis. Similarly, we did not find an improvement is survival in those who were on long-term rifaxamin as reported previously in a study involving patients with alcohol-related decompensated cirrhosis.15 Contrary to prior studies,14, 35 the use of beta-blockers did not have an impact on survival including those who were admitted with refractory ascites.
In addition to corroborating the findings of previous studies in sub-group of patients (infections, ascites or intensive care unit settings) with complications of cirrhosis, we have shown that the increased mortality associated with serum creatinine increments after admission is applicable to all hospitalized patients with cirrhosis irrespective for the reason for admission.36, 37, 38, 39, 40, 41, 42, 43, 44, 45
As one might expect that those with AKI required lengthier hospital stays than those who did not develop AKI. We did not determine the hospital costs associated with the increased hospital stay, but we would expect that the costs will be significantly higher in those who develop AKI. One limitation of our study was that it is a single center study, but it is also one of the strengths since the management was more standardized. We did not use potentially more sensitive markers of reduced glomerular filtration rate such as cystatin-C46 or NGAL47 in our study. Despite some of these limitations, our findings further establish that AKI has a significant effect on outcomes of all patients who are hospitalized, irrespective of the reason for admission. Moreover, we have clearly shown that any incremental change in serum creatinine within 48-h relative to the admission serum creatinine is significant provided the peak serum creatinine within 48 h after admission is above the normal range.
Author Contributions
Anantha Nuthalapati—study concept, data collection and entry, analysis of data and writing the manuscript; Nicholas Schluterman—data analysis and review of manuscript; Anuj Khanna—data collection and entry, review of manuscript; Deborah Greenberg—data analysis and review of manuscript; Paul J. Thuluvath—study concept, data analysis, writing and review of the manuscript.
Conflicts of Interest
The authors have none to declare.
References
- 1.Asrani S.K., Larson J., Yawn B., Therneau T., Kim R. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375–382. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim W.R., Brown R.S., Jr., Terrault N.A., El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36(July (1)):227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Ginés P., Quintero E., Arroyo V. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(January–February (1)):122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 5.Singal A.K., Salameh H., Kamath P.S. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalized patients in the United States. Aliment Pharmacol Ther. 2014;40(July (1)):105–112. doi: 10.1111/apt.12797. [DOI] [PubMed] [Google Scholar]
- 6.Mellinger J.L., Richardson C.R., Mathur A.K. Variation among US hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.09.038. pii:S1542-3565(14)01387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand F., Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(suppl 1):S100–S107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Botta F., Giannini E., Romagnoli P. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52(January (1)):134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degre D., Bourgeois N., Boon N. Aminopyrine breath test compared to the MELD and Child-Pugh scores for predicting mortality among cirrhotic patients awaiting liver transplantation. Transpl Int. 2004;17(January (1)):31–38. doi: 10.1007/s00147-003-0655-6. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(January (1)):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Serste T., Gustot T., Rautou P.E. Severe hyponatremia is a better predictor of mortality than MELD-Na in patients with cirrhosis and refractory ascites. J Hepatol. 2012;57(August (2)):274–280. doi: 10.1016/j.jhep.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj J.S., Ananthakrishnan A.N., Hafeezullah M. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: a national and tertiary center perspective. Am J Gastroenterol. 2010;105(January (1)):106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj J.S., Zadvornova Y., Heuman D.M. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104(May (5)):1130–1134. doi: 10.1038/ajg.2009.80. [DOI] [PubMed] [Google Scholar]
- 14.Sersté T., Melot C., Francoz C. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52(September (3)):1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 15.Vlachogiannakos J., Viazis N., Vasianopoulou P. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(March (3)):450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 16.Mehta R.L., Kellum J.A., Shah S.V. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong F., Nadim M.K., Kellum J.A. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60(May (5)):702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 18.Orlando R., Floreani M., Padrini R. Evaluation of measured and calculated creatinine clearances as glomerular filtration markers in different stages of liver cirrhosis. Clin Nephrol. 1999;51(June (6)):341–347. [PubMed] [Google Scholar]
- 19.Sherman D.S., Fish D.N., Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41(February (2)):269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 20.Belcher J.M., Gracía-Tsao G., Sanyal A.J., TRIBE-AKI Consortium Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57(February (2)):753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsien C.D., Rabie R., Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62(January (1)):131–137. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho J.R., Villela-Nogueira C.A., Luiz R.R. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46(March (3)):e21–e26. doi: 10.1097/MCG.0b013e31822e8e12. [DOI] [PubMed] [Google Scholar]
- 23.Cholongitas E., Calvaruso V., Senzolo M. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24(October (10)):1639–1647. doi: 10.1111/j.1440-1746.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopes J.A., Melo M.J., Costa A.C. Acute kidney injury and in-hospital mortality in critically ill patients with cirrhosis: a cohort study. Gut. 2012;61(June (6)):955–956. doi: 10.1136/gutjnl-2011-301190. [DOI] [PubMed] [Google Scholar]
- 25.Wong F., O’Leary J.G., Reddy K.R. North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(December (6)) doi: 10.1053/j.gastro.2013.08.051. 1280-8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piano S., Rosi S., Maresio G. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59(September (3)):482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Siew E.D., Ikizler T.A., Matheny M.E. Estimated baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(May (5)):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagundes C., Barreto R., Guevara M. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59(September (3)):474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Scott R.A., Austin A.S., Kolhe N.V. Acute kidney injury is independently associated with death in patients with cirrhosis. Frontline Gastroenterol. 2013;4(July (3)):191–197. doi: 10.1136/flgastro-2012-100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuman D.M., Abou-Assi S.G., Habib A. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 31.Biggins S.W., Kim W.R., Terrault N.A. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Biggins S.W., Rodriguez H.J., Bacchetti P. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 33.Londono M.C., Cardenas A., Guevara M. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283–1290. doi: 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruf A.E., Kremers W.K., Chavez L.L. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 35.Ge P.S., Runyon B.A. The changing role of beta-blocker therapy in patients with cirrhosis. J Hepatol. 2014;60(March (3)):643–653. doi: 10.1016/j.jhep.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Ginès P., Schrier R.W. Renal failure in cirrhosis. N Engl J Med. 2009;361(September (13)):1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 37.Angeli P., Merkel C. Pathogenesis and management of hepatorenal syndrome in patients with cirrhosis. J Hepatol. 2008;48:S93–S103. doi: 10.1016/j.jhep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Moreau R., Lebrec D. The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology. 2006;43:385–394. doi: 10.1002/hep.21094. [DOI] [PubMed] [Google Scholar]
- 39.Ginès P., Guevara M., Arroyo V., Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 40.Llach J., Gines P., Arroyo V. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482–487. doi: 10.1016/0016-5085(88)90441-6. [DOI] [PubMed] [Google Scholar]
- 41.Fasolato S., Angeli P., Dallagnese L. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 42.Thabut D., Massard J., Gangloff A. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 43.Montoliu S., Balleste B., Planas R. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616–622. doi: 10.1016/j.cgh.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Fede G., D’Amico G., Arvaniti V. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810–818. doi: 10.1016/j.jhep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Llahi M., Guevara M., Torre A. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–496. doi: 10.1053/j.gastro.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 46.Gerbes A.L., Gülberg V., Bilzer M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50(January (1)):106–110. doi: 10.1136/gut.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerbes A.L., Benesic A., Vogeser M. Serum neutrophil gelatinase-associated lipocalin – a sensitive novel marker of renal impairment in liver cirrhosis? Digestion. 2011;84(1):82–83. doi: 10.1159/000324881. [DOI] [PubMed] [Google Scholar]