Abstract
Purpose of the review
The specialized microenvironments of lymphoid tissue affect immune cell function and progression of disease. However, current animal models and conventional 2D cultures either lack the accessibility or physiological relevance to study precise microenvironmental interactions. Thus, artificial tissues are being developed to study these interactions in the context of immune development, function, and disease.
Recent findings
New bone marrow and secondary lymph node models have been created to respectively study microenvironmental interactions in hematopoiesis and germinal center-like biology. These models have also been extended to understand the effect of these interactions on the progression and therapeutic response in leukemia, multiple myeloma, and lymphoma.
Summary
3D in vitro immune models have elucidated new cellular, biochemical, and biophysical interactions as potential regulatory mechanisms, therapeutic targets, or biomarkers that previously could not be studied in animal models and conventional 2D cultures. Incorporation of advanced biomaterials, genome editing, and single cell analysis tools will enable further studies of function, driver mutations, and tumor heterogeneity. Continual refinement will help inform the development of antibody and cell-based immunotherapeutics and patient-specific treatment plans.
Keywords: microenvironment, in vitro, secondary lymph node, bone marrow, B cell malignancies
INTRODUCTION
Immune cell function is highly dependent on communication with the surrounding microenvironment. Specialized microenvironments, such as primary lymphoid organs and secondary lymphoid tissues, efficiently inform immune cells to combat pathogens and repair tissue. Individual changes in the surrounding microenvironmental cellular components, soluble signals, extracellular matrix (ECM) composition, and stiffness have been shown to affect immune cell maturation, migration, and function. These interactions are crucial to homeostatic regulation, tolerance, and mounting adaptive immune responses, while their dysregulation can lead to the progression of disease and affect ensuing therapeutic responses.
The study of these specialized microenvironments and their individual components have primarily relied on regular and genetically engineered animal models. However, these models are limited by their inherent complexity, inaccessibility, cost, and restricted biological control. On the other hand, conventional 2D in vitro models afford needed biological control, but present an oversimplification of the natural microenvironment. To ultimately bridge the gap between the high-throughput, cost-effective nature of in vitro systems with the physiological relevance of in vivo models, the creation of artificial tissue models has garnered interest (Fig. 1).
Figure 1. The need for artificial tissue models.
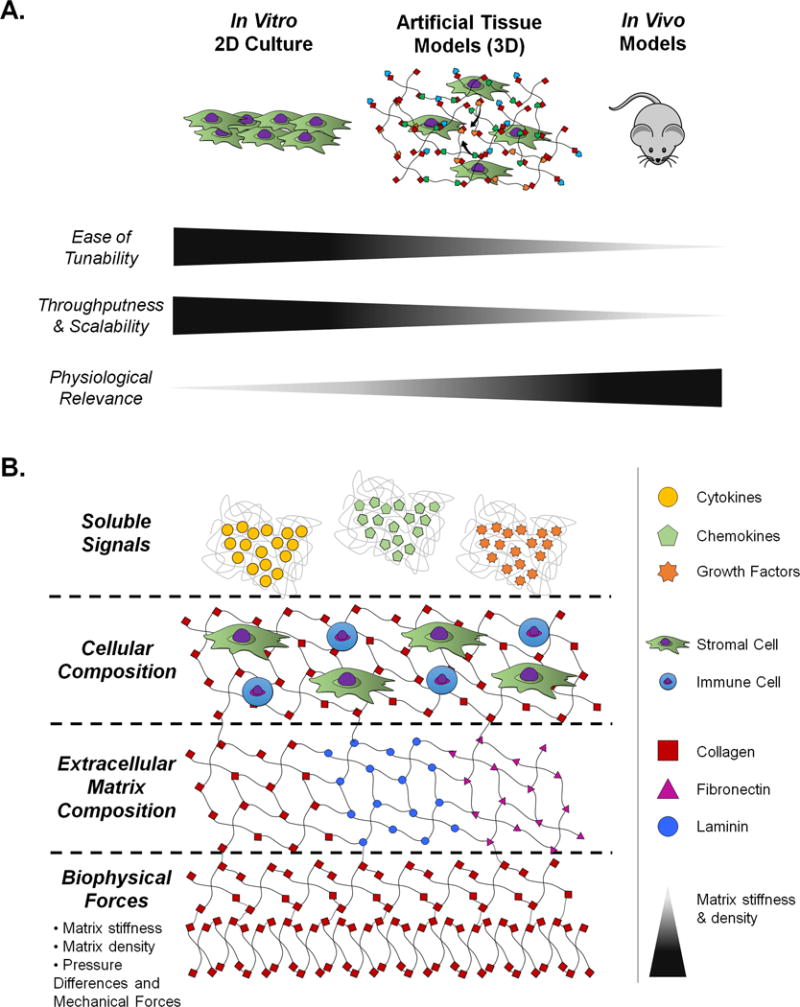
A.) Conventional in vitro 2D cell culture models and animal models are compared to artificial tissue models with respect to tunability, throughputness, scalability, and physiological relevance. Relative increases are indicated by increases in the triangle next to each category title. B.) The biological control afforded by artificial tissue models is summarized in terms of its primary tunable components. These components are divided into soluble signals, cellular composition, extracellular matrix composition, and biophysical forces.
In particular, immune-based microenvironments, including that of the bone marrow and secondary lymph node, are being developed to effectively culture primary immune cells for a better understanding of immune cell maturation and function. These models are also used to test the efficacy of immunomodulatory agents and inform the development of antibody and cell-based immunotherapeutics. In this review, we explore bone marrow and secondary lymph node models used to respectively study hematopoiesis and germinal center-like biology. We also discuss how these models are being extended to study the progression and therapeutic response of bone-marrow- and secondary lymph node-based hematological malignancies, including leukemia, multiple myeloma, and lymphoma.
BONE MARROW
For bone marrow models, the primary aim has been to elucidate how the microenvironment facilitates hematopoiesis. The hope is that this understanding will lead to increases in in vitro expansion yields, engraftment efficiencies, and overall long-term homeostasis of clinically relevant hematopoietic stem cell (HSC) populations for use in hematopoietic stem cell transplantation. Additional biomimetic models have been created to evaluate drug toxicity on the hematopoietic system.
Lately, these models have shifted from expanding HSCs in static 2D culture to biomaterial-based 3D cultures to provide greater spatiotemporal control of multiplexed cell, biophysical, and biomolecular signals reminiscent of the native environment. In the native microenvironment, putative quiescent HSCs localize close to the endosteal bone surface, while actively cycling HSCs are found near the sinusoidal endothelium. Although these niches are close in proximity and may even overlap, decreasing gradients of calcium and matrix stiffness along with increases in CXCL12 expression have been found between them. The endosteam is also associated with high levels of fibronectin as opposed to high levels of laminin in the perivascular space [1,2].
In modeling these differences, initial 3D culture studies have primarily focused on comparing material composition, mechanical properties, architecture, topology, and overall culture conditions for increased yields of HSC ex vivo [3–9]. Several elegant reviews have summarized the findings for each model and future directions to better understand the native endosteal or perivascular niche[2,10].
To better design systems for increasing HSC yield, Choi and Harley studied the effect of marrow-inspired matrix cues and biophysical signals on HSC fate decisions. They coated polyacrylamide surfaces with either type I collagen, laminin, or fibronectin of varying stiffness. Within 24 hours, matrix ligand type and stiffness regulated HSC morphology, proliferation, and myeloid lineage specification based on integrin engagement and the myosin II activation processes. In particular, high fibronectin content with greater stiffness maintained HSC stemness, most likely via α5β1 integrin activation, while high laminin content promoted myeloid specification, especially that of the erythrocyte lineage when high intracellular tension was induced. These findings mirror the biophysical properties and expected biological outcomes present in native endosteal and perivascular niche and dictate the explicit role matrix ligands play in regulating HSC fate **[11]. Building upon this finding for increased HSC yields, fibronectin presentation can be combined with spatiotemporal control of biophysical signals by using photolabile and photoactivatable crosslinkers to either soften or harden specific matrix locales. Multiplexed biochemical gradients, including that of CXCL12, can also be added with the use of dynamic flow-based approaches, such as microfluidic devices. Further incorporation of downstream single-cell analysis tools could be used to decode the nuances of heterogeneous HSC responses in both regulation and function [12,13].
Instead of recreating the entire niche, researchers have also focused on modeling certain features of the bone marrow niche for the clinical expansion of HSC-derived blood and immune cells. Di Buduo et al. modeled the walls of venous sinusoids for platelet formation. They created programmable silk scaffolds that supported endothelial cells and megakaryocyte growth on opposite sites, and then modulated the surface topography, stiffness, extracellular matrix composition, and external shear forces to form millions of functional human platelets **[14]. This elaborate system not only enables the study of megakaryopoiesis but also allows for the expansion of clinically translatable human platelets.
Coincident efforts have also similarly extended these models to create biomimetic niche-like analogs to evaluate the drug toxicity on HSC populations. Torisawa and colleagues created a “bone-marrow-on-chip” platform by first implanting a collagen scaffold infused with demineralized bone powder, BMP2, and BMP4 into mice to form bone in vivo, and then harvesting the bone and perfusing it with a microfluidic device for long-term culture [15]. The ensuing engineered bone marrow displayed radiation injury responses similar to those found in native marrow. They also recently extended this platform to more closely model hematopoiesis by maintaining HSCs and forming blood cells for 2 weeks in culture. This platform responded positively to possible radiation countermeasure drugs, granulocyte-colony stimulating factor or bactericidal/permeability-increasing protein, post radiation injury *[16]. However, to allow for translation of these preclinical findings from bench to bedside, differences between mice and human in morphological and functional niche features and cellular biology must be considered. Decellularization approaches combined with the use of human induced pluripotent stem cells can be a step forward into furthering preparing this platform for clinical translation.
SECONDARY LYMPH NODES
The microenvironment of secondary lymph nodes consists of spatially patterned ECM and stromal matrix of follicular dendritic and reticular cells to enable the migration of B, T, and dendritic cells [17]. These dynamic migration processes facilitate communication between the outer sampling zone, B cell activation, and T cell activation zones during adaptive immune responses [18]. These migration processes, especially that of T cells, have previously been studied using 3D scaffolds [19]. However, current models of the secondary lymph node are primarily focused on understanding germinal center (GC) physiology and pathophysiology to inform the creation of antibody-based therapeutics. As a post-developmental process, GCs are highly affected by cellular-microenvironmental interactions. GC B cells also naturally must be rescued by anti-apoptotic signals, further limiting their expansion ex vivo with 2D cultures. Slight improvements in 2D culture were made when Nojima et al. co-cultured naïve B cells with “40LB” cells, their genetically modified stromal cell line engineered to present CD40L and BAFF [20]. Although activated B cells were reportedly formed, memory B cell formation was dependent on transplantation into mice.
Our group has therefore aimed to create GCs ex vivo by emulating the lymphoid microenvironment. Purwada et al. first engineered gelatin hydrogels reinforced with polyionic silicate nanoparticles to encapsulate murine naïve B cells and 40LB cells in the presence of exogenous IL-4. The silicate nanoparticles enabled us to mirror the stiffness, porosity, and architecture of native lymphoid tissue, while RGD motifs present in the gelatin also mimicked native ECM. By imitating the lymphoid microenvironment, these “immune organoids” produced ~100 fold increase in GC B cells when compared to 2D cultures and induced antibody class switching. They also found that proliferation of GC B cells were dependent on the interaction between B cell integrin αvβ3 and RGD-motifs in the gelatin [21,**,22]. To investigate further integrin-ECM interactions, we encapsulated naïve B and 40LB cells in immune organoids composed of 4-arm polyethylene glycol (PEG-MAL) conjugated with ligands specific to the integrin αvβ3 or α4β1. These integrins were chosen as αvβ3 expression changes throughout the GC, while the expression level of α4β1 remains constant. When compared to αvβ3-specific organoids, α4β1-specific organoids resulted in a greater number of early GC-like B cells in a ligand concentration-dependent manner. Simultaneous presentations of both ligands paralleled results found in αvβ3-specific organoids, suggesting a regulatory role for αvβ3 in the B cell niche **[23]. This mechanistic understanding will instruct future biomaterial model design to produce long-lived, high affinity activated B cells and antibody-based therapeutics.
BONE MARROW MALIGNANCIES
Crosstalk between the microenvironment and malignant cells often regulates the initiation, progression, and ensuing therapeutic response of disease. The pathophysiology is often tied to dysregulation of microenvironmental interactions. Therefore, understanding and modeling these changes can significantly inform development of personalized, microenvironment-based therapies and help overcome environment-mediated and acquired resistance to current therapeutics.
Within the bone marrow niche, there is particular interest in modeling leukemia and multiple myeloma. In the case of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML), changes in interactions with the bone marrow stromal microenvironment has been shown to promote leukemic cell survival and affect therapeutic response[24]. These interactions have also been implicated in immunosuppression and reduced T cell activation, thereby minimizing the efficacy of T cell redirection therapies [25]. Therapeutics can also alter the stromal microenvironment after treatment, potentially changing the progression of disease and mediating environment-mediated resistance [26]. Microenvironment-induced increased vascular permeability and PIM kinase expression have also been noted in AML and CLL, respectively [27–29]. Recently, a 3D microfluidic ALL cell culture platform was created to study how changes in the microenvironment affected drug response. Primary human bone marrow stromal cells, leukemic cells, and osteoblasts were encapsulated in a 3D collagen gel and then exposed to physiological flow. 2D-static, 3D-static, and 3D-flow cultures were exposed to cytarabine, an antimetabolite chemotherapeutic agent. Decreased sensitivity in the 3D models alluded to the microenvironment’s protective role for tumor cell survival *[30].
For multiple myeloma (MM), interactions with the microenvironment are tied to disease progression and relapse due to its pro-survival effect on reservoirs of residual MM cells. Distinguishing characteristics of the pro-survival microenvironment, including cell membrane protein Junctional adhesion molecule-A (JAM-A), high expression of transmembrane receptor Roundabout 1 (ROBO1), and absolute lymphocyte/monocyte ratio, can serve as clinical biomarkers and potential therapeutic targets [31–33]. To identify additional potential targets in MM cells induced by the microenvironment, Meads et al. recently conducted activity-based protein profiling along with a high-throughput protein kinase inhibitor (PKI) screen on MM patient specimens. This screen resulted in 8 PKIs that showed significant activity, and it displayed how this method can pinpoint changes in tumor cells in situ to help overcome therapy-induced resistance [34]. Microenvironmental changes in MM have also been recently studied using 3D scaffolds derived from the bone marrow of MM patients. Fibrinogen present in the plasma of the supernatant was cross-linked with calcium and encapsulated the patient’s MM cells, stromal cells, and endothelial cells. These models mimicked native MM growth, interactions with the microenvironment, and biochemical gradients, including that of oxygen and drug availability. They also induced greater drug resistance in comparison to conventional 2D and commercial 3D cultures. Therefore, these cultures can further our understanding of the pathophysiology of MM and have the potential to inform personalized therapeutic plans **[35].
SECONDARY LYMPH NODE MALIGNANCIES
Models of the secondary lymph node have been extended to elucidate the relationship between the microenvironment and lymphoma – lymphoproliferative cancer of B and T cells. Initial lymphoma cellular aggregates alluded to the importance of 3D culture as the expression level of 7,000 genes and therapeutic response to chemo- and antibody-based therapeutics differed between 3D and 2D cultures [36–38]. In comparison to 2D cultures, virally infected lymphoma aggregates grown in 3D microwells also displayed a larger viral genome copy number and higher rate of lytic reactivation. [39] While these multicellular aggregates are informative, they do not account for the effect of cell-cell interactions, cellular-ECM interactions, and tumor heterogeneity.
Mannino et al. recently concentrated on cell-cell communication between endothelial and lymphoma cells. They created a “lymphoma-on-chip” model by encapsulating tumor cells in a hyaluronic acid-based hydrogel that was traversed by a perfusable, fully endothelized microchannel. Tumor cells increased endothelial cell permeability and responded to anti-CSF-1R antibodies with expected macrophage death. This chip also enabled cells to be extracted for mechanistic studies. With additional modifications, distinct microenvironments can be connected to understand the downstream effects of tumor cells and therapeutics [40].
This chip is a promising initial step to inform drug delivery within tumor vasculature. However, for clinical translation, intra-tumor changes within matrix density, composition, and even stiffness and their effect on vascular outgrowth, branching, and integrity must be modeled and considered [41].
Within cellular-ECM interactions, integrin-mediated crosstalk between lymphoma cells and the ECM affects survival and chemo-resistance [42,43]. Inhibition of integrin αvβ3, in particular, decreased NF-κB mediated VEGFA and VEGFB upregulation and induced lymphoma cell death in vitro and in patient-derived xenograft mouse models [43]. To further understand integrin signaling within lymphomas, Tian et al. used the aforementioned integrin ligand-specific PEGMAL system to encapsulate either malignant B or T lymphoma cells with follicular dendritic cells **[44]. Through these “lymphoma organoids”, B and T cell lymphomas were found to have different integrin-signaling requirements for survival. When compared to 2D cultures, these 3D organoids also displayed enhanced proliferation, clustering, and drug resistance to common chemotherapeutics and Pabinostat, an epigenetic histone deacetylase inhibitor. In diffuse large B cell lymphoma cell lines, integrin ligand presentation upregulated B cell receptor (BCR) expression, plausibly explaining the enhanced survival.
Lymphoma tumors heterogeneity within cell and ECM composition is further complicated by biophysical gradients. Kiran et al. focused on the heterogeneity of biophysical forces in the tumor microenvironment by using the previously described gelatin system. They found that lymphoma survival, proliferation, drug response, and BCR expression are affected by stiffness in a molecular subtype dependent manner **[45].
The changes in drug resistance, drug delivery, and gene expression within these three models seem to better capture the heterogeneity of lymphoma tumors and show potential for uses in drug screening and development.
CONCLUSION
Overall, the creation of 3D has identified key microenvironment interactions in immune function, development, and disease that previously could not be studied in animal models and conventional 2D cultures. Incorporation of advancement biomaterials will improve the reproducibility and functional of these model for additional mechanistic understanding [46]. Future work should consider including patient-derived immune cells to enable formation of an immune synapse. Similarly, the use of genome editing and single-cell analysis tools will also answer questions regarding driver genetic and epigenetic mutations as well as tumor heterogeneity. Continual refinement will enable the identification of potential therapeutic targets and biomarkers along with informing the development of antibody and cell-based immunotherapeutics and patient-specific treatment plans.
KEY POINTS.
-
-
Bone marrow and secondary lymph node platforms have been developed to study microenvironmental interactions in hematopoiesis and germinal center-like biology.
-
-
Dysregulation of these interactions have been noted and modeled for leukemia, multiple myeloma, and lymphoma.
-
-
Advances in biomaterials, genome editing, and single-cell analysis technologies will further improve the reproducibility and functionality of these models.
Acknowledgments
None.
Financial support and sponsorship
The authors acknowledge financial support from the National Institute of Health/National Cancer Institute’s Innovative Molecular Analysis Technologies (IMAT) program (1R33CA212968-01) and academic scholarship support from the U.S. Department of Education’s Graduate Assistance in National Need Fellowship. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflicts of Inerest
Both authors declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
- 1.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi JS, Mahadik BP, Harley BA. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol J. 2015;10(10):1529–1545. doi: 10.1002/biot.201400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortera-Blanco T, Mantalaris A, Bismarck A, Aqel N, Panoskaltsis N. Long-term cytokine-free expansion of cord blood mononuclear cells in three-dimensional scaffolds. Biomaterials. 2011;32(35):9263–9270. doi: 10.1016/j.biomaterials.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 4.Leisten I, Kramann R, Ventura Ferreira MS, Bovi M, Neuss S, Ziegler P, Wagner W, Knuchel R, Schneider RK. 3d co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials. 2012;33(6):1736–1747. doi: 10.1016/j.biomaterials.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira MS, Jahnen-Dechent W, Labude N, Bovi M, Hieronymus T, Zenke M, Schneider RK, Neuss S. Cord blood-hematopoietic stem cell expansion in 3d fibrin scaffolds with stromal support. Biomaterials. 2012;33(29):6987–6997. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Raic A, Rodling L, Kalbacher H, Lee-Thedieck C. Biomimetic macroporous peg hydrogels as 3d scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35(3):929–940. doi: 10.1016/j.biomaterials.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Nichols JE, Cortiella J, Lee J, Niles JA, Cuddihy M, Wang S, Bielitzki J, Cantu A, Mlcak R, Valdivia E, Yancy R, et al. In vitro analog of human bone marrow from 3d scaffolds with biomimetic inverted colloidal crystal geometry. Biomaterials. 2009;30(6):1071–1079. doi: 10.1016/j.biomaterials.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyoshi H, Murao M, Ohshima N, Tun T. Three-dimensional culture of mouse bone marrow cells within a porous polymer scaffold: Effects of oxygen concentration and stromal layer on expansion of haematopoietic progenitor cells. J Tissue Eng Regen Med. 2011;5(2):112–118. doi: 10.1002/term.295. [DOI] [PubMed] [Google Scholar]
- 9.Braccini A, Wendt D, Jaquiery C, Jakob M, Heberer M, Kenins L, Wodnar-Filipowicz A, Quarto R, Martin I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23(8):1066–1072. doi: 10.1634/stemcells.2005-0002. [DOI] [PubMed] [Google Scholar]
- 10.Di Maggio N, Piccinini E, Jaworski M, Trumpp A, Wendt DJ, Martin I. Toward modeling the bone marrow niche using scaffold-based 3d culture systems. Biomaterials. 2011;32(2):321–329. doi: 10.1016/j.biomaterials.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 11**.Choi JS, Harley BAC. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Science Advances. 2017;3(1) doi: 10.1126/sciadv.1600455. Marrow-inspired matrix stiffness and ligand type regulate early HSC specification. Fibronectin with physiological stiffness promoted HSC maintenance and can be incorporated in future systems for HSC expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadik BP, Wheeler TD, Skertich LJ, Kenis PJ, Harley BA. Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv Healthc Mater. 2014;3(3):449–458. doi: 10.1002/adhm.201300263. [DOI] [PubMed] [Google Scholar]
- 13.Lecault V, Vaninsberghe M, Sekulovic S, Knapp DJ, Wohrer S, Bowden W, Viel F, McLaughlin T, Jarandehei A, Miller M, Falconnet D, et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat Methods. 2011;8(7):581–586. doi: 10.1038/nmeth.1614. [DOI] [PubMed] [Google Scholar]
- 14**.Di Buduo CA, Wray LS, Tozzi L. Alessandro Malara, 2 Ying Chen,Programmable 3d silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood. 2015;125(14) doi: 10.1182/blood-2014-08-595561. By modeling the walls of venous sinusoids using programmable silk scaffolds, the authors created a method to study megakaryopoiesis to expand human platelets for clinical translation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11(6):663–669. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 16*.Torisawa YS, Mammoto T, Jiang E, Jiang A, Mammoto A, Watters AL, Bahinski A, Ingber DE. Modeling hematopoiesis and responses to radiation countermeasures in a bone marrow-on-a-chip. Tissue Eng Part C Methods. 2016;22(5):509–515. doi: 10.1089/ten.TEC.2015.0507. This “bone-marrow-on-chip” platform enables the study of drug toxicity and countermeasure therapeutics on the hematopoietic system. [DOI] [PubMed] [Google Scholar]
- 17.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50 doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 18.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachowiak AN, Irvine DJ. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J Biomed Mater Res A. 2008;85(3):815–828. doi: 10.1002/jbm.a.31661. [DOI] [PubMed] [Google Scholar]
- 20.Nojima T, Haniuda K, Moutai T, Matsudaira M, Mizokawa S, Shiratori I, Azuma T, Kitamura D. In-vitro derived germinal centre b cells differentially generate memory b or plasma cells in vivo. Nat Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 21.Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, Cerchietti L, Singh A. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. doi: 10.1016/j.biomaterials.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of b-cell development and antibody production. Nat Protoc. 2017;12(1):168–182. doi: 10.1038/nprot.2016.157. This article presents a method to create 3D immune organoids for the study and modulation germinal center dynamics ex vivo. This system enables regulation of environment’s microarchitecture, mechanical forces, and biochemical composition to affect the magnitude and rate of the germinal center response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Purwada A, Shah SB, Melnick A, Singh A. Modular immune organoids with integrin ligand specificity differentially regulate ex vivo b cell activation. ACS Biomaterials and Engineering. 2016 doi: 10.1021/acsbiomaterials.6b00474. Differential integrin ligand presentation was found to affect the initiation of early-GC like phenotype, with α4β1 inducing a robust response and αvβ3 possibly playing a regulatory role in the niche. This study presents how specific biochemical cues can alter antibody production. [DOI] [PubMed] [Google Scholar]
- 24.Sison EA, Brown P. The bone marrow microenvironment and leukemia: Biology and therapeutic targeting. Expert Rev Hematol. 2011;4(3):271–283. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair-Gupta P, Rudnick S, Luistro L, Chin D, Smith M, McDaid R, Li Y, Pillarisetti K, Baldwin E, Packman K, Elsayed Y, et al. The impact of the bone marrow microenvironment on t cell redirection therapeutics. doi: 10.1038/s41408-020-0331-4. https://ash.confex.com/ash/2016/webprogram/Paper91923.html. [DOI] [PMC free article] [PubMed]
- 26.Sorokina T, Shipounova I, Bigildeev A, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Alterations of the bone marrow stromal microenvironment in adult patients with leukemia before and after the treatment. doi: 10.1080/10428194.2016.1187277. https://ash.confex.com/ash/2016/webprogram/Paper91202.html. [DOI] [PubMed]
- 27.Passaro D, Tullio AD, Abarrategi A, Rouault-Pierre K, Foster K, McNaughton LA, Lassailly F, Bonnet D. Increased vascular permeability in the bone marrow microenvironment contributes to acute myeloid leukemia progression and drug response. doi: 10.1016/j.ccell.2017.08.001. https://ash.confex.com/ash/2016/webprogram/Paper90896.html. [DOI] [PMC free article] [PubMed]
- 28.Decker S, Kissel S, Aumann K, Zenz T, Zirlik K, Claus R, Duyster J, Dierks C. The pan-pim kinase inhibitor lgb321 affects apoptotic pathways and microenvironmental interactions in cll. https://ash.confex.com/ash/2016/webprogram/Paper94256.html.
- 29.Bialopiotrowicz E, Gorniak P, Pula B, Noyszewska-Kania M, Makuch-Lasica H, Nowak G, Szydlowski M, Jablonska E, Sewastianik T, Polak A, Lech-Maranda E, et al. Microenvironment-induced expression of pim kinases supports chronic lymphocytic leukemia cells survival and promotes cxcr4-mtor pathway dependent migration. doi: 10.1111/jcmm.13632. https://ash.confex.com/ash/2016/webprogram/Paper93712.html. [DOI] [PMC free article] [PubMed]
- 30*.Bruce A, Evans R, Mezan R, Shi L, Moses BS, Martin KH, Gibson LF, Yang Y. Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS One. 2015;10(10):e0140506. doi: 10.1371/journal.pone.0140506. 3D microfluidic ALL cell culture platform displayed how mechanical forces, such as flow, and biochemical cues present in the microenvironment affect drug response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solimando A, Brandl A, Katharina M, Graf C, Ritz M, Ruckdeschel A, Stühmer T, Rudelius M, Frassanito MA, Andreas R, Jakob F, et al. Jam-a as a prognostic factor and new therapeutic target in multiple myeloma. doi: 10.1038/leu.2017.287. https://ash.confex.com/ash/2016/webprogram/Paper94942.html. [DOI] [PMC free article] [PubMed]
- 32.Bianchi G, Czarnecki PG, Roccaro AM, Sacco A, Kawano Y, Samur MK, Gulla A, Tai Y-T, Ghobrial IM, Carrasco RD, Anderson KC. Roundabout 1 (robo1)/slit2 is a novel signaling pathway in multiple myeloma promoting survival and bone marrow niche interaction. https://ash.confex.com/ash/2016/webprogram/Paper92513.html.
- 33.Malek E, Dosani T, Pinto R, Covut F, Akabane H, Driscoll JJ, Lima MD. Immunologic status evaluated by the absolute lymphocyte/monocyte ratio provides a powerful prognostic tool for newly diagnosed multiple myeloma. https://ash.confex.com/ash/2016/webprogram/Paper90474.html.
- 34.Meads MB, Oliveira P, Distler A, Silva M, Burger K, Fang B, Collins A, Cleveland JL, Koomen JM, Silva A, Rebatchouk D, et al. Identification of target pathways induced by the multiple myeloma tumor microenvironment using activity-based protein profiling and ex vivo protein kinase inhibitor screening. https://ash.confex.com/ash/2016/webprogram/Paper97909.html.
- 35**.de la Puente P, Muz B, Gilson RC, Azab F, Luderer M, King J, Achilefu S, Vij R, Azab AK. 3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma. Biomaterials. 2015;73:70–84. doi: 10.1016/j.biomaterials.2015.09.017. Bone marrow from multiple myeloma patients were created into scaffolds that mimicked native MM growth, interactions with the microenvironment, and biochemical gradients, including that of oxygen and drug availability. They also induced greater drug resistance in comparison to conventional cultures, alluding to their use in understanding MM pathology and creating personalized therapeutic plans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravelle P, Jean C, Familiades J, Decaup E, Blanc A, Bezombes-Cagnac C, Laurent C, Savina A, Fournie JJ, Laurent G. Cell growth in aggregates determines gene expression, proliferation, survival, chemoresistance, and sensitivity to immune effectors in follicular lymphoma. Am J Pathol. 2014;184(1):282–295. doi: 10.1016/j.ajpath.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Decaup E, Gravelle P, Jean C, Laurent C, Klein C, Varoqueaux N, Savina A, Fournié J-J, Laurent G. Multicellular aggregates of lymphoma cells (malc): An invaluable model for studying follicular lymphoma biology and mechanisms of action of therapeutic drugs such as anti-cd20 antibodies. Blood. 2013;122(21):4410–4410. [Google Scholar]
- 38.Decaup E, Jean C, Laurent C, Gravelle P, Fruchon S, Capilla F, Marrot A, Al Saati T, Frenois FX, Laurent G, Klein C, et al. Anti-tumor activity of obinutuzumab and rituximab in a follicular lymphoma 3d model. Blood Cancer J. 2013;3:e131. doi: 10.1038/bcj.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Assal R, Gurkan UA, Chen P, Juillard F, Tocchio A, Chinnasamy T, Beauchemin C, Unluisler S, Canikyan S, Holman A, Srivatsa S, et al. 3-d microwell array system for culturing virus infected tumor cells. Sci Rep. 2016;6:39144. doi: 10.1038/srep39144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mannino RG, Santiago-Miranda AN, Pradhan P, Qiu Y, Mejias JC, Neelapu SS, Roy K, Lam WA. 3d microvascular model recapitulates the diffuse large b-cell lymphoma tumor microenvironment in vitro. Lab Chip. 2017;17(3):407–414. doi: 10.1039/c6lc01204c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc Natl Acad Sci U S A. 2017;114(3):492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damiano JS, Dalton WS. Integrin-mediated drug resistance in multiple myeloma. Leuk Lymphoma. 2000;38(1–2):71–81. doi: 10.3109/10428190009060320. [DOI] [PubMed] [Google Scholar]
- 43.Cayrol F, Diaz Flaque MC, Fernando T, Yang SN, Sterle HA, Bolontrade M, Amoros M, Isse B, Farias RN, Ahn H, Tian YF, et al. Integrin alphavbeta3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant t cells. Blood. 2015;125(5):841–851. doi: 10.1182/blood-2014-07-587337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Tian YF, Ahn H, Schneider RS, Yang SN, Roman-Gonzalez L, Melnick AM, Cerchietti L, Singh A. Integrin-specific hydrogels as adaptable tumor organoids for malignant b and t cells. Biomaterials. 2015;73:110–119. doi: 10.1016/j.biomaterials.2015.09.007. B and T lymphoma cells were found to have differential integrin-signaling requirements for survival. In the presence of these ligands, the lymphoma cells displayed enhanced proliferation, clustering, and drug response, thereby displaying the key of integrin signaling and revealing a potential therapeutic target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Apoorva F, Tian YF, Pierpont TM, Bassen DM, Cerchietti L, Butcher JT, Weiss RS, Singh A. Lymph node stiffness mimicking hydrogels regulate human b cell lymphoma growth and cell surface receptor expression in a molecular subtype-specific manner. J Biomed Mater Res A. 2017 doi: 10.1002/jbm.a.36031. Differences in biomechanical forces affected lymphoma survival, proliferation, drug response, and BCR expression in a molecular subtype manner. This model better captures the progression of lymphoma and heterogeneity within the tumor. [DOI] [PubMed] [Google Scholar]
- 46.Shah SB, Singh A. Cellular self-assembly and biomaterials-based organoid models of development and diseases. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.01.075. [DOI] [PubMed] [Google Scholar]


