Abstract
The vast majority of cancer associated deaths result from metastasis, yet the behaviors of its most potent cellular driver, circulating tumor cell clusters, are only beginning to be revealed. This review highlights recent advances to our understanding of tumor cell clusters with emphasis on enabling technologies. The importance of intercellular adhesions among cells in clusters have begun to be unraveled with the aid of promising microfluidic strategies for isolating clusters from patient blood. Due to their metastatic potency, the utility of circulating tumor cell clusters for cancer diagnosis, drug screening, precision oncology and as targets of antimetastatic therapeutics are being explored. The continued development of tools for exploring circulating tumor cell clusters will enhance our fundamental understanding of the metastatic process and may be instrumental in devising new strategies to suppress and eliminate metastasis.
Keywords: circulating tumor cell clusters, tumor microemboli, ctm, metastasis, cancer, biophysics, biomechanics, microfluidics, intercellular adhesions, capture, isolation, drug screening, precision medicine, antimetastatic therapeutics, review
Ninety percent of cancer associated deaths are a result of metastasis. The conventional study of the metastatic process focused on individual circulating tumor cells (CTCs), which disseminate from primary tumors to colonize distant organs[1]. However, evidence[2] suggests that individual CTCs may not be the only drivers of metastasis. Instead, multicellular aggregates of CTCs (CTC Clusters) appear to play a large role in metastasis and their potential utility in the clinic has begun to be explored (Figure). Clues to the greater potency of CTC clusters in establishing tumors were first discovered over four decades ago in experiments where cancer cells were injected into the circulation of mice to study their abilities to generate metastases. Fidler et al.[3] found that when cancer cells were aggregated into clusters before injection, they established several-fold more tumors than equal numbers of individual cancer cells, a finding that was later replicated by other groups[4,5]. Further work exploring the biology and behavior of cancer cell aggregates was hampered for many years due to the lack of available technologies for the efficient isolation of CTC clusters from blood and their subsequent analyses.
Figure.
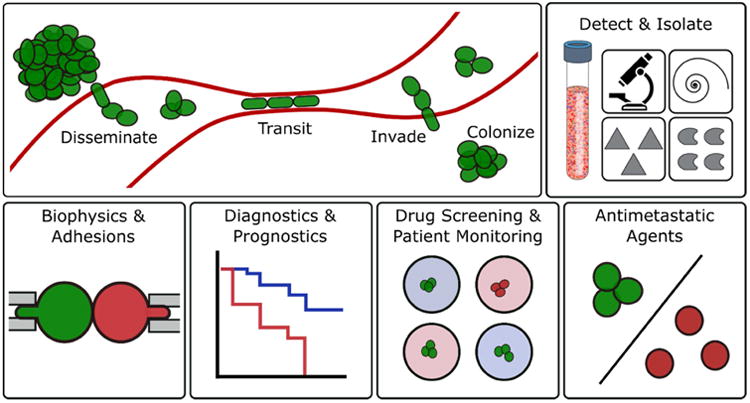
Topics covered in this review. Schematic of formation, transit, invasion and colonization of distant organs by circulating tumor cell clusters (top left). Microfluidic strategies for isolating CTC clusters from blood which may be useful for: biophysical studies, diagnostics and prognostics, drug screening and the development of antimetastatic agents.
Emergent technologies[6-10] have reinvigorated this research, leading to the efficient isolation of CTCs from patient blood[10-14] and to numerous surprising discoveries[2] such as reports that these clusters are responsible for seeding ∼50-97% of metastatic tumors in mouse models[15,16]. Alarmingly, studies also revealed that the isolation of one or more CTC clusters in patient blood at any given time point correlated with significantly worse survival rates in patients with prostate[15], breast[15], colorectal[17] and small-cell lung[18] cancers. It is quite likely that CTC clusters play a far greater role in the metastatic process than previously believed.
Much of the biology and biophysical characteristics of CTC clusters however, remains poorly understood. Here, we review recent advancements in the field of CTC clusters with an emphasis on enabling technologies and strategies that have the potential to accelerate the study and potential clinical utility of clusters (Figure). Recent literature elucidating their cellular characteristics and role in metastatic progression will be highlighted, with a focus on the importance of cellular biophysics. We will then review tools and techniques for isolating these rare cellular aggregates from blood. Finally, we will discuss their utility as diagnostic or prognostic markers and ultimately their potential as targets of anti-metastatic interventions.
Intercellular Adhesions and Biophysics of Metastasis
Studies have begun to reveal the extent to which clusters contribute to the formation of new tumors in patients with cancer. Metastatic tumors were traditionally believed to be established by the invasion and proliferation of individual CTCs into distant organs. Three recent studies[15,16,19] have challenged this assumption by exploring the abilities of CTC clusters to transit through the circulation to reach distant organs and to establish secondary tumors. A common element among these studies is the important biophysical role that intercellular adhesions, a defining feature of multicellular aggregates, play in their metastatic potentials.
Perhaps the most prominent rationale for the belief that CTC clusters were incapable of seeding metastatic tumors was that these clusters, which sometimes contain over one hundred cells[14], are incapable of transiting through capillaries of 5-10 μm in diameter, and therefore immediately arrest in circulation leading to the rupture of vessel walls[1,20,21]. Using microfluidic constrictions that modelled the microcirculation, we demonstrated that clusters containing over 20 cells could travel through capillary-sized constrictions under physiological pressures by reorganizing into chains of single cells[19]. This behavior was then recapitulated in true blood vessels by injecting clusters into the circulation of zebrafish, which have vascular geometries and physiology similar to the human microcirculation. Importantly, it was the strengths of intercellular adhesions within clusters that dictated whether clusters were capable of rearranging into intact chains at constrictions. Computational simulations demonstrated that weakening or strengthening the adhesive energies among cells in CTC clusters to levels outside the range found in typical cancer clusters resulted in dissociation of clusters into single cells or complete occlusion, respectively. These findings have dissuaded us from using the traditional nomenclature of “circulating tumor microemboli” when referring to clusters since these aggregates are often no more likely to occlude capillaries than individual CTCs and therefore the label of “embolism” is ill-suited.
After transiting through the circulation to reach distant organs, CTC clusters need to proliferate to establish macrometastases and secondary tumors. Aceto et al.[15] and Cheung et al.[16] explored the colonization ability of clusters through the use of fluorescently tagged cancer cells injected into immunodeficient mice in various configurations. Both groups found that CTC clusters were not only capable of establishing macrometastases, but that these clusters may be 50-100-fold[15,16] more likely to seed metastatic tumors than equal numbers of individual cells. By injecting cells labelled with two different fluorescent proteins and later examining established metastases and isolated CTC clusters for the presence of one or both colors, these groups: a) ruled out tumor reseeding or serial seeding as causes of tumor establishment and b) demonstrated that CTC clusters likely disseminate from primary tumors as aggregates, instead of being formed by the aggregation of individual CTCs inside vessels. Interestingly, both groups found that proteins involved in intercellular adhesion complexes played important roles in the ability of clusters to seed metastases. Aceto et al. found that plakoglobin, an intercellular anchor protein involved in desmosomes and adherens junctions, had 219-fold greater expression in CTC clusters than single CTCs, and its expression in primary tumors was associated with significantly reduced metastasis-free survival rates in patients. Cheung et al. extensively characterized the presence and role of keratin 14 (K14), an intermediate filament protein also involved in desmosomes and hemidesmosomes, and was found to be enriched in cells at the periphery of cancer aggregates and tumors. Interestingly, primary tumors with reduced K14 expression presented a seven-fold reduction in the mean number of metastases vs. control tumors in animal models suggesting that K14 is important in the formation of distant organ metastases. The role of K14 in metastasis is likely related to its effects on the strengths of intercellular adhesions and coordination of collective cell migration events[22], but further studies are needed on its influence on other potential biophysical properties such as stiffness[23] and mechanical force-induced migration[24].
Altogether, these biophysical and biological studies indicate that intercellular adhesions play a dynamic role in CTC cluster cohesion, transit, collective migration and tumor seeding. Yet, much work remains to be done in this field. It is likely that intercellular adhesion complexes such as desmosomes play a strong role in cluster-driven metastasis, but it is unclear to what extent other cell-cell, cell-matrix adhesion proteins and their associated anchor proteins are involved in the ability of CTC clusters to perform all the steps relevant to metastases. These adhesion molecules may eventually play key roles in advancing our ability to predict, treat and eventually prevent the development of metastases in patients. As we develop technologies to isolate clusters with greater efficiency and ease, we will more rapidly advance our understanding of cluster biophysics and its relation to metastatic progression.
Isolation Strategies
A significant challenge for scientists and clinicians working with CTCs and CTC clusters is their rarity. It is currently estimated that a 10 mL sample of blood from the peripheral circulation of a patient with metastatic cancer typically contains 0-100's of individual CTCs and roughly 0-5 CTC clusters among approximately 50 billion red blood cells, 80 million leukocytes and 3 billion platelets. These estimates can vary greatly depending on cancer type[25,26], blood collection site[27,28] and treatment stage[29] and currently rely on the isolation and enumeration of these cells from sampled patient blood instead of direct in vivo measurements. Nonetheless, the numerous strategies for isolating individual CTCs from whole blood that have been developed have been greatly enabling for many studies in the CTC field[30]. Most studies of CTC clusters however, have thus far relied on the strategies designed for individual CTCs, which are capable of capturing clusters with varying efficiencies.
One simple technique for detecting the presence of CTCs and CTC clusters in patient blood is to lyse red blood cells (RBCs) and then fix, stain and plate the remaining nucleated cells[9,13,31]. Antibodies against cytokeratin and CD45 are then used to discriminate cancerous from cells from leukocytes. The lack of enrichment in this strategy minimizes potential bias in the population of detected tumor cells as a result of the selection method. Furthermore, because cells are fixed onto glass slides and stained as part of detection, this technique can be easily integrated into downstream molecular, morphologic and phenotypic analyses by pathologists or automated image-processing tools. However, this technique is incompatible with applications that require the recovery of viable CTC clusters, such as culture and drug screening, because cells are fixed during processing.
A different strategy for isolating CTCs and CTC clusters was developed by Warkiani et al.[32]. This microfluidic platform uses trapezoidal cross-section microchannels arranged into spiral geometries to separate cancer cells based on size differences, and is capable of capturing CTCs and CTC clusters from patients with breast[32], lung[32] and head & neck cancers[33]. This method is particularly attractive because of its simplicity and its very high processing rates, ∼0.5-1 mL/min for cells within RBC-lysed blood, which makes it beneficial for applications that require the isolation of CTCs from large volumes of patient blood such as early detection. CTC isolation strategies, such as this one, that rely on size differences to discriminate cancer cells from leukocytes and RBCs[6,30,32,34,35] may have limitations when applied to isolating CTC clusters. Although clusters are on average larger than individual CTCs and normal blood cells, strategies that rely solely on size-based separation are hindered by the geometries and biomechanics of CTC clusters. First, the sizes of individual CTCs vary dramatically, ranging from 4-30 μm within a single patient[36], which overlaps with the diameters of leukocytes (∼12-15 μm) and the disc diameters of red blood cells (∼8 μm). Second, the majority of clusters exist as small 2-4 cell aggregates[37], reducing the average size disparities between large single cells and most clusters. Third, cells and clusters often assume alignments that “mask” their longest axes during size-based sorting by microfluidics[38-40] or filtration[41]. Therefore, strategies that rely solely on size-based separation likely have difficulty isolating a significant fraction of CTC clusters from normal blood cells and single CTCs.
Affinity-based isolation, which relies on the expression of cellular markers to either isolate cancer cells based on known tumor markers (positive selection) or to remove normal blood cells thereby enriching for cancer cells (negative selection), also has its limitations. Positive selection may have challenges targeting cancer cells with reduced expression of cancer associated markers, such as those lost during epithelial-mesenchymal transition[42] and often irreversibly attaches CTCs/clusters to magnetic beads[7] or substrates[8]. In addition, antibody cocktails need to be customized for specific cancer subtypes such as melanoma[43] and non-small cell lung cancer[44] that lack the expression of more ubiquitous cancer markers like epithelial cell adhesion molecule (EpCAM). Negative selection is a promising technique for isolating individual CTCs[45], but because leukocytes are sometimes found attached to CTCs and CTC clusters[14,26,46] and because cancer-associated leukocytes play important roles in metastatic progression[47], negative selection may exclude an invaluable subpopulation of clusters from detection.
The other concern with using existing CTC isolation strategies for clusters is that processing may cause irreversible cellular damage or disrupt clusters into single cells and cluster fragments. The greater than physiological shear stresses applied to cells during processing are often a direct consequence of the desire to process greater volumes of blood within suitable time-frames[32] or the need to achieve desired hydrodynamic phenomena required for sorting[48]. Furthermore, methods that require cellular permeation[7,9,30,31] render clusters unsuitable for many applications such as ex vivo culture and subsequent drug screening. Overall, the great disparities in the number of reported CTC clusters sampled from patient blood are likely attributable to both the variable efficiencies of previous CTC capture technologies and the disruption of clusters into single cells during blood processing[10,12,30,49].
In response to these concerns, Sarioglu et al. developed the first strategy to specifically isolate CTC clusters (and not single CTCs) from blood by immobilizing them on arrays of triangular micropillars[37]. While simple in principle, this technique capitalizes on the fact that clusters are composed of discrete cellular units connected by intercellular adhesions. Different cells within clusters attempt to simultaneously travel down each path at “Y” junctions defined by the rows of the triangular micropillars. As a result, CTC clusters are immobilized while individual cells pass through. This strategy is effective at discriminating CTC clusters from other blood cells, capturing CTC clusters from ∼30-40% of patients with metastatic breast cancer, prostate cancer or melanoma at flow rates of 2.5 mL/hr. This cluster chip bypasses many of the drawbacks of strategies developed for single CTC capture described above, most notably the potential for cluster disruption. While effective for capture and enumeration, the recovery of clusters immobilized on micropillar arrays is challenging because of its bulk operation and requires operation at 4°C and flows that exerts significantly greater than physiological shear stresses to release CTC Clusters.
To address this limitation, we developed a continuous-flow microfluidic strategy for isolating CTC clusters that relies on a two-stage deterministic lateral displacement (DLD) approach, integrated into a single device[40].The first stage separates larger clusters based solely on size-based separation using standard cylindrical DLD micropillar arrays, while the second stage uses asymmetric pillars to discriminate smaller clusters from single CTCs based on their inherent asymmetry. The continuous flow strategy isolates 99% of clusters containing ∼9 or more cells and 66% of smaller clusters from whole blood. Importantly, clusters experience physiological or lower shear stresses during processing, remain intact and have short (∼10 s) residence times resulting in clusters with cell viabilities over 87% and unhindered proliferative abilities without significant induction of apoptosis vs. controls. This strategy is limited by its relatively slow blood processing rate, 1.0 mL/hr, a design consideration that was chosen to limit applied shear stresses to levels below those experienced in the human circulation.
Altogether, the unique challenge of isolating CTC clusters from blood has led to the development of cluster-specific isolation strategies that will be beneficial to groups studying metastasis and developing microfluidics. Ultimately, the most advantageous isolation strategies will enable a wide range of future studies exploring the cellular characteristics and clinical potentials of these rare cellular aggregates.
Towards Clinical Utility
Because of their high metastatic potential, CTC clusters have the potential to be valuable markers and targets in oncology[2]. A number of studies have revealed a strong correlation between the presence of CTC clusters recovered from venous patient blood and significantly reduced survival rates[15,17,18,50-52]. More work needs to be done to determine if this relationship is causal or simply associative, but the isolation of these clusters may still provide beneficial information for clinicians for diagnostic and prognostic purposes[13,53]. Efficient and standardized CTC cluster isolation and processing strategies will be vital in attempts to translate these findings to the clinic.
Another potential application of CTC clusters is in precision cancer medicine, where the aim is to customize the best course of treatment for each individual patient. This may be especially useful during the course of treatment since tumors rapidly evolve, developing drug resistance to therapeutic agents. The screening of CTC clusters may enable clinicians to rapidly identify mutations in primary tumors and/or CTC populations. Bithi and Vanapalli recently developed a microfluidic microdroplet array for trapping individual CTCs and CTC clusters and subsequently screening them for drug responsiveness[54]. An important consideration for this technology is that the platform requires a high concentration of purified cancer cells (5-50×103/mL) in buffer meaning that even though the device is capable of “isolating” individual cells and clusters within droplets, another upstream strategy is required to first isolate these cells from blood. Nonetheless, this technology may be valuable in oncology because it enables the viability of CTCs and CTC clusters to be monitored alongside drug uptake kinetics. The latter may be useful since a substantial fraction of tumor drug resistance is caused by the acquisition of enhanced drug efflux capabilities[55]. Interestingly, it was found that clustered MCF-7 cancer cells had greater resistance to doxorubicin than individual cells which suggests that this may be another pathway that provides clusters a survival advantage over single cells in patients.
Finally, the majority of anti-cancer drugs that enter the market are modest improvements over previous standards of care. The mean overall survival benefit of approved new drug regimens has been only 1-2 months over the last few decades[56,57]. A major contributor to these modest gains is that these drugs were not developed to directly target agents responsible for metastasis. A novel strategy for combating metastasis may be to dissociate CTC clusters into less potent individual CTCs in the circulation (e.g. by weakening the adhesion energies among cancer cells within clusters)[19]. Choi et al. reported the development of such a strategy using urokinase, which they claimed reduced the incidence of metastasis in animal models by lysing fibrin to dissociate CTC clusters[46]. Some have expressed skepticism in the potential use of urokinase for this application however, since urokinase has significant off-target and potentially detrimental effects in many patients[58]. Furthermore, since there is little evidence that fibrin is a significant component of CTC cluster adhesion, the mechanism of action in these studies may not in fact be related to cluster dissociation[58]. These points highlight how important it is that antimetastatic strategies targeting CTC clusters be highly specific and have minimal off-target effects. An optimal agent of this sort would likely have a long half-life in the circulation, but exhibits negligible toxicity and/or diffusion into healthy tissue and cells.
This is an intriguing time to be studying metastasis. Further advancements in CTC cluster isolation technologies will only accelerate our understanding of these potent multicellular aggregates and their contribution to metastatic progression. The development of therapeutic agents that specifically target CTC clusters could lead to dramatic reductions in the incidence of metastasis and the mortality rates across many cancers.
Highlights.
CTC clusters establish secondary tumors with high efficiency
Clusters travel through narrow capillaries by rearranging into single file chains
Intercellular adhesions are important for cluster transit and tumor seeding
Isolation strategies designed for clusters improve capture efficiency
Clusters are promising clinical tools and targets due to high metastatic potentials
Acknowledgments
We are grateful for the contributions from the many current and past faculty, students and staff of the BioMEMS research center and Massachusetts General Hospital Cancer Center whose contributions and discussions make our work possible. This work was financially supported by National Institute of Biomedical Imaging and Bioengineering Quantum Grant (MT and DAH), National Institutes of Health P41 EB002503-11 (MT), the Howard Hughes Medical Institute (DAH) and the Executive Committee on Research of Massachusetts General Hospital (SHA).
Abbreviations
- CTC
Circulating tumor cell
- DLD
Deterministic lateral displacement
- K14
Keratin 14
- RBC
Red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Aceto N, Toner M, Maheswaran S, Haber DA. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends in Cancer. 2015;1:44–52. doi: 10.1016/j.trecan.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Relationship of Embolic Homogeneity, Number, Size and Viability to Incidence of Experimental Metastasis. European Journal of Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Kleinerman J, Saidel GM. Significance of Hematogenous Tumor-cell Clumps in Metastatic Process. Cancer Research. 1976;36:889–894. [PubMed] [Google Scholar]
- 5.Lione A, Bosmann HB. Quantitative Relationship Between Volume of Tumor-cell Units and their Intra-vascular Survival. British Journal of Cancer. 1978;37:248–253. doi: 10.1038/bjc.1978.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al. Isolation by size of epithelial tumor cells - A new method for the immunomorphological and molecular characterization of circulating tumor cells. American Journal of Pathology. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen L, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 8.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–U1210. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, Voigt K, Lazar D, Nieva J, Bazhenova L, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Physical Biology. 2012;9 doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clinical Cancer Research. 2001;7:4080–4085. [PubMed] [Google Scholar]
- 12.Hou JM, Krebs M, Sloane R, Ward T, Priest L, Blackhall F, Dive C. Molecular features and clinical relevance of circulating tumor cells (CTC) and circulating tumor microemboli (CTM) in patients with small cell lung cancer (SCLC) Clinical & Experimental Metastasis. 2011;28:221–222. [Google Scholar]
- 13.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, Schram E, Nieva J, Bazhenova L, Morgan A, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Physical Biology. 2012;9:6. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu M, Bardia A, Wittner B, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••16.Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proceedings of the National Academy of Sciences. 2016;113:E854–E863. doi: 10.1073/pnas.1508541113. This work explored the formation of CTC clusters and their ability to establish metastases with a particular focus on the importance of keratin 14, an intermediate filament protein which was found to be enable CTC clusters to establish metastases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang DJ, Zhao L, Zhou PF, Ma H, Huang F, Jin M, Dai XM, Zheng XM, Huang SY, Zhang T. Circulating tumor microemboli (CTM) and vimentin plus circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell International. 2017;17 doi: 10.1186/s12935-016-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJC, Greystoke A, Zhou C, Morris K, et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients With Small-Cell Lung Cancer. Journal of Clinical Oncology. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- ••19.Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O'Keefe R, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. Microfluidic and animal models of capillaries were used to demonstrate that CTC clusters can transit through constrictions as narrow as five microns by rearranging into single-file chains suggesting a greater role for clusters in the seeding of metastases than previous believed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balzer EM, Konstantopoulos K. Intercellular adhesion: mechanisms for growth and metastasis of epithelial cancers. Wiley Interdisciplinary Reviews-Systems Biology and Medicine. 2012;4:171–181. doi: 10.1002/wsbm.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong B, Zu YL. Detecting Circulating Tumor Cells: Current Challenges and New Trends. Theranostics. 2013;3:377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective Invasion in Breast Cancer Requires a Conserved Basal Epithelial Program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seltmann K, Fritsch AW, Kas JA, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18507–18512. doi: 10.1073/pnas.1310493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber GF, Bjerke MA, DeSimone DW. A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Developmental Cell. 2012;22:104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Therapeutic Advances in Medical Oncology. 2010;2:351–365. doi: 10.1177/1758834010378414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong YP, Fang F, Zhang Q. Circulating tumor cell clusters: What we know and what we expect (Review) International Journal of Oncology. 2016;49:2206–2216. doi: 10.3892/ijo.2016.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosbie PAJ, Shah R, Krysiak P, Zhou C, Morris K, Tugwood J, Booton R, Blackhall F, Dive C. Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. Journal of Thoracic Oncology. 2016;11:1793–1797. doi: 10.1016/j.jtho.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deneve E, Riethdorf S, Ramos J, Nocca D, Coffy A, Daures JP, Maudelonde T, Fabre JM, Pantel K, Alix-Panabieres C. Capture of Viable Circulating Tumor Cells in the Liver of Colorectal Cancer Patients. Clinical Chemistry. 2013;59:1384–1392. doi: 10.1373/clinchem.2013.202846. [DOI] [PubMed] [Google Scholar]
- 29.Toss A, Mu Z, Fernandez S, Cristofanilli M. CTC enumeration and characterization: moving toward personalized medicine. Annals of Translational Medicine. 2014;2:108. doi: 10.3978/j.issn.2305-5839.2014.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian WY, Zhang Y, Chen WQ. Capturing Cancer: Emerging Microfluidic Technologies for the Capture and Characterization of Circulating Tumor Cells. Small. 2015;11:3850–3872. doi: 10.1002/smll.201403658. [DOI] [PubMed] [Google Scholar]
- 31.Nair VS, Keu KV, Luttgen MS, Kolatkar A, Vasanawala M, Kuschner W, Bethel K, Iagaru AH, Hoh C, Shrager JB, et al. An Observational Study of Circulating Tumor Cells and F-18-FDG PET Uptake in Patients with Treatment-Naive Non-Small Cell Lung Cancer. Plos One. 2013;8 doi: 10.1371/journal.pone.0067733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warkiani ME, Guan GF, Luan KB, Lee WC, Bhagat AAS, Chaudhuri PK, Tan DSW, Lim WT, Lee SC, Chen PCY, et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab on a Chip. 2014;14:128–137. doi: 10.1039/c3lc50617g. [DOI] [PubMed] [Google Scholar]
- •33.Kulasinghe A, Tran THP, Blick T, O'Byrne K, Thompson EW, Warkiani ME, Nelson C, Kenny L, Punyadeera C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Scientific Reports. 2017;7 doi: 10.1038/srep42517. The spiral microfluidic technology used in this paper has the advantage of significantly higher blood processing rates in comparison to most other microscale methods of CTC & CTC cluster isolation. Here, the authors isolated CTC clusters from over half of CTC-positive patients with head and neck cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan SJ, Yobas L, Lee GYH, Ong CN, Lim CT. Microdevice for the isolation and enumeration of cancer cells from blood. Biomedical Microdevices. 2009;11:883–892. doi: 10.1007/s10544-009-9305-9. [DOI] [PubMed] [Google Scholar]
- 35.Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT, et al. Flexible Micro Spring Array Device for High-Throughput Enrichment of Viable Circulating Tumor Cells. Clinical Chemistry. 2014;60:323–333. doi: 10.1373/clinchem.2013.206805. [DOI] [PubMed] [Google Scholar]
- 36.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen L. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical Cancer Research. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- •37.Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nature Methods. 2015;12:685–691. doi: 10.1038/nmeth.3404. - The paper reported the first microfluidic device designed specifically to capture CTC clusters through the use of triangular micropillars arrays that defined numerous fluid bifurcations for trapping clusters out of whole blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beech JP, Holm SH, Adolfsson K, Tegenfeldt JO. Sorting cells by size, shape and deformability. Lab on a Chip. 2012;12:1048–1051. doi: 10.1039/c2lc21083e. [DOI] [PubMed] [Google Scholar]
- 39.Holm SH, Beech JP, Barrett MP, Tegenfeldt JO. Separation of parasites from human blood using deterministic lateral displacement. Lab on a Chip. 2011;11:1326–1332. doi: 10.1039/c0lc00560f. [DOI] [PubMed] [Google Scholar]
- •40.Au SH, Edd J, Stoddard AE, Wong KHK, Fachin F, Maheswaran S, Haber DA, Stott SL, Kapur R, Toner M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Scientific Reports. 2017;7:2433. doi: 10.1038/s41598-017-01150-3. This two-stage continuous-flow platform utilized deterministic lateral displacement to isolate CTC clusters from whole blood based on differences in both the size and asymmetry of clusters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madsen R, Meltzer T, Jornitz M. How Pore and Fibrous Interstice Structure Influence Filter Performance. Bioprocess International. 2010 [Google Scholar]
- 42.Hyun KA, Goo KB, Han H, Sohn J, Choi W, Kim SI, Jung HI, Kim YS. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7:24677–24687. doi: 10.18632/oncotarget.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odashiro DN, Odashiro AN, Pereira PR, Godeiro K, Antecka E, Di Cesare S, Burnier MN. Expression of EpCAM in uveal melanoma. Cancer Cell International. 2006;6 doi: 10.1186/1475-2867-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, et al. Analysis of Circulating Tumor Cells in Patients with Non-small Cell Lung Cancer Using Epithelial Marker-Dependent and -Independent Approaches. Journal of Thoracic Oncology. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 45.Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen PI, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nature Protocols. 2014;9:694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH, Yun SH. Urokinase Exerts Antimetastatic Effects by Dissociating Clusters of Circulating Tumor Cells. Cancer Research. 2015;75:4474–4482. doi: 10.1158/0008-5472.CAN-15-0684. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nature Reviews Immunology. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martel JM, Toner M. Inertial Focusing in Microfluidics. In: Yarmush ML, editor. Annual Review of Biomedical Engineering. Vol. 16. 2014. pp. 371–396. Annual Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating Tumor Cells as a Window on Metastasis Biology in Lung Cancer. American Journal of Pathology. 2011;178:989–996. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Mu ZM, Chervoneva I, Austin L, Ye Z, Rossi G, Palazzo JP, Sun C, Abu-Khalaf M, Myers RE, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Research and Treatment. 2017;161:83–94. doi: 10.1007/s10549-016-4026-2. [DOI] [PubMed] [Google Scholar]
- 51.Mu ZM, Wang C, Ye Z, Austin L, Civan J, Hyslop T, Palazzo JP, Jaslow R, Li BS, Myers RE, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Research and Treatment. 2015;154:563–571. doi: 10.1007/s10549-015-3636-4. [DOI] [PubMed] [Google Scholar]
- 52.Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. Bmc Cancer. 2016;16 doi: 10.1186/s12885-016-2406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsson A, Nair VS, Luttgen MS, Keu KV, Horng G, Vasanawala M, Kolatkar A, Jamali M, Iagaru AH, Kuschner W, et al. Circulating Tumor Microemboli Diagnostics for Patients with Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology. 2014;9:1111–1119. doi: 10.1097/JTO.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •54.Bithi SS, Vanapalli SA. Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Scientific Reports. 2017;7:41707. doi: 10.1038/srep41707. This work describes a microfluidic platform for arraying individual CTCs and CTC clusters within microdroplets for screening the effectiveness of anti-cancer drugs. CTC clusters were found to be more resistant to doxorubicin than individual CTCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug Resistance in Cancer: An Overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apolone G, Joppi R, Bertele V, Garattini S. Ten years of marketing approvals of anticancer drugs in Europe: regulatory policy and guidance documents need to find a balance between different pressures. British Journal of Cancer. 2005;93:504–509. doi: 10.1038/sj.bjc.6602750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fojo T, Mailankody S, Lo A. Unintended Consequences of Expensive Cancer Therapeutics-The Pursuit of Marginal Indications and a Me-Too Mentality That Stifles Innovation and Creativity The John Conley Lecture. Jama Otolaryngology-Head & Neck Surgery. 2014;140:1225–1236. doi: 10.1001/jamaoto.2014.1570. [DOI] [PubMed] [Google Scholar]
- 58.Mirshahi S, Pujade-Lauraine E, Soria C, Pocard M, Mirshahi M, Soria J. Urokinase Antimetastatic Effects-Letter. Cancer Research. 2016;76:4909–4909. doi: 10.1158/0008-5472.CAN-16-0138. [DOI] [PubMed] [Google Scholar]


