Abstract
OBJECTIVE
To examine baseline clinical and psychosocial characteristics that predict 12- month symptom change in men and women with urologic chronic pelvic pain syndromes (UCPPS).
METHODS
221 female and 176 male UCPPS patients were recruited from 6 academic medical centers in the United States and evaluated at baseline with a comprehensive battery of symptom, psychosocial, and illness-impact measures. Based on biweekly symptom reports, a functional clustering procedure classified participant’s outcome as worse, stable, or improved on pain and urinary symptom severity. Cumulative logistic modeling was used to examine individual predictors associated with symptom change as well as multiple predictor combinations and interactions.
RESULTS
About 60% of participants had stable symptoms with smaller numbers (13% to 22%) showing clear symptom worsening or improvement. For both pain and urinary outcomes the extent of widespread pain, amount of non-urological symptoms and poorer overall health were predictive of worsening outcomes. Anxiety, depression and general mental health were not significant predictors of outcomes, but pain catastrophizing and self-reported stress were associated with pain outcome. Prediction models did not differ between men and women and for the most part were independent of symptom duration and age.
CONCLUSION
These results demonstrate for the first time in a large multisite prospective study that presence of widespread pain, non-urological symptoms and poorer general health are risk factors for poorer pain and urinary outcomes in both men and women. The results point to the importance of broad based assessment in UCPPS and future studies of mechanisms that underlie these findings.
Keywords: interstitial cystitis, chronic prostatitis, chronic overlapping pain conditions, quality of life
Introduction
Urologic chronic pelvic pain syndromes (UCPPS) include idiopathic chronic pelvic pain in both men and women and what have in the past been considered separate bladder and prostate syndromes1. Interstitial cystitis/bladder pain syndrome (IC/BPS)2 has been diagnosed primarily in women whereas chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS)3 is a diagnosis exclusive to men. Historically these conditions have been studied separately, but recent cross-sectional studies have shown many common features for men and women with UCPPS including prevalence of key symptoms, psychosocial impact and some neurobiological mechanisms.4–6
Longitudinal usual-care and natural history studies have suggested that IC/BPS is a relatively stable disorder with significant short term fluctuations in severity. Only a small minority of patients are free of symptoms at follow-up periods of 1 to 3 years.7 Similar findings have been reported in the few longitudinal studies of CP/CPPS, with few remissions over a 1 to 2 year period in patients with persistent symptoms.8–10
There is little data on prognostic factors that might influence the course of IC/BPS or CP/CPPS although some studies suggest greater severity and persistence of symptoms are associated with continuing symptoms at follow up 1–3 years later.11,7, 8 Most of the previous studies have examined IC/BPS and CP/CPPS separately, and have not evaluated non-UCPPS risk factors at baseline. Thus, it is not known if non-urological and psychosocial factors such as sex, presence of non-urological symptoms, widespread pain, life stress, or negative affect are predictive of longitudinal symptom change in UCPPS.
The present study, as part of the MAPP network longitudinal study of UCPPS, examines a variety of demographic and clinical characteristics as predictors of 12-month symptom change to test the following hypotheses. 1) Independent of baseline symptom severity, presence of non-urological symptoms including widespread pain, poor general physical well-being and mood disturbance will be associated with a greater probability of symptom worsening over 12 months. 2) Similar predictors will be obtained for males and females with UCPPS and older and younger patients, and 3) Longer chronicity of symptoms will be associated with a greater probability of symptom worsening.
Materials and Methods
The MAPP network is a multisite, NIDDK-funded prospective study to improve understanding of IC/BPS and CP/CPPS. Overall goals and design of the MAPP network have been previously described.1, 12 Males and females with UCPPS enrolled at six US discovery sites were followed for 52 weeks so the data describe a prospectively studied, usual care cohort.
Participants
Details of recruitment methods and inclusion and exclusion criteria have been published.12 Participants had to have a clinical diagnosis of IC/BPS or CP/CPPS, pain severity of at least 1 on a 0–10 Likert pain scale, be over age 18, and have urinary symptoms present the majority of the time during 3 of the previous 6 months. Enrollment was monitored throughout to encourage recruitment of substantial numbers of both men and women and participants with shorter (less than 2 years) and longer (greater than 2 years) symptom duration.
Design
Following consent and enrollment, participants filled out all the study assessments via computer during a single baseline visit. They were subsequently contacted every two weeks for the next 52 weeks for online ratings of current symptoms on the urinary and pain severity outcomes and any changes in medications.
Measures
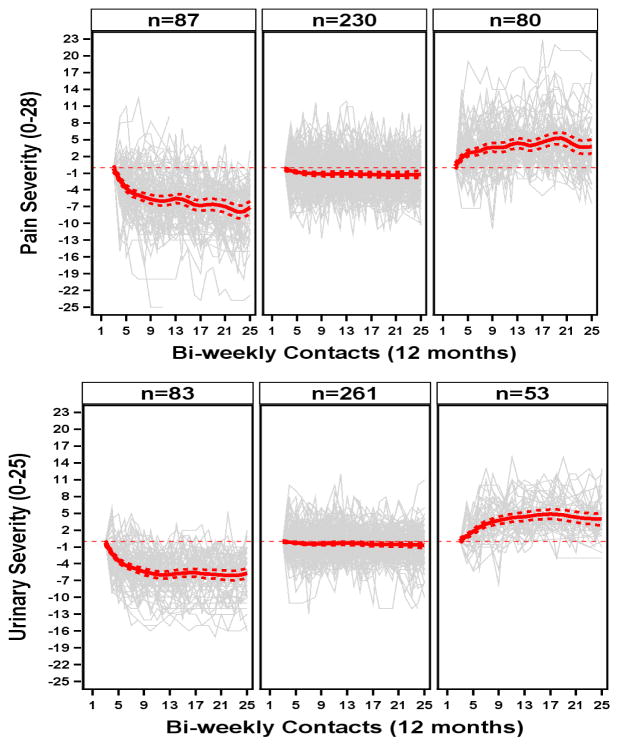
The MAPP patient reported outcomes used in this study are described in prior publications5, 12 and detailed in Supplemental Appendix B. UCPPS severity was characterized on two distinct dimensions, pain and urinary symptoms, based on a recent psychometric analysis of the MAPP baseline data.13 A functional clustering procedure was applied to the biweekly pain and urinary severity scores to classify the overall symptom trajectory for each participant as worsening, stable or improving over the study period (for details see Supplemental Appendix C). To decrease the influence of study entry effects on outcome classification the first month of symptom ratings were not used in the clustering algorithm 14 and the symptom ratings at study week 4 were used as the baseline symptom level for the modeling. Figure 1 shows individual symptom trajectories by cluster, overlaid by the average symptom trajectory within the cluster.
Figure 1.
Functional clustering of participants based on biweekly symptom ratings (in grey), classified into one of three clusters (Improved, Stable, or Worse). Clustering was done separately for pain severity (top) and urinary severity (bottom). Red lines show the mean symptom ratings for each cluster. For details of the clustering methods see Supplemental Appendix C.
Widespread non-urological symptoms were assessed with the Complex Multi-Symptom Inventory (CMSI)15 and widespread pain from the sum of pain locations (including headache) marked on a standardized body map.16 Relationship satisfaction with the Self-Esteem And Relationship questionnaire (SEAR:M & F), physical (PCS) and mental (MCS) health quality of life with the SF-12,17 fatigue and sleep disturbance with the NIH Patient Reported Outcomes Measurement Information System (PROMIS) questionnaires, perceived life stress with the Perceived Stress Scale (PSS),18 anxiety and depression with the Hospital Anxiety and Depression Scale (HADS),19 and pain catastrophizing with Coping Strategies Questionnaire.20
Statistical Analyses
Examination of predictors of symptom change used cumulative logistic modeling. For each analysis a significant positive relationship indicated a greater likelihood of symptom improvement, i.e. likelihood of being stable vs worse or improved vs stable. A preliminary analysis indicated higher baseline levels of pain and urinary severity were strongly predictive of greater likelihood of pain or urinary symptom improvement respectively. Therefore in all subsequent analyses the baseline level was included in the model to control for this relationship. A second analysis step examined individually the association of each of the potential predictor variables with outcome controlling for baseline severity. This was followed by a third set of analyses that examined interactions between predictor variables and patient’s sex, age, and duration of illness to clarify how these variables may impact the strength of significant outcome predictors. A final set of regressions tested whether a combination of baseline variables would improve prediction of outcomes. The threshold for statistical significance was set at a conservative level of p<.01 to minimize Type 1 error. Data imputation was used to handle missing data for cluster determination. C statistic is provided to show relative strength of the predictive model.
Results
Descriptive data
The initial sample consisted of 233 female and 191 male UCPPS patients; 27 were excluded from the analysis due to insufficient longitudinal data. Table 1 shows baseline values stratified by sex.
Table 1.
Comparison of Baseline Variables for Males (n=176) and Females (n=221)
| Males (N=176) | Females (N=221) | ||
|---|---|---|---|
| Demographics | |||
| Age | Mean(SD) | 47.7 (15.5) | 40.6 (14.3) |
| Race | White | 157 (89.2%) | 194 (87.8%) |
| Black | 9 (5.1%) | 7 (3.2%) | |
| Asian | 4 (2.3%) | 4 (1.8%) | |
| Multi Race | 1 (0.6%) | 10 (4.5%) | |
| Other | 4 (2.3%) | 5 (2.3%) | |
| Ethnicity | Hispanic | 6 (3.4%) | 15 (6.8%) |
| Non-Hispanic | 169 (96.0%) | 206 (93.2%) | |
| Income | $10,000 or less | 9 (5.1%) | 28 (12.7%) |
| $10,001 to $25,000 | 10 (5.7%) | 22 (10.0%) | |
| $25,001 to $50,000 | 22 (12.5%) | 42 (19.0%) | |
| $50,001 to $100,000 | 57 (32.4%) | 59 (26.7%) | |
| More than $100,000 | 65 (36.9%) | 48 (21.7%) | |
| Duration of symptom (yrs) | Mean(SD) | 8.1 (10.9) | 9.1 (10.3) |
| Duration of symptom (n) | <2 yr | 81 (46.0%) | 83 (37.6%) |
| >2yr | 95 (54.0%) | 138 (62.4%) | |
| Symptom Severity | |||
| Pain Severity at Baseline | Mean(SD) | 11.8 (5.6) | 14.3 (5.8) |
| Urinary Severity at Baseline | Mean(SD) | 10.0 (5.9) | 12.2 (6.1) |
| CMSI | |||
| CMSI Non-Urological Symptoms | Mean(SD) | 5.9 (4.7) | 9.2 (7.7) |
| Body Map | |||
| Total Sites Non-pelvic (0–42) | Mean(SD) | 2.9 (3.5) | 5.4 (7.3) |
| Head pain (n) | none | 123 (69.9%) | 115 (52.0%) |
| present | 53 (30.1%) | 106 (48.0%) | |
| SF-12 | |||
| Physical Component (PCS) (0–100) | Mean(SD) | 50.0 (9.1) | 45.4 (10.7) |
| Mental Component (MCS) (0–100) | Mean(SD) | 45.0 (10.5) | 43.7 (10.0) |
| PROMIS Scales | |||
| Fatigue (0–100) | Mean(SD) | 52.2 (7.7) | 56.5 (8.9) |
| Sleep disturbance(0–100) | Mean(SD) | 52.5 (8.9) | 55.6 (9.3) |
| HADS | |||
| HADS: Depression (0–21) | Mean(SD) | 5.3 (4.0) | 5.2 (4.2) |
| HADS: Anxiety (0–21) | Mean(SD) | 7.3 (4.2) | 7.8 (4.7) |
| CSQ: Catastrophizing (CAT) | |||
| CSQ: CAT (0–36) | Mean(SD) | 10.4 (8.5) | 13.9 (8.6) |
| PSS | |||
| PSS total (0–40) | Mean(SD) | 15.0 (7.0) | 17.0 (8.2) |
| SEAR | |||
| Total Score (0–100) | Mean(SD) | 47.6 (16.4) | 38.2 (15.1) |
Univariate predictors
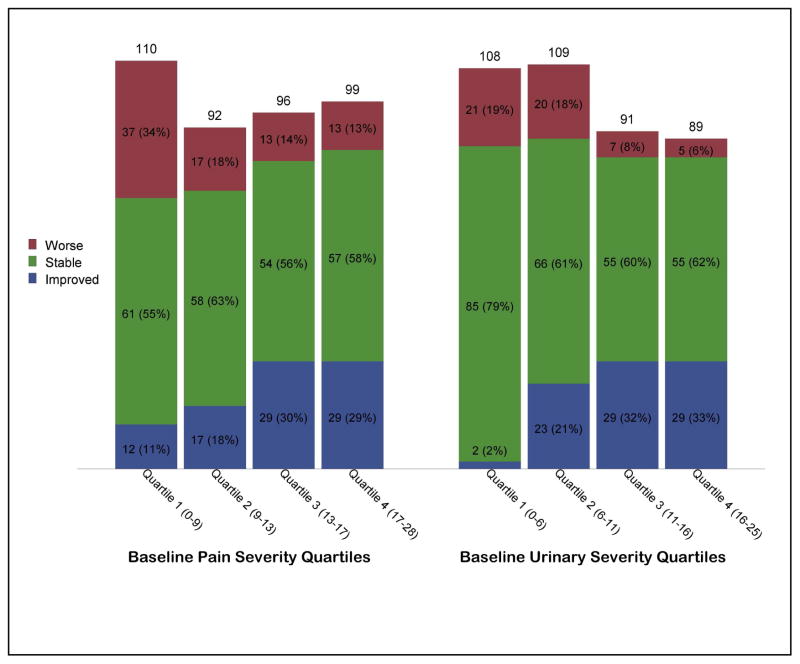
For pain severity 87 (21.9%), 230 (57.9%), and 80 (20.1%) were classified as improved, stable or worse, respectively; for urinary severity 83 (20.9%), 261 (65.7%), and 53 (13.3%) were improved, stable, or worse. Figure 2 shows the distribution of outcomes stratified by quartiles of baseline severity. Baseline severity was highly positively related to likelihood of a more favorable outcome for both pain (OR=1.1, p<0.001) and urinary symptoms (OR=1.1, p<0.001).
Figure 2.
Predicted percent of participants Improved, Stable, and Worse stratified by baseline severity. For illustration purposes baseline severity is divided into quartiles. Pain severity outcome results are shown in the left panel and urinary severity in the right panel.
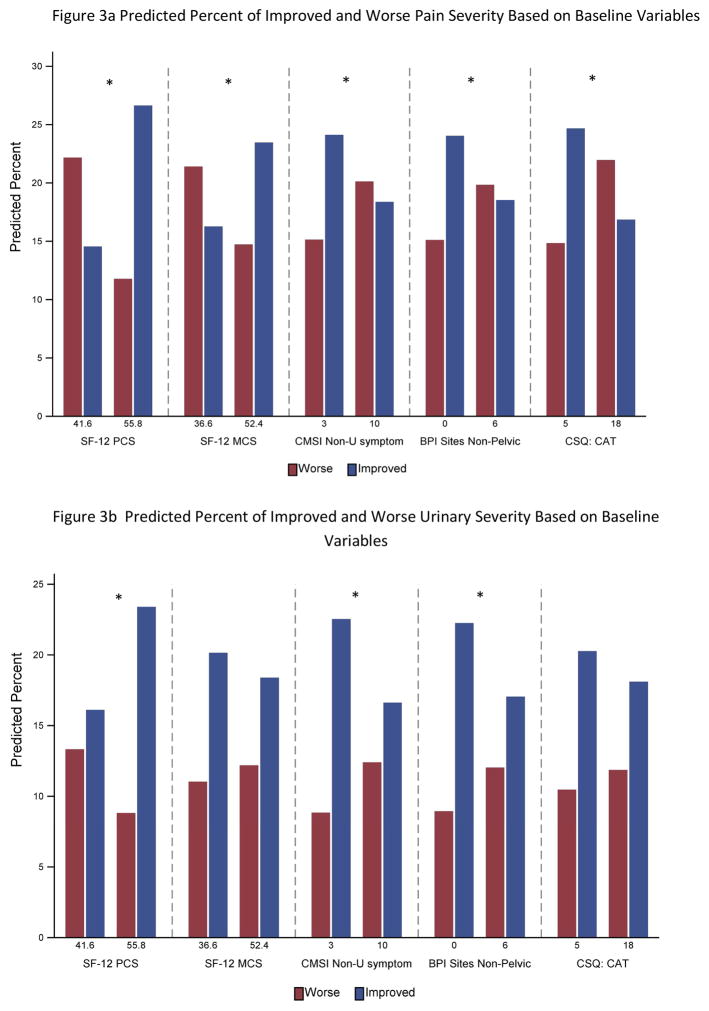
The univariate predictor analyses are summarized in Table 2 and selected variables illustrated in Figure 3. For pain, significant predictors of decreased likelihood of improvement included greater widespread symptoms (CMSI) (OR=0.95), body map total of non-pelvic sites (OR=0.95) and presence of headache (OR=0.52), poorer sleep (OR=0.97) and greater fatigue (OR=0.96). Better physical health and mental health (higher SF12-PCS and -MCS) were associated with an increased likelihood of improvement (PCS OR=1.06; MCS OR=1.03). Worse pain catastrophizing and perceived stress were also significantly associated with poorer outcome (Catastrophizing OR=0.96; Stress OR=0.96). Greater number of medication changes was associated with decreased likelihood of improvement (OR=0.90). Older age was associated with better outcomes (OR=1.02) but sex, severity of urinary symptoms, and duration of symptoms were not significantly predictive of pain severity change.
Table 2a.
Ordinal Logistic Regression - Pain Severity (Adjusted for Baseline Severity)
| Obs Used | Variable | Estimate | P-value | OR (99% CI) | C statistic |
|---|---|---|---|---|---|
| 397 | Age | 0.019 | 0.004 | 1.020 (1.002–1.037) | 0.644 |
| 397 | Sex (0=Males, 1=Females) | −0.348 | 0.089 | 0.706 (0.417–1.196) | 0.636 |
| 397 | Race: White | −0.117 | 0.705 | 0.890 (0.402–1.971) | 0.633 |
| 397 | Non-Hispanic | −0.071 | 0.870 | 0.932 (0.305–2.844) | 0.633 |
| 362 | Income | 0.022 | 0.791 | 1.022 (0.824–1.269) | 0.629 |
| 389 | Duration of symptom(yrs) | 0.005 | 0.576 | 1.005 (0.981–1.030) | 0.634 |
| 397 | Duration of symptom: 0:<2yr,1:>=2yrs | −0.051 | 0.802 | 0.951 (0.566–1.597) | 0.634 |
| 397 | Urinary Severity at Baseline | −0.035 | 0.079 | 0.966 (0.918–1.016) | 0.638 |
| 396 | CMSI Non-Uro symptoms | −0.049 | 0.002 | 0.952 (0.914–0.992) | 0.653 |
| 397 | Body Map Sites Non-pelvic (0–42) | −0.055 | 0.002 | 0.947 (0.905–0.990) | 0.655 |
| 397 | Body Map: Head (y/n) | −0.660 | 0.001 | 0.517 (0.304–0.878) | 0.659 |
| 377 | SF12-PCS (0–100) | 0.054 | <.0001 | 1.055 (1.024–1.087) | 0.680 |
| 377 | SF12-MCS (0–100) | 0.029 | 0.006 | 1.029 (1.002–1.058) | 0.653 |
| 396 | Fatigue Score (29.4 – 83.2) | −0.041 | 0.001 | 0.960 (0.929–0.992) | 0.655 |
| 396 | Sleep disturbance(28.9 – 76.5) | −0.036 | 0.003 | 0.965 (0.935–0.995) | 0.647 |
| 395 | HADS: Depression (0–21) | −0.048 | 0.064 | 0.953 (0.891–1.019) | 0.641 |
| 395 | HADS: Anxiety (0–21) | −0.021 | 0.353 | 0.979 (0.923–1.039) | 0.633 |
| 395 | CSQ: CAT total score (0–36) | −0.037 | 0.003 | 0.964 (0.933–0.996) | 0.648 |
| 394 | PSS total (0–40) | −0.039 | 0.004 | 0.962 (0.929–0.996) | 0.652 |
| 359 | SEAR Total Score (0–100) | 0.009 | 0.185 | 1.009 (0.992–1.027) | 0.636 |
| 397 | Number of Medication Changes | −0.101 | 0.004 | 0.904 (0.826–0.989) | 0.647 |
Figure 3.
Predicted percent of participants Improved vs Worse on pain severity (Figure 3a) and urinary severity (Figure 3b) outcomes, based on selected baseline variables. For illustration purposes the Stable category subjects are not plotted. To simplify the presentation, predicted percentages are shown at the 25th and 75th percentile for each predictor. An * indicates a significant (p<.01) estimate for the variable in the full analysis.
Fewer baseline measures were significant predictors for urinary symptom outcome. Greater widespread symptoms from the CMSI (OR=0.95) and body map (OR=0.95) predicted worse urinary outcome as did greater fatigue (OR=0.96) and sleep disturbance (OR=.97). The SF-12 PCS predicted greater likelihood of urinary symptom improvement (OR=1.03). Sex, duration of symptoms, baseline pain severity and the psychosocial variables were unrelated to urinary symptom change.
Predictor interactions
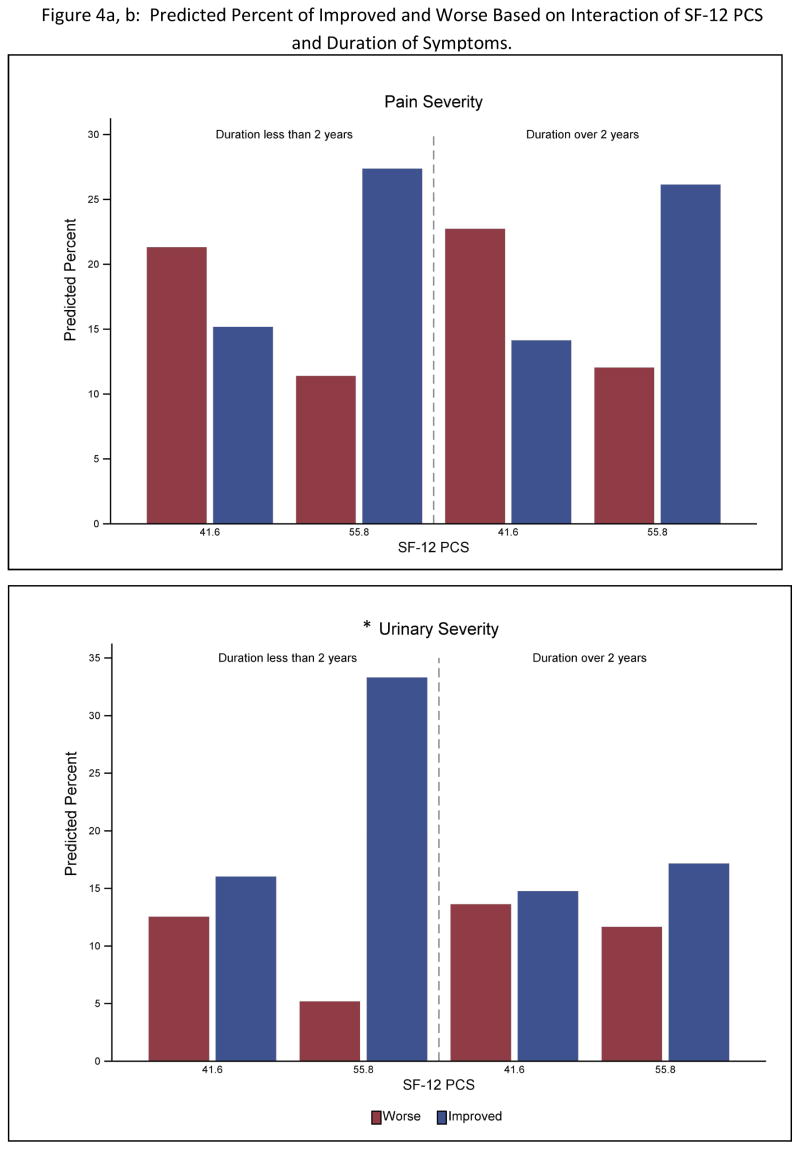
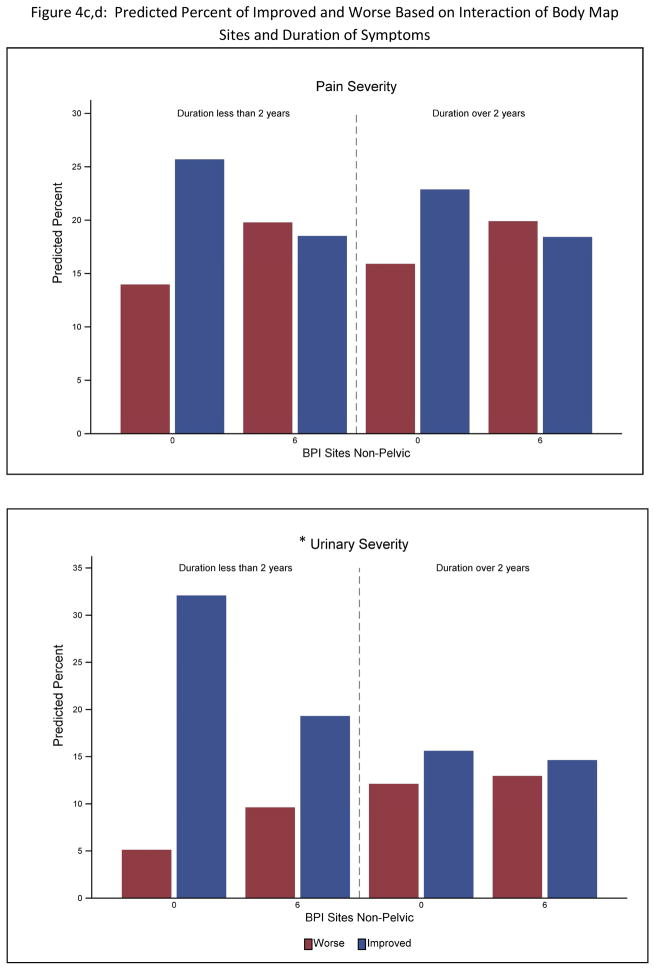
For the pain outcome there were no significant interactions between the variables identified as significant univariate predictors and participant’s sex or duration of symptoms. For urinary symptom outcome, symptom duration but not sex interacted with both number of non-pelvic body sites (p<.0001) and the SF12 PCS (p<.0001) (Table 3). Greater widespread pain and decreased physical QOL was associated with poorer outcome only for participants with a less than 2 year history of UCPPS (see Figure 4).
Table 3.
Ordinal Logistic Regression Models: Urinary Severity Interactions (Adjusted for Baseline Severity)a
| Model | Dependent Variable | n | Variable | Estimate | p-value | Odds Ratio (CI) | C Statistic |
|---|---|---|---|---|---|---|---|
| 1 | Urinary Severity | 377 | Urinary Severity at Baseline | 0.140 | <.0001 | 1.151 (1.104–1.199) | 0.711 |
| Duration of symptom: 0:<2yr,1:>=2yrs | −0.420 | 0.060 | 0.657 (0.424–1.017) | ||||
| SF12 - PCS (Duration < 2yrs) | 0.068 | <.0001 | 1.070 (1.032–1.110) | ||||
| SF12 - PCS (Duration ≥ 2yrs) | 0.013 | 0.374 | 1.013 (0.985–1.041) | ||||
| 2 | Urinary Severity | 397 | Urinary Severity at Baseline | 0.132 | <.0001 | 1.141 (1.099–1.185) | 0.714 |
| Duration of symptom: 0:<2yr,1:>=2yrs | −0.508 | 0.021 | 0.602 (0.392–0.925) | ||||
| BPI Sites, Non-pelvic (Duration < 2yrs) | −0.113 | <.0001 | 0.893 (0.845–0.944) | ||||
| BPI Sites, Non-pelvic (Duration ≥ 2yrs) | −0.013 | 0.595 | 0.987 (0.942–1.035) |
SF12 PCS and BPI sites variables were centered prior to analysis to facilitate interpretation of the direction of the interactions.
Figure 4.
Predicted percent of participants Improved vs Worse on pain severity (top) and urinary severity (bottom). Percentages are stratified on outcomes based on the interaction of SF-12 PCS with symptom duration and number of Body Map sites. To simplify the presentation predicted percentages are shown at the 25th and 75th percentile for SF-12 PCS and Body Map variables. An * indicates a significant (p<.01) interaction in the full analysis.
Multivariable prediction
All of the predictor variables described above as well as baseline severity were entered into exploratory multivariable, stepwise ordinal logistic regression analyses for pain and urinary outcomes (Table 4). For pain severity only the SF-12 PCS and age were left in the final model in addition to baseline pain severity. For urinary severity only the SF- 12 PCS was left in the model in addition to baseline urinary severity. Thus the other variables did not show enough independent association with the outcomes to be included after baseline severity and SF-12 PCS were included.
Table 4.
Multivariable Ordinal Logistic Regression Models (Adjusted for Respective Baseline Severity)
| Dependent Variable | Variable | Est | p-value | Odds Ratio (CI) | C Statistic |
|---|---|---|---|---|---|
| Pain Severity | Pain Severity (Baseline) | 0.169 | <.0001 | 1.184 (1.117–1.254) | 0.703 |
| Age | 0.027 | <.0001 | 1.028 (1.009–1.047) | ||
| SF12 PCS | 0.061 | <.0001 | 1.063 (1.031–1.096) | ||
| Urinary Severity | Urinary Severity (Baseline) | 0.136 | <.0001 | 1.145 (1.085–1.208) | 0.695 |
| SF12_PCS | 0.033 | 0.0049 | 1.033 (1.003–1.065) |
Note: p< 0.1 was required to include a variable and p< 0.05 required for a variable to stay in the model.
Discussion
The current study is the first to prospectively examine a broad range of clinical and psychosocial patient characteristics as predictors of longitudinal symptom change in men and women with UCPPS. About 60% of participants had stable symptoms with smaller numbers (13% to 22%) showing clear symptom worsening or improvement. These results using biweekly sampling and a sophisticated empirical subgrouping of outcomes are consistent with prior studies done separately in IC/BPS and CP/CPPS. There was a general persistence of established UCPPS symptoms with a minority of patients displaying sustained improvement or worsening over a one year period.
It is well known that enrollment in a clinical study is typically followed by symptom improvement.21 This may be due to enrollment bias, i.e. participants seek out studies when symptoms are relatively elevated so declines in the following weeks are expected due to the pattern of normal symptom fluctuation, or to study-related expectation/placebo effects even if no specific treatments are involved. Symptom levels even at week 4 were highly positively related to 12 month improvement suggesting a long lasting impact of enrollment bias that should be considered in design of future UCPPS trials. This baseline-outcome relationship was statistically controlled for in all the prediction models.
As hypothesized, the extent of widespread pain and non-urological symptoms were predictive of poorer outcomes. Presence of more widespread, non-urological problems may be a marker for ‘centralization’ of symptoms thought to involve a greater role of failing central nervous system modulatory mechanisms vs worsening peripheral symptom generators.22, 23 A significant role for ‘centralization’ has been previously suggested for UCPPS based on the persistence of symptoms in the absence of obvious peripheral pathophysiology and the high degree of co-morbidity with other chronic pain conditions such as fibromyalgia and irritable bowel syndrome (IBS) 24, 25 and affective disorders. 26
A second and related set of significant predictor variables for pain and urinary outcomes were those describing a participant’s sense of their general health. These include the Physical Health component of the SF-12 quality of life measure along with the PROMIS Sleep and Fatigue scales. Taken together with recent studies showing poorer outcome associated with increased inflammatory markers in UCPPS27 and altered HPA axis function in CP28, this data suggest inflammatory processes as part of an overall stress response may be a part of the ‘centralization’ mechanisms involved in UCPPS, perhaps recursively as both a driver and responder to peripheral events. Future MAPP analyses of inflammatory markers and brain imaging will be critical to test these potential mechanisms.
Contrary to our initial hypothesis, severity of anxiety and depression symptoms was not significantly associated with the likelihood of 12 month improvement or worsening, perhaps due to the small number of participants with severe psychiatric symptomology. However, several other psychological measures including report of current life stressors and the degree to which pain is felt to be uncontrollable and devastating (pain catastrophizing) as well as an overall lowered sense of mental well-being (SF-12 Mental component score) had moderate negative relationships with the likelihood of UCPPS pain improvement. None of these measures were related to urinary symptom outcome suggesting cognitive variables (unlike presence of non-urological symptoms) are less important for determining the clinical course of urinary symptoms compared to pain. This data suggest the potential of targeting psychosocial interventions on issues of general stress and illness coping rather than psychiatric symptoms as done in other chronic painful conditions.
The current study provides the first longitudinal data in UCPPS showing that clinical characteristics predictive of symptom outcomes are not differentially influenced by sex suggesting that the biological mechanisms underlying these relationships may also not be sex dimorphic. Other MAPP analyses examining cross-sectional clinical and neuroimaging data have also found more similarity than differences across men and women with UCPPS5, 29,30. Also, neither income level (as a marker of social economic status) nor UCPPS chronicity were significant predictors of outcome. However, for the urinary but not the pain severity outcome, only participants with recent onset of UCPPS showed a relationship of outcome with widespread symptoms and QOL, indicating that more chronic symptoms are highly persistent and less influenced by non-urological morbidity.
Despite the large and diverse sample and high retention rate for the MAPP longitudinal data the current study does have several limitations. It is important to recognize that UCPPS is a long lasting chronic disorder and the 12 month study period is a relatively short period of time when considering the full natural history of the disorder. Thus, variables predicting symptom change in the current study are those related to relatively short term change. In addition, this study focused on how baseline non-urological variables predicted clinical outcome, but it is also possible, even likely, that severity of UCPPS symptoms are also a cause of psychological and biological stress, which can be impacted by symptomatic treatment. The next set of MAPP studies will examine a longer time frame (3–5 years) and will therefore provide a more complete test of the hypotheses generated here including treatment by predictor interactions.
Conclusions
These results demonstrate in a large multisite prospective study that presence of widespread pain, non-urological symptoms and poorer general health are risk factors for pain and urinary UCPPS symptom change in both men and women over the following year. Clinically, this study points to the importance of broad based assessment and intervention in UCPPS and future studies aimed at determining mechanisms that underlie the relationship of widespread symptoms with UCPPS.
Supplementary Material
Appendix A - MAPP Investigators
Appendix B – Patient Reported Outcomes
Appendix C – Functional Clustering Methodology
Table 2b.
Ordinal Logistic Regression - Urinary Severity (Adjusted for Baseline Severity)
| Obs Used | Variable | Estimate | P-value | OR (99% CI) | C statistic |
|---|---|---|---|---|---|
| 397 | Age | −0.005 | 0.451 | 0.995 (0.977–1.013) | 0.679 |
| 397 | Sex (0=Males, 1=Females) | −0.294 | 0.172 | 0.745 (0.428–1.298) | 0.679 |
| 397 | Race: White | 0.292 | 0.374 | 1.339 (0.575–3.119) | 0.680 |
| 397 | Non-Hispanic | −0.028 | 0.951 | 0.972 (0.299–3.163) | 0.677 |
| 362 | Income | 0.011 | 0.899 | 1.011 (0.809–1.264) | 0.676 |
| 389 | Duration of symptom(yrs) | −0.017 | 0.098 | 0.983 (0.958–1.009) | 0.682 |
| 397 | Duration of symptom: 0:<2yr,1:>=2yrs | −0.506 | 0.020 | 0.603 (0.345–1.055) | 0.688 |
| 397 | Pain Severity at Baseline | −0.010 | 0.646 | 0.990 (0.935–1.048) | 0.677 |
| 396 | CMSI Non-Uro symptoms | −0.054 | 0.001 | 0.947 (0.907–0.990) | 0.694 |
| 397 | Body Map Sites Non-pelvic (0–42) | −0.055 | 0.002 | 0.946 (0.903–0.991) | 0.693 |
| 397 | Body Map: Head (y/n) | −0.448 | 0.039 | 0.639 (0.366–1.116) | 0.681 |
| 377 | PCS (0–100) | 0.033 | 0.005 | 1.033 (1.003–1.065) | 0.695 |
| 377 | MCS (0–100) | −0.007 | 0.499 | 0.993 (0.966–1.020) | 0.677 |
| 396 | Fatigue Score (29.4 – 83.2) | −0.035 | 0.008 | 0.966 (0.934–0.999) | 0.688 |
| 396 | Sleep disturbance(28.9 – 76.5) | −0.035 | 0.006 | 0.965 (0.934–0.998) | 0.694 |
| 396 | Anger (32.4 – 85.2) | −0.003 | 0.803 | 0.997 (0.966–1.029) | 0.677 |
| 395 | HADS: Depression (0–21) | 0.003 | 0.902 | 1.003 (0.937–1.074) | 0.678 |
| 395 | HADS: Anxiety (0–21) | −0.005 | 0.832 | 0.995 (0.936–1.058) | 0.677 |
| 395 | CSQ: CAT total score (0–36) | −0.011 | 0.394 | 0.989 (0.958–1.022) | 0.677 |
| 394 | PSS total (0–40) | −0.011 | 0.446 | 0.989 (0.955–1.025) | 0.677 |
| 359 | SEAR Total Score (0–100) | −0.001 | 0.910 | 0.999 (0.982–1.017) | 0.677 |
| 384 | % of Visits with Medication Change | −0.012 | 0.054 | 0.988 (0.972–1.004) | 0.681 |
Negative estimates indicate lower likelihood of improvement with higher value of predictor variable.
Bold values indicate significant (p<.01) estimates.
Acknowledgments
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316.).
Abbreviations
- CP/CPPS
chronic prostatitis/chronic pelvic pain syndrome
- IC/BPS
interstitial cystitis/bladder pain syndrome
- MAPP
Multi-disciplinary Approach to the Study of Chronic Pelvic Pain
- UCPPS
urologic chronic pelvic pain syndromes
- CMSI
Complex Multi-Symptom Inventory
- SF-PCS
Short Form – Physical Component Score
- SF-MCS
Short Form – Mental Component Score
Footnotes
Registration Number and Registry Name: ClinicalTrials.gov identifier : NCT01098279 “Chronic Pelvic Pain Study of Individuals with Diagnoses or Symptoms of Interstitial Cystitis and/or Chronic Prostatitis (MAPP-EP)”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185:2162–70. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. Jama. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Clemens JQ, Clauw DJ, Kreder K, et al. Comparison of baseline urological symptoms in men and women in the MAPP research cohort. J Urol. 2015;193:1554–8. doi: 10.1016/j.juro.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naliboff BD, Stephens AJ, Afari N, et al. Widespread Psychosocial Difficulties in Men and Women With Urologic Chronic Pelvic Pain Syndromes: Case-control Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015;85:1319–27. doi: 10.1016/j.urology.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suskind AM, Berry SH, Ewing BA, et al. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–5. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suskind AM, Berry SH, Suttorp MJ, et al. Symptom persistence in a community cohort of women with interstitial cystitis/bladder pain syndrome (IC/BPS): 3-, 6-, 9-, and 12- month follow-up from the RICE cohort. Int Urogynecol J. 2014;25:1639–43. doi: 10.1007/s00192-014-2420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JA, Ciol MA, Von Korff M, et al. Prognosis of patients with new prostatitis/pelvic pain syndrome episodes. J Urol. 2004;172:538–41. doi: 10.1097/01.ju.0000132797.63480.44. [DOI] [PubMed] [Google Scholar]
- 9.Propert KJ, McNaughton-Collins M, Leiby BE, et al. A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol. 2006;175:619–23. doi: 10.1016/S0022-5347(05)00233-8. discussion 623. [DOI] [PubMed] [Google Scholar]
- 10.Tripp DA, Nickel JC, Shoskes D, et al. A 2-year follow-up of quality of life, pain, and psychosocial factors in patients with chronic prostatitis/chronic pelvic pain syndrome and their spouses. World J Urol. 2013;31:733–9. doi: 10.1007/s00345-013-1067-6. [DOI] [PubMed] [Google Scholar]
- 11.Warren JW, Clauw DJ, Langenberg P. Prognostic factors for recent-onset interstitial cystitis/painful bladder syndrome. BJU Int. 2013;111:E92–7. doi: 10.1111/j.1464-410X.2012.11422.x. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith JW, Stephens-Shields AJ, Hou X, et al. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol. 2016;195:949–54. doi: 10.1016/j.juro.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens-Shields AJ, Clemens JQ, Jemielita T, et al. Symptom Variability and Early Symptom Regression in the MAPP Study: A Prospective Study of Urological Chronic Pelvic Pain Syndrome. J Urol. 2016;196:1450–1455. doi: 10.1016/j.juro.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35:339–57. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62:1188–94. doi: 10.1016/j.eururo.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Keefe FJ, Brown GK, Wallston KA, et al. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 21.Stephens-Shields AJ, Clemens JQ, Jemielita T, et al. Symptom Variability and Early Symptom Regression in the MAPP Study, a Prospective Study of Urologic Chronic Pelvic Pain Syndrome. J Urol. 2016 doi: 10.1016/j.juro.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders - pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s) Best Pract Res Clin Rheumatol. 2015;29:6–19. doi: 10.1016/j.berh.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Krieger JN, Stephens AJ, Landis JR, et al. Relationship between chronic non-urological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. J Urol. 2015;193:1254–62. doi: 10.1016/j.juro.2014.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leue C, Kruimel J, Vrijens D, et al. Functional urological disorders: a sensitized defence response in the bladder-gut-brain axis. Nat Rev Urol. 2016 doi: 10.1038/nrurol.2016.227. [DOI] [PubMed] [Google Scholar]
- 26.Chuang YC, Weng SF, Hsu YW, et al. Increased risks of healthcare-seeking behaviors of anxiety, depression and insomnia among patients with bladder pain syndrome/interstitial cystitis: a nationwide population-based study. Int Urol Nephrol. 2015;47:275–81. doi: 10.1007/s11255-014-0908-6. [DOI] [PubMed] [Google Scholar]
- 27.Schrepf A, O’Donnell MA, Luo Y, et al. Inflammation and Symptom Change in Interstitial Cystitis or Bladder Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Study. Urology. 2016;90:56–61. doi: 10.1016/j.urology.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RU, Orenberg EK, Morey A, et al. Stress induced hypothalamus-pituitary-adrenal axis responses and disturbances in psychological profiles in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2009;182:2319–24. doi: 10.1016/j.juro.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Woodworth D, Mayer E, Leu K, et al. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS One. 2015;10:e0140250. doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai HH, Krieger JN, Pontari MA, et al. Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J Urol. 2015;194:1634–41. doi: 10.1016/j.juro.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A - MAPP Investigators
Appendix B – Patient Reported Outcomes
Appendix C – Functional Clustering Methodology