Abstract
Several cyanotryptophans have been shown to be useful biological fluorophores. However, how their fluorescence lifetimes vary with solvent has not been examined. In this regard, herein we measure the fluorescence decay kinetics as well as the absorption and emission spectra of six cyanoindoles in different solvents. In particular, we find, among other results, that only 4-cyanoindole affords a long fluorescence lifetime and hence high quantum yield in H2O. Therefore, our measurements provide not only a guide for choosing which cyanotryptophan to use in practice but also data for computational modeling of the substitution effect on the electronic transitions of indole.
Graphical abstract
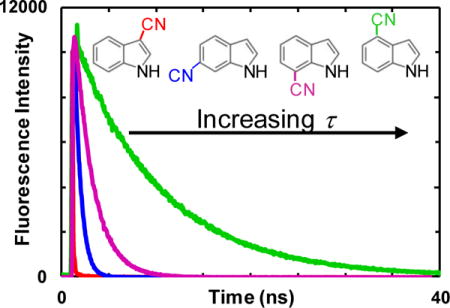
1. Introduction
As a naturally occurring amino acid, tryptophan (Trp) is arguably the most widely used fluorescence probe in the investigation of protein structure, function, and dynamics. This is mainly due to the fact that, in comparison to other native fluorescent amino acids, Trp has a relatively large extinction coefficient and fluorescence quantum yield (QY), and that its fluorescent properties depend on environment [1,2]. Despite its wide utility, however, in practice application of Trp fluorescence suffers from several limitations or drawbacks. For example, (1) it often exhibits complex decay kinetics [3], complicating data interpretation; (2) its absorption spectrum overlaps with those of other aromatic amino acids, making it difficult to be selectively excited [3]; and (3) its fluorescence spectrum and QY typically do not change in a large extent in response to an environmental change, for instance, from a hydrated to a dehydrated one, resulting in a relatively low signal contrast [4]. Therefore, previous efforts have been dedicated to the development of Trp-based amino acid fluorophores that could overcome some or all of those shortcomings [4–9]. In particular, several recent studies have indicated that certain cyanotryptophans (CN-Trp), such as 4CN-Trp [9], 5CN-Trp [4], 6CN-Trp [8], and 7CN-Trp [8], could afford improved photophysical properties, compared to those of Trp, in the context of making it a better fluorophore in biological studies. However, except in the case of 5CN-Trp, a systematic examination of how the florescence decay kinetics of CN-Trp derivatives varies with environment (i.e., solvent) has not been done. To this end and as a first step towards this direction, we studied the fluorescence decay kinetics of six nitrile-derivatized indole variants (referred to as n-CNI, where n = 2 – 7 denotes the carbon position on the indole ring where the CN substitution is located, as shown in Fig. 1 inset) in a variety of solvents.
Figure 1.
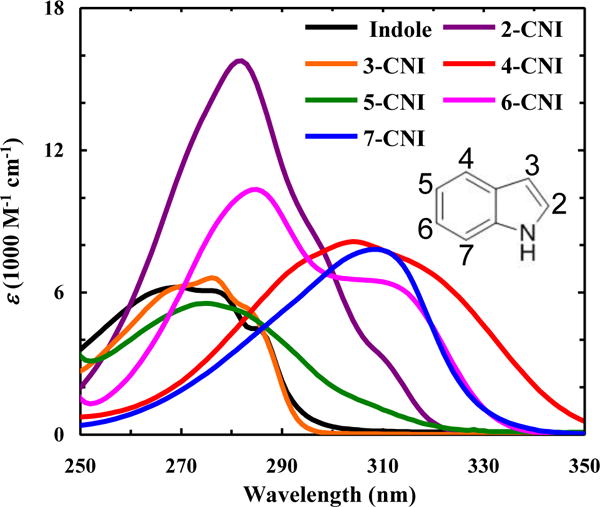
Absorption spectra of indole and n-CNI in H2O at room temperature, as indicated.
We chose these CNI derivatives for the following reasons. (1) Unlike their amino acid counterparts, they are commercially available. (2) Indole is an intercellular and interspecies bacterial signaling molecule that is involved in a variety of bacterial processes, including antibiotic resistance, biofilm formation, quorum sensing, and acid tolerance, among others [10–12]. Therefore, by investigating the photophysical properties of these cyanoindoles, we hope to identify an indole derivative that could be used to investigate these bacterial processes via fluorescence spectroscopy and/or microscopy, provided that the corresponding CN substitution does not significantly alter the biological activity in question. (3) One of these CNI derivatives (i.e., 5-CNI) has become a popular model in recent theoretical and computational interrogations of how a simple substitution affects the electronic structures and energetics of the indole ring [13–18]. In this regard, we believe that the photophysical results obtained from our study will not only provide new experimental results for comparison but can also be used to benchmark various quantum mechanical calculation methods. This is because, as shown below, the absorption and emission spectra, fluorescence QY, and fluorescence lifetime of a cyanoindole in a given solvent (e.g., H2O) exhibit a sensitive dependence on the position of the nitrile group.
2. Experimental
2.1. Materials and Sample Preparation
The compounds indole (Sigma), 2-cyanoindole (2-CNI, 97%, Alfa Aesar), 3-cyanoindole (3-CNI, 98.6%, Chem-Impex International, Inc.), 4-cyanoindole (4-CNI, 97%, Acros Organics), 5-cyanoindole (5-CNI, 99%, Acros Organics), 6-cyanoindole (6-CNI, 98+%, Alfa Aesar), and 7-cyanoindole (7-CNI, 95+%, Ark Pharm Inc.) were used as received. The solvents 1,4-dioxane (Alfa Aesar), 2-propanol (Alfa Aesar), acetonitrile (ACN, Acros Organics), D2O (99.9%, Cambridge Isotopes), dimethyl sulfoxide (DMSO, Acros Organics), ethanol (EtOH, Decon Laboratories, Inc.), methanol (MeOH, Acros Organics), tetrahydrofuran (THF, Acros Organics), trifluoroethanol (TFE, Chem-Impex International Inc.), deuterated CF3CD2OD (TFE-d3, Sigma), and formamide (FA, Acros Organics) were of spectroscopic grade and used as received. Solutions of all indole derivatives were made by dissolving the respective compound in the desired solvent.
2.2 Absorption Measurements
UV-Vis absorption spectra were collected on a Jasco V-650 UV-Vis spectrophotometer using a 1.0 cm quartz cuvette at room temperature.
2.3 Static and Time-Resolved Fluorescence Measurements
Static fluorescence spectra were collected on a Jobin Yvon Horiba Fluorolog 3.10 fluorometer at 25 °C in a 1.0 cm quartz cuvette at a resolution of 1.0 nm, an integration time of 1 s/nm, with an OD of 0.05 – 0.2 at the excitation wavelength (λex) of 270 or 280 nm, as indicated. In addition, for all the fluorescence spectra presented, the difference in the optical densities of the n-CNI samples at λex has been appropriately accounted for.
Time-resolved measurements were collected on a time-correlated single photon counting (TCSPC) system in a 0.4 cm quartz cuvette at room temperature with a fluorophore OD at 270 nm in the range of 0.05 – 0.3. The details of the system have been described elsewhere [4]. Briefly, a Ti:Sapphire oscillator (800 nm, 85.0 MHz) was used to derive the 270 nm excitation pulse through third harmonic generation while an electro-optical pulse picking system (Conoptics, Inc.) was used to reduce the repetition rate of the pulse to 21 MHz. To minimize any inner filter effect, the excitation beam was positioned near the edge of the sample cuvette that faces the fluorescence collection optics. Selective emission was collected under magic angle polarization using a short band pass 355/45 nm filter (Semrock) and a long band pass 300 nm filter (Semrock) and was detected with a MCP-PMT detector (Hamamatsu R2809U) and a TCSPC board (Becker and Hickl SPC-730). The fluorescence decays were deconvoluted with the experimental instrument response function (IRF) and were fit to single-exponential functions using the FLUOFIT (Picoquant GmbH) program.
3. Results and Discussion
3.1. Absorption spectra
As shown (Fig. 1), the position of the nitrile group on the indole ring has a distinct effect on the absorption spectra of the cyanoindoles in H2O, as manifested by the differences in spectral shape, wavelength of the absorption maxima (λabs) and molar extinction coefficient (ε) (Table 1). In comparison to that of indole, the λabs values of all the nitrile-derivatives are red-shifted, with the smallest shifts (5 – 6 nm) observed for 3-CNI and 5-CNI, moderate shifts (12 – 15 nm) observed for 2-CNI and 6-CNI, and largest shifts (>35 nm) observed for 4-CNI and 7-CNI. These results are in qualitative agreement with the study of Juszczak and coworkers [20], which found that an electron-withdrawing group (e.g., CN or F) on the indole ring leads to a red-shift in λabs, whereas an electron-donating moiety (e.g., CH3, OCH3, or OH) results in a blue-shift. In addition, these substitution effects indicate that the electronic transitions of indole show a certain level of symmetry along the short and long molecular axes, despite the fact that the molecule itself does not possess any symmetry along these directions. However, these crude comparisons do not take into account the spectral shapes, which reveal a more complex substitution effect on the absorption spectrum. For example, the spectral shape and ε of 3-CNI and 5-CNI are similar to those of indole, whereas a nitrile substitution at other positions leads to a much large change in these properties (Fig. 1). Indole has two nearly orthogonal, low-lying, and almost degenerate excited states, denoted 1Lb and 1La [21]. However, the directions of the transition dipole moments corresponding to transitions from the ground electronic state to these excited states are different, resulting in different electronic distributions in the 1Lb and 1La states. Therefore, it is expected that the absorption spectrum of n-CNI is not only different from that of indole but also depends on n. However, without further high-level quantum mechanics calculations and modeling, which is outside the scope of the current study, it is impossible to explain such dependence. Nevertheless, based on those spectra one could make several qualitative assessments. For example, (1) the absorption spectrum of 6-CNI has two distinct maxima, centered at ~280 and 310 nm, respectively, likely due to a larger separation between the 1Lb and 1La states in this molecule; (2) the absorption spectrum of 7-CNI is similar to that of 6-CNI, except the intensity of the higher energy band is significantly decreased; and (3) the absorption spectrum of 4-CNI is found to be much broader than that of indole and extends beyond 350 nm. While 4-CNI has a similar ε value at λabs to indole, its red-shifted and broadened spectrum would result in a larger radiative rate constant (kR), as the latter is proportional to , where ε(ν) is the molar extinction coefficient at frequency ν [22]. In other words, this spectral shift and broadening suggests that 4-CNI will have a larger fluorescence QY than indole, provided that the corresponding nitrile substitution does not significantly increase the nonradiative rate constant of the excited state(s).
Table 1.
The absorption (λabs) and emission (λem) maxima and the molar extinction coefficient (ε) at λabs in H2O for indole and n-CNI.
As shown (Fig. S2 in Supporting Information), the absorption spectra of these cyanoindoles in other polar solvents do not show significant deviation from their respective spectrum in H2O. However, like that observed for indole [20], their absorption spectra in cyclohexane become narrower, exposing the underlying vibronic bands and also the position of the 0-0 transition.
3.2. Fluorescence spectra
As shown (Fig. 2), the fluorescence spectra of those cyanoindoles obtained in H2O also show that the position of the nitrile group on the indole ring has a significant effect on the fluorescent properties of the fluorophore. It is clear that the maximum emission wavelengths (λem) for all fluorophores except 3-CNI are red-shifted compared to that of indole (Table 1), with a 5 nm shift for 2-CNI and much larger shifts (30 – 60 nm) for the others. In polar solvents like H2O, it is generally believed that the emissive state of indole is 1La, which is one of the two transitions from the ground state to the excited state [23]. The transition from the ground electronic state to the 1La state leads to a decrease in the π-electron density at positions 1 and 3, an increase at positions 4 and 7, and an overall charge transfer of ~0.33e from the pyrrole to the benzene ring [21,24]. Therefore, when an electron-withdrawing group (e.g., CN) is placed at the benzene ring, particularly at position 4 or 7, this charge separation is enhanced, due to an internal Stark effect [25,26], which, in turn, results in a red-shift in the fluorescence spectrum, as observed. Conversely and as expected, the λem of 3-CNI is blue-shifted ~25 nm compared to that of indole.
Figure 2.
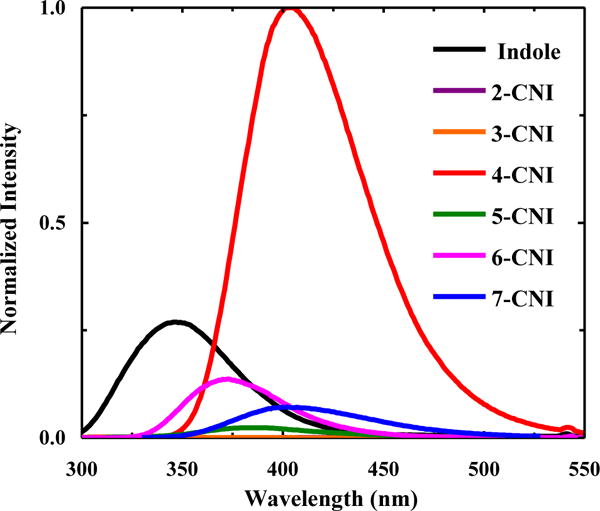
Fluorescence spectra of indole and n-CNI in H2O at 25 °C, as indicated. The excitation wavelength (λex) was 270 nm. To facilitate an easy comparison between the fluorescence intensities of different n-CNI molecules for a given solvent, the data have been ‘normalized’ against the maximum fluorescence intensity of 4-CNI in each case.
Furthermore, these spectra reveal that all these cyanoindoles, except 4-CNI, have a smaller fluorescence QY than indole in H2O. In particular, the maximum intensity of the fluorescence spectra of 2-CNI and 3-CNI is less than 0.1% of that of 4-CNI, making them practically non-fluorescent. This result is consistent with the study of Sulkes and coworkers [27], which showed that substitution with an electron-withdrawing group (i.e., -COOH or -F) on either the pyrrole or benzene ring of indole will lead to an increase in the nonradiative decay rate and hence a decrease in the fluorescence QY in comparison to those of indole. It is apparent that 4-CNI does not follow this trend and has a much higher fluorescence QY than indole, which indicates, for reasons that yet need to be determined, that this particular nitrile substitution is able to decrease the overall nonradiative decay rate of the fluorescent state.
As shown (Fig. S3 and Table S1 in Supporting Information), the λem of 3-CNI shows little, if any, dependence on solvent. This suggests that the emissive state of 3-CNI is Lb-like. On the contrary, the λem of any other n-CNI exhibits a much greater dependence on solvent, with a general trend that λem increases with increasing solvent polarity (Fig. S3 and Table S1 in Supporting Information). This behavior is similar to that of indole and suggests that the fluorescent states of these cyanoindoles are La-like [23]. In addition, these solvent dependences, as judged by the difference between the maximum emission wavelengths measured in THF and H2O, which is 22, 30, 32, 34, and 41 nm for 2-CNI, 6-CNI, 7-CNI, 4-CNI and 5-CNI, indicate that the energy of the emissive state of 5-CNI is most sensitive to environment. Furthermore, like H2O, TFE is found to significantly reduce the fluorescence intensities of these cyanoindoles, except that of 4-CNI (Fig. S3 in Supporting Information). It is well known that reabsorption of the emitted light (i.e., the inner filter effect) can potentially cause the fluorescence spectrum to red-shift. Therefore, to confirm that the n-CNI solutions used to determine the λem values (Table 1) are dilute enough so that the inner filter effect is insignificant, we carried out additional fluorescence measurements in three representative solvents (i.e., THF, EtOH, and DMSO) using a solute concentration that was approximately 10 times smaller than that used in the above experiment. As shown (Fig. S4 and Table S2 in Supporting Information), the λem values obtained with less concentrated n-CNI samples (1 – 5 μM) are within experimental uncertainty identical to those reported in Table 1. Thus, these results indicate that the reported λem values are not significantly affected by the inner filter effect.
3.3. Fluorescence lifetimes
The fluorescence lifetimes of these cyanoindoles, except 5-CNI, [4] have not been previously investigated. Therefore, in an attempt to provide a more comprehensive understanding of the effect of nitrile substitution on the photophysical properties of indole, we next measured the fluorescence decay kinetics of 2-CNI, 3-CNI, 4-CNI, 6-CNI, and 7-CNI in a series of solvents that differ in polarity and/or hydrogen-bonding (H-bonding) ability. As indicated (Fig. S5 in Supporting Information), the fluorescence decay kinetics of 2-CNI, 3-CNI, 4-CNI, 6-CNI, and 7-CNI in H2O are well described by a single-exponential function, with lifetimes (Table 2) consistent with the equilibrium measurements (Fig. 2). For example, 4-CNI has the longest lifetime of 9.1 ns while the lifetimes of 2-CNI and 3-CNI are shorter than the time resolution (~50 ps) of our experimental setup. Interestingly, like 5-CNI, both 6-CNI and 7-CNI, whose amino acid forms have been used previously as biological fluorescence reporters [8], have a shorter excited-state decay time constant (i.e., 0.6 and 2.0 ns, respectively) than indole (4.0 – 4.5 ns) [28,29].
Table 2.
Fluorescence lifetime (τ) of n-CNI in different solvents, as indicated.
| Solvent | 2-CNI τ (ns) |
3-CNI τ (ns) |
4-CNI τ (ns) |
5-CNI τ (ns)a |
6-CNI τ (ns) |
7-CNI τ (ns) |
|---|---|---|---|---|---|---|
| THF | 2.9 | 3.1b | 5.3 | 4.4 | 3.7 | 6.9 |
| 1,4-Dioxane | 2.9 | 3.0b | 5.3 | 4.2 | 3.9 | 7.5 |
| ACN | 0.9 | 1.8 | 5.4 | 4.4 | 3.8 | 8.0 |
| 2-Propanol | ---- | ---- | 5.7 | 4.6 | 3.7 | 9.0 |
| EtOH | ---- | ---- | 5.7 | 4.3 | 3.7 | 8.7 |
| MeOH | ---- | ---- | 5.8 | 2.9 | 3.5 | 8.2 |
| FA | ---- | ---- | 7.8 | 5.4 | 4.2 | 15.7 |
| TFE | ---- | ---- | 6.3 | 0.1 | 0.8 | 1.2 |
| TFE-d3 | ---- | ---- | ---- | ---- | 1.1 | ---- |
| DMSO | 0.4 | 0.2 | 7.4 | 7.1 | 4.3 | 14.5 |
| H2O | <0.05c | <0.05c | 9.1 | 0.3 | 0.6 | 2.0 |
| D2O | ---- | ---- | 9.3 | 0.4 | 0.8 | 2.3 |
Fluorescence lifetimes of 5-CNI in all solvents, except 2-propanol and FA, were obtained from Markiewicz et al. [4]. The reported values for 5-CNI in H2O, D2O, and TFE are amplitude-averaged lifetimes.
Amplitude-averaged lifetimes resulting from a fit to a biexponential function.
Unresolved due to the IRF (~50 ps).
Similar to that observed for other indole derivatives [30–34], the fluorescence lifetimes of these cyanoindoles are dependent on solvent (Table 2). A cursory inspection of the results indicates that among all the solvents used, H2O, TFE, and DMSO appear to have a more pronounced effect with 5-CNI, 6-CNI, and 7-CNI exhibiting the longest (shortest) fluorescence lifetime in DMSO (H2O and TFE). This is an interesting result as the fluorescence lifetime of indole in H2O (4.0 – 4.5 ns) [28,29] is similar to that in DMSO (4.8 – 5.4 ns) [24,26]. Traditionally, studies concerning how solvent affects the fluorescence properties of a fluorophore often seek to use an empirical solvent parameter, such as ET(30) or ETN [35], to correlate between the solvent and the fluorescence property in question. Following this practice, we tried to find out whether a simple correlation exists between the fluorescence lifetime and an empirical solvent parameter for those cyanoindoles. Recently, Zhang et al. [36] have shown that the nitrile stretching vibrational frequency of 5-CNI exhibits a linear dependence on the solvent parameter σ = π* + β − α, where π*, β, and α are the Kamlet-Taft parameters that characterize its polarizability (π*), its H-bond accepting ability (β) and its H-bond donating ability (α), respectively. Specifically, the π* parameter manifests the nonspecific electrostatic interactions between the solute (5-CNI) and solvent molecules, whereas the β and α parameters signify the specific H-bonding interactions between the solvent and the nitrile (β) and NH (α) groups respectively. Since the nitrile stretching frequency is dependent on the electronic densities of the carbon and nitrogen atoms of the CN group, which will change upon photoexcitation to the excited states, this linear correlation suggests that σ could be a useful proxy of the fluorescence lifetime of n-CNI molecules.
As shown (Fig. 3), the fluorescence lifetime of 5-CNI, excluding those obtained in H2O and TFE, indeed exhibits a reasonable linear correlation with σ with a positive slope. This trend suggests that increasing either the solvent’s polarizability (i.e., π*) or its H-bonding interactions (i.e., β) with the NH group would lead to an increase in the fluorescence lifetime of 5-CNI, whereas increasing H-bonding interactions with the CN group (i.e., α) would have an opposite effect. In addition, as expected, the fluorescence lifetimes of 5-CNI in these solvents are shorter than that measured in the gas phase (16.9 ns) [37].
Figure 3.
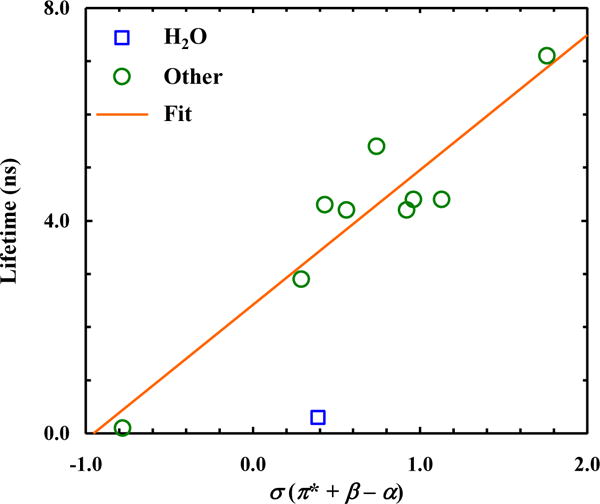
Dependence of the fluorescence lifetime of 5-CNI on the empirical solvent parameter σ. Line corresponds to a linear fit of the data (R2 = 0.88), excluding that obtained in H2O, to the following equation: τ (ns) = 2.4 + 2.5×σ.
As indicated (Fig. 4), the results obtained with 6-CNI show a similar linear correlation when those obtained in H2O and TFE are excluded. Previous studies have shown that, upon transition to the first electronic excited state, a partial negative/positive charge will be accumulated at the CN/NH group of 5-CNI [13, 18], which, in turn, will increase the H-bonding interactions between these sites and solvent having non-zero β and/or α values. Therefore, it is not surprising that a solvent’s H-bonding ability (as measured by β and α) will play a role in the excited-state decay kinetics of 5-CNI. It is worth noting that the fluorescence lifetimes of 5-CNI and 6-CNI in H2O and TFE are comparatively much shorter than those obtained in other solvents (Table 2). Since both H2O and TFE have a large α value and hence can form strong H-bonds with a nitrile group, these results support the aforementioned notion that H-bonding interactions with the CN group of 5-CNI or 6-CNI will decrease its fluorescence lifetime. However, the significantly shorter lifetimes obtained in these solvents indicate that the empirical σ solvent parameter in this case is incapable of correlating the respective solvent effect on the excited-state decay kinetics of 5-CNI and 6-CNI. There are two possible interpretations of this phenomenon. The first possibility is that both H2O and TFE can engage other (and perhaps more specific) interactions with these cyanoindoles that cannot be fully reflected by their σ parameters. For example, it has been shown that H2O and TFE can quench indole fluorescence via excited-state proton transfer between the solvent and various aromatic carbons, due to their large α values [29]. To test whether this process also occurs at the excited state of these cyanoindoles, we carried out, as has been done for indole [29], fluorescence lifetime measurements in D2O and deuterated TFE (TFE-d3). As indicated (Table 2), the fluorescence decay kinetics of both 5-CNI and 6-CNI exhibit a measurable isotopic effect; however, the increase in the fluorescence lifetime in all cases is less than 40%, which is significantly smaller than that observed for indole (>45%) [29]. Another possibility is that H2O and TFE can form stable complexes with 5-CNI and 6-CNI at their excited states, which, for example, can allow a more efficient energy flow to the solvent due to the increased number of vibrational and rotational degrees of freedom in the system, and hence resulting in an enhanced nonradiative decay rate.
Figure 4.
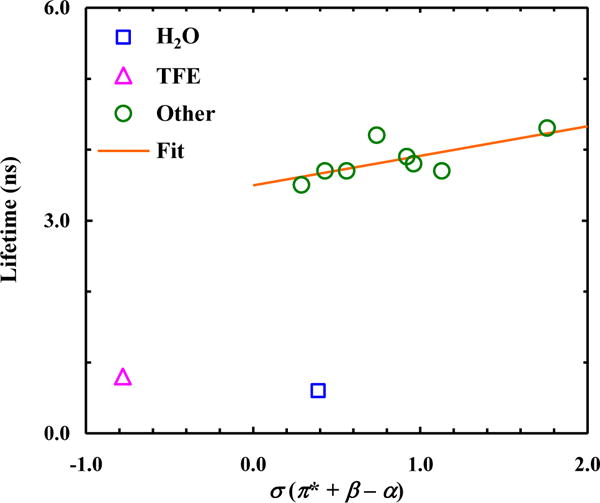
Dependence of the fluorescence lifetime of 6-CNI on the empirical solvent parameter σ. Line corresponds to a linear fit of the data (R2 = 0.50), excluding those obtained in H2O and TFE, to the following equation: τ (ns) = 3.5 + 0.42×σ.
As shown (Table 2 and Fig. S5 in Supporting Information), H2O and TFE also significantly reduce the fluorescence lifetime of 7-CNI in comparison to other solvents. Also, similar to that observed for 5-CNI and 6-CNI, the lifetime of 7-CNI is not significantly increased (~15%) in a deuterated solvent (i.e., D2O), indicating a small solvent isotopic effect. Interestingly, however, the fluorescence lifetimes of 7-CNI obtained in FA and DMSO are much longer than those obtained in other solvents. A possible interpretation of this result is that these solvent molecules can engage in more specific or preferential interactions with the aromatic ring of 7-CNI, leading to the formation of a solute-solvent complex with a decreased nonradiative relaxation rate. In addition, the lifetimes obtained in the remainder solvents exhibit a weak correlation with σ. However, unlike that observed for 5-CNI and 6-CNI, the corresponding σ dependence has a negative slope. As indicated (Fig. 6), the solvent dependence of the fluorescence lifetime of 4-CNI is similar to that of 7-CNI, except the aforementioned effect of H2O and TFE. This may arise from the similarity betweens the C-4 and C-7 atoms in the indole ring; as discussed above, upon excitation to the La state of indole, both carbons will accumulate π-electron density on the order of 0.1e [21].
Figure 6.
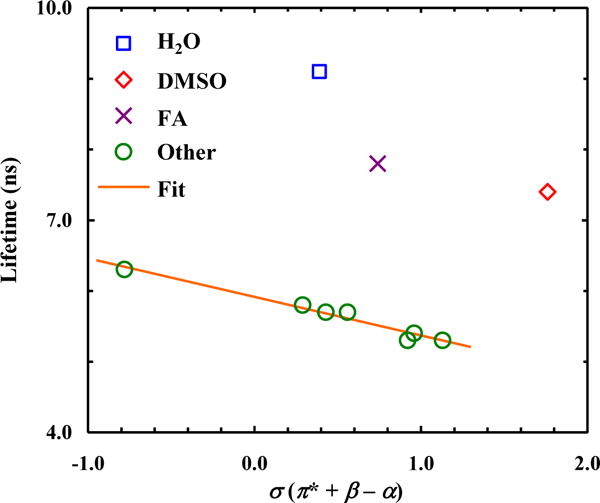
Dependence of the fluorescence lifetime of 4-CNI on the empirical solvent parameter σ. Line corresponds to a linear fit of the data (R2 = 0.97), excluding those obtained in H2O, FA and DMSO, to the following equation: τ (ns) = 5.9 − 0.54×σ.
From a biological application point of view, what is more important is that among all the cyanoindoles, 4-CNI has the longest fluorescence lifetime (i.e., 9.1 ns) in H2O. While we do not know all the factors underlying this phenomenon, one reason, as suggested above, is that a nitrile substitution at the 4th position would lead to an increase in the radiative rate constant (kr). With the availability of the fluorescence lifetime, we can verify this suggestion. The fluorescence QY of a fluorophore is equal to krτ, where τ is the fluorescence lifetime. In addition, for two fluorophores studied under identical conditions, the ratio of their fluorescence QYs is equal to that of the integrated areas of their fluorescence spectra. Using the fluorescence lifetimes of 4-CNI (9.1 ns) and indole (4.0 ns) [29] in H2O and their spectra in Fig. 2, which gives rise to a QY(4-CNI)/QY(indole) ratio of ~4.1, we determined that the kr of 4-CNI is approximately 1.8 times that of indole. While this result confirms that 4-CNI indeed has a larger radiative rate constant than indole, this factor alone, however, does not account for its much increased fluorescence QY from that of indole. This is because using the published kr and knr (nonradiative rate constant) values of indole [29], one can easily calculate that increasing kr by a factor of 1.8 while keeping knr unchanged only results in an increase of the fluorescence QY by ~51%. In other words, the highly fluorescent behavior of 4-CNI in H2O is due mostly to a decreased knr, in comparison to that of indole. It is well known that several mechanisms, including internal conversion, intersystem crossing, photo-ionization, and excited-state proton transfer or exchange (in H2O), contribute significantly to its nonradiative decay rate [1]; however, identifying why and to what extent these processes are affected by a nitrile substitution at the 4th position is nontrivial and requires further experimental and computational studies.
4. Conclusion
Several cyanotryptophans have been shown to be useful fluorophores in the study of protein conformations and conformational dynamics [4,8,9]. Due to the limited commercial availability of these tryptophan analogs, however, a thorough examination of how their fluorescence lifetimes, except that of 5-cyanotryptophan, vary with solvent has not been done before. In this regard, herein we report the fluorescence decay kinetics as well as the absorption and emission spectra of six cyanoindoles (referred to as n-CNI, with n = 2–7) in a series of solvents. We find that (1) the absorption spectra of these cyanoindoles, except that of 3-CNI, are significantly different from that of indole, (2) 2-CNI and 3-CNI are only weakly fluorescent, (3) solvents with strong hydrogen-bond donating ability, such as H2O and trifluoroethanol (TFE), significantly reduce the fluorescence lifetimes of 5-CNI, 6-CNI and 7-CNI, whereas in solvents with strong hydrogen-bond accepting ability, such as dimethyl sulfoxide (DMSO) and formamide (FA), these indole derivatives exhibit the longest fluorescence decay kinetics, and (4) the fluorescence lifetime of 4-CNI affords a unique dependence on solvent, with that measured in H2O being the longest. Taken together, we believe that these spectroscopic and kinetic results will not only help identify which cyanotryptophan to use for a given biological application, but also provide valuable experimental data for QM calculations of the substitution effect [38] to compare. Moreover, like that demonstrated previously for 5-CNI (and 5-cyanotryptophan) [39], we believe that the nitrile stretching vibrations of the other cyanoindoles also afford useful infrared applications [40].
Supplementary Material
Figure 5.
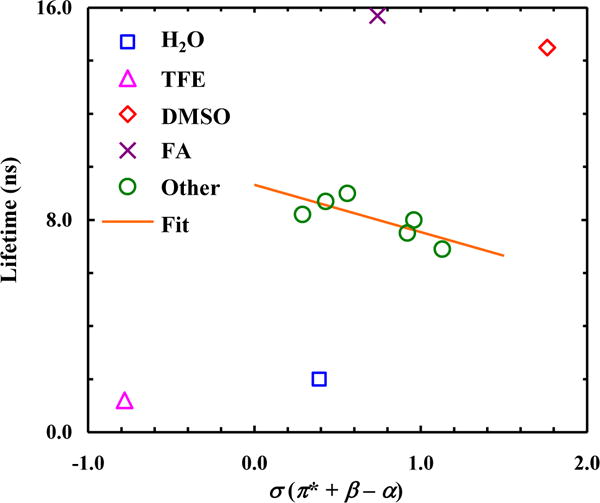
Dependence of the fluorescence lifetime of 7-CNI on the empirical solvent parameter σ. Line corresponds to a linear fit of the data (R2 = 0.60), excluding those obtained in H2O, TFE, FA, and DMSO, to the following equation: τ (ns) = 9.3 − 1.8×σ.
Highlights.
We report the photophysical properties of 6 cyanoindoles.
The absorption spectra of 4-, 6-, and 7-cyanoindoles are significantly red-shifted from that of indole.
4-cyanoindole has the longest fluorescence lifetime in water.
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (P41-GM104605). M.R.H. is supported by a National Science Foundation Graduate Research Fellowship (DGE-1321851).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Electronic Supplemental Information with absorption and fluorescence spectra of all cyanoindoles in all solvents is available. See DOI: xxxxx.
References
- 1.Chen Y, Barkley MD. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 2.Royer CA. Chem Rev. 2006;106:1769–1784. doi: 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- 3.Lakowicz JR. Principles of Fluorescence Spectroscopy. Second. Springer; New York, NY, U.S.A: 1999. [Google Scholar]
- 4.Markiewicz BN, Mukherjee D, Troxler T, Gai F. J Phys Chem B. 2016;120:936–944. doi: 10.1021/acs.jpcb.5b12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross JBA, Szabo AG, Hogue CWV. Methods Enzymol. 1997:151. doi: 10.1016/s0076-6879(97)78010-8. [DOI] [PubMed] [Google Scholar]
- 6.Smirnov AV, English DS, Rich RL, Lane J, Teyton L, Schwabacher AW, Luo S, Thornburg RW, Petrich JW. J Phys Chem B. 1997;101:2758–2769. [Google Scholar]
- 7.Lotte K, Plessow R, Brockhinke A. Photochem Photobiol Sci. 2004;3:348–359. doi: 10.1039/b312436c. [DOI] [PubMed] [Google Scholar]
- 8.Talukder P, Chen S, Roy B, Yakovchuk P, Spiering MM, Alam MP, Madathil MM, Bhattacharya C, Benkovic SJ, Hecht SM. Biochemistry. 2015;54:7457–7469. doi: 10.1021/acs.biochem.5b01085. [DOI] [PubMed] [Google Scholar]
- 9.Hilaire MR, Ahmed IA, Lin CW, Jo H, Degrado WF, Gai F. Proc Natl Acad Sci U S A. 2017;114:6005–6009. doi: 10.1073/pnas.1705586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Lee J. FEMS Microbiol Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 11.Melander RJ, Minvielle MJ, Melander C. Tetrahedron. 2014;70:6363–6372. doi: 10.1016/j.tet.2014.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Wood TK, Lee J. Trends Microbiol. 2015;23:707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Oeltermann O, Brand C, Engels B, Tatchen J, Schmitt M. Phys Chem Chem Phys. 2012;14:10266. doi: 10.1039/c2cp41094j. [DOI] [PubMed] [Google Scholar]
- 14.Stuhlmann B, Gmerek F, Krügler D, Schmitt M. Phys Chem Chem Phys. 2014;16:899–905. doi: 10.1039/c3cp51795k. [DOI] [PubMed] [Google Scholar]
- 15.Min A, Moon CJ, Ahn A, Lee JH, Kim SK, Choi MY. Chem Phys Lett. 2016;658:63–70. [Google Scholar]
- 16.Lopes Jesus AJ, Redinha JS. Struct Chem. 2016;27:809–820. [Google Scholar]
- 17.Wilke J, Wilke M, Brand C, Meerts WL, Schmitt M. ChemPhysChem. 2016:2736–2743. doi: 10.1002/cphc.201600420. [DOI] [PubMed] [Google Scholar]
- 18.Yokogawa D. J Chem Phys. 2016;145:94101. doi: 10.1063/1.4962062. [DOI] [PubMed] [Google Scholar]
- 19.Creed D. Photochem Photobiol. 1984;39:577–583. [Google Scholar]
- 20.Meng X, Harricharran T, Juszczak LJ. Photochem Photobiol. 2013;89:40–50. doi: 10.1111/j.1751-1097.2012.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callis PR. J Chem Phys. 1991;95:4230–4240. [Google Scholar]
- 22.Strickler SJ, Berg RA. J Chem Phys. 1962;37:814–822. [Google Scholar]
- 23.Lami H, Glasser N. J Chem Phys. 1986;84:597–604. [Google Scholar]
- 24.Callis PR. Methods Enzymol. 1997;278:113–150. doi: 10.1016/s0076-6879(97)78009-1. [DOI] [PubMed] [Google Scholar]
- 25.Callis PR, Burgess BK. J Phys Chem B. 1997;101:9429–9432. [Google Scholar]
- 26.Vivian JT, Callis PR. Biophys J. 2001;80:2093–2109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold S, Tong L, Sulkes M. J Phys Chem. 1994;98:2325–2327. [Google Scholar]
- 28.Chen Y, Gai F, Petrich JW. J Phys Chem. 1994;98:2203–2209. [Google Scholar]
- 29.Chen Y, Liu B, Barkley MD. J Am Chem Soc. 1995;117:5608–5609. [Google Scholar]
- 30.Kirby EP, Steiner RF. J Phys Chem. 1970;26:4480–4490. [Google Scholar]
- 31.Roy Meech S, Phillips D, Lee AG. Chem Phys. 1983;80:317–328. [Google Scholar]
- 32.Hager JW, Demmer DR, Wallace SC. J Phys Chem. 1987;91:1375–1382. [Google Scholar]
- 33.Demmer DR, Leach GW, Wallace SC. J Phys Chem. 1994;98:12834–12843. [Google Scholar]
- 34.Tine A, Valat P, Aaron JJ. J Lumin. 1986;36:109–113. [Google Scholar]
- 35.Reichardt C. Chem Rev. 1994;94:2319–2358. [Google Scholar]
- 36.Zhang W, Markiewicz BN, Doerksen RS, Smith AB, III, Gai F. Phys Chem Chem Phys. 2016;18:7027–7034. doi: 10.1039/c5cp04413h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Sulkes M. Chem Phys Lett. 1996;254:242–248. [Google Scholar]
- 38.Abou-Hatab S, Spata VA, Matsika S. J Phys Chem A. 2017;121:1213–1222. doi: 10.1021/acs.jpca.6b12031. [DOI] [PubMed] [Google Scholar]
- 39.Waegele MM, Tucker MJ, Gai F. Chem Phys Lett. 2009;478:249–253. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Pazos IM, Zhang W, Culik RM, Gai F. Annu Rev Phys Chem. 2015;66:357–377. doi: 10.1146/annurev-physchem-040214-121802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


