Abstract
Impaired insight into illness (IMP-INS) is common among individuals with schizophrenia spectrum disorders (SSD), contributing to medication nonadherence and poor clinical outcomes. Caloric vestibular simulation (CVS) is typically used to assess peripheral vestibular system function. Left cold CVS is also a transiently effective treatment for IMP-INS and hemineglect secondary to right brain hemisphere stroke, and possibly for IMP-INS and mood stabilization in patients with SSD. Participants with SSD and moderate-to-severe IMP-INS participated in an exploratory double blind, crossover, randomized controlled study of the effects of CVS on IMP-INS. Participants sequentially received all experimental conditions—left cold (4°C), right cold, and body temperature/sham CVS—in a random order. Repeated measures ANOVA were performed to compare changes in IMP-INS, mood and positive symptom severity pre and 30 min post CVS. A significant interaction was found between CVS condition, time, and body temperature nystagmus peak slow phase velocity (PSPV) for IMP-INS, indicating that single session left cold CVS transiently improved IMP-INS while right cold CVS may have worsened IMP-INS, particularly in participants with greater vestibular reactivity (i.e. higher PSPV) to body temperature CVS. The procedure’s effectiveness is attributed to stimulation of underactive right hemisphere circuits via vestibular nuclei projections to the contralateral hemisphere.
Keywords: Schizophrenia, Insight into illness, Illness denial, Anosognosia, Vestibular stimulation
1. Introduction
Impaired insight into illness (IMP-INS) in schizophrenia, arguably the most treatment resistant manifestation of the disorder, is common (Buckley, 2007; Jablensky, 1992; Olfson, 2006) and contributes to medication non-adherence (Amador, 1994), worsening symptoms (Robinson, 1999), hospitalization (Svarstad, 2001), housing instability (Opler, 1994), and violence (Swanson, 2000). Moreover, IMP-INS in schizophrenia is unresponsive to commonly used psychological interventions, including psychoeducation and cognitive behavioural therapy (O’Donnell, 2003; Olfson, 2006; Pekkala, 2002; Zygmunt, 2002). Novel methods of intervention are required to address this clinical phenomenon.
IMP-INS is commonly associated with right hemisphere brain damage secondary to stroke, but can also occur with right hemisphere lesions due to neurodegeneration or brain injury (Orfei et al., 2008). Lesions typically involve the parietotemporal regions, but are frequently reported in the prefrontal cortex, insula and thalamus. IMP-INS in these contexts is thought to arise from interhemispheric imbalance leading to left hemisphere dominance, and serves as a model for understanding IMP-INS in other neuropsychiatric disorders, such as schizophrenia (Ramachandran, 1995; Ramachandran et al., 2007; Shad et al., 2007).
Structural imaging studies of IMP-INS in schizophrenia support the interhemispheric imbalance neuroanatomical model of IMP-INS by reporting an association with reduced right hemisphere grey matter volume (Flashman et al., 2001; Gerretsen et al., 2012; Shad, 2007; Shad et al., 2007; Shad et al., 2004; Shad et al., 2006). However, voxel-based morphometry studies using a whole brain approach have produced mixed results (Bassitt et al., 2007; Berge et al., 2011; Cooke et al., 2008; Ha et al., 2004; Morgan et al., 2010). The results of functional MRI studies produced by our group suggest IMP-INS is related to left hemisphere dominance in schizophrenia. Specifically, IMP-INS was associated with left hemisphere activations in the prefrontal and parietal regions during an illness awareness task (Gerretsen et al., 2015), and increased functional connectivity in the default mode network with the left parietal lobe (Gerretsen et al., 2014a).
Caloric vestibular stimulation (CVS), which involves the infusion of cold or warm water into the external ear canal, induces a temperature gradient across the semicircular canals of the vestibular apparatus stimulating the vestibular nerve. It is commonly used in otolaryngology to assess vestibular function and neurology to elicit the vestibuloocular reflex (VOR) to test brain stem function. CVS is shown to be an effective, albeit transient, treatment for IMP-INS, somatoparaphrenia, and hemineglect secondary to right hemisphere strokes (Bisiach, 1991; Cappa, 1987; Vallar, 1990). Functional imaging studies demonstrate that cold CVS of the left ear activates a number of areas in the right hemisphere, including the temporoparietal junction, posterior insula, putamen, anterior cingulate, and primary somatosensory cortex (Bottini, 1994; Naito, 2003; Suzuki, 2001). The procedure’s effectiveness is attributed to the stimulation of underactive right hemisphere circuits via vestibulo-cortical projections from the thalamus and brain stem vestibular nuclei (Ramachandran, 1996).
A number of studies have used CVS to investigate the relationship between vestibular pathology and schizophrenia (Fish and Dixon, 1978; Levy et al., 1978, 1983). Another study explored the effects of CVS on language in schizophrenia (Bailey, 1978). There are a few case reports of improved IMP-INS and mood stabilization with left cold CVS in patients with schizophrenia spectrum or bipolar disorders (Dodson, 2004; Levine et al., 2012). One case study of refractory bipolar disorder, unresponsive to mood stabilizers, antipsychotics and electroconvulsive therapy, demonstrated significant sustained improvement in symptoms of mania with left cold CVS (Dodson, 2004). In another case series, left versus right cold CVS reportedly had a transient beneficial effect on IMP-INS in one patient with schizoaffective disorder and two others with schizophrenia (Levine et al., 2012). We are not aware of any studies that have explored the effects of CVS on IMP-INS in schizophrenia spectrum disorders using a randomized controlled design in combination with physiological markers of vestibular system reactivity.
As such, the aim of the present study was to determine whether left cold CVS transiently improves IMP-INS in participants with a schizophrenia spectrum disorder as measured by changes on validated measures of IMP-INS using a within subject design. Comparator and control conditions consisted of right cold and sham (i.e. body temperature) CVS, respectively. The physiological response of vestibular stimulation was assessed with electronystagmography. The intensity of CVS can be indirectly measured by the velocity of nystagmus (i.e. peak slow phase velocity (PSPV)), which is an essential component of the VOR. Based on the neurological and psychiatric literature, we hypothesized left cold CVS would transiently improve IMP-INS by stimulating ‘underactive’ right hemisphere brain regions, in turn, temporarily restoring interhemispheric balance in regions associated with illness awareness. Positive results would demonstrate that IMP-INS in schizophrenia spectrum disorders involves a similar brain network to IMP-INS in patients with brain lesions, and can be similarly modulated with CVS.
Impaired illness awareness is a complex and controversial phenomenon for which a variety of models and theorizations exist. We adopt the most widely accepted conceptualization of insight into illness as a multidimensional construct that consists of core domains, namely general illness awareness (e.g. “I have schizophrenia”), accurate symptom attribution (e.g. “the voices are imaginary due to my mind playing tricks on me”), awareness of need for treatment (“I need an antipsychotic in order to stay mentally healthy”), and awareness of negative consequences attributable to the illness (“I have difficulty working because of schizophrenia” or “I was in jail because I was psychotic”). For in depth reviews on the topic, the reader is directed to Gerretsen et al. (2014) (Gerretsen et al., 2014b) and Chakraborty and Basu (2010) (Chakraborty and Basu, 2010).
2. Methods
2.1. Participants
A total of 16 participants with diagnoses of schizophrenia or schizoaffective disorder with moderate to severe IMP-INS completed the study. An additional participant discontinued the study after a single Right cold CVS condition for undisclosed reasons. Participants were recruited from the Schizophrenia Research Registry and from outpatient and inpatient units of the Schizophrenia Division at the Centre for Addiction & Mental Health (CAMH). Written informed consent was obtained after full explanation of the study procedures and risks. Capacity to consent was confirmed for all participants with the MacArthur Test of Competence (MacCAT) (Stroup et al., 2005). Participants were not informed of the rationale for the different CVS conditions or the hypothesized direction of effects. An assessment of psychiatric disorders was performed using the MINI-Plus 5.0 structured interview (Sheehan et al., 1998). Inclusion criteria for patients were as follows: (i) age 18–65; (ii) fluency in English; (iii) DSM-IV diagnosis of schizophrenia or schizoaffective disorder; (iv) outpatients or inpatients with voluntary status; (v) capable of consenting to participation in the research study; and (vi) moderate to severe IMP-INS (≥3 on Positive and Negative Syndrome Scale, PANSS, G12 Insight and Judgment item) (Kay et al., 1987). Exclusion criteria included: (i) serious, unstable medical illness or any concomitant major medical or neurological illness; (ii) acute suicidal and/or homicidal ideation; (iii) formal thought disorder rating >2 on the Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984); (iv) DSM-IV substance dependence (except caffeine and nicotine) within one month prior to entering the study; (v) pregnant women; (vi) history of external or middle ear pathology; (vii) history or signs of middle ear surgery (e.g. tympanoplasty, mastoidectomy); and (viii) signs of active ear disease. Urine toxicology screens were done as part of the initial assessment. The study was approved by the Research Ethics Boards of CAMH and the University Health Network.
2.2. Study measures
The Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984), the Scale of the Assessment of Negative Symptoms (SANS) (Andreasen, 1989) and PANSS item G12 (Kay et al., 1987) were used to assess symptoms of schizophrenia and to confirm eligibility. IMP-INS was measured at baseline using the clinician-rated Schedule for the Assessment of Insight – Expanded (SAI-E) (David, 1990) and the VAGUS Self-report version (VAGUS-SR) (Gerretsen et al., 2014c). VAGUS-SR was used to measure changes in IMP-INS following CVS due to its temporal sensitivity in comparison with the SAI-E. VAGUS-SR (www.vagusonline.com) is a 10 item self-report measure designed to be easy to administer, sensitive to small changes, and, like the SAI-E, inclusive of the core dimensions of clinical insight into psychosis, i.e. (i) Illness awareness or acceptance, (ii) symptom awareness and accurate symptom attribution, (iii) awareness of need for treatment, and (iv) awareness of negative consequences of the illness (Gerretsen et al., 2014c). Lower scores on both SAI-E and VAGUS-SR represent greater impairment of insight into illness. The Wide Range Achievement Test (WRAT-3) reading subtest was used to measure Premorbid IQ (Wechsler, 2001). Cognitive insight was evaluated with the Beck Insight Scale (BIS), which provides subscale scores for the concepts self-reflectiveness and self-certainty (Beck et al., 2004). Mood was assessed using a 10-point Likert scale, with ‘10’ representing the best mood the participant recalled experiencing, and ‘0’ representing the worst mood the participant recalled experiencing. Mood scores were missing for the first four participants as the importance of assessing mood emerged following the initial participants’ subjective reports of CVS on their mood states.
2.3. Caloric vestibular stimulation experiment
All subjects were randomized and sequentially participated in the experimental, comparator and control conditions, which consisted of: (1) cold CVS of the left ear (experimental); (2) cold CVS of the right ear (comparator), and (3) body temperature (37°C)/sham vestibular stimulation of either the right or left ear (control).
Pre-CVS, participants were administered the SAPS and VAGUS-SR to determine their baseline level of positive symptom severity and IMP-INS, respectively. An otoscopic examination was performed by an otolaryngologist to determine if the eardrum was obscured by cerumen or had any active pathology that may be exacerbated by caloric irrigation. Following thorough cleaning of peri-orbital skin with NUPREP (Weaver and Company, USA) and/or alcohol preparation, patients had surface, disposable, silver chloride electrodes placed lateral to the outer canthus of both eyes and a ground electrode on the forehead to enable electrooculography recording of lateral eye movement. Electrodes were also placed above and below the right eye to record vertical eye movements and blinking. Participants lied supine with their head at a 30-degree angle. Calibration of horizontal eye movements was performed using LED lights. Participants were asked to close their eyes and to answer a series of mentally alerting questions during which their eye movement was recorded to check for spontaneous nystagmus. Participants also received standard bilateral caloric testing to establish vestibular function.
To produce a maximum caloric stimulation for the Left and Right cold CVS conditions, iced water (4 C) was irrigated into each ear for 30 seconds or until the stimulus could not be tolerated owing to vertigo, nausea, or discomfort. For the sham CVS condition, body temperature water (37 C) was irrigated into either the left or right ear for 30 seconds in a randomized fashion. Participants were asked to indicate the onset and conclusion of the rotational feeling (vertigo). Following the resolution of vertigo (roughly 3–5 minutes) or 5 min post CVS a post-irrigation otoscopic examination was performed.
Each participant received repeat administration of the VAGUS-SR and SAPS at 30 min post CVS.
2.4. Peak slow phase velocity (PSPV)
Nystagmus induced by the caloric stimulus is made up of a slow phase and a fast phase. By conventional electrooculography, nystagmus is measured according to the velocity of the slow phase component (slow-phase velocity). The slow-phase velocity of each beat of nystagmus is obtained by calculating the slope (rise over run) of the slow-phase component from a plot displaying position across time. This is a semi-automated procedure completed under the supervision of an examiner (i.e. audiologist) (Banchi and Beattie, 1991; Kavanagh and Babin, 1986).
After a caloric stimulus is presented, the slow phase velocity of the nystagmus will gradually increase until it reaches a peak intensity (at approximately 60 seconds), after which the slow phase velocity of the nystagmus will decrease, eventually returning to baseline. Individual beats of nystagmus will typically cluster around the same velocity for a given point in time on the plot. In the present study, this pattern was captured with a Gould Universal Amplifer (Model: 13–4615–56). A custom software-generated plot was used to display the velocity of each measured beat of nystagmus across time. The audiologist visually identified and excluded outliers distinct form the cluster, and then selected the beat of nystagmus from the cluster with PSPV (Kavanagh and Babin, 1986). This process was repeated for each caloric stimulus.
2.5. Statistical analyses
Statistical analyses of clinical, demographic and behavioral variables were carried out with PASW software, formerly SPSS Statistics (Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc). Means and standard deviations were calculated for the demographic and clinical data. Bivariate Pearson correlations and Mann-Whitney U tests were performed between VAGUS-SR scores and relevant demographic and clinical variables where appropriate. The significance level for tests was established at p<0.05. Bonferroni correction for multiple comparisons was applied (p<0.0004, 0.05/125).
Repeated measures analysis of variance were carried out comparing changes in IMP-INS, mood and positive symptom severity for assessments performed pre-CVS and 30 min post-CVS. Order of condition, laterality of Body temperature CVS (i.e. left versus right), and nystagmus PSPV were entered into the analysis as between subject factors or covariates, as appropriate, if determined to have an effect.
Post-hoc regression analyses were performed to determine the following: (1) baseline predictors of change in insight into illness with Left Cold CVS; and (2) Baseline predictors of Body temperature PSPV.
3. Results
3.1. Relationships at baseline between VAGUS-SR, subscales and participants’ characteristics
Table 1 presents participants’ demographic and clinical characteristics, and the relationship between these characteristics and IMP-INS. VAGUS-SR Average Total score was positively associated with SAI-E, BCIS composite, and BCIS self-reflectiveness subscale scores. Premorbid IQ was positively associated with VAGUS-SR Symptom Attribution subscale and negatively associated with BCIS self-certainty subscale scores. Female gender was associated with lower VAGUS-SR Awareness of Need for Treatment scores. Of these, only the positive association between VAGUS-SR and SAI-E survived Bonferroni correction for multiple comparisons.
Table 1.
Relationship between baseline VAGUS-SR, subscales and participant characteristics.
| VAGU S-SR Total |
p- value |
Illness Awareness |
p- value |
Symptom Attribution |
p- value |
Treatment Awareness |
p- value |
Negative Consequences |
p- value |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 16 | ||||||||||
| Male:Female1 | 12:4 | z=−1.64 | 0.101 | z=−0.43 | 0.669 | z=−0.12 | 0.903 | z=−2.51 | 0.012* | z=−0.26 | 0.798 |
| Schizophrenia:Schizoa ffective1 | 9:7 | z=−2.05 | 0.040* | z=−1.38 | 0.169 | z=−1.58 | 0.115 | z=−2.81 | 0.005* | z=−1.49 | 0.135 |
| Clozapine:Non-clozapine | 4:12 | z=−0.97 | 0.332 | z=−1.41 | 0.160 | z=1.03 | 0.302 | z=−0.18 | 0.854 | z=−0.45 | 0.655 |
| Mean (SD) | r | r | r | r | r | ||||||
| Age, range (years) | 43.1 (11.6), 26–62 | −0.13 | 0.639 | 0.06 | 0.814 | 0.05 | 0.869 | 0.08 | 0.761 | −0.27 | 0.307 |
| Age of illness onset | 28.3 (10.5) | −0.34 | 0.204 | −0.19 | 0.481 | −0.30 | 0.253 | −0.37 | 0.162 | 0.07 | 0.803 |
| Duration (years) of Illness | 14.9 (10.5) | 0.35 | 0.191 | 0.26 | 0.329 | 0.35 | 0.179 | 0.46 | 0.074 | −0.37 | 0.160 |
| Antipsychotic CPZ equivalents | 272.6 (124.6) | 0.21 | 0.432 | 0.10 | 0.711 | 0.10 | 0.725 | 0.24 | 0.380 | −0.01 | 0.977 |
| Education | 14.1 (2.0) | −0.03 | 0.901 | 0.15 | 0.569 | 0.28 | 0.289 | −0.00 | 0.994 | −0.13 | 0.627 |
| Test score | |||||||||||
| Premorbid IQ (WRAT-3) | 102.6 (11.5) | 0.26 | 0.330 | 0.31 | 0.238 | 0.51 | 0.042* | −0.15 | 0.592 | 0.06 | 0.833 |
| Degree of Right Handedness2 | 85.6 (22.9) | 0.20 | 0.476 | −0.20 | 0.470 | 0.43 | 0.113 | −0.03 | 0.927 | 0.60 | 0.017* |
| Insight into Illness | |||||||||||
| SAI-E Total (max = 28), range | 12.3 (4.0), 5–19 | 0.86 | 0.0003** | 0.75 | 0.001** | 0.38 | 0.142 | 0.75 | 0.004* | −0.36 | 0.171 |
| VAGUS-SR Total Average3 | 5.9 (1.5) | - | - | 0.70 | 0.002* | 0.57 | 0.022* | 0.79 | 0.0003 | −0.20 | 0.460 |
| VAGUS-SR Illness Awareness3 | 5.5 (3.6) | 0.70 | 0.002* | - | - | 0.20 | 0.459 | 0.44 | 0.089 | −0.48 | 0.057 |
| VAGUS-SR Symptom Attribution3 | 3.2 (1.8) | 0.57 | 0.022* | 0.20 | 0.459 | - | - | 0.36 | 0.176 | −0.17 | 0.530 |
| VAGUS-SR Treatment Awareness3 | 6.9 (3.2) | 0.79 | 0.0003** | 0.44 | 0.089 | 0.36 | 0.176 | - | - | −0.49 | 0.057 |
| VAGUS-SR Negative Consequences3 | 8.2 (2.6) | −0.20 | 0.460 | −0.48 | 0.057 | −0.17 | 0.530 | −0.49 | 0.057 | - | - |
| Psychotic/Positive Symptoms | |||||||||||
| SAPS Summary (max = 20) | 6.8 (3.2) | −0.15 | 0.593 | 0.01 | 0.964 | 0.31 | 0.243 | −0.22 | 0.420 | −0.25 | 0.357 |
| Negative Symptoms | |||||||||||
| SANS Summary score (max = 25) | 6.9 (2.9) | 0.05 | 0.861 | −0.02 | 0.954 | 0.45 | 0.079 | −0.41 | 0.113 | 0.32 | 0.225 |
| Cognitive Insight | |||||||||||
| Self-reflectiveness | 10.9 (5.4) | 0.52 | 0.039* | 0.39 | 0.138 | 0.31 | 0.242 | 0.24 | 0.373 | 0.09 | 0.746 |
| Self-certainty | 7.9 (4.2) | −0.46 | 0.075 | −0.03 | 0.916 | −0.58 | 0.020* | −0.32 | 0.232 | −0.12 | 0.653 |
| Composite score | 3.1 (8.2) | 0.58 | 0.019* | 0.27 | 0.312 | 0.50 | 0.049 | 0.32 | 0.227 | 0.12 | 0.656 |
| Nystagmus PSPV | |||||||||||
| Body temperature, range | 4.2 (5.2), 0–17.1 | −0.09 | 0.730 | −0.14 | 0.615 | 0.14 | 0.611 | −0.20 | 0.449 | 0.22 | 0.407 |
| Left cold (4°C), range | 47.1 (17.6), 21.7–78.5 | −0.49 | 0.057 | −0.17 | 0.524 | −0.26 | 0.325 | −0.65 | 0.006* | 0.20 | 0.460 |
| Right cold (4°C), range | 49.0 (17.3), 17.6–83.5 | −0.45 | 0.082 | −0.26 | 0.331 | −0.01 | 0.980 | −0.45 | 0.079 | 0.01 | 0.972 |
| Left-Right cold asymmetry, range | −2.1 (24.9), −44.6–45.2 | −0.05 | 0.861 | 0.09 | 0.748 | −0.33 | 0.214 | −0.16 | 0.564 | 0.18 | 0.496 |
SCZ, schizophrenia; SCZ-AFF, schizoaffective disorder; IQ, intelligence quotient; WRAT-III, Wide Range Achievement Test, 3rd Edition; SAI-E, Schedule for the Assessment of Insight - Extended Version; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; PSPV, nystagmus peak slow phase velocity
Mann-Whitney U test
Edinburgh Handedness Inventory
For the VAGUS Total score and each domain score the maximum is 10.
p≤0.05
p<0.0004, after Bonferroni correction for multiple comparisons (0.05/125)
3.2. Caloric vestibular stimulation
Significant interactions were found between CVS condition (i.e. Left cold CVS, Right cold, and Body temperature CVS), time (i.e. pre- and 30 min post-CVS), and Body temperature PSPV for the VAGUS-SR Average Total score, which was largely driven by the effects of CVS condition on the Illness Awareness subscale. As such, below we present the results for the effects of CVS condition on the VAGUS-SR Average Total score and the Illness Awareness subscale score. The results for the effects of CVS condition on Symptom Attribution, Awareness of Need for Treatment, and Awareness of Negative Consequences subscales are available as supplementary materials. For all three-way ANOVAs, the Greenhouse-Geisser correction was used when the assumption of sphericity was violated (i.e. Mauchly’s test of sphericity<0.05). The adjusted degrees of freedom were reported only when the assumption of sphericity was violated.
3.2.1. VAGUS-SR Average Total score
There was no statistically significant interaction between CVS condition and time (F(2,30)=0.53, p=0.595). There was a significant interaction between CVS condition, time, and Body temperature PSPV (F(2,28)=7.70, p=0.002).
There was no effect of order (F(2,28)=2.42, p=0.074), Body temperature CVS laterality (F(2,28)=0.97, p=0.392), Left cold PSPV (F(2,28)=0.25, p=0.780), or Right cold PSPV (F(2,28)=0.65, p=0.530).
The pairwise analyses between conditions revealed a significant interaction between Left cold vs. Right cold CVS, Time, and Body temperature PSPV (F(1,14)=18.11, p=0.001), and Right cold vs. Body temperature CVS, Time, and Body temperature PSPV (F(1,14)=5.02, p=0.042), indicating Left cold and Body temperature CVS improved IMP-INS in relation to Right cold CVS in those with higher Body temperature PSPV. There was no interaction between Left cold vs. Body temperature CVS, Time, and Body temperature PSPV (F(1,10)=1.57, p=0.231).
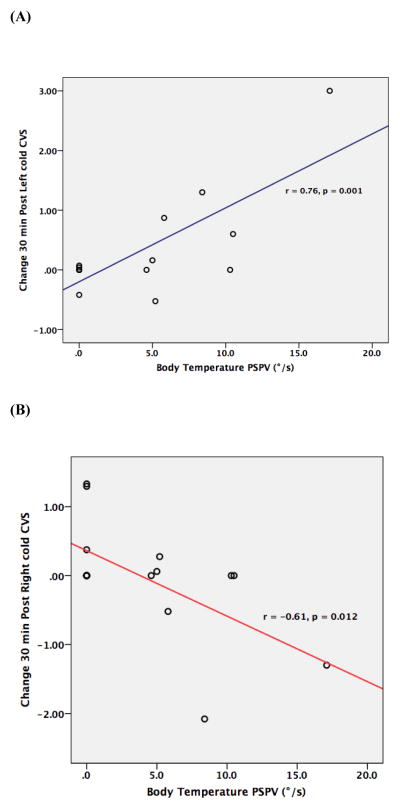
Table 2 and Figure 1 present the mean changes and effect sizes for the VAGUS-SR Average Total scores at 30 min post CVS, and the relationships with nystagmus PSPV. The change in VAGUS-SR Average Total score 30 min post Left cold CVS was significant and positively associated with Body temperature PSPV. Conversely, the change in VAGUS-SR Average Total score 30 min post Right cold CVS was significant and negatively associated with Body temperature PSPV, but not after correction for multiple comparisons. No association was found between VAGUS-SR change 30 min post CVS and Left or Right cold PSPV.
Table 2.
Relationship between changes in VAGUS-SR Average Total scores and vestibular reactivity to CVS.
| VAGUS-SR | Effect sizea | Body Temperature PSPV | Left cold PSPV | Right cold PSPV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Δ (SD) | Cohen’s d | B | ηp2 | r | p-value | r | p-value | r | p-value | |
| Average Total | ||||||||||
| Δ 30 min post Sham CVS | 0.09 (0.92) | 0.05 | 0.06 | 0.13 | 0.36 | 0.169 | 0.09 | 0.754 | 0.19 | 0.488 |
| Δ 30 min post Left cold CVS | 0.32 (0.84) | 0.14 | 0.12 | 0.58 | 0.76 | 0.001** | 0.06 | 0.833 | −0.28 | 0.303 |
| Δ 30 min post Right cold CVS | −0.04 (0.81) | 0.02 | −0.10 | 0.37 | −0.61 | 0.012* | −0.20 | 0.459 | 0.07 | 0.797 |
| General Illness Awareness | ||||||||||
| Δ 30 min post Sham CVS | 0.03 (0.81) | 0.01 | 0.05 | 0.09 | 0.30 | 0.262 | −0.34 | 0.201 | −0.02 | 0.938 |
| Δ 30 min post Left cold CVS | 0.63 (1.5) | 0.18 | 0.19 | 0.47 | 0.69 | 0.003** | 0.05 | 0.859 | −0.14 | 0.612 |
| Δ 30 min post Right cold CVS | −0.13 (1.4) | 0.01 | −0.04 | 0.02 | −0.13 | 0.625 | 0.10 | 0.702 | 0.13 | 0.635 |
VAGUS-SR, VAGUS–Self-report; CVS, caloric vestibular stimulation; PSPV, nystagmus peak slow phase velocity
Cohen’s d values do not account for Body temperature PSPV, while Beta coefficient and partial eta squared values include Body temperature PSPV as a covariate.
p<0.05
p≤0.004, after Bonferroni correction for multiple comparisons (0.05/12)
Figure 1. The relationship between the change in VAGUS-SR Average Total scores at 30 min post CVS with Body temperature peak slow phase velocity (PSPV), a physiological marker of vestibular stimulation reactivity.
(A) Left cold CVS. Improvement in insight into illness with Left cold CVS occurs in relation to increased vestibular reactivity.
(B) Right cold CVS. Greater impairment of insight into illness with Right cold CVS occurs in relation to increased vestibular reactivity.
3.2.2. VAGUS-SR Illness Awareness subscale
There was no interaction between CVS condition and time (F(1.31,19.57)=1.57, p=0.230). There was a significant interaction between CVS condition, time, and Body temperature PSPV (F(1.34,18.68)=4.14, p=0.046).
There was no effect of order (F(2.77,18.02)=1.59, p=0.229), Body temperature laterality (F(1.28,18.02)=0.72, p=0.443), Left cold PSPV (F(1.24,17.39)=0.451, p=0.553) or Right cold PSPV (F(1.33,18.56)=1.01, p=0.353).
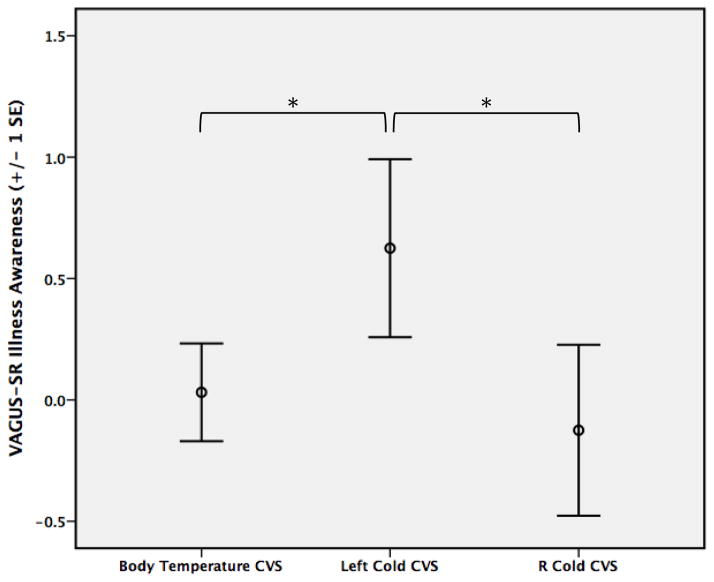
The pairwise analyses between conditions revealed a significant interaction between Left cold vs. Body temperature CVS, Time, and Body temperature PSPV (F(1,14)=7.72, p=0.015), and Left cold vs. Right cold CVS, Time, and Body temperature PSPV (F(1,14)=4.90, p=0.044), suggesting improved illness awareness with Left cold CVS and Body temperature CVS vs. Right cold CVS in those with higher Body temperature PSPV (Figure 2). There was no interaction between Right cold vs. Body temperature CVS, Time, and Body temperature PSPV (F(1,14)=1.12, p=0.308).
Figure 2. Differences in mean change in VAGUS-SR Illness.
Awareness scores at 30 min post CVS for each condition. Left Cold CVS > R Cold (F(1,14)=4.896, p=0.044) and Left Cold CVS > Body temperature CVS (F(1,14)=7.722, p=0.015) when accounting for vestibular reactivity as measured by Body temperature nystagmus peak slow phase velocity (PSPV). * = Significant difference (p<0.05) between conditions.
Table 2 and Figure 1 present the mean changes and effect sizes for the VAGUS-SR Illness Awareness subscale scores at 30 min post CVS, and the relationships with nystagmus PSPV. The change in VAGUS-SR Illness Awareness subscale score 30 min post Left cold CVS was significant and positively associated with Body temperature PSPV. No association was found between VAGUS-SR change 30 min post CVS and Left or Right cold PSPV.
3.2.3. Mood and positive symptom severity
There was no interaction between CVS condition and time for changes in mood or positive symptom severity. Although, the direction of response for mood and positive symptom severity was similar to changes in IMP-INS, i.e. at 30 min post CVS, Left cold CVS was accompanied by mood and positive symptom improvement while Right cold CVS was accompanied by worsening mood and positive symptom severity.
3.3. Baseline predictors of change in insight into illness with Left Cold CVS
Only illness duration (r=0.52, p=0.023) was associated with VAGUS-SR Average Total score change at 30 min post Left cold CVS, but not after Bonferroni correction for multiple comparisons (p<0.0004).
3.4 Baseline predictors of Body temperature PSPV
Only WRAT-3 (r=0.49, p=0.032) was associated with Body temperature PSPV, but not after Bonferroni correction for multiple comparisons (p<0.001).
3.5 Adverse effects
CVS was well tolerated by all of the participants with expected transient symptoms of dizziness/vertigo and minor nausea (<5 minutes) in relation to the vestibular stimuli. No participants vomited. One participant discontinued the study after a single Right cold CVS condition for undisclosed reasons.
4. Discussion
Only a few prior case studies exist exploring the effects of CVS on IMP-INS in schizophrenia spectrum and bipolar disorder (Dodson, 2004; Levine et al., 2012). These reports consistently suggest left cold CVS may be a means to improve IMP-INS and stabilize mood in schizophrenia and bipolar disorder, respectively. This is the first study we are aware of to systematically address this through an exploratory randomized controlled approach in participants with a schizophrenia spectrum disorder. Our main results indicate that single session left cold CVS (iced water at 4°C) transiently improves IMP-INS while right cold CVS may worsen IMP-INS. These converse effects of left and right cold CVS were most evident in participants with greater vestibular reactivity to body temperature PSPV (Table 2 and Figures 1 and 2). Intriguingly, 37°C CVS should not theoretically produce a significant vestibuloocular response due to the stimulus’ approximation to body temperature (Schmal et al., 2005). In schizophrenia, there is no conclusive link between psychopathology and vestibular dysfunction (Levy et al., 1983), and we are not aware of any data that exists on the range of responses to body temperature CVS in persons with schizophrenia. Increased vestibular reactivity to 37°C CVS in schizophrenia spectrum disorders may represent heightened sensitivity to subtle temperature differences between body temperature and the 37°C stimulus. Alternatively, increased vestibular reactivity may be related to a failure of normal frontal VOR suppression akin to frontal dysfunction manifesting clinically as frontal release signs/primitive reflexes (e.g. Glabellar reflex, snout reflex, etc.). Studies are required to compare vestibular reactivity between schizophrenia spectrum disorder patients and a healthy comparison group. The preliminary results from the present study suggest increased reactivity may predict response to CVS (Figure 1).
We did not observe differences between treatment conditions for mood or positive symptom severity; however, the direction of response was similar to changes in IMP-INS, i.e. at 30 min post CVS, Left cold CVS was accompanied by mood and positive symptom improvement while Right cold CVS was accompanied by worsening mood and positive symptom severity.
The vestibular system is the principal balance system of the body. It integrates sensory inputs in a complex bilaterally organized neuronal network involving the peripheral and central nervous system (Dieterich and Brandt, 2008; Pfeiffer et al., 2014). This system receives information from the vestibular organs (the utricle and saccule) located bilaterally in the inner ear. Together, these organs perceive head position, movement in three-dimensional space, and drive the VOR. The VOR integrates multiple brain structures, including brainstem nuclei, the thalamus, cerebellum, spinal cord and a number of cortical areas, including the temporoparietal vestibular cortex, insula, superior temporal gyrus, inferior parietal lobule, precuneus, inferior frontal gyrus, hippocampus, and anterior cingulate cortex (Dieterich and Brandt, 2008). The VOR is elicited in opposite directions by warm and cold CVS, with greater temperature differences from body temperature producing stronger stimuli. For example, warm water (44°C) causes an increased rate of firing in the vestibular afferent nerve, mimicking a head turn to the ipsilateral side. The eyes will turn toward the contralateral ear, with horizontal nystagmus (quick horizontal eye movements) toward the ipsilateral ear. Conversely, cold water (≤30°C) decreases the rate of vestibular afferent firing, causing the eyes to turn toward the ipsilateral ear, with horizontal nystagmus to the contralateral ear (Bottini, 1994; Naito, 2003; Suzuki, 2001). Stronger stimuli can be produced with cold calorics (i.e. 4°C produces a temperature gradient of 33°C) than with warm calorics (i.e. 44°C produces a maximum temperature gradient of 7°C) due to the greater temperature gradients that can be safely obtained with colder temperatures (33°C vs. 7°C) (Batuecas-Caletrio et al., 2009; Schmal et al., 2005). Warm and cold CVS activate the cerebral cortex via ascending cortical vestibular projections, but with opposite effects. Functional imaging studies demonstrate that warm CVS primarily stimulates the ipsilateral brain hemisphere (Dieterich et al., 2003), while cold CVS predominantly stimulates the contralateral hemisphere. Specifically, cold CVS of the left ear activates right hemisphere brain regions, including the temporoparietal junction, posterior insula, putamen, anterior cingulate, and primary somatosensory cortex (Bottini, 1994; Naito, 2003; Suzuki, 2001). Our results suggest that the effectiveness of left cold CVS to improve IMP-INS in schizophrenia may be due to the stimulation of the ‘underactive’ right hemisphere circuits in regions implicated in IMP-INS in schizophrenia spectrum disorders (e.g. posterior parietal and medial prefrontal cortex) (Gerretsen et al., 2015; Gerretsen et al., 2014a; Levine et al., 2012) via cortical vestibular projections from the thalamus and brain stem vestibular nuclei re-establishing hemispheric balance (Ramachandran, 1996). According with this mechanism, right cold CVS’s worsening of IMP-INS is possibly due to overactivation of the left hemisphere, contributing to left hemisphere dominance (Arshad et al., 2014). In the present study, the physiological effects of CVS were demonstrated by measuring the VOR (i.e. nystagmus PSPV), suggesting that the changes in IMP-INS were secondary to the brain’s response to CVS.
Although there is no direct evidence linking vestibular pathology with psychosis or other psychiatric conditions, there appears to be a bidirectional relationship between vestibular dysfunction and affective symptoms (Gurvich et al., 2013). That is, many patients with vestibular dysfunction have co-morbid anxiety, depression, agoraphobia, and dissociative or spatial disturbances (e.g. altered perceptions of self, derealization, out of body experiences); whereas, symptoms associated with the vestibular system (e.g. dizziness), are frequent symptoms of anxiety and affective disorders (Jauregui Renaud, 2015; Staab, 2012). This interaction may be attributable to the well-described connections between the vestibular system and varied brain regions, both cortically and subcortically, associated with emotional and cognitive processing, in particular spatial abilities (Gurvich et al., 2013). CVS, appears to take advantage of these diffuse connections with prior studies indicating CVS modulates mood, affective control (Preuss et al., 2014a), and pain perception (Ferre et al., 2015) in healthy participants. CVS may also influencing decision making by attenuating the rewarding effects of acquiring items (Preuss et al., 2014b). Further, CVS appears to effect body schema and perception (Lopez et al., 2012; Schonherr and May, 2016). Taken together, vestibular stimulation modulates space, body and self-awareness, and somatosensory and emotional processing, highlighting the fundamental contribution of the vestibular system to self-body integration and promoting an egocentric frame of reference (Lopez, 2016).
Our study is limited by the inclusion of only those participants with moderately IMP-INS. Although this study was designed to include participants with moderate to severe IMP-INS, for the most part, participants were clinically stable with minimal positive symptoms and good awareness of the need for treatment (Table 1). That being said, minimal symptom severity helps ensure the specificity of CVS for IMP-INS as symptom severity and IMP-INS are strongly correlated (Gerretsen et al., 2014b). Relatedly, it is difficult to determine the clinical significance of impaired illness awareness in a sample that accepts the need for treatment. Arguably, Awareness of Need for Treatment is the domain of insight into illness that has the greatest influence on clinical outcomes. Future studies should attempt to include acutely ill patients with schizophrenia and those with greater impairment of awareness of the need for treatment. However, the limited treatment decision-making capacity that frequently accompanies acute psychosis represents a challenge for study participation. Our study is also limited by the short period of observation (i.e. 30 min) post CVS condition. It is possible that the effects of single session CVS may have persisted for longer than 30 min duration, as was observed in a case of difficult to treat mania where sustained beneficial effects were observed for approximately 24 hours (Dodson, 2004). However, a case series of 3 patients with a schizophrenia spectrum disorder (one with schizoaffective disorder, current episode manic, and two with schizophrenia) found the effect to last 20 minutes, but not more than 60 minutes (Levine et al., 2012), which is generally consistent with the duration of effect observed in patients with anosognosia attributable to stroke (Cappa, 1987). Other limitations of our study include the modest sample size and short study duration (i.e. single session). Nonetheless, the within subject design and the positive results can be used to justify larger randomized clinical trials using a multisession approach.
In conclusion, single session left cold CVS transiently improved IMP-INS in participants with schizophrenia spectrum disorders, particularly in those with greater vestibular system reactivity to body temperature CVS. Future studies should consider other methods of vestibular stimulation, such as galvanic vestibular stimulation (GVS) (Lopez, 2016), including subliminal (subsensory) GVS, which reportedly does not produce nystagmus or vertigo (Oppenlander et al., 2015), or air caloric vestibular stimulation (Zapala et al., 2008), to determine if results are replicable across treatment approaches. Future studies should also employ multisession designs (e.g. 10 consecutive days) to determine if repeated CVS can lead to sustained improvement in IMP-INS in schizophrenia. The addition of noninvasive neurostimulation, such as transcranial direct current stimulation or transcranial magnetic stimulation, should also be considered to determine if it can facilitate the effects of CVS (Arshad et al., 2014; Been, 2007; Daskalakis et al., 2006; George et al., 1999). If proven effective, this easy to administer, safe, non-invasive intervention would have the potential to alter individuals’ attitude towards their illness and medication, leading ultimately to an improvement in individuals’ capacity for illness recognition and engagement in treatment, which would in turn, significantly impact the management of this devastating mental disorder.
5. Patient perspective
Participant #9 was a 29-year-old single man in a common-law relationship with a diagnosis of schizophrenia. He was on long-term disability from his job as a customer service representative for a government agency. He was no longer able to work at his previous position due to unrelenting critical auditory hallucinations he experienced over the telephone, which he confused for customer complaints. He had accompanying persistent vague persecutory delusions that his employer and colleagues were conspiring against him and self-recrimination for not being able to decipher God’s intended mission for him. He maintained he did not have a major mental illness and the veracity of his psychotic experiences. He was prescribed aripirazole 20 mg/day. Although ambivalent about whether or not he should be taking an antipsychotic medication, he subjectively felt the aripiprazole alleviated the intensity of his auditory hallucinations and persecutory delusions, and improved his sleep.
In response to left cold CVS, the patient reported a change in the certainty he had about his illness beliefs, stating, “I’m more unsure [whether I have schizophrenia or not]. My thoughts are racing. I’m going back and forth”. Prior to the stimulus he was certain that he was mentally well and did not have schizophrenia. He appeared elated, laughed, and said, “That was an upper…I feel giddy. I feel more self-confident”. Shortly thereafter, he unsatisfyingly disclosed, “I can’t believe I feel better from a stupid jet of water than from my treatment”. In relation to his symptoms, he continued to misattribute his delusional beliefs to objective reality, whereas with regard to his treatment he felt he was more “certain [he] should take his antipsychotic medication”. Conversely, in response to right cold CVS, the patient described the procedure as “disappointing” because of the lack of improvement in his mood, and added, “I know this is silly, but I feel kind of sad”. His affect was congruently dysphoric. The improvement in the participant’s illness awareness and acceptance of treatment had returned to baseline.
Supplementary Material
Highlights.
Single session left cold (4°C) caloric vestibular stimulation (CVS) transiently improved impaired insight into illness (IMP-INS) while right cold CVS appeared to worsen IMP-INS, particularly in participants with greater vestibular reactivity to body temperature CVS.
The procedure’s effectiveness for IMP-INS in schizophrenia and other conditions that feature IMP-INS (e.g. stroke) is attributed to stimulation of underactive right hemisphere circuits via vestibular nuclei projections to the contralateral hemisphere.
Future studies in schizophrenia spectrum disorders should consider: (1) multi-session designs (e.g. 10 consecutive days) to determine if repeated CVS can lead to sustained improvement in IMP-INS; and (2) other methods of vestibular stimulation, such as galvanic vestibular stimulation or air caloric vestibular stimulation, to determine if results are replicable across treatment approaches.
Acknowledgments
Funding Sources
The research was partially supported by Ontario Mental Health Foundation grant (OMHF)–Type A Grant (AG), National Institutes of Health RO1MH084886-01A2 (AG and DM), the Clinician Scientist Program, Department of Psychiatry, University of Toronto (PG), and by Canadian Institutes of Health Research (CIHR), OMHF and Centre for Addiction and Mental Health (CAMH) fellowship awards (PG).
Wanna Mar, research coordinator; Kathryn Kalahani-Bargis, co-op student; Matt Kim, volunteer; and Zhe Feng, volunteer.
Footnotes
Disclosures
P Gerretsen reports receiving fellowship awards from CIHR, OMHF and CAMH.
DD Pothier reports no conflicts of interest.
C Falls reports no conflicts of interest.
M Armstrong reports no conflicts of interest.
T Balakumar reports no conflicts of interest.
D Mamo has received research support from the NIH and the Canadian Institutes of Health Research. He has received investigator-initiated research support from Pfizer Canada over the past three years.
H Uchida has received grants from Astellas Pharmaceutical, Eisai, Otsuka Pharmaceutical, GlaxoSmithKline, Shionogi, Dainippon-Sumitomo Pharma, Eli Lilly, Mochida Pharmaceutical, Meiji-Seika Pharma, and Yoshitomi Yakuhin and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Shionogi, Pfizer, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma, Meiji-Seika Pharma, Abbvie, MSD, and Janssen Pharmaceutical within the past three years.
BG Pollock receives research support from the National Institute of Health and the Canadian Institutes of Health Research.
A Graff has received support from Brain Canada, Canadian Foundation for Innovation, Canadian Institutes of Health Research (CIHR), Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), Ontario Mental Health Foundation (OMHF), Consejo Nacional de Ciencia y Tecnologia (CONACyT), Instituto de Ciencia y Tecnología del DF (ICyTDF), and Brain & Behavior Research Foundation. Dr. Graff-Guerrero reports no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, Gorman JM. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 1994;51:826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Dept of Psychiatry, University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49–58. [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Roberts RE, Bhrugubanda V, Asavarut P, Bronstein AM. Left cathodal trans-cranial direct current stimulation of the parietal cortex leads to an asymmetrical modulation of the vestibular-ocular reflex. Brain Stimul. 2014;7(1):85–91. doi: 10.1016/j.brs.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM. The effects of vestibular stimulation on verbalization in chronic schizophrenics. Am J Occup Ther. 1978;32(7):445–450. [PubMed] [Google Scholar]
- Banchi CA, Beattie RC. Effects of stimulus duration on air caloric slow phase velocity. J Am Acad Audiol. 1991;2(4):246–252. [PubMed] [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257(1):58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Batuecas-Caletrio A, Montes-Jovellar L, Boleas-Aguirre MS, Perez-Fernandez N. The ice-water caloric test. Acta Otolaryngol. 2009;129(12):1414–1419. doi: 10.3109/00016480902791686. [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2–3):319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Been G, Ngo TT, Miller SM, Fitzgerald PB. The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res Rev. 2007;56:346–361. doi: 10.1016/j.brainresrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Berge D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123(6):431–439. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Rusconi ML, Vallar G. Remission of somatoparaphrenic delusion through vestibular stimulation. Neuropsychologia. 1991;29:1029–1031. doi: 10.1016/0028-3932(91)90066-h. [DOI] [PubMed] [Google Scholar]
- Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, Passingham RE, Frith CD, Frackowiak RS. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res. 1994;99:164–169. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Wirshing DA, Bhushan P, Pierre JM, Resnick SA, Wirshing WC. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21:129–141. doi: 10.2165/00023210-200721020-00004. [DOI] [PubMed] [Google Scholar]
- Cappa S, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987;25:775–782. doi: 10.1016/0028-3932(87)90115-1. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Basu D. Insight in schizophrenia-A comprehensive update. German Journal of Psychiatry. 2010;13(1):17–30. [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res. 2008;103(1–3):40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174(3):403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131(Pt 10):2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
- Dodson MJ. Vestibular stimulation in mania: a case report. J Neurol Neurosurg Psychiatry. 2004;75(1):168–169. [PMC free article] [PubMed] [Google Scholar]
- Ferre ER, Haggard P, Bottini G, Iannetti GD. Caloric vestibular stimulation modulates nociceptive evoked potentials. Exp Brain Res. 2015;233(12):3393–3401. doi: 10.1007/s00221-015-4412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish B, Dixon WJ. Vestibular hyporeactivity in infants at risk for schizophrenia. Association with critical developmental disorders. Arch Gen Psychiatry. 1978;35(8):963–971. doi: 10.1001/archpsyc.1978.01770320057004. [DOI] [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13(2):255–257. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry. 1999;56(4):300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK, Graff-Guerrero A. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp. 2012 doi: 10.1002/hbm.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Chakravarty MM, Lerch JP, Mamo DC, Remington G, Pollock BG, Graff-Guerrero A. Illness denial in schizophrenia spectrum disorders: a function of left hemisphere dominance. Hum Brain Mapp. 2015;36(1):213–225. doi: 10.1002/hbm.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: Resting state functional connectivity. Schizophr Res. 2014a;160(1–3):43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Plitman E, Rajji TK, Graff-Guerrero A. The effects of aging on insight into illness in schizophrenia: a review. Int J Geriatr Psychiatry. 2014b;29(11):1145–1161. doi: 10.1002/gps.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Remington G, Borlido C, Quilty L, Hassan S, Polsinelli G, Teo C, Mar W, Simon R, Menon M, Pothier DD, Nakajima S, Caravaggio F, Mamo DC, Rajji TK, Mulsant BH, Deluca V, Ganguli R, Pollock BG, Graff-Guerrero A. The VAGUS insight into psychosis scale - Self-report and clinician-rated versions. Psychiatry Res. 2014c;220(3):1084–1089. doi: 10.1016/j.psychres.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich C, Maller JJ, Lithgow B, Haghgooie S, Kulkarni J. Vestibular insights into cognition and psychiatry. Brain Res. 2013;1537:244–259. doi: 10.1016/j.brainres.2013.08.058. [DOI] [PubMed] [Google Scholar]
- Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132(3):251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;22:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Jauregui Renaud K. Vestibular Function and Depersonalization/Derealization Symptoms. Multisens Res. 2015;28(5–6):637–651. doi: 10.1163/22134808-00002480. [DOI] [PubMed] [Google Scholar]
- Kavanagh KT, Babin RW. Definitions and types of nystagmus and calculations. Ear Hear. 1986;7(3):157–166. doi: 10.1097/00003446-198606000-00007. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Levine J, Toder D, Geller V, Kraus M, Gauchman T, Puterman M, Grisaru N. Beneficial effects of caloric vestibular stimulation on denial of illness and manic delusions in schizoaffective disorder: a case report. Brain Stimul. 2012;5(3):267–273. doi: 10.1016/j.brs.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Proctor LR. Vestibular responses in schizophrenia. Arch Gen Psychiatry. 1978;35(8):972–981. doi: 10.1001/archpsyc.1978.01770320066005. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Proctor LR. Vestibular dysfunction and psychopathology. Schizophr Bull. 1983;9(3):383–438. doi: 10.1093/schbul/9.3.383. [DOI] [PubMed] [Google Scholar]
- Lopez C. The vestibular system: balancing more than just the body. Curr Opin Neurol. 2016;29(1):74–83. doi: 10.1097/WCO.0000000000000286. [DOI] [PubMed] [Google Scholar]
- Lopez C, Schreyer HM, Preuss N, Mast FW. Vestibular stimulation modifies the body schema. Neuropsychologia. 2012;50(8):1830–1837. doi: 10.1016/j.neuropsychologia.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS. Insight, grey matter and cognitive function in first-onset psychosis. Br J Psychiatry. 2010;197:141–148. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- Naito Y, Tateya I, Hirano S, Inoue M, Funabiki K, Toyoda H, Ueno M, Ishizu K, Nagahama Y, Fukuyama H, Ito J. Cortical correlates of vestibulo-ocular reflex modulation: a PET study. Brain. 2003;126:1562–1578. doi: 10.1093/brain/awg165. [DOI] [PubMed] [Google Scholar]
- O’Donnell C, Donohoe G, Sharkey L, Owens N, Migone M, Harries R, Kinsella A, Larkin C, O’Callaghan E. Compliance therapy: a randomised controlled trial in schizophrenia. BMJ. 2003;327(7419):834. doi: 10.1136/bmj.327.7419.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Wilk J, West JC. Awareness of illness and nonadherence to antipsychotic medications among persons with schizophrenia. Psychiatr Serv. 2006;57:205–211. doi: 10.1176/appi.ps.57.2.205. [DOI] [PubMed] [Google Scholar]
- Opler LA, Caton CL, Shrout P, Dominguez B, Kass FI. Symptom profiles and homelessness in schizophrenia. J Nerv Ment Dis. 1994;182:174–178. doi: 10.1097/00005053-199403000-00008. [DOI] [PubMed] [Google Scholar]
- Oppenlander K, Keller I, Karbach J, Schindler I, Kerkhoff G, Reinhart S. Subliminal galvanic-vestibular stimulation influences ego- and object-centred components of visual neglect. Neuropsychologia. 2015;74:170–177. doi: 10.1016/j.neuropsychologia.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14(2):203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Pekkala E, Merinder L. Psychoeducation for schizophrenia. Cochrane Database Syst Rev. 2002;(2):CD002831. doi: 10.1002/14651858.CD002831. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C, Serino A, Blanke O. The vestibular system: a spatial reference for bodily self-consciousness. Front Integr Neurosci. 2014;8:31. doi: 10.3389/fnint.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss N, Hasler G, Mast FW. Caloric vestibular stimulation modulates affective control and mood. Brain Stimul. 2014a;7(1):133–140. doi: 10.1016/j.brs.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Preuss N, Mast FW, Hasler G. Purchase decision-making is modulated by vestibular stimulation. Front Behav Neurosci. 2014b;8:51. [Google Scholar]
- Ramachandran VS. Anosognosia in parietal lobe syndrome. Conscious Cogn. 1995;4(1):22–51. doi: 10.1006/ccog.1995.1002. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. The evolutionary biology of self-deception, laughter, dreaming and depression: some clues from anosognosia. Med Hypotheses. 1996;47:347–362. doi: 10.1016/s0306-9877(96)90215-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, McGeoch PD, Williams L. Can vestibular caloric stimulation be used to treat Dejerine-Roussy Syndrome? Med Hypotheses. 2007;69(3):486–488. doi: 10.1016/j.mehy.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Woerner MG, Alvir JM, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544–549. doi: 10.1176/ajp.156.4.544. [DOI] [PubMed] [Google Scholar]
- Schmal F, Lubben B, Weiberg K, Stoll W. The minimal ice water caloric test compared with established vestibular caloric test procedures. J Vestib Res. 2005;15(4):215–224. [PubMed] [Google Scholar]
- Schonherr A, May CA. Influence of Caloric Vestibular Stimulation on Body Experience in Healthy Humans. Front Integr Neurosci. 2016;10:14. doi: 10.3389/fnint.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry. 2007;19(4):437–446. doi: 10.1080/09540260701486324. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry. 2007;19:437–446. doi: 10.1080/09540260701486324. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode Schizophrenia. Neuroimage. 2004;22(3):1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86(1–3):54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Staab JP. Chronic subjective dizziness. Continuum (Minneap Minn) 2012;18(5 Neuro-otology):1118–1141. doi: 10.1212/01.CON.0000421622.56525.58. [DOI] [PubMed] [Google Scholar]
- Stroup S, Appelbaum P, Swartz M, Patel M, Davis S, Jeste D, Kim S, Keefe R, Manschreck T, McEvoy J, Lieberman J. Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res. 2005;80(1):1–8. doi: 10.1016/j.schres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001;2001:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52:805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Borum R, Hiday VA, Wagner HR, Burns BJ. Involuntary out-patient commitment and reduction of violent behaviour in persons with severe mental illness. Br J Psychiatry. 2000;176:324–331. doi: 10.1192/bjp.176.4.324. [DOI] [PubMed] [Google Scholar]
- Vallar G, Sterzi R, Bottini G, Cappa S, Rusconi ML. Temporary remission of left hemianesthesia after vestibular stimulation. A sensory neglect phenomenon. Cortex. 1990;26:123–131. doi: 10.1016/s0010-9452(13)80078-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading - UK Adaptation (WTAR—UK) The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Zapala DA, Olsholt KF, Lundy LB. A comparison of water and air caloric responses and their ability to distinguish between patients with normal and impaired ears. Ear Hear. 2008;29(4):585–600. doi: 10.1097/AUD.0b013e3181734ed0. [DOI] [PubMed] [Google Scholar]
- Zygmunt A, Olfson M, Boyer CA, Mechanic D. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653–1664. doi: 10.1176/appi.ajp.159.10.1653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.