Abstract
The adipocyte-released hormone-like cytokine/adipokine leptin behaves differently in obesity compared to its functions in the normal healthy state. In obese individuals, elevated leptin levels act as a pro-inflammatory adipokine and are associated with certain types of cancers. Further, a growing body of evidence suggests that higher circulating leptin concentrations and/or elevated expression of leptin receptors (Ob-R) in tumors may be poor prognostic factors. Although the underlying pathological mechanisms of leptin’s association with poor prognosis are not clear, leptin can impact the tumor microenvironment in several ways. For example, leptin is associated with a number of biological components that could lead to tumor cell invasion and distant metastasis. This includes interactions with carcinoma-associated fibroblasts, tumor promoting effects of infiltrating macrophages, activation of matrix metalloproteinases, transforming growth factor-β signaling, etc. Recent studies also have shown that leptin plays a role in the epithelial-mesenchymal transition, an important phenomenon for cancer cell migration and/or metastasis. Furthermore, leptin’s potentiating effects on insulin-like growth factor-I, epidermal growth factor receptor and HER2/neu have been reported. Regarding unfavorable prognosis, leptin has been shown to influence both adenocarcinomas and squamous cell carcinomas. Features of poor prognosis such as tumor invasion, lymph node involvement and distant metastasis have been recorded in several cancer types with higher levels of leptin and/or Ob-R. This review will describe the current scenario in a precise manner. In general, obesity indicates poor prognosis in cancer patients.
Keywords: Invasive cancer, Leptin, Metastasis, Obesity, Prognosis
Graphical Abstract
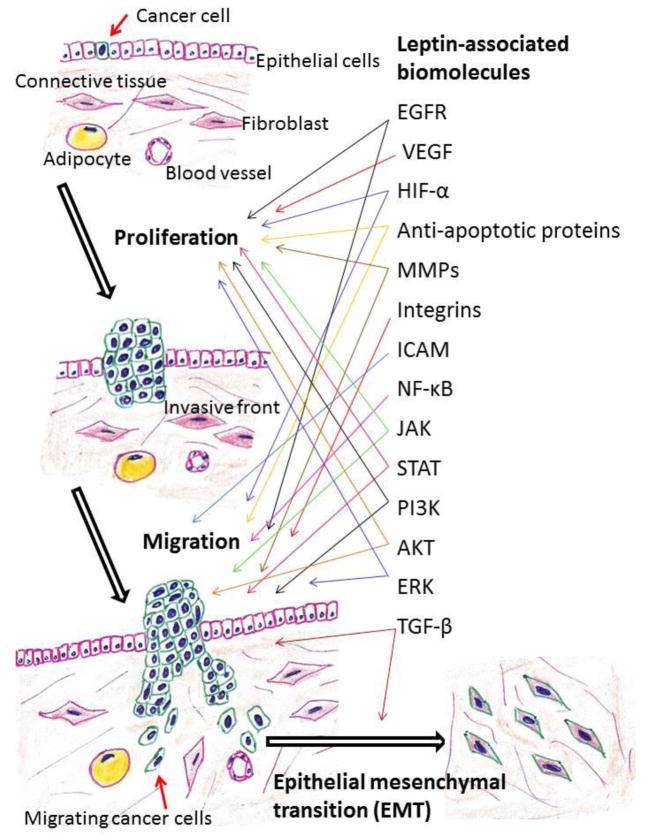
Leptin-linked Pathological Mechanisms in Tumor Proliferation and Migration
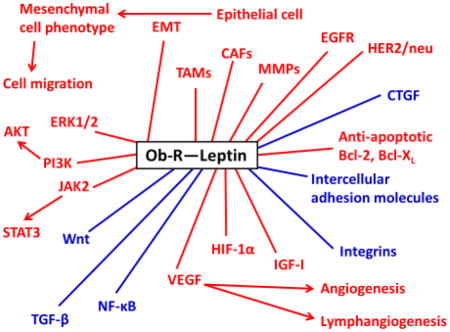
Red: Tumor-promoting effect
Blue: Dual role (tumor–suppressor or –promoting effect depending on the situation)
CAFs: Cancer-associated fibroblasts, CTGF: Connective tissue growth factor, EGFR: Epidermal growth factor receptor, EMT: Epithelial–mesenchymal transition, ERK1/2: Extracellular signal-regulated kinases 1 and 2, HER2: Human epidermal growth factor receptor 2, HIF-1α: Hypoxia-inducible factor-1 alpha, IGF-I: Insulin-like growth factor-I, JAK2: Janus kinase 2, MMPs: Matrix metalloproteinases, NF-κB: Nuclear factor-kappa B, Ob-R: Leptin receptor, PI3K: Phosphatidylinositol 3-kinase, STAT3: Signal transducer and activator of transcription 3, TAMs: Tumor-associated macrophages, TGF-β: Transforming growth factor-beta, VEGF: Vascular endothelial growth factor
1. Introduction
Obesity is associated with poor prognosis among cancer patients, apart from its link to other disease processes such as type-2 diabetes, cardiovascular disorders and increased risk of certain malignancies. Hormone-like cytokines or adipokines released from the expanded adipose tissue mass in obesity are thought to be responsible for these pathophysiological changes. One of the important adipokines is leptin, which may influence several biological processes in both normal and disease conditions. Leptin is a 16 kDa protein mainly synthesized by adipocytes that maintains energy homeostasis by influencing the central anorexigenic pathway [1]. However, in obesity, leptin displays diverse abnormal functions. Leptin acts via transmembrane receptors (Ob-R), which include at least 6 alternatively spliced isoforms. The long form Ob-Rb appears to be most important for leptin’s weight regulating effects.
The tumor microenvironment, which consists of extracellular matrix (ECM) and various populations of stromal cells such as fibroblasts, adipocytes and macrophages, may influence the behavior of cancer cells through expression of cytokines, growth factors, and proteases [2]. For instance, both cancer-associated fibroblasts (CAFs) and matrix metalloproteinases (MMPs) are involved in different phases of cancer progression including invasiveness, and metastasis [3,4]. In fact, cancer cells and CAFs crosstalk enhances the production of growth factors, cytokines, chemokines, MMPs, and inflammatory mediators, which eventually facilitates tumor growth [3]. Interestingly, leptin has been demonstrated to be closely associated with activities of CAFs and expression levels of MMPs [5–7]. On the other hand, many reports have revealed leptin’s link with a number of ECM components such as fibronectin, transforming growth factor-beta (TGF-β), and connective tissue growth factor (CTGF) as well as several intracellular signaling molecules including AKT, phosphatidylinositol 3-kinase (PI3K), and signal transducer and activator of transcription (STAT) [8–10]. Therefore, leptin is capable of inducing the local autocrine/paracrine cascades within the tumor microenvironment and likely of stimulating neoplastic growth and progression.
The epithelial-mesenchymal transition (EMT) is an intricate biological process by which epithelial cells acquire mesenchymal morphology. This phenomenon is observed during tumor invasion and metastasis, apart from normal embryological development and wound healing. Interestingly, blocking leptin’s availability to estrogen receptor positive (ER+) MCF-7 breast cancer cells significantly decreased the number of metastatic lesions in SCID/beige mice [11]. A growing body of evidence shows that leptin may promote EMT, and a number of mechanisms have been proposed. For example, Choi et al. found that leptin activated hedgehog signaling in hepatic stellate cells, which in turn promoted EMT [12]. Further, a potential cross-talk between leptin and Wnt signaling in EMT has been reported in breast cancer cell-lines [13]. This was demonstrated by an increased accumulation and nuclear translocation of β-catenin by leptin due to a decrease in the formation of glycogen synthase kinase 3β (GSK3β)-liver kinase B1 (LKB1)-axin complex in MCF-7, MDA-MB-468 and MDA-MB-231 breast cancer cells. In addition, using A549 human lung cancer cells, Feng and colleagues reported that leptin significantly upregulated TGF-β that may play an important role in inducing EMT [14]. Leptin-mediated activation of intracellular signaling molecules such as STAT3, AKT, and PI3K could contribute to EMT-linked mechanisms [15,16].
Invasion and distant metastases by cancer cells are the final event that leads to the majority of cancer deaths. Leptin potentially has a potent impact on the spread of cancer to distinct organs. Apart from the pathological effects that have been mentioned above, leptin may influence disease processes through a number of mechanisms such as enhanced cellular proliferation, angiogenesis, and interaction with various cytokines (Fig. 1). In this review, we analyze leptin’s role in tumor invasion and metastasis.
Fig. 1. Leptin-associated biomolecules that may promote cancer cell proliferation and migration.
EGFR: Epidermal growth factor receptor, ERK: Extracellular signal-regulated kinases, HIF-1α: Hypoxia-inducible factor-1 alpha, ICAM: Intercellular adhesion molecule, JAK: Janus kinase, MMPs: Matrix metalloproteinases, NF-κB: Nuclear factor-kappa B, PI3K: Phosphatidylinositol 3-kinase, STAT: Signal transducer and activator of transcription, TGF-β: Transforming growth factor-beta, VEGF: Vascular endothelial growth factor
2. Obesity-related microenvironment and its influence on tumor progression
A growing body of evidence suggests that adipocytes in the tumor microenvironment play a crucial role in disease progression by providing fatty acids, pro-inflammatory cytokines and proteases [17,18]. Interestingly, cancer cells utilize adipocyte-released fatty acids for energy production through β-oxidation. Therefore, adequate supply of fatty acids/lipids from cancer-associated adipocytes favors uncontrolled growth and progression of malignancy [17]. A recent study showed that after co-culture with isolated omental adipocytes, MKN-45 and AGS gastric cancer cells exhibited a significant increase in lipid uptake and enhanced invasiveness [19]. Similarly, co-culture of adipocytes with SW480 and DLD1 human colon cancer cells led to a transfer of free fatty acids from the adipocytes to the cancer cells [20]. Furthermore, this study found an accumulation of adipocytes in close association with invasive tumor cells in colon cancer patients. In an in vitro study, murine 3T3L1 adipocytes were co-cultured with pancreatic intraepithelial neoplasia and ductal adenocarcinoma cells derived from PKCY mice. Adipocytes promoted proliferation of both cell types [21].
Prostate cancer progression may also be impacted by adipose tissue microenvironment. For example, androgen independent RM1 mouse prostate carcinoma cells were co-cultured with pre-adipocytes or adipocytes as well as with their respective conditioned medium [22]. Both adipocytes and its conditioned media significantly increased RM1 cell proliferation and greater cell migration was observed with pre-adipocyte and adipocyte conditioned media. Moreover, RM1 cell invasion was significantly increased after co-culture with pre-adipocytes and adipocytes. Another study using human prostate cancer cell-lines (DU145, LNCaP, and PC-3) found that conditioned medium from pre-adipocytes increased cell proliferation and invasive activity [23]. Interestingly, Ribeiro et al. reported higher MMP-2 activity in periprostatic adipose tissue compared to pre-peritoneal visceral adipose tissue [24]. In addition, increased proliferation and migration of hormone-refractory PC-3 cells were detected after stimulation with conditioned medium from periprostatic adipose tissue explants.
A number of studies have been conducted on the interactions between breast tumors and adipocytes in the tumor microenvironment. For example, Zhu et al. analyzed 294 cases and concluded that invasive portions of breast cancer were generally situated adjacent to breast adipose tissue [25]. Intriguingly, Fletcher et al. noted that adipocytes associated with the invasive front are reduced in size compared to adipocytes that are farther away [26]. Many investigators have recorded considerable morphological and functional changes of adipocytes which are adjacent to tumors and referred to as cancer-associated adipocytes [27,28].
Additional evidence for an interaction of adipocytes with breast cancer development comes from studies with co-cultures of human breast cancer lines with adipocytes. When MDA-MB-231, MCF-7 and ZR-75-1 human breast cancer cell lines were co-cultured in the presence of adipocytes significant increases in proliferation rate were determined [29]. Furthermore, an enhanced MDA-MB-231 cancer cell invasiveness has been documented when co-cultured with adipocytes [30]. Similarly, Lee et al. observed that adipocytes induced migration and invasion in MCF-7, MDA-MB-435S, and MDA-MB-231 breast cancer cells after co-culture with adipocytes [31]. In this study, increased cell migration and invasion were accompanied by the upregulation of MMP-9 and TWIST1. In an interesting study, MCF-7 cells were co-cultured with SGBS adipocytes (which originated from a patient with Simpson-Golabi-Behmel syndrome, an overgrowth disorder). Gene expression levels of EMT-inducing transcription factors FOXC2 and TWIST1 were increased in the MCF-7 cells while in the SGBS cells hypoxia-inducible factor-1α (HIF-1α), TGF-β, and lectin-type oxidized LDL receptor 1 (LOX1) mRNA levels were increased [32].
In a somewhat different approach to evaluate the potential impact of adipose tissue in the microenvironment on breast cancer development experiments have been conducted using conditioned medium from isolated normal breast adipocytes as well as cancer associated adipocytes. MCF-7 and MDA-MB-231 cells were used for the purpose of detecting phenotypic changes resulting from culture with the different media. Both breast cancer cell-lines had higher migration in conditioned medium obtained from the cancer-associated adipocytes in comparison to the media from normal breast adipocytes. The conditioned medium from the cancer associated adipocytes had higher levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) than that from the normal breast adipocytes [33].
In the breast tumor microenvironment, adipocytes have been reported to produce a number of biologically active compounds including different cytokines, e.g., MMP-11, plasminogen activator inhibitor-1 (PAI-1), collagen VI, IL-1β, IL-6, tumor necrosis factor – α (TNF-α), insulin-like growth factor-I (IGF-I), and leptin; many of which are metastasis-associated proteins [34–36]. In particular, Wolfson et al. suggested that leptin has a key effect on breast cancer; and in adipocyte-adjacent breast cancer cells, due to stimulation of various regulatory pathways such as Notch, Wnt and Nanog signaling axis [37]. Clearly, the significance of leptin at the adipocyte-breast cancer cell interface, and its effects in the neighboring breast cancer cells are complex (Fig. 2). A potential role of leptin acting to increase aromatase activity in adipose tissue associated with breast cancer is supported by interesting in vivo studies. Liu et al. co-injected pre-adipocytes (F442A) with MCF-7 breast cancer cells subcutaneously into SCID mice [38]. The pre-adipocytes developed into fat pads and interestingly tumors also developed in these mice while those with only the MCF-7 cells injected did not. Further investigation indicated that leptin signaling in these adipocytes increased aromatase expression and further that direct injection of leptin into these fat pads increased mRNA for aromatase 6-fold [38]. Their findings suggest that leptin in the tumor microenvironment could play a crucial role in the progress of hormone-dependent breast cancer.
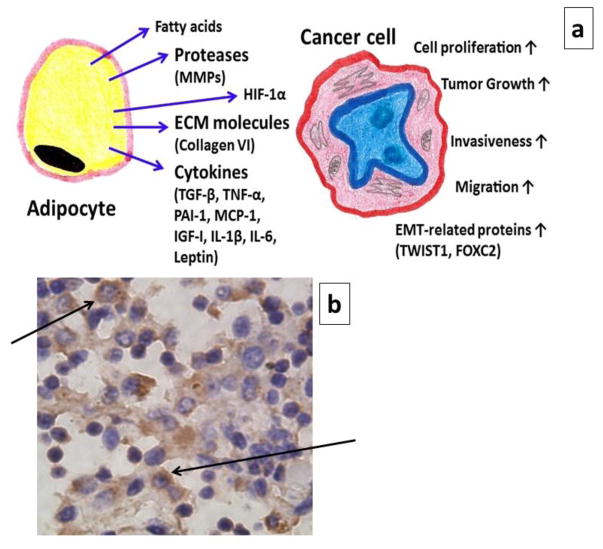
Fig. 2. Adipocytes in tumor microenvironment.
(a) A schematic diagram to show the influence of adipocytes on the progression of cancer. (b) Immunohistochemical expression of leptin in tumor-adjacent adipose tissue, which was collected from the mammary fat pad of a CD-1 female mouse. In this xenograft model, T47D breast cancer cells were inoculated. The arrows indicate the presence of leptin in the cytoplasm of cells. (↑ - Increase)
3. Leptin biology and molecules of relevant signal transduction pathways
The leptin gene (ob) is located on the long arm of chromosome 7. Initially a 167 amino acid peptide is translated, which forms the mature 146 amino acid peptide after processing through the endoplasmic reticulum [39]. Leptin protein sequences are highly conserved among mammals; however, it exhibits considerable sequence variation among other vertebrates [40]. Due to poor solubility of natural leptin, a mutant leptin has been constructed with a single amino acid substitution of glutamic acid for tryptophan at position 100 [41]. For improved solubility, it has been possible to crystallize this leptin analog W100E. The crystallographic structure has displayed the secondary and tertiary structure of the leptin molecule, which consists of four antiparallel α-helices (A, B, C, and D) and two relatively long interconnected loops [39,41,42]. Two conserved cysteine residues (C96 in the CD loop and C146 as the C-terminal residue) form a disulfide bridge that is essential for structural stability and thus biological activity [43].
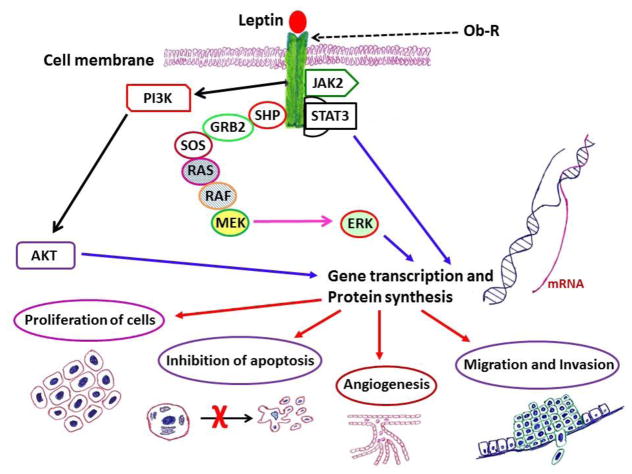
Leptin belongs to the family of class-I cytokines, which also includes biomolecules such as IL-6, leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (CNTF) [44,45]. For this reason, like other members, Ob-R mainly functions via the activation of Janus kinases (JAK) and STAT, particularly through JAK2-STAT3 signal transduction pathway (Fig. 3 and Fig. 4a–c). Finally, activated/phosphorylated STAT3 (p-STAT3) translocate to the nucleus and stimulates transcription. Other pathways that support neoplastic growth include PI3K and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathways [46,47]. Evidence to support the role of these proteins in cancer progression will be presented next.
Fig. 3.
Leptin-related intracellular signaling molecules and their association with survival/anti-apoptotic and proliferative potential of cells.
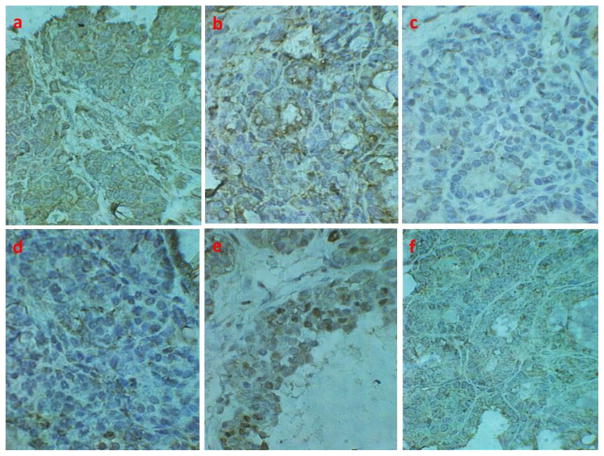
Fig. 4. Immunohistochemical expression of leptin receptor and relevant effector molecules in tissue sections from mammary tumors of TGFα mice.
Expression of (a) Ob-Rb, (b) STAT3, (c) p-STAT3, (d) PI3K, (e) cell proliferation marker proliferating cell nuclear antigen (PCNA), and (f) anti-apoptotic protein Bcl-2. Presence of yellowish- to dark-brown color indicates positive staining.
3.1. STAT3 activation in neoplastic process
STAT3 activation has been documented in both solid and hematological malignancies [48,49]. It has been suggested that aberrant signaling of STAT3 is associated with different biological processes of tumor progression such as angiogenesis, cellular proliferation and survival of cells or inhibition of apoptosis leading to invasion and metastasis. Furthermore, STAT3 is involved in EMT by regulating various EMT-related proteins, e.g., Twist, Snail, and vimentin [50–52]. A recent study on intrahepatic cholangiocarcinoma found that patients with high STAT3 expression levels had a poor overall and disease-free survival [53]. After analyzing a large number of gastric cancer cases, both STAT3 and p-STAT3 were shown to be associated with an increased mortality risk [54,55]. In another study, Deng et al. reported that 62.6% (67/107) and 38% (41/107) of gastric cancer tissues exhibited positive immunostaining for STAT3 and p-STAT3, respectively [56]. In a study on gastric cancer conducted by Song et al., immunostaining for STAT3 and p-STAT3 was 81.7% (49/60) and 58% (35/60), respectively [57]. Moreover, their study showed that p-STAT3 played a significant role in prognosis. Similarly, in colon cancer, positive immunostaining rates for STAT3 and p-STAT3 were 72% (36/50) and 76% (38/50), respectively [58]. After reviewing 17 studies and a total of 2,346 colon cancer patients, the authors revealed a positive association between p-STAT3 expression and lymph node metastasis [59].
Likewise, results of another meta-analysis on non-small-cell lung cancer (NSCLC), which included 17 retrospective trials and 1,793 patients, suggested that high STAT3 or p-STAT3 expression was a strong predictor of poor prognosis [60]. Of note, the majority of lung cancers are NSCLC, which includes predominant squamous cell carcinomas and adenocarcinomas. Nevertheless, in a study conducted by Zhao et al., STAT3, and p-STAT3 expressions were observed in 72.1% (49/68) and 58.8% (40/68) of NSCLC cases, respectively [61]. Furthermore, immunohistochemical analyses on 82 surgically resected NSCLC tissues revealed p-STAT3 expression in 59.7% of tumors [62]. The investigators also recorded that p-STAT3 expression was correlated with tumor’s degree of differentiation, lymph node metastasis, clinical staging and prognosis after surgical resection.
Liu et al. analyzed 208 breast cancer tissues, and they noticed high level of STAT3 immunoreactivity in 72% (n=150) of cancer samples; whereas high p-STAT3 expression was present in 43.8% (n=91) cases [63]. The expression levels of both STAT3 and p-STAT3 were increased with higher TNM stage and lymph node metastasis. In another study on 76 breast cancer cases, 66% (n=50) of the cancer specimens showed positive immuno-expression of STAT3, which was significantly associated with the clinical stage, tumor differentiation and lymph node metastasis [64]. Additionally, a key role of STAT3 in the regulation of invasion and metastasis has been revealed by a recent study [65].
3.2. PI3K involvement in cancers
An important intermediate, which functions in diverse hormone signaling pathways including insulin and leptin, is PI3K (Fig. 4d). Possibly, the PI3K signaling pathway is a significant link between obesity, leptin and an increased risk of cancer [66]. In a nutshell, activation of PI3K causes phosphorylation of AKT and stimulation of the mammalian target of rapamycin (mTOR); while the phosphatase and tensin homolog (PTEN) is a negative regulator. Remarkably, PI3K pathway regulates various biological processes such as cellular metabolism, proliferation (Fig. 4e), and apoptosis (Fig. 4f); aberrant responses of these phenomena trigger the malignant pathology (Fig. 3). It is noteworthy that PI3K is composed of a regulatory subunit and a 110 kDa catalytic subunit. The gene encoding PI3K catalytic subunit α (PIK3CA/p110α) is frequently activated by mutation in cancers [67].
PIK3CA mutations represent one of the most common genetic aberrations in breast cancer [68]. In a study on 241 patients with breast cancer, PIK3CA mutations were found in 15.8% cases [69]. On the other hand, Firoozinia et al. analyzed 50 breast cancer samples; 72% samples showed significant amplification of PIK3CA gene, while 24% exhibited protein overexpression [70]. In another study that examined 95 breast cancer tissue samples, PIK3CA gene mutations were present in 25.3% cases [71]; and in a recent study, mutations of PIK3CA were found in 28% (21/75) cases [72].
A number of studies have shown the involvement of PI3K pathway in carcinomas of the gastrointestinal tract. In a recent study on Japanese patients with gastric cancer, PIK3CA mutations were detected in 12% (25/208) [73]. In another study on gastric cancer patients, both PIK3CA gene mutations and amplification were assessed in connection with clinicopathological characteristics and disease outcome [74]. PIK3CA mutations and amplification were found in 7.1% (8/113) and 67% (88/131), respectively. Furthermore, patients with PIK3CA amplification had significantly shorter survival times. A study, which analyzed PIK3CA mRNA levels in both gastric cancer tissues and metastases, observed 2 fold higher mRNA in lymph node metastasis compared to primary tumors [75]. On the other hand, an immunohistochemical analysis of tissue samples from 70 patients with gastric cancer revealed that expression level of PI3K protein was significantly correlated with TNM stage, differentiation grade, lymph node metastasis and distant metastases [76]. In the same way, after analyzing 60 colon cancer specimens immunohistochemically, the investigators concluded that high PI3K expression was associated with cancer metastasis [77]. They also noticed a significant difference in the proportion of PI3K in primary lesions (30%, 18/60) vs metastatic lesions (46.7%, 28/60). Overall, PI3K pathway alteration is common in human cancers, and overexpression of this pathway is associated with the uncontrollable cellular proliferation and growth.
3.3. Overexpression of AKT and ERK in cancers
Different investigators have reported an interaction between PI3K-AKT and ERK signaling pathways [78–80]. A number of studies, which evaluated both AKT and ERK in tumors, recorded similar frequencies of expression [81–83]. In various cancers, high expression rates of both AKT and ERK have been documented [84–88]. Regarding overexpression of AKT and its association with patients’ prognosis, conflicting reports have been reported. For example, David et al. noticed that lung cancer patients with strong phosphorylated AKT (p-AKT) expression were more likely to die early [89]. In a study that analyzed endometrial adenocarcinomas, p-AKT expression was significantly higher in women with positive lymph nodes, and higher expression was significantly associated with poor survival [81]. Jia et al. recorded that p-AKT expression correlated with higher histological grade and resistance to chemotherapy in ovarian cancer [90]. On the other hand, a study in colorectal cancer patients showed that p-AKT expression was associated with lower stage and good prognosis [91]. Interestingly, in renal cell carcinoma, Pantuck et al. found that high nuclear p-AKT expression was associated with a favorable prognosis, while high cytoplasmic p-AKT expression was associated with a poor prognosis [92]. Conversely, a study in patients with non-small cell lung cancer observed that higher levels of cytoplasmic p-AKT had a trend toward longer overall survival; whereas higher levels of nuclear p-AKT had a poorer prognosis [93]. In general, investigators have identified an association between ERK activation and poor prognosis such as aggressive neoplastic features, metastasis, and reduced survival [94,95]. In a study among breast carcinoma patients, p-ERK was shown to be associated with higher TNM stage and lymph node metastasis [96]. Similar pathologic characteristics with regard to ERK expression/activation have also been detected in digestive tract cancers. In esophageal carcinoma, increasing p-ERK nuclear and cytoplasmic expression was found to be significantly correlated with tumor grade [97]. Likewise, high nuclear p-ERK expression in colorectal cancer was associated with highly invasive disease [98]. In addition, the investigators showed that nuclear but not cytoplasmic p-ERK expression correlated with the patients’ overall survival rate. Perhaps, activation of ERK may increase the metastatic potentiality of cancer cells.
4. Leptin and tumor behavior of the gastrointestinal tract
4.1. Upper gastrointestinal tract
An association between high levels of leptin and risk of esophageal adenocarcinoma in obese individuals has been reported [99]. Adenocarcinoma in the lower part of esophagus most often arises from the epithelium of intestinal metaplasia (Barrett’s); while squamous cell carcinoma occurs mainly in the middle and lower regions and originates from non-keratinized stratified epithelium [100]. In a study of esophageal adenocarcinoma increased peri-tumoral adipocyte size was associated with higher leptin expression, angiogenesis, lymphangiogenesis and nodal metastasis [101]. These findings suggest that leptin secreted from peri-tumoral adipocytes may play a key role in the progression of cancer. In esophageal squamous cell carcinoma, leptin immunoexpression also has been demonstrated to be upregulated. Moreover, the expression of leptin was significantly correlated with lymph node involvement and advanced tumor stage in esophageal squamous cell carcinoma [102].
With respect to gastric carcinoma Ob-R expression was correlated with poor survival in 47% of patients with lymph node metastasis, 60% of the patients with advanced gastric cancer, and 41% of the cases defined as Lauren diffuse pathology [103] (Table 1). Of note, gastric cancer has been classified in many different ways such as Lauren, World Health Organization (WHO), and Borrmann classifications [104]. Poorly cohesive carcinomas belong to the diffuse type according to the Lauren classification. By analyzing 61 gastric cancer cases, Zhao et al. observed a significant association between leptin expression and vascular endothelial growth factor (VEGF) expression [105]. Further, leptin expression in these gastric cancer tissues was significantly associated with histology, Borrmann classification, lymph node metastasis and stage. Moreover, in patients with poorly differentiated gastric cancer, a poor prognosis was found for those with a strong expression of leptin. Similarly, Geng et al. recorded that the survival of gastric cancer patients with leptin positive expression was significantly poorer than those with negative expression [106]. They also noted a correlation between the expression of leptin and HER2, both of which were significantly associated with invasion depth, lymph node metastasis, tumor stage and expression of VEGF. Interestingly, Ishikawa et al. found that the immunohistochemical expression levels of both leptin and Ob-R tended to increase as the depth of either gastric tumor invasion or TNM stage increased [107]. Among stages I and II, 31% and 27% of the cases exhibited significant immunostaining for leptin and Ob-R, respectively. Whereas, in stages III and IV, 48% of the cases were strongly positive for leptin and 47% expressed Ob-R. In this study, lymph node metastasis was detected in approximately half of the leptin strong positive and Ob-R positive cases. Both venous and lymphatic invasion was more frequently observed in tumors with high leptin and positive Ob-R expression.
Table 1.
Summary of selected clinical studies that recorded a positive association between overexpression of leptin and/or its receptor proteins in tumor tissue and cancer cell invasion or metastasis
| Site | Subjects | Findings | Investigators |
|---|---|---|---|
| Breast | 76 invasive ductal carcinomas and 32 normal control samples. | Distant metastasis was detected in 21 (34%) of 61 Ob-R-positive tumors with leptin overexpression, but in none of the 15 tumors that lacked Ob-R expression or leptin overexpression. | Ishikawa et al. 2004 [104] |
| Colon | 68 cases of colorectal carcinoma tissue, tumor adjacent tissue and normal colorectal tissue. | Expression of leptin and Ob-R in tumor was correlated with depth of bowel wall invasion, lymph node metastasis, and distant metastasis. | Liu et al. 2011 [117] |
| Colon | 108 patients with colorectal carcinoma. | Leptin/Ob-R expression was significantly associated with TNM stage, lymph node metastasis, and distant metastasis. | Wang et al. 2012 [116] |
| Esophagus | 122 cases of esophageal squamous cell carcinoma and 40 normal esophageal mucosa tissue. | Expression of leptin was significantly correlated with lymph node involvement and advanced tumor stage. | Duan et al. 2014 [102] |
| Lung | 50 patients with lung cancer along with another 50 lung cancer patients with metastatic bone lesions. | Leptin was present at higher levels in bone metastasis cases. | Feng et al. 2013 [14] |
| Ovary | 70 ovarian cancer cases. | Ob-Rb was highly overexpressed in metastases compared to primary tumors. | Kato et al. 2015 [16] |
| Pancreas | 60 pancreatic cancer tissue specimens. | Expression of Ob-Rb was significantly stronger in patients with lymph node metastasis than in those without lymph node metastasis. | Fan et al. 2015 [90] |
| Stomach | 343 cases of gastric carcinoma. | Ob-R expression was correlated with poor survival in 207 patients with advanced gastric cancer (muscle or deeper invasion), 139 of the Lauren diffuse group (poorly differentiated tumor cells scatter throughout the organ), and in 160 patients with lymph node metastasis. | Choi et al. 2015 [103] |
| Stomach | 84 cases from primary gastric carcinoma. | Moderate to strong immnoreactivity for leptin was identified in 57% (n=48) cases. Leptin was positively correlated with lymph node metastasis and clinical stage. | Dong et al. 2014 [109] |
| Stomach | 110 gastric cancer specimens and 96 normal gastric mucosa were analyzed. | Expression of leptin was associated with tumor invasion depth, lymph node metastasis, and expression of HER2 and VEGF. | Geng et al. 2012 [106] |
| Stomach | 207 gastric carcinomas (100 early and 107 advanced carcinomas) | Expression levels of both leptin and Ob-R tended to increase as the depth of tumor invasion or TNM stage increased. | Ishikawa et al. 2006 [107] |
| Stomach | 61 gastric cancer specimens. | Leptin expression was significantly associated with lymph node metastasis and higher stage. | Zhao et al. 2007 [105] |
| Thyroid | 49 papillary thyroid carcinomas. | Coexpression of leptin and Ob-R in primary neoplasms had greater incidence of lymph node metastasis. | Cheng et al. 2010 [95] |
| Thyroid | 93 cases with papillary thyroid carcinoma and 25 cases with medullary thyroid carcinoma. | For papillary thyroid carcinoma, expression of leptin and Ob-R was significantly correlated with lymph node metastasis and advanced stage. For medullary thyroid carcinoma, Ob-R was significantly correlated with lymph node metastasis and advanced stage. | Fan and Li 2015 [93] |
To explain the mechanisms of leptin-mediated invasion and metastasis in gastric cancer, Dong and colleagues reported that leptin promoted gastric cancer cell invasion by upregulating the expression of membrane type-1 matrix metalloproteinase (MT1-MMP or MMP14), which is an ECM degrading protease which plays crucial roles in tumor invasion [108]. Furthermore, they observed that leptin and intercellular adhesion molecule-1 (ICAM-1) were both overexpressed in gastric cancer tissue, and there was a strong positive correlation between them [109]. Both were related with clinical stage and lymph node metastasis. In addition, they demonstrated that leptin induced migration of human gastric carcinoma cells (AGS and MKN-45 cell-lines) by upregulating ICAM-1. It is known that ICAM-1 or CD54 is a transmembrane glycoprotein of the immunoglobulin superfamily and it can facilitate the metastasis of cancer cells by escaping the recognition and attack of immunocytes [110]. Interestingly, leptin expression in gastric adenocarcinoma cell-lines was reported to be correlated with resistance to the chemotherapeutic agent cisplatin [111]. Furthermore, including a leptin antagonist increased the sensitivity of both the cisplatin-resistant gastric cancer AGS Cis5 and esophageal adenocarcinoma OE33 cell-lines to cisplatin, which greatly illustrated the impact of leptin in the disease course (Table 2 [101,111,112]).
Table 2.
Relevant leptin antagonists that have been exhibited to prevent aggressive tumor behavior
| Authors/Tissue | Antagonist used | Experimental design | Major observations |
|---|---|---|---|
| Bain et al. 2014 [111] Gastroesophageal adenocarcinomas (clinical samples) as well as in vitro experiments on gastric cancer cells and esophageal cancer cells. |
Recombinant super human leptin antagonist (SHLA), which is a polypeptide chain containing 146 amino acids. | Cisplatin-resistant gastric cancer cell-line AGS Cis5 and esophageal adenocarcinoma cell-line OE33 were used. Originally, AGS cell-line was derived from a gastric adenocarcinoma of a Caucasian female. In a 96-well plate, cells were grown. Cisplatin and/or SHLA were added. Following incubation, MTT cell proliferation assay was performed. | Leptin antagonist SHLA increased the sensitivity of AGS Cis5 and OE33 cell-lines to cisplatin. SHLA inhibited the growth of OE33 cells when given alone. In both AGS Cis5 and OE33, SHLA inhibited leptin-induced cell proliferation. |
| Trevellin et al. 2015 [101] Esophageal adenocarcinoma patients, and in vitro study on esophageal adenocarcinoma cells. |
Super human leptin antagonist (SHLA). | Treatment of OE33 cells with conditioned media (CM) collected from cultured biopsies of adipose tissue and peritumoral adipose tissue of patients with lymph node metastasis. | After treatment with CM, mRNA levels of two key EMT regulator genes, alpha-smooth muscle actin (α-SMA) and E-cadherin, were increased. However, SHLA diminished the mRNA expression of α-SMA and E-cadherin. |
| Catalano et al. 2015 [112] In vitro and in vivo experimental models using ERα-positive (MCF-7) and -negative (SK-BR-3) breast cancer cells. |
Pegylated LDFI (LDFI-PEG): polyethylene glycol (PEG)-attached to tetrapeptide that mimics the sequence of leptin binding site I. Leptin interacts with Ob-R through 3 different binding sites: I–III. Site I is crucial for the formation of an active leptin–Ob-R complex and in its subsequent activation. Amino acids 39–42 (Leu-Asp-Phe-Ile-LDFI) were shown to contribute to leptin binding site I. | Injected SK-BR-3 breast cancer cells into the intrascapular region of female nude mice (nu/nu Swiss) and followed tumor growth after administration of LDFI-PEG. | After LDFI-PEG treatment, tumor volumes continued to reduce over control for the duration of experiment. At the end of treatment (28 days) it was observed that PEG-LDFI induced a significant tumor growth inhibition compared to vehicle-treated mice. Sections of tumors from PEG-LDFI-treated mice exhibited a reduction in the expression of proliferation marker Ki-67, and phosphorylated levels of STAT3, MAPK and AKT than controls. |
AKT: Protein kinase B/a serine/threonine kinase, EMT: Epithelial-mesenchymal transition, MAPK: Mitogen-activated protein kinase, STAT3: Signal transducer and activator of transcription 3
Leptin may also be involved with the severity of gall bladder cancer. For example, in a recent in vitro study using gallbladder cancer cells the addition of leptin increased the levels of MMPs (−3 and −9) and VEGF (-C and -D, responsible for lymphangiogenesis) [113]. Since these proteins increase proliferation, migration and invasion of cancer cells, leptin could act through them to eventually promote metastasis.
4.2. Lower gastrointestinal tract
At another gastrointestinal cancer site, i.e., colorectal cancer, a relationship between higher serum leptin levels and lymph node involvement has been documented [114,115]. Moreover, after analyzing the data of 130 colorectal cancer patients, Healy et al. found that serum leptin level was associated with microvascular invasion and advanced tumor stage [115]. Similarly, leptin and Ob-R protein expressions in tumor tissue have been found to be correlated with aggressive characteristics of cancer, e.g., tumor grade and stage, depth of bowel wall invasion, lymph node metastasis and distant metastasis [116,117]. Pro-angiogenic and pro-migratory effects of leptin are possibly important in progression of colon cancer. In a study conducted by Ratke and colleagues using SW480, SW620 and HCT116 human colon carcinoma cell-lines, leptin significantly enhanced the migratory activity of these cell-lines through the activation of STAT3 and JAK [118]. The investigators performed three-dimensional collagen-based migration assays, and carcinoma cells exhibited a significant increase of their migratory activity in response to 100 ng/ml of human leptin. It is noteworthy that a positive correlation was observed among leptin, Ob-R, VEGF, and microvessel density (MVD) in colorectal carcinoma [117]. In accordance with the protein expression (i.e., Ob-R) in tumor tissue that has been discussed above, Erkasap et al. documented higher mRNA expression of Ob-R in metastatic growth compared to primary colorectal cancer [119].
Cancers of the gastrointestinal tract are common causes of death worldwide. High levels of leptin released from excessive adipose tissue in obesity may play a key role in different stages of cancer. It is known that leptin can affect several aspects of the gastrointestinal tract such as interactions with local hormones, food absorption and inflammatory condition. Therefore, it is important to understand the precise role of leptin in gastrointestinal tumors for better management and patient outcome.
5. Tumors of endocrine and associated organs
5.1. Pancreas
Adipocyte-derived leptin and insulin which is secreted from the pancreas have fascinating similarities and complex interactions in normal health and conditions such as obesity, leptin resistance and insulin resistance. Overall, an intricate relationship has been observed in energy homeostasis including glucose and lipid metabolism. Leptin resistance in obesity markedly influences the status of insulin sensitivity, and vice versa. Therefore, it is imperative to understand the role of leptin in various disorders of the pancreas, which is a storehouse of several exocrine and endocrine factors.
Fan et al. found an increased expression of leptin’s functional receptor Ob-Rb in pancreatic tumor tissue which was significantly associated with the level of MMP-13, lymph node metastasis and TNM stage [120]. Further, in in vitro experiments these investigators showed that addition of leptin stimulated the expression of MMP-13 as well as increased the migration and invasion of PANC-1 human pancreatic cancer cells. Likewise, in a xenograft model with the PANC-1 cell-line, they observed that the overexpression of leptin in tumor tissue significantly increased tumor growth and lymph node metastasis. In this experiment, the investigators used the PANC-1 cells that were transfected with the recombinant lentivirus carrying the human leptin gene; the cell-line stably overexpressed leptin. In another study, Ren et al. found that HIF-1α expression was correlated with Ob-R in pancreatic cancer tissue in xenograft mouse model that was inoculated with PANC-1 cells [121]. This study included human clinical data indicating that overexpression of HIF-1 was associated with lymph node metastasis and overall survival. These findings are consistent with the fact that HIF-1α appears to be involved in tumorigenesis and angiogenesis [122].
5.2. Thyroid gland
Recently there has been an increasing incidence of thyroid cancer. One of the suspected risk factors is the increasing prevalence of obesity [123,124]. Since the level of leptin is elevated in obesity, this adipokine could be involved in the development and progression of this malignancy [124]. The thyroid gland contains two types of endocrine cells – follicular cells that synthesize thyroid hormone, and parafollicular or C cells that produce calcitonin. Papillary carcinoma, the most common type of thyroid cancer, originates from follicular cells; whereas medullary carcinoma develops from C cells. A study on different subtypes of thyroid carcinoma revealed that immunohistochemical expression of Ob-R was significantly correlated with nodal metastasis and advanced stage in medullary thyroid carcinoma. On the other hand, both leptin and Ob-R levels were strongly associated with larger tumor size, nodal metastasis and advanced stage in papillary thyroid carcinoma [125]. Several studies on patients with papillary thyroid cancer documented that expression of leptin and/or Ob-R was directly linked with disease aggressiveness including increased tumor size and lymph node metastasis [126–128]. In addition, it has been found that Ob-R overexpression was significantly associated with the overexpression of anti-apoptotic XIAP and Bcl-XL proteins as well as with poor disease-free survival in cases with papillary thyroid cancer [128]. In a study on K1 and BCPAP human papillary thyroid cancer cell-lines, tumor cell migration was significantly enhanced by leptin in a dose-dependent manner [129].
6. Female reproductive system cancers
6.1. Gynecologic cancers
Malignancies of the female reproductive system are responsible for significant morbidity and mortality throughout the world. Among all gynecological malignancies, ovarian cancer is the most deadly disease. Due to unique anatomical position of the ovary, dissemination of cancer cells within the peritoneal cavity is common in ovarian cancer. The disease is generally diagnosed at a late stage and prognosis is poor.
Similar to colon cancer [119], metastases from ovarian cancers were found to have higher Ob-Rb expression than what was associated with primary tumors [16]. In vitro studies with various ovarian cancer cell-lines further demonstrated that leptin stimulated cell migration and invasion. In a study on endometrial cancer, elevated levels of patients’ serum leptin were associated with invasiveness [114]. Similar findings were observed with regard to immunohistochemical expression of both leptin and Ob-R in endometrial cancer tissue. The expressions of leptin and Ob-R were positively correlated with the invasiveness of cancer, lymph node metastasis, and poorer prognosis [130].
6.2. Breast cancer
Higher breast cancer incidence has been shown to be attributable to increased body mass index (BMI) [131,132]. Leptin is considered as an important adipokine that may modulate this disease process. A number of studies on breast cancer patients have noted an association of higher serum leptin levels with aggressive malignant features. For instance, in Italian women, serum leptin concentrations significantly correlated with tumor size, lymph node involvement and metastasis among ER+ breast cancer cases [133]. Similarly in another study, serum leptin levels were positively correlated with TNM staging, tumor size, lymph node metastasis, and histological grading among postmenopausal women with breast cancer [134]. A number of investigators also included measurement of adiponectin and expressed results in relation to leptin [134–136]. Adiponectin is an adipokine with anti-inflammatory effects unlike the pathophysiological functions of leptin [137]. Nonetheless, Hou et al. observed that reduced serum levels of adiponectin and elevated leptin were associated with lymph node metastasis in breast cancer patients [135]. Chen et al. also reported that an increased serum ratio of leptin/adiponectin (L/A ratio) was positively correlated with tumor size [136].
Similar to serum levels, tumor tissue expressions of leptin and/or Ob-R also indicate the presence of aggressive behavior. For example, Ishikawa and colleagues reported that distant metastasis was detected in Ob-R-positive tumors with leptin overexpression compared to tumors that lacked Ob-R expression or leptin overexpression [138]. Similarly in another study, overexpression of leptin and Ob-R in breast cancer was positively correlated to lymph node metastasis [139]. In this study, analyses of 60 cases of breast cancer and adjacent normal tissue along with benign breast disease tissues revealed that immunostaining intensity for both parameters (co-expression) in cancer tissue was directly proportional to the degree of cancer cell dissemination. Interestingly, Alshaker et al. detected that Ob-R genes were elevated in metastases of ER-negative breast cancer patients [140]. Results from a mouse model have also provided evidence for a role of leptin in the metastatic process in breast cancer. Park et al. observed an absence of Ob-R attenuated tumor progression and lung metastasis through a reduction of ERK1/2 and JAK2/STAT3 pathways in the MMTV-PyMT mammary tumor model of breast cancer [141]. For this purpose, the brain-specific long form of leptin receptor (NseLEPR-B) transgenic mouse was crossed with the MMTV-PyMT mouse strain. Normally, MMTV-PyMT female mice develop mammary adenocarcinomas that metastasize to the lung. Additional support comes from in vitro studies. For example, in mouse (4T1, EMT6 and MMT) mammary cancer cell-lines, the addition of leptin induced cell proliferation and migration, as well as upregulated VEGF and its receptor VEGFR-2 [142]. McMurtry et al. reported that leptin increased the invasiveness and MMP-2 activity of the ER+ MCF-7 human breast cancer cell-line [143]. Additionally, in an experiment using breast cancer cells and tumor-associated macrophages (TAMs), leptin was shown to stimulate the expression of IL-18, which is closely linked with inflammatory reactions [144]. In this study, THP-1 human monocytic cell-line and MCF-7, SK-BR-3 and MDA-MB-231 human breast cancer cell-lines were used. Furthermore, the study documented that leptin could induce IL-8 expression in both breast cancer cells and TAMs.
It is interesting to note leptin’s influence on TAMs, which are a crucial component in the tumor microenvironment and the principal source of inflammatory cytokines [144]. Moreover, clinical evidence has revealed a strong correlation between a high density of TAMs and poor prognosis in breast cancer [144]. As such, TAMs could play key roles in cancer progression including EMT. It is noteworthy that macrophages can be classified into two major categories – classically activated M1 macrophages are linked with tumoricidal functions, and alternatively activated M2 macrophages are involved in tumor promotion [145]. Fascinatingly, TAMs have been considered as M2 macrophages, which could promote the EMT [145–147]. A recent study has shown that leptin may promote breast cancer progression by stimulating M2 macrophages [148]. Using a different approach Strong et al. [11] co-cultured adipose stromal/stem cells isolated from obese women with either ER+ MCF-7, ZR75, or T47D human breast cancer cells, which resulted in enhanced expression of EMT and metastasis related genes, SERPINE1, MMP-2, and IL-6. Of note, adipose stromal/stem cells have the capability to differentiate along the adipocyte and other lineage pathways. Nevertheless, these investigators concluded that leptin from adipose stromal/stem cells could contribute to the aggressiveness of breast cancer in obese women [11]. In a similar way, migration and colonization patterns of MDA-MB-231 and MCF-7 breast cancer cells were studied in co-culture with cancellous or spongy bone tissue fragments. The results showed that breast cancer cells migrated to human bone tissue-conditioned medium in association with increasing levels of leptin and IL-1β, and colonized the bone marrow adipose tissue compartment of cultured fragments [149].
Leptin has been demonstrated to influence numerous biological elements such as signal transduction proteins like JAK and PI3K, cellular receptors such as epidermal growth factor receptor (EGFR) and androgen receptor, and the enzymes aromatase and cytochrome P450 1B1 (CYP1B1): all of these biomolecules could be associated with neoplastic process [10,137]. Perera et al. identified a number of leptin-regulated genes in MCF-7 cells by using a microarray system; and observed that leptin up-regulated the expression of CTGF and the anti-apoptotic genes Bcl-2 and survivin, and also reduced the expression of apoptotic genes [150]. Furthermore, experiments demonstrated a bidirectional crosstalk between leptin and IGF-I signalling that transactivated EGFR and promoted the migration of breast cancer cells [151]. Overall, the results of different clinical, in vivo and in vitro studies have suggested that leptin could contribute to the aggressiveness of breast cancer.
7. Tumors of other organs
Leptin has been shown to stimulate the migration of human prostate cancer and chondrosarcoma cells [152,153]. In both human prostate cancer and chondrosarcoma cells, one of the underlying mechanisms of leptin-influenced migration was transcriptional up-regulation of αvβ3 integrin expression through Ob-R and activation of associated signaling pathways such as PI3K, AKT, and nuclear factor-κB (NF-κB). Of note, integrins are cell adhesion molecules that connect the cytoskeleton with ECM/other cells. The integrin αvβ3 or vitronectin receptor is linked to many biological processes such as angiogenesis and tumor metastasis [154]. It may be worth mentioning that obesity has not been shown to be involved in prostate cancer development per se. However, obesity is associated with more aggressive prostate cancer, and this could be mediated by leptin [155].
In a study on patients with renal cell carcinoma, it was observed that serum leptin levels were significantly higher in those with venous invasion. There were significant associations between high Ob-R expression in tumor tissue and the presence of venous invasion, tumor histology, and lymph node metastasis. Patients with higher serum leptin had significantly shorter progression-free survival than patients with lower levels [156].
Investigators have observed that leptin levels were significantly higher in melanoma patients with positive sentinel nodes [157]. Their follow-up analysis revealed more aggressive disease in diabetic patients. It may be worth mentioning that melanoma is a highly aggressive form of skin cancer and the condition is frequently fatal if the tumor is not diagnosed early. An excess of adipose tissue may influence the development of melanoma and disease progression by different obesity-linked phenomena such as type 2 diabetes and enhanced release of leptin [158–161]. In an interesting study on melanoma, RNA was extracted from nodal tissue of 13 tumor-negative sentinel nodes, 10 tumor-positive sentinel nodes (micro-metastases<2 mm), and 11 tumor-negative non-sentinel nodes [162]. Expression levels of leptin were significantly higher in tumor-positive as compared with tumor-negative sentinel nodes.
In a study on lung cancer patients that included both primary and metastatic bone lesions, leptin was present at higher levels in samples associated with diagnosis of lung cancer bone metastastic tissue than lung cancer tissue [14]. In another study, the authors determined the association between the expression level of leptin and the survival of patients suffering from NSCLC [163]. They observed that leptin expression in NSCLC tissue affected the survival time of patients. Therefore it has been suggested that the expression of leptin may be an independent prognostic factor for NSCLC. On the other hand, in a syngeneic murine model study of Lewis lung carcinoma, consumption of a high-fat diet significantly increased plasma leptin in male C57BL/6 mice and resulted in a two-fold increase in the number of lung metastases along with a 50% increase in tumor volume compared to mice fed a low fat diet [164]. These studies have clearly shown that higher concentrations of leptin were associated with tumor progression and this adipokine could serve as an additional parameter for prognosis.
8. Leptin in tumor progression
Deregulated cell proliferation is the key feature in all phases of cancer development. Uncontrolled cell proliferation associated with suppression of apoptosis, proper supply of oxygen and nutrients, and removal of metabolic wastes provide an appropriate condition to support disease progression from in situ neoplastic growth to tissue invasion and metastasis. Remarkably in normal and cancer cells, leptin has been shown to stimulate proliferation. In a study of normal uterine smooth muscle cells collected from myometrial biopsies, leptin induced cell proliferation at both low and high doses (6.25 and 50 ng/ml) [165]. In the same way, leptin was documented to stimulate the proliferation of normal intestinal epithelial cells (IEC-6 cells) [166]. With regard to the proliferation of cancer cells, the pertinent information has been provided in Table 3 along with a brief etiologic account [116,167–180].
Table 3.
Possible mechanisms of leptin-induced cancer cell proliferation
| Investigators and Cancer types | Involved intracellular signaling pathways/Mechanisms | Principal mediating factors/phenomena |
|---|---|---|
| Blanquer-Rosselló Mdel et al. 2016 [167] MCF-7 breast cancer cells |
Lipid metabolism pathways, AMPK | Leptin favored the use of glucose for biosynthesis and lipids for energy production |
| Chen et al. 2013 [168] OVCAR-3 ovarian cancer cells |
PI3K/AKT and MEK/ERK1/2 pathways | Leptin enhanced the expression of regulators of cell proliferation and apoptosis inhibition, cyclin D1 and Mcl-1 |
| Chin et al. 2017 [169] SKOV-3 and OVCAR-3 ovarian cancer cells |
PI3K, STAT3, ERK1/2 | Leptin increased FSH, stimulated ERα and the expression of ERα-responsive genes |
| Dubois et al. 2014 [170] MCF-7 and T47D breast cancer cells |
Estrogenic pathway, leptin autocrine/paracrine signaling loop | Leptin stimulated proliferation, overexpression of leptin, Ob-R, ER, and aromatase |
| Habib et al. 2015 [171] PC-3 prostate cancer cells |
Estrogenic pathway | Leptin increased the expression of ERα, aromatase and CYP1B1, and decreased the expression of ERβ and COMT |
| Harbuzariu et al. 2017 [172] BxPC-3, MiaPaCa-2, Panc-1, AsPC-1 pancreatic cancer cells |
Notch signaling pathway, leptin autocrine/paracrine signaling loop | Leptin increased cell cycle progression and proliferation |
| Kim et al. 2017 [173] MCF-7 breast cancer cells |
STAT3, AKT, ERK, JNK, PKA, cAMP | Leptin increased COX-2 expression and PGE2 production, increased aromatase expression |
| Liu et al. 2013 [174] Ishikawa endometrial cancer cells |
Estrogenic pathway | Leptin stimulated cell proliferation via enhancing aromatase expression and estradiol synthesis |
| Nepal et al. 2015 [175] HepG2 hepatoma and MCF-7 breast cancer cells |
p53/FoxO3A axis | Leptin augmented the expression of autophagy-related genes, including beclin-1, Atg5 and LC3 II, which caused increase in cell number and suppression of apoptosis |
| Ptak et al. 2013 [176] OVCAR-3 ovarian cancer cells |
Increase in cyclin D1, cyclin A2, and a decrease in p21WAF1/CIP1, Bad, TNFR1, and caspase 6 | Leptin promoted cell proliferation and downregulated apoptotic pathway |
| Qian et al. 2015 [177] MCF-7 and tamoxifen-resistant cells |
ERK1/2, STAT3 | Leptin enhanced proliferation, and CCND1 (cyclin D1) gene transcription by inducing the binding of ERα to the promoter of CCND1 gene |
| Shouman et al. 2016 [178] MCF-7 breast cancer cells |
Estrogenic pathway | Leptin increased CYP1B1 expression and DNA adducts, and diminished COMT protein expression |
| Wang et al. 2012 [116] HCT-116 colon cancer cells |
PI3K/AKT/mTOR pathway | Leptin stimulated proliferation and inhibited apoptosis |
| Xu et al. 2013 [179] SKOV3 and A2780 ovarian cancer cells |
STAT5 | Leptin promoted cell proliferation in cooperation with microRNA-182 and microRNA-96 (targeting FoxO3) |
| Yoon et al. 2014 [180] LS174T, HCT-116 and CaCo-2 colon cancer cells |
JAK and ERK signaling pathways | Leptin increased the number of cells |
AKT: v-Akt murine thymoma viral oncogene or protein kinase B (a serine/threonine kinase), AMPK: AMP-activated protein kinase, Atg5: autophagy protein 5, cAMP: cyclic AMP, COMT: catechol-o-methyltransferase, COX-2: cyclooxygenase-2, CYP1B1: cytochrome P450 1B1, ER: estrogen receptor, ERK: extracellular signal-regulated kinase, FoxO3A: forkhead box O3, FSH: follicle-stimulating hormone, JAK: Janus kinase, JNK: c-Jun N-terminal kinase, LC3: light chain 3 (microtubule-associated), Mcl-1: myeloid cell leukemia 1, MEK: mitogen-activated protein kinase kinase, mTOR: mechanistic/mammalian target of rapamycin, Ob-R: leptin receptor, p21WAF1/CIP1: cyclin-dependent kinase inhibitor, PGE2: prostaglandin E2, PI3K: phosphatidylinositol-3-kinase, PKA: protein kinase A, STAT3: signal transducer and activator of transcription 3, TNFR1: tumor necrosis factor receptor 1
As mentioned above, both inhibition of apoptosis and formation of new blood vessels for adequate supply of oxygen with nutrients and removal of cellular wastes are the essential requirements for the progression of neoplastic disease. Anti-apoptotic effect of leptin in cancer is a frequent observation of many investigators [181–185]. Similarly, several reports have documented that leptin can facilitate the formation of new blood vessels or angiogenesis, along with biosynthesis of pro-angiogenic factors like VEGF [185–187]. It is worth mentioning that without angiogenesis usually a tumor cannot grow beyond 2 mm in diameter [188]. Nonetheless, the presence of vascular channels in the tumor microenvironment definitely promotes the process of metastasis.
Metastasis is a highly complex process, which leads to a number of hypotheses such as progression, transient compartment, and fusion models [189]. The progression model proposes the development of metastatic subpopulations or clone of mutated cells with metastatic potential within the primary tumor. It is known that tumor aneuploidy (chromosome number variation) and genetic heterogeneity/diversity can influence the disease behavior including metastatic capability [190–192]. Apart from genetic changes, epigenetic alterations such as DNA methylation and histone modifications could play a significant role in metastasis [193,194]. Accumulating evidence suggests that both genetic and epigenetic changes can occur in obesity [195–199]. Interestingly, epigenetic regulation of the leptin signaling pathway may cause leptin resistance in obesity [200]. It is noteworthy that an epigenetic phenomenon is the notion of the transient metastatic compartment model [189]. On the other hand, an important aspect of the genetic events is the role of metastasis suppressor genes in secondary tumors. Metastasis suppressors are molecules that inhibit metastasis formation without affecting primary tumor growth [201]. Functionally, leptin is associated with a number of metastasis suppressor molecules, e.g., caspase 8, CD44, kisspeptin, and thioredoxin interacting protein (TXNIP) [172,202–204].
For the development of secondary tumors, cancer cells undergo various steps from local invasion to distant metastasis. This process is involved with numerous intracellular signaling pathways and associated transduction molecules. Some of the well-studied signaling pathways/molecules include: MAPK/ERK, PI3K/AKT, STAT, TGF-β, and Wnt/β-Catenin [205]. Fascinatingly, many of these molecules are linked with the signal transduction pathways of leptin (Table 4 [14,108,109,113,118,120,129,142,144,148,152,153,206–222]).
Table 4.
Proposed mechanisms of cancer cell migration and metastasis under the influence of leptin
| Investigators and Cancer types | Nature of neoplastic progression | Mechanisms or involved intracellular signaling pathways | Observed phenomena |
|---|---|---|---|
| Cao et al. 2016 [148] MCF-7 and MDA-MB-231 breast cancer cells, and xenograft model |
Migration and metastasis | p38 MAPK and ERK1/2 | Leptin-induced tumor-associated M2 macrophage-derived IL-8 promoted the migration and invasion/metastasis of cancer cells in both in vitro and xenograft model |
| Cheng et al. 2011 [129] K1 and B-CPAP papillary thyroid cancer cells |
Migration | AKT and ERK pathways | Leptin enhanced the migratory activity |
| Ding et al. 2016 [206] HepG2 hepatocellular carcinoma and MCF-7 breast cancer cells |
Migration | STAT3, ERK1/2, and AKT | APPL1 positively mediated leptin signaling and promoted leptin-induced proliferation and migration of cancer cells |
| Dong et al. 2013 [108] AGS, MKN-28 and MKN-45 gastric cancer cells |
Migration | AKT and ERK1/2 | Leptin upregulated MT1-MMP expression and enhanced the interaction of MT1-MMP with KIF1B, which contributed to cancer cell invasion |
| Dong et al. 2014 [109] AGS and MKN-45 gastric cancer cells |
Migration | Rho/ROCK pathway | Leptin enhanced cancer cell migration by upregulating ICAM-1 |
| Fan et al. 2015 [120] PANC-1 and AsPC-1 pancreatic cancer cells, and clinical samples |
Migration and metastasis | JAK2/STAT3 pathway | Leptin upregulated the expression of MMP-13 |
| Fava et al. 2008 [207] Intrahepatic cholangiocarcinoma HuH-28 cells |
Migration | STAT3 and ERK1/2 | Leptin increased the growth and migration of cancer cells |
| Feng et al. 2013 [14] A549 lung cancer cells |
Metastasis | TGF-β | Leptin promoted the metastasis by inducing EMT in a TGF-β-dependent manner |
| Frankenberry et al. 2004 [208] androgen-independent DU145 and PC-3 prostate cancer cells |
Migration | MAPK and PI3K (mainly) | Leptin enhanced cancer cell migration, and induced expression of VEGF, TGF-β1, and bFGF |
| Ghasemi et al. 2017 [209] OVCAR3, SKOV3 and CaoV-3 ovarian cancer cells |
Migration | RhoA/ROCK, PI3K/AKT, JAK/STAT pathways and NF-κB activation | Leptin induced cancer cell invasion via upregulating uPA |
| Ghasemi et al. 2017 [210] SKOV3 and OVCAR3 ovarian cancer cells |
Migration | ERK and JNK pathways | Leptin regulated cancer cell invasion by promoting MMP-7 expression |
| Guo and Gonzalez-Perez 2011 [142] Mouse 4T1, EMT6 and MMT breast cancer cells |
Migration | JAK2/STAT3, MAPK, PI3K/mTOR, p38 MAPK and JNK signaling pathways | Leptin upregulated cell proliferation/migration and pro-angiogenic factors Notch, IL-1 and VEGF/VEGFR-2 |
| Huang et al. 2011 [152] PC-3, DU145, and LnCaP prostate cancer cells |
Migration | IRS-1, PI3K, AKT, and NF-κB | Leptin increased the migration of cancer cells. Moreover, leptin upregulated αvβ3 integrin expression |
| Huang et al. 2017 [211] MCF-7 and T47D breast cancer cells |
Migration | PI3K/AKT/SREBP2 pathway | Leptin enhanced the proliferation, migration and invasion of cancer cells via ACAT2 upregulation |
| Knight et al. 2011 [212] MCF-7 and MDA-MB-231 breast cancer cells |
Migration | EGFR, Notch1, and survivin | Leptin increased the migration potential of cancer cells |
| Li et al. 2016 [144] THP1 human monocytes, and MCF-7, SK-BR-3 and MDA-MB-231 breast cancer cells, and xenograft model |
Migration and metastasis | NF-κB signaling in TAMs, and PI3K-AKT/ATF-2 signaling in cancer cells | Leptin stimulated IL-18 expression in both TAMs and breast cancer cells |
| Martín et al. 2017 [213] Human astrocytoma 1321N1 cells |
Migration | EGFR, and ERK, AKT/mTOR pathways | Leptin and sPLA2-IIA increased growth and migration in these cells |
| Mendonsa et al. 2015 [214] Murine Panc02 and human Panc1 pancreatic cancer cells |
Migration | PI3K/AKT pathway | Leptin increased migration of cancer cells |
| Mishra et al. 2017 [215] Breast epithelial and cancer cells: MCF10A, MCF10AT1, MCF-7 and MDA-MB-231 |
Metastasis | TGF-β1 pathway | Leptin-mediated changes represented EMT and a cancer stem cell-like phenotype |
| Noda et al. 2015 [216] LNCaP, DU145 and PC-3 prostate cancer cells |
Migration | PI3K/AKT | Leptin increased the cell proliferation, migration, and invasion. Leptin also increased the phosphorylation of FOXO1, the expression of cyclin D1, and decreased the expression of p21 protein |
| Ratke et al. 2010 [118] SW480, SW620, and HCT-116 colon cancer cells |
Migration | JAK, STAT3, Src kinase, FAK, PI3K, PKCδ | Leptin enhanced the migratory activity of cancer cells |
| Saxena et al. 2007 [217] HepG2 and Huh7 hepatocellular carcinoma cells |
Migration | JAK/STAT3, ERK, and PI3K | Leptin promoted cancer cell growth, invasiveness, and migration |
| Sobrinho Santos et al. 2017 [218] SCC-9 and SCC-4 oral squamous cell cancer cells, and clinical samples |
Migration | E-cadherin, Col1A1, MMP-2 and -9, mir-210 and HIF-1α | Leptin favored higher cell proliferation, migration, and reduced apoptosis. Furthermore, leptin decreased caspase-3 mRNA expression |
| Wang et al. 2013 [219] MCF-7 breast cancer cells |
Migration | JAK/STAT and PI3K/AKT signaling pathways | Leptin promoted migration and invasion. Leptin also increased the expression of MMP-9 and TGF-β |
| Wei et al. 2016 [220] MCF-7, SK-BR-3, and MDA-MB-468 breast cancer cells |
Migration and metastasis | PI3K/AKT signaling pathway | Leptin promoted EMT in breast cancer cells via upregulation of PKM2 |
| Yang et al. 2009 [153] JJ012 and SW1353 chondrosarcoma cells |
Migration | IRS-1, PI3K, AKT and NF-κB | Leptin increased migration and expression of αvβ3 integrin in human chondrosarcoma cells |
| Yeh et al. 2009 [221] C6 glioma cells |
Migration | p38 MAPK and NF-κB pathways | Leptin enhanced the migration and invasion of glioma cells. Leptin also upregulated the expression of MMP-13 |
| Yuan et al. 2014 [222] MCF-7 breast cancer cells |
Migration | ERK pathway | Leptin increased the proliferation and migration of cancer cells |
| Zou et al. 2016 [113] Gallbladder cancer cell subline GBC-SD, xenograft model, and clinical samples |
Migration | JAK2/STAT3/SOCS3 | Leptin promoted the proliferation, migration and invasion of cancer cells. Leptin upregulated VEGF-C and VEGF-D levels and activated MMP-3 and MMP-9 |
ACAT2: acetyl-CoA acetyltransferase 2, AKT: v-Akt murine thymoma viral oncogene or protein kinase B (a serine/threonine kinase), APPL1: adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (involved in the regulation of cell proliferation), ATF-2: activating transcription factor 2, bFGF: basic fibroblast growth factor, Col1A1: collagen type I alpha 1, EGFR: epidermal growth factor receptor, EMT: epithelial-mesenchymal transition, ERK: extracellular signal-regulated kinase, FAK: focal adhesion kinase, FOXO1: forkhead box O1, HIF-1α: hypoxia-inducible factor 1α, ICAM-1: Intercellular adhesion molecule-1, IL: interleukin, IRS: insulin receptor substrate, JAK: Janus kinase, JNK: c-Jun N-terminal kinase, KIF1B: kinesin family member 1B (involved in the transport of materials), MAPK: mitogen-activated protein kinase, mir-210: mir-210 microRNA (predominant hypoxia-responsive miRNA), MMP: matrix metalloproteinase, MT1-MMP: membrane type 1-matrix metalloproteinase, mTOR: mechanistic/mammalian target of rapamycin, NF-κB: nuclear factor-kappa B, PI3K: phosphatidylinositol-3-kinase, PKC: protein kinase C, PKM2: pyruvate kinase M2 isoform, Rho: G protein under the Ras homologue gene family, RhoA: Rho family small GTPase-member A, ROCK: Rho-associated coiled-coil-forming protein kinase, SOCS3: suppressor of cytokine signaling-3, sPLA2-IIA: secreted phospholipase A2-IIA, Src: avian sarcoma viral oncogene homolog, SREBP2: sterol regulatory element-binding protein 2, STAT3: signal transducer and activator of transcription 3, TAM: tumor-associated macrophage, TGF-β: transforming growth factor beta, uPA: urokinase plasminogen activator, VEGF: vascular endothelial growth factor, VEGFR-2: vascular endothelial growth factor receptor 2.
A highly complex role is played by the tumor infiltrating immune cells, particularly macrophages. In general, studies have indicated that the presence of macrophages within the tumor microenvironment (i.e., TAMs) was associated with poor prognosis in cancer patients [223,224]. Often TAMs display M2 phenotype [225–227]. On the other hand, evidence suggests that obesity can favor M2 polarization [228,229]. Furthermore, leptin has been shown to influence M2 macrophages [148,230]. Nonetheless, macrophages are highly specialized cells having character of plasticity/adaptability in diverse microenvironments. Interestingly, many features of macrophages are also seen in metastatic cancer cells [231]. For instance, carcinomas of the lining epithelial cells can form a solid mass like mesenchymal tissue. Of note, macrophages arise from the myeloid lineage that originates from mesenchymal cells (derivative of mesoderm). Moreover, many metastatic cancers express aerobic glycolysis (Warburg effect), which may occur in activated macrophages in chronic inflammatory lesions. Additionally, acquiring mesenchymal characteristics in the process of EMT happens during metastasis. Other interesting macrophage-like features of metastatic cells are: they can migrate and extravasate/infiltrate into the surrounding tissues, show phagocytic behavior, and release various pro-angiogenic molecules. All of these features have led to the proposition for the fusion model, which suggests the formation of fusion hybrids between cancer cells and macrophages [189,231]. So, there could be generation of fusion subclones with varying dissemination potentials.
In a recent report, Clawson and colleagues described macrophage-tumor cell fusions that were isolated from the blood of pancreatic ductal adenocarcinoma patients [232]. They observed that fusion cells expressed markers characteristic of pancreatic ductal adenocarcinoma and stem cells, as well as M2-polarized macrophages. In another study, M2 macrophages (derived from U937 cells) were fused with MCF-7 and MDA-MB-231 breast cancer cells [233]. The investigators found that the fusion hybrids had a more aggressive phenotype. It is worth noting that macrophages have the capacity to form fusion cells in physiologic and different pathologic circumstances. Multinucleated cells such as osteoclasts and foreign-body giant cells are thought to be generated by fusion of macrophages. Although the dichotomy of M1 and M2 macrophages is not based on very clear ideas, it has been suggested that M2 macrophages appear at the later phase of a lesion, they stimulate angiogenesis, synthesize ECM components and work towards resolution of inflammation [234]. Obviously, these properties are beneficial for the development of secondary tumors. Overall, excess adipose tissue is an attractive place for macrophages – because, obesity is considered as a state of low grade chronic inflammation. Like other inflammatory conditions, macrophages may influence the disease process or contribute to disease progression in obesity.
9. Conclusions
Leptin could influence cancer cells through numerous phenomena, e.g., inflammation and oxidative stress, cell proliferation, inhibition of apoptosis, angiogenesis, immune modulation, etc. In obesity, cancer cells constantly receive growth promoting stimuli in an environment where pro-inflammatory cytokines or adipokines in particular leptin dominate. Obviously, manipulation of this environment surrounding the neoplastic growth or tumor microenvironment is a challenging task. However, already some potential directions have been proposed such as increased biosynthesis of anti-inflammatory substances like adiponectin, leptin antagonists, and other pharmaceutical agents like metformin.
Leptin antagonists can be divided into 2 major groups: leptin analogs and antibodies against leptin or Ob-R. Leptin interacts with Ob-R through three different binding sites: I-III. Different analogs or muteins are formed by changing the amino acid sequence such as LDFI (in binding site I), LPrA2 (II), and Allo-aca (III). In general, administration of antagonists has been reported to cause weight gain. It is anticipated that orexigenic neuropeptide Y (NPY) will dominate in the hypothalamus in the absence of appropriate leptin signaling. However, some adverse effects are serious and could be dangerous, e.g., insulin resistance, increased bone mass, disrupted locomotor activity, depressive behavior, and peripheral inflammation [235,236]. All these studies were conducted in experimental animals and for a relatively short duration. In real clinical situations, the treatment is expected to continue for a considerable time period and thus the adverse effects could be substantial. Furthermore, malignancies are often associated with comorbidities, which are common in patients with colon, breast, and lung cancers [237]. Consequently complications in such patients may aggravate in additional disorders such as obesity, insulin resistance or any inflammatory conditions. Development of methods for the local delivery of leptin antagonists at the tumor site is definitely a superior alternative.
Highlights.
The multifaceted action of leptin involves multiple cell types in the tumor microenvironment and intracellular signaling molecules; cooperation/interactions between these biomolecules in obesity could accelerate the process of cancer cell migration and metastasis.
Approximately half of the cancer burden and two-fifths of cancer deaths are attributable to cancers of the digestive and reproductive systems: usually leptin’s role is important in these cancers.
Leptin has been demonstrated to affect the prognosis of both adenocarcinomas and squamous cell carcinomas, although obesity is not a risk factor for squamous cell carcinomas.
Several cancer dissemination mechanisms such as the involvement of matrix metalloproteinases (MMPs), M2 macrophages, and epithelial-mesenchymal transition (EMT) have been documented to be linked with leptin.
Acknowledgments
Funding
This work was supported by NIH NCI-CA157012, Paint the Town Pink and The Hormel Foundation.
Abbreviations
- AKT
protein kinase B (a serine/threonine kinase)
- CAF
cancer-associated fibroblast
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- HIF
hypoxia-inducible factor
- ICAM
intercellular adhesion molecule
- IGF
insulin-like growth factor
- IL
interleukin
- JAK
Janus kinase
- MCP-1
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinase
- NSCLC
non-small-cell lung cancer
- Ob-R
leptin receptor
- PI3K
phosphatidylinositol 3-kinase
- STAT
signal transducer and activator of transcription
- TAM
tumor-associated macrophage
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TNM
tumor-lymph node-distant metastasis
- VEGF
vascular endothelial growth factor
Biographies

Amitabha Ray
Amitabha Ray completed his graduation in medicine (M.B.B.S.) from University of Calcutta, postgraduation (M.D.) from B.H.U., Varanasi, and Ph.D. from J.M.I., New Delhi, India. He pursued his postdoctoral research in Dr. Cleary’s laboratory at the Hormel Institute, University of Minnesota. Currently, he is working with LECOM at Seton Hill University, Greensburg, PA, as an Associate Professor.

Margot P. Cleary
Dr. Margot P. Cleary is a professor at the Hormel Institute, University of Minnesota. She received an undergraduate degree in Chemistry from Regis College in Weston, MA and then did graduate work at Columbia University at the Institute of Human Nutrition earning MS, MPhil and Ph.D. degrees. Dr. Cleary’s laboratory was one of the first to study the interactions of body weight with the development of breast and prostate cancers using preclinical models. In addition, she carried out extensive studies on the potential role of leptin as a growth factor linking obesity with breast cancer and proposed that the leptin to adiponectin ratio may be an important determinate in the development of some cancers.
In an interesting study, a leptin antagonist was demonstrated to stimulate appetite and weight gain in chronic kidney disease-associated cachexia in experimental animals [238]. The investigators also observed that the leptin antagonist normalized the expression of pro-inflammatory cytokines such as IL-6 and TNF-α. Cachexia in cancer is associated progressive wasting of body tissues, systemic inflammation, and poor prognosis. Leptin antagonists could be a promising inclusion in the therapy.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ray A. Adipokine leptin in obesity-related pathology of breast cancer. J Biosci. 2012;37:289–294. doi: 10.1007/s12038-012-9191-9. [DOI] [PubMed] [Google Scholar]
- 2.McSherry EA, Donatello S, Hopkins AM, McDonnell S. Molecular basis of invasion in breast cancer. Cell Mol Life Sci. 2007;64:3201–3218. doi: 10.1007/s00018-007-7388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 5.Giordano C, Barone I, Vircillo V, Panza S, Malivindi R, Gelsomino L, et al. Activated FXR inhibits leptin signaling and counteracts tumor-promoting activities of cancer-associated fibroblasts in breast malignancy. Sci Rep. 2016;6:21782. doi: 10.1038/srep21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn JH, Choi YS, Choi JH. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod. 2015;21:792–802. doi: 10.1093/molehr/gav039. [DOI] [PubMed] [Google Scholar]
- 7.Berg G, Schreier L, Miksztowicz V. Circulating and adipose tissue matrix metalloproteinases in cardiometabolic risk environments: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17:79–87. doi: 10.1515/hmbci-2013-0069. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Martínez E, Miana M, Jurado-López R, Bartolomé MV, Souza Neto FV, Salaices M, et al. The potential role of leptin in the vascular remodeling associated with obesity. Int J Obes (Lond) 2014;38:1565–1572. doi: 10.1038/ijo.2014.37. [DOI] [PubMed] [Google Scholar]
- 9.Cui W, Maimaitiyiming H, Qi X, Norman H, Wang S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol. 2013;305:F871–880. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray A, Cleary MP. Obesity and breast cancer: a clinical biochemistry perspective. Clin Biochem. 2012;45:189–197. doi: 10.1016/j.clinbiochem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, Witek RP, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287:8598–8612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, et al. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol Res. 2013;21:165–171. doi: 10.3727/096504014X13887748696662. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, et al. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16:1220–1230. doi: 10.1080/15384047.2015.1056409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato S, Abarzua-Catalan L, Trigo C, Delpiano A, Sanhueza C, García K, et al. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget. 2015;6:21100–21119. doi: 10.18632/oncotarget.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, McDonald LT, Russell DL, Kelly RR, Wilson KR, Mehrotra M, et al. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment. World J Stem Cells. 2015;7:253–265. doi: 10.4252/wjsc.v7.i2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang F, Wu K, Liu Y, Shi L, Wang D, Li G, et al. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int J Biochem Cell Biol. 2016;84:14–21. doi: 10.1016/j.biocel.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer KA, Neeley CK, Baker NA, Washabaugh AR, Flesher CG, Nelson BS, et al. Adipocytes promote pancreatic cancer cell proliferation via glutamine transfer. Biochem Biophys Rep. 2016;7:144–149. doi: 10.1016/j.bbrep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira A, Pereira SS, Costa M, Morais T, Pinto A, Fernandes R, et al. Adipocyte secreted factors enhance aggressiveness of prostate carcinoma cells. PLoS One. 2015;10:e0123217. doi: 10.1371/journal.pone.0123217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y, Ishiguro H, Kobayashi N, Hasumi H, Watanabe M, Yao M, et al. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. Prostate. 2015;75:1009–1019. doi: 10.1002/pros.22972. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31:32. doi: 10.1186/1756-9966-31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W, Harvey S, Macura KJ, Euhus DM, Artemov D. Invasive breast cancer preferably and predominantly occurs at the interface between fibroglandular and adipose tissue. Clin Breast Cancer. 2017;17:e11–e18. doi: 10.1016/j.clbc.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher SJ, Sacca PA, Pistone-Creydt M, Coló FA, Serra MF, Santino FE, et al. Human breast adipose tissue: characterization of factors that change during tumor progression in human breast cancer. J Exp Clin Cancer Res. 2017;36:26. doi: 10.1186/s13046-017-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7:2346–2359. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197–210. doi: 10.1016/j.pharmthera.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massa M, Gasparini S, Baldelli I, Scarabelli L, Santi P, Quarto R, et al. Interaction between breast cancer cells and adipose tissue cells derived from fat grafting. Aesthet Surg J. 2016;36:358–363. doi: 10.1093/asj/sjv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Esposito V, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7:24495–24509. doi: 10.18632/oncotarget.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat. 2015;153:323–335. doi: 10.1007/s10549-015-3550-3559. [DOI] [PubMed] [Google Scholar]
- 32.Yao-Borengasser A, Monzavi-Karbassi B, Hedges RA, Rogers LJ, Kadlubar SA, Kieber-Emmons T. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol Rep. 2015;33:2689–2694. doi: 10.3892/or.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisaki K, Fujimoto H, Sangai T, Nagashima T, Sakakibara M, Shiina N, et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat. 2015;150:255–263. doi: 10.1007/s10549-015-3318-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Gao C, Meng K, Qiao H, Wang Y. Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS One. 2015;10:e0119348. doi: 10.1371/journal.pone.0119348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550–70. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 37.Wolfson B, Eades G, Zhou Q. Adipocyte activation of cancer stem cell signaling in breast cancer. World J Biol Chem. 2015;6:39–47. doi: 10.4331/wjbc.v6.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu E, Samad F, Mueller BM. Local adipocytes enable estrogen-dependent breast cancer growth: Role of leptin and aromatase. Adipocyte. 2013;2:165–169. doi: 10.4161/adip.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marwarha G, Ghribi O. Leptin signaling and Alzheimer’s disease. Am J Neurodegener Dis. 2012;1:245–265. [PMC free article] [PubMed] [Google Scholar]
- 40.Prokop JW, Duff RJ, Ball HC, Copeland DL, Londraville RL. Leptin and leptin receptor: analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides. 2012;38:326–336. doi: 10.1016/j.peptides.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–372. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 42.Chimal-Vega B, Paniagua-Castro N, Carrillo Vazquez J, Rosas-Trigueros JL, Zamorano-Carrillo A, Benítez-Cardoza CG. Exploring the structure and conformational landscape of human leptin. A molecular dynamics approach. J Theor Biol. 2015;385:90–101. doi: 10.1016/j.jtbi.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Peelman F, Zabeau L, Moharana K, Savvides SN, Tavernier J. 20 years of leptin: insights into signaling assemblies of the leptin receptor. J Endocrinol. 2014;223:T9–23. doi: 10.1530/JOE-14-0264. [DOI] [PubMed] [Google Scholar]
- 44.Dozio E, Ruscica M, Galliera E, Corsi MM, Magni P. Leptin, ciliary neurotrophic factor, leukemia inhibitory factor and interleukin-6: class-I cytokines involved in the neuroendocrine regulation of the reproductive function. Curr Protein Pept Sci. 2009;10:577–584. doi: 10.2174/138920309789630561. [DOI] [PubMed] [Google Scholar]
- 45.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han TJ, Wang X. Leptin and its receptor in hematologic malignancies. Int J Clin Exp Med. 2015;8:19840–19849. [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang N, Sun R, Sun Q. Leptin signaling molecular actions and drug target in hepatocellular carcinoma. Drug Des Devel Ther. 2014;8:2295–2302. doi: 10.2147/DDDT.S69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Kim BH, Yi EH, Ye SK. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch Pharm Res. 2016;39:1085–1099. doi: 10.1007/s12272-016-0795-8. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Huang C. Regulation of EMT by STAT3 in gastrointestinal cancer (review) Int J Oncol. 2017;50:753–767. doi: 10.3892/ijo.2017.3846. [DOI] [PubMed] [Google Scholar]
- 51.Yoon J, Ko YS, Cho SJ, Park J, Choi YS, Choi Y, et al. Signal transducers and activators of transcription 3-induced metastatic potential in gastric cancer cells is enhanced by glycogen synthase kinase-3β. APMIS. 2015;123:373–382. doi: 10.1111/apm.12370. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C, Guo F, Xu G, Ma J, Shao F. STAT3 cooperates with Twist to mediate epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Rep. 2015;33:1872–1882. doi: 10.3892/or.2015.3783. [DOI] [PubMed] [Google Scholar]
- 53.Yang XW, Li L, Hou GJ, Yan XZ, Xu QG, Chen L, et al. STAT3 overexpression promotes metastasis in intrahepatic cholangiocarcinoma and correlates negatively with surgical outcome. Oncotarget. 2017;8:7710–7721. doi: 10.18632/oncotarget.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Liu X, Jiao H, Peng L, Huo Z, Yang W, et al. Prognostic and clinical significance of STAT3 and MMP9 in patients with gastric cancer: a meta-analysis of a Chinese cohort. Int J Clin Exp Med. 2015;8:546–557. [PMC free article] [PubMed] [Google Scholar]
- 55.Yu S, Li G, Wang Z, Wang Z, Chen C, Cai S, et al. The prognostic value of pSTAT3 in gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2016;142:649–657. doi: 10.1007/s00432-015-2023-1. [DOI] [PubMed] [Google Scholar]
- 56.Deng J, Cui J, Jiang N, Zhang R, Zhang L, Hao X, et al. STAT3 regulation the expression of VEGF-D in HGC-27 gastric cancer cell. Am J Transl Res. 2014;6:756–767. [PMC free article] [PubMed] [Google Scholar]
- 57.Song YY, Sun LD, Liu ML, Liu ZL, Chen F, Zhang YZ, et al. STAT3, p-STAT3 and HIF-1α are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma. Oncol Lett. 2014;8:431–437. doi: 10.3892/ol.2014.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong B, Liu Q, Liu Y, Xiong X, Liu Y. Expressions of STAT3, p-STAT3 and E-cadherin in colorectal cancer and clinical implications. Zhonghua Wei Chang Wai Ke Za Zhi (Chinese Journal of Gastrointestinal Surgery) 2014;17:594–597. [PubMed] [Google Scholar]
- 59.Ji K, Zhang M, Chu Q, Gan Y, Ren H, Zhang L, et al. The role of p-STAT3 as a prognostic and clinicopathological marker in colorectal cancer: A systematic review and meta-analysis. PLoS One. 2016;11:e0160125. doi: 10.1371/journal.pone.0160125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol. 2014;40:311–317. doi: 10.1016/j.ejso.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Zhao M, Gao FH, Wang JY, Liu F, Yuan HH, Zhang WY, et al. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73:366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Yu Y, Zhao Q, Wang Z, Liu XY. Activated STAT3 correlates with prognosis of non-small cell lung cancer and indicates new anticancer strategies. Cancer Chemother Pharmacol. 2015;75:917–922. doi: 10.1007/s00280-015-2710-2. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H, et al. Activation of STAT3 is involved in malignancy mediated by CXCL12-CXCR4 signaling in human breast cancer. Oncol Rep. 2014;32:2760–2768. doi: 10.3892/or.2014.3536. [DOI] [PubMed] [Google Scholar]
- 64.Wei G, Mingliang Z, Yong C, Suyang G. Expression of signal transducer and activator of transcription 3 in breast cancer and its clinical significance. J Cancer Res Ther. 2015;11:C56–58. doi: 10.4103/0973-1482.163840. [DOI] [PubMed] [Google Scholar]
- 65.McDaniel JM, Varley KE, Gertz J, Savic DS, Roberts BS, Bailey SK, et al. Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget. 2017;8:8226–8238. doi: 10.18632/oncotarget.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donato J, Jr, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591–602. doi: 10.1590/S0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- 67.Sheen MR, Marotti JD, Allegrezza MJ, Rutkowski M, Conejo-Garcia JR, Fiering S. Constitutively activated PI3K accelerates tumor initiation and modifies histopathology of breast cancer. Oncogenesis. 2016;5:e267. doi: 10.1038/oncsis.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014;16:201. doi: 10.1186/bcr3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arsenic R, Lehmann A, Budczies J, Koch I, Prinzler J, Kleine-Tebbe A, et al. Analysis of PIK3CA mutations in breast cancer subtypes. Appl Immunohistochem Mol Morphol. 2014;22:50–56. doi: 10.1097/PDM.0b013e318297afea. [DOI] [PubMed] [Google Scholar]
- 70.Firoozinia M, Zareian Jahromi M, Moghadamtousi SZ, Nikzad S, Abdul Kadir H. PIK3CA gene amplification and PI3K p110α protein expression in breast carcinoma. Int J Med Sci. 2014;11:620–625. doi: 10.7150/ijms.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendelová A, Jezková E, Zubor P, Holubeková V, Lasabová Z, Plank L, et al. Correlation between the incidence of PIK3CA mutations in breast cancer and histopathological characteristics of the tumor. Ceska Gynekol. 2014;79:283–288. [PubMed] [Google Scholar]
- 72.Tserga A, Chatziandreou I, Michalopoulos NV, Patsouris E, Saetta AA. Mutation of genes of the PI3K/AKT pathway in breast cancer supports their potential importance as biomarker for breast cancer aggressiveness. Virchows Arch. 2016;469:35–43. doi: 10.1007/s00428-016-1938-5. [DOI] [PubMed] [Google Scholar]
- 73.Harada K, Baba Y, Shigaki H, Ishimoto T, Miyake K, Kosumi K, et al. Prognostic and clinical impact of PIK3CA mutation in gastric cancer: pyrosequencing technology and literature review. BMC Cancer. 2016;16:400. doi: 10.1186/s12885-016-2422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi J, Yao D, Liu W, Wang N, Lv H, Zhang G, et al. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC Cancer. 2012;12:50. doi: 10.1186/1471-2407-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, et al. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol. 2010;16:4986–4991. doi: 10.3748/WJG.v16.i39.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu Y, Jin S, Wang F, Hua Y, Yang L, Shu Y, et al. Clinicopathological significance of PI3K, Akt and survivin expression in gastric cancer. Biomed Pharmacother. 2014;68:471–475. doi: 10.1016/j.biopha.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012;18:3745–3751. doi: 10.3748/wjg.v18.i28.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15:245–246. doi: 10.4161/cbt.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dai J, Bercury KK, Macklin WB. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia. 2014;62:2096–2109. doi: 10.1002/glia.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946–1954. doi: 10.1039/c5mb00101c. [DOI] [PubMed] [Google Scholar]
- 81.Gungorduk K, Ertas IE, Sahbaz A, Ozvural S, Sarica Y, Ozdemir A, et al. Immunolocalization of ERK1/2 and p-AKT in normal endometrium, endometrial hyperplasia, and early and advanced stage endometrioid endometrial adenocancer and their prognostic significance in malignant group. Eur J Obstet Gynecol Reprod Biol. 2014;179:147–152. doi: 10.1016/j.ejogrb.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 82.Li XP, Zhang XW, Zheng LZ, Guo WJ. Expression of CD44 in pancreatic cancer and its significance. Int J Clin Exp Pathol. 2015;8:6724–6731. [PMC free article] [PubMed] [Google Scholar]
- 83.Wang CY, Deng JY, Cai XW, Fu XL, Li Y, Zhou XY, et al. High EGFR and low p-Akt expression is associated with better outcome after nimotuzumab-containing treatment in esophageal cancer patients: preliminary clinical result and testable hypothesis. Oncotarget. 2015;6:18674–18682. doi: 10.18632/oncotarget.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin F, Zhang PL, Yang XJ, Prichard JW, Lun M, Brown RE. Morphoproteomic and molecular concomitants of an overexpressed and activated mTOR pathway in renal cell carcinomas. Ann Clin Lab Sci. 2006;36:283–293. [PubMed] [Google Scholar]
- 85.Aleskandarany MA, Rakha EA, Ahmed MA, Powe DG, Ellis IO, Green AR. Clinicopathologic and molecular significance of phospho-Akt expression in early invasive breast cancer. Breast Cancer Res Treat. 2011;127:407–416. doi: 10.1007/s10549-010-1012-y. [DOI] [PubMed] [Google Scholar]
- 86.Yun F, Jia Y, Li X, Yuan L, Sun Q, Yu H, et al. Clinicopathological significance of PTEN and PI3K/AKT signal transduction pathway in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2112–2120. [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Lu HS, Gan MF, Chen LX, He K, Fan GM, et al. Expression and prognostic role of MEKK3 and pERK in patients with renal clear cell carcinoma. Asian Pac J Cancer Prev. 2015;16:2495–2499. doi: 10.7314/apjcp.2015.16.6.2495. [DOI] [PubMed] [Google Scholar]
- 88.Holck S, Bonde J, Pedersen H, Petersen AA, Chaube A, Nielsen HJ, et al. Localization of active, dually phosphorylated extracellular signal-regulated kinase 1 and 2 in colorectal cancer with or without activating BRAF and KRAS mutations. Hum Pathol. 2016;54:37–46. doi: 10.1016/j.humpath.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 89.David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, et al. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res. 2004;10:6865–6871. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 90.Jia W, Chang B, Sun L, Zhu H, Pang L, Tao L, et al. REDD1 and p-AKT over-expression may predict poor prognosis in ovarian cancer. Int J Clin Exp Pathol. 2014;7:5940–5949. [PMC free article] [PubMed] [Google Scholar]
- 91.Baba Y, Nosho K, Shima K, Hayashi M, Meyerhardt JA, Chan AT, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer. 2011;117:1399–1408. doi: 10.1002/cncr.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 93.Yip PY, Cooper WA, Kohonen-Corish MR, Lin BP, McCaughan BC, Boyer MJ, et al. Phosphorylated Akt expression is a prognostic marker in early-stage non-small cell lung cancer. J Clin Pathol. 2014;67:333–340. doi: 10.1136/jclinpath-2013-201870. [DOI] [PubMed] [Google Scholar]
- 94.Campbell L, Nuttall R, Griffiths D, Gumbleton M. Activated extracellular signal-regulated kinase is an independent prognostic factor in clinically confined renal cell carcinoma. Cancer. 2009;115:3457–3467. doi: 10.1002/cncr.24389. [DOI] [PubMed] [Google Scholar]
- 95.Tsujino I, Nakanishi Y, Hiranuma H, Shimizu T, Hirotani Y, Ohni S, et al. Increased phosphorylation of ERK1/2 is associated with worse chemotherapeutic outcome and a poor prognosis in advanced lung adenocarcinoma. Med Mol Morphol. 2016;49:98–109. doi: 10.1007/s00795-015-0130-3. [DOI] [PubMed] [Google Scholar]
- 96.Ma L, Lan F, Zheng Z, Xie F, Wang L, Liu W, et al. Epidermal growth factor (EGF) and interleukin (IL)-1β synergistically promote ERK1/2-mediated invasive breast ductal cancer cell migration and invasion. Mol Cancer. 2012;11:79. doi: 10.1186/1476-4598-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tasioudi KE, Saetta AA, Sakellariou S, Levidou G, Michalopoulos NV, Theodorou D, et al. pERK activation in esophageal carcinomas: clinicopathological associations. Pathol Res Pract. 2012;208:398–404. doi: 10.1016/j.prp.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Tai CJ, Lee CH, Chen HC, Wang HK, Jiang MC, Su TC, et al. High nuclear expression of phosphorylated extracellular signal-regulated kinase in tumor cells in colorectal glands is associated with poor outcome in colorectal cancer. Ann Diagn Pathol. 2013;17:165–171. doi: 10.1016/j.anndiagpath.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Thrift AP. Determination of risk for Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Gastroenterol. 2016;32:319–324. doi: 10.1097/MOG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 100.Tercioti-Junior V, Lopes LR, Coelho-Neto JS. Adenocarcinoma versus squamous cell carcinoma: analysis of 306 patients in university hospital. Arq Bras Cir Dig. 2011;24:272–276. [Google Scholar]
- 101.Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A, et al. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget. 2015;6:11203–11215. doi: 10.18632/oncotarget.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duan X, Tang P, Zhang H, Yu Z. Expression of leptin and adiponectin in esophageal squamous cell carcinoma and their clinical significance. Zhonghua Zhong Liu Za Zhi (Chinese Journal of Oncology) 2014;36:839–843. [PubMed] [Google Scholar]
- 103.Choi E, Byeon SJ, Kim SH, Lee HJ, Kwon HJ, Ahn H, et al. Implication of leptin-signaling proteins and epstein-barr virus in gastric carcinomas. PLoS One. 2015;10:e0130839. doi: 10.1371/journal.pone.0130839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Espejo Romero H, Navarrete Siancas J. Classification of stomach adenocarcinomas. Rev Gastroenterol Peru. 2003;23:199–212. [PubMed] [Google Scholar]
- 105.Zhao X, Huang K, Zhu Z, Chen S, Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–1321. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 106.Geng Y, Wang J, Wang R, Wang K, Xu Y, Song G, et al. Leptin and HER-2 are associated with gastric cancer progression and prognosis of patients. Biomed Pharmacother. 2012;66:419–424. doi: 10.1016/j.biopha.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Ishikawa M, Kitayama J, Nagawa H. Expression pattern of leptin and leptin receptor (OB-R) in human gastric cancer. World J Gastroenterol. 2006;12:5517–5522. doi: 10.3748/wjg.v12.i34.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong Z, Xu X, Du L, Yang Y, Cheng H, Zhang X, et al. Leptin-mediated regulation of MT1-MMP localization is KIF1B dependent and enhances gastric cancer cell invasion. Carcinogenesis. 2013;34:974–983. doi: 10.1093/carcin/bgt028. [DOI] [PubMed] [Google Scholar]
- 109.Dong Z, Fu S, Xu X, Yang Y, Du L, Li W, et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer. 2014;110:1801–1810. doi: 10.1038/bjc.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 111.Bain GH, Collie-Duguid E, Murray GI, Gilbert FJ, Denison A, McKiddie F, et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110:1525–1534. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Catalano S, Leggio A, Barone I, De Marco R, Gelsomino L, Campana A, et al. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J Cell Mol Med. 2015;19:1122–1132. doi: 10.1111/jcmm.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zou H, Liu Y, Wei D, Wang T, Wang K, Huang S, et al. Leptin promotes proliferation and metastasis of human gallbladder cancer through OB-Rb leptin receptor. Int J Oncol. 2016;49:197–206. doi: 10.3892/ijo.2016.3530. [DOI] [PubMed] [Google Scholar]
- 114.Yunusova NV, Kondakova IV, Kolomiets LA, Afanasiev SG, Chernyshova AL, Shatokhina OV, et al. Serum adipokines and their receptors in endometrial and colon cancer patients: relationship with tumor invasion and metastasis. Vopr Onkol. 2015;61:619–623. [PubMed] [Google Scholar]
- 115.Healy LA, Howard JM, Ryan AM, Beddy P, Mehigan B, Stephens R, et al. Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis. 2012;14:157–165. doi: 10.1111/j.1463-1318.2011.02562.x. [DOI] [PubMed] [Google Scholar]
- 116.Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei M, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37:91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 117.Liu H, Wan D, Pan Z, Cao L, Wu X, Lu Z, et al. Expression and biological significance of leptin, leptin receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochem Biophys. 2011;60:241–244. doi: 10.1007/s12013-010-9145-5. [DOI] [PubMed] [Google Scholar]
- 118.Ratke J, Entschladen F, Niggemann B, Zänker KS, Lang K. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocr Relat Cancer. 2010;17:179–189. doi: 10.1677/ERC-09-0225. [DOI] [PubMed] [Google Scholar]
- 119.Erkasap N, Ozkurt M, Erkasap S, Yasar F, Uzuner K, Ihtiyar E, et al. Leptin receptor (Ob-R) mRNA expression and serum leptin concentration in patients with colorectal and metastatic colorectal cancer. Braz J Med Biol Res. 2013;46:306–310. doi: 10.1590/1414-431x20122559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao F, et al. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ren H, Jia L, Zhao T, Zhang H, Chen J, Yang S, et al. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014;354:172–180. doi: 10.1016/j.canlet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16:1042–1054. doi: 10.1111/obr.12321. [DOI] [PubMed] [Google Scholar]
- 124.Pappa T, Alevizaki M. Obesity and thyroid cancer: a clinical update. Thyroid. 2014;24:190–199. doi: 10.1089/thy.2013.0232. [DOI] [PubMed] [Google Scholar]
- 125.Fan YL, Li XQ. Expression of leptin and its receptor in thyroid carcinoma: distinctive prognostic significance in different subtypes. Clin Endocrinol (Oxf) 2015;83:261–267. doi: 10.1111/cen.12598. [DOI] [PubMed] [Google Scholar]
- 126.Zhang GA, Hou S, Han S, Zhou J, Wang X, Cui W. Clinicopathological implications of leptin and leptin receptor expression in papillary thyroid cancer. Oncol Lett. 2013;5:797–800. doi: 10.3892/ol.2013.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng SP, Chi CW, Tzen CY, Yang TL, Lee JJ, Liu TP, et al. Clinicopathologic significance of leptin and leptin receptor expressions in papillary thyroid carcinoma. Surgery. 2010;147:847–853. doi: 10.1016/j.surg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 128.Uddin S, Bavi P, Siraj AK, Ahmed M, Al-Rasheed M, Hussain AR, et al. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17:191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]
- 129.Cheng SP, Yin PH, Hsu YC, Chang YC, Huang SY, Lee JJ, et al. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26:1265–1271. doi: 10.3892/or.2011.1388. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y, Liu L, Li C, Ai H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark. 2014;14:353–359. doi: 10.3233/CBM-140415. [DOI] [PubMed] [Google Scholar]
- 131.Gui Y, Pan Q, Chen X, Xu S, Luo X, Chen L. The association between obesity related adipokines and risk of breast cancer: a systematic review and meta-analysis. Oncotarget. 2017 doi: 10.18632/oncotarget.17853. (Online May 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Engin A. Obesity-associated breast cancer: Analysis of risk factors. Adv Exp Med Biol. 2017;960:571–606. doi: 10.1007/978-3-319-48382-5_25. [DOI] [PubMed] [Google Scholar]
- 133.Madeddu C, Gramignano G, Floris C, Murenu G, Sollai G, Macciò A. Role of inflammation and oxidative stress in post-menopausal oestrogen-dependent breast cancer. J Cell Mol Med. 2014;18:2519–2529. doi: 10.1111/jcmm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Assiri AM, Kamel HF, Hassanien MF. Resistin, visfatin, adiponectin, and leptin: risk of breast cancer in pre- and postmenopausal Saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis Markers. 2015;2015:253519. doi: 10.1155/2015/253519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu CL, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–1596. [PubMed] [Google Scholar]
- 136.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 137.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 138.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 139.Xia XH, Gu JC, Bai QT, Yu W. Overexpression of leptin and leptin receptors in breast cancer positively correlates with clinicopathological features. Chin Med J (Engl) 2009;122:3078–3081. [PubMed] [Google Scholar]
- 140.Alshaker H, Krell J, Frampton AE, Waxman J, Blyuss O, Zaikin A, et al. Leptin induces upregulation of sphingosine kinase 1 in oestrogen receptor-negative breast cancer via Src family kinase-mediated, janus kinase 2-independent pathway. Breast Cancer Res. 2014;16:426. doi: 10.1186/s13058-014-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am J Pathol. 2010;177:3133–3144. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McMurtry V, Simeone AM, Nieves-Alicea R, Tari AM. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin Exp Metastasis. 2009;26:197–204. doi: 10.1007/s10585-008-9231-x. [DOI] [PubMed] [Google Scholar]
- 144.Li K, Wei L, Huang Y, Wu Y, Su M, Pang X, et al. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol. 2016;48:2479–2487. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 145.Zhang J, Yao H, Song G, Liao X, Xian Y, Li W. Regulation of epithelial-mesenchymal transition by tumor-associated macrophages in cancer. Am J Transl Res. 2015;7:1699–1711. [PMC free article] [PubMed] [Google Scholar]
- 146.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang M, Ma B, Shao H, Clark AM, Wells A. Macrophage phenotypic subtypes diametrically regulate epithelial-mesenchymal plasticity in breast cancer cells. BMC Cancer. 2016;16:419. doi: 10.1186/s12885-016-2411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cao H, Huang Y, Wang L, Wang H, Pang X, Li K, et al. Leptin promotes migration and invasion of breast cancer cells by stimulating IL-8 production in M2 macrophages. Oncotarget. 2016;7:65441–65453. doi: 10.18632/oncotarget.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Templeton ZS, Lie WR, Wang W, Rosenberg-Hasson Y, Alluri RV, Tamaresis JS, et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia. 2015;17:849–861. doi: 10.1016/j.neo.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–233. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 151.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang CY, Yu HS, Lai TY, Yeh YL, Su CC, Hsu HH, et al. Leptin increases motility and integrin up-regulation in human prostate cancer cells. J Cell Physiol. 2011;226:1274–1282. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 153.Yang SN, Chen HT, Tsou HK, Huang CY, Yang WH, Su CM, et al. Leptin enhances cell migration in human chondrosarcoma cells through OBRl leptin receptor. Carcinogenesis. 2009;30:566–574. doi: 10.1093/carcin/bgp023. [DOI] [PubMed] [Google Scholar]
- 154.Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-targeted cancer therapy. Drug Dev Res. 2008;69:329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grossmann ME, Mizuno NK, Bonorden MJ, Ray A, Sokolchik I, Narasimhan ML, et al. Role of the adiponectin leptin ratio in prostate cancer. Oncol Res. 2009;18:269–277. doi: 10.3727/096504009x12596189659367. [DOI] [PubMed] [Google Scholar]
- 156.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Asano T, et al. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176:1631–1635. doi: 10.1016/j.juro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 157.Oba J, Wei W, Gershenwald JE, Johnson MM, Wyatt CM, Ellerhorst JA, et al. Elevated serum leptin levels are associated with an increased risk of sentinel lymph node metastasis in cutaneous melanoma. Medicine (Baltimore) 2016;95:e3073. doi: 10.1097/MD.0000000000003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clement E, Lazar I, Muller C, Nieto L. Obesity and melanoma: could fat be fueling malignancy? Pigment Cell Melanoma Res. 2017;30:294–306. doi: 10.1111/pcmr.12584. [DOI] [PubMed] [Google Scholar]
- 159.Font-Clos F, Zapperi S, La Porta CAM. Integrative analysis of pathway deregulation in obesity. N P J Syst Biol Appl. 2017;3:18. doi: 10.1038/s41540-017-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Malvi P, Chaube B, Pandey V, Vijayakumar MV, Boreddy PR, Mohammad N, et al. Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: role of adipokines. Mol Oncol. 2015;9:689–703. doi: 10.1016/j.molonc.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qi L, Qi X, Xiong H, Liu Q, Li J, Zhang Y, et al. Type 2 diabetes mellitus and risk of malignant melanoma: a systematic review and meta-analysis of cohort studies. Iran J Public Health. 2014;43:857–866. [PMC free article] [PubMed] [Google Scholar]
- 162.Torisu-Itakura H, Lee JH, Scheri RP, Huynh Y, Ye X, Essner R, et al. Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res. 2007;13:3125–3132. doi: 10.1158/1078-0432.CCR-06-2645. [DOI] [PubMed] [Google Scholar]
- 163.Xu YJ, Shao YF, Zhao X, Geng YT, Wang K, Yin YM. Expression and clinical significance of leptin, the functional receptor of leptin (OB-Rb) and HER-2 in non-small-cell lung cancer: a retrospective analysis. J Cancer Res Clin Oncol. 2011;137:1841–1848. doi: 10.1007/s00432-011-1054-5. [DOI] [PubMed] [Google Scholar]
- 164.Yan L, DeMars LC. Effects of dietary fat on spontaneous metastasis of Lewis lung carcinoma in mice. Clin Exp Metastasis. 2010;27:581–590. doi: 10.1007/s10585-010-9347-7. [DOI] [PubMed] [Google Scholar]
- 165.Barrichon M, Hadi T, Wendremaire M, Ptasinski C, Seigneuric R, Marcion G, et al. Dose-dependent biphasic leptin-induced proliferation is caused by non-specific IL-6/NF-κB pathway activation in human myometrial cells. Br J Pharmacol. 2015;172:2974–2990. doi: 10.1111/bph.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667–2676. doi: 10.1080/15384101.2015.1041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.del Blanquer-Rosselló MM, Oliver J, Sastre-Serra J, Valle A, Roca P. Leptin regulates energy metabolism in MCF-7 breast cancer cells. Int J Biochem Cell Biol. 2016;72:18–26. doi: 10.1016/j.biocel.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 168.Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- 169.Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, et al. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J Biomed Sci. 2017;24:51. doi: 10.1186/s12929-017-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dubois V, Jardé T, Delort L, Billard H, Bernard-Gallon D, Berger E, et al. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer. 2014;66:645–655. doi: 10.1080/01635581.2014.894104. [DOI] [PubMed] [Google Scholar]
- 171.Habib CN, Al-Abd AM, Tolba MF, Khalifa AE, Khedr A, Mosli HA, et al. Leptin influences estrogen metabolism and accelerates prostate cell proliferation. Life Sci. 2015;121:10–15. doi: 10.1016/j.lfs.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 172.Harbuzariu A, Rampoldi A, Daley-Brown DS, Candelaria P, Harmon TL, Lipsey CC, et al. Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget. 2017;8:7740–7752. doi: 10.18632/oncotarget.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim HG, Jin SW, Kim YA, Khanal T, Lee GH, Kim SJ, et al. Leptin induces CREB-dependent aromatase activation through COX-2 expression in breast cancer cells. Food Chem Toxicol. 2017;106:232–241. doi: 10.1016/j.fct.2017.05.058. [DOI] [PubMed] [Google Scholar]
- 174.Liu L, Wang L, Zheng J, Tang G. Leptin promotes human endometrial carcinoma cell proliferation by enhancing aromatase (P450arom) expression and estradiol formation. Eur J Obstet Gynecol Reprod Biol. 2013;170:198–201. doi: 10.1016/j.ejogrb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 175.Nepal S, Kim MJ, Hong JT, Kim SH, Sohn DH, Lee SH, et al. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: involvement of p53/FoxO3A axis. Oncotarget. 2015;6:7166–7181. doi: 10.18632/oncotarget.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013;43:394–403. doi: 10.1007/s12020-012-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qian Y, Shi D, Qiu J, Zhu F, Qian J, He S, et al. ObRb downregulation increases breast cancer cell sensitivity to tamoxifen. Tumour Biol. 2015;36:6813–6821. doi: 10.1007/s13277-015-3375-5. [DOI] [PubMed] [Google Scholar]
- 178.Shouman S, Wagih M, Kamel M. Leptin influences estrogen metabolism and increases DNA adduct formation in breast cancer cells. Cancer Biol Med. 2016;13:505–513. doi: 10.20892/j.issn.2095-3941.2016.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu X, Dong Z, Li Y, Yang Y, Yuan Z, Qu X, et al. The upregulation of signal transducer and activator of transcription 5-dependent microRNA-182 and microRNA-96 promotes ovarian cancer cell proliferation by targeting forkhead box O3 upon leptin stimulation. Int J Biochem Cell Biol. 2013;45:536–545. doi: 10.1016/j.biocel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 180.Yoon KW, Park SY, Kim JY, Lee SM, Park CH, Cho SB, et al. Leptin-induced adhesion and invasion in colorectal cancer cell lines. Oncol Rep. 2014;31:2493–2498. doi: 10.3892/or.2014.3128. [DOI] [PubMed] [Google Scholar]
- 181.Yu W, Cao DD, Li QB, Mei HL, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7:86075–86086. doi: 10.18632/oncotarget.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou X, Li H, Chai Y, Liu Z. Leptin inhibits the apoptosis of endometrial carcinoma cells through activation of the nuclear factor κB-inducing kinase/IκB kinase pathway. Int J Gynecol Cancer. 2015;25:770–778. doi: 10.1097/IGC.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 183.Valladares M, Corsini G, Romero C. Association between obesity and ovarian cancer. Rev Med Chil. 2014;142:593–598. doi: 10.4067/S0034-98872014000500007. [DOI] [PubMed] [Google Scholar]
- 184.Yuan Y, Zhang J, Cai L, Ding C, Wang X, Chen H, et al. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol Rep. 2013;29:2291–2296. doi: 10.3892/or.2013.2390. [DOI] [PubMed] [Google Scholar]
- 185.Shen Y, Wang Q, Zhao Q, Zhou J. Leptin promotes the immune escape of lung cancer by inducing proinflammatory cytokines and resistance to apoptosis. Mol Med Rep. 2009;2:295–299. doi: 10.3892/mmr_00000099. [DOI] [PubMed] [Google Scholar]
- 186.Gonzalez-Perez RR, Lanier V, Newman G. Leptin’s pro-angiogenic signature in breast cancer. Cancers (Basel) 2013;5:1140–1162. doi: 10.3390/cancers5031140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin-leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29:1596–1602. doi: 10.1016/j.peptides.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 188.Wicki A, Christofori G. The angiogenic switch in tumorigenesis. In: Marme D, Fusenig N, editors. Tumor Angiogenesis: Basic Mechanisms and Cancer Therapy. Springer-Verlag; Berlin: 2008. pp. 67–88. [Google Scholar]
- 189.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10:S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bloomfield M, Duesberg P. Inherent variability of cancer-specific aneuploidy generates metastases. Mol Cytogenet. 2016;9:90. doi: 10.1186/s13039-016-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017;15:133. doi: 10.1186/s12916-017-0900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang F, Wang Y, Li Q, Cao L, Sun Z, Jin J, et al. Intratumor heterogeneity predicts metastasis of triple-negative breast cancer. Carcinogenesis. 2017;38:900–909. doi: 10.1093/carcin/bgx071. [DOI] [PubMed] [Google Scholar]
- 193.Werner RJ, Kelly AD, Issa JJ. Epigenetics and precision oncology. Cancer J. 2017;23:262–269. doi: 10.1097/PPO.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ell B, Kang Y. Transcriptional control of cancer metastasis. Trends Cell Biol. 2013;23:603–611. doi: 10.1016/j.tcb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Uriarte G, Paternain L, Milagro FI, Martínez JA, Campion J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem. 2013;69:601–611. doi: 10.1007/s13105-012-0231-6. [DOI] [PubMed] [Google Scholar]
- 197.Pokrywka M, Kieć-Wilk B, Polus A, Wybrańska I. DNA methylation in obesity. Postepy Hig Med Dosw (Online) 2014;68:1383–1391. doi: 10.5604/17322693.1130084. [DOI] [PubMed] [Google Scholar]
- 198.Hair BY, Xu Z, Kirk EL, Harlid S, Sandhu R, Robinson WR, et al. Body mass index associated with genome-wide methylation in breast tissue. Breast Cancer Res Treat. 2015;151:453–463. doi: 10.1007/s10549-015-3401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Del Carmen Martínez-Jiménez V, Méndez-Mancilla A, Patricia Portales-Pérez D. miRNAs in nutrition, obesity, and cancer: The biology of miRNAs in metabolic disorders and its relationship with cancer development. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600994. (Online Jun 8) [DOI] [PubMed] [Google Scholar]
- 200.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: An epigenetic landscape. Life Sci. 2015;140:57–63. doi: 10.1016/j.lfs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 201.Yan J, Yang Q, Huang Q. Metastasis suppressor genes. Histol Histopathol. 2013;28:285–292. doi: 10.14670/HH-28.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–8096. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 203.Hajagos-Tóth J, Ducza E, Samavati R, Vari SG, Gaspar R. Obesity in pregnancy: a novel concept on the roles of adipokines in uterine contractility. Croat Med J. 2017;58:96–104. doi: 10.3325/cmj.2017.58.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Blouet C, Liu SM, Jo YH, Chua S, Schwartz GI. TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity. J Neurosci. 2012;32:9870–9877. doi: 10.1523/JNEUROSCI.0353-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pachmayr E, Treese C, Stein U. Underlying mechanisms for distant metastasis - Molecular biology. Visc Med. 2017;33:11–20. doi: 10.1159/000454696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ding Y, Cao Y, Wang B, Wang L, Zhang Y, Zhang D, et al. APPL1-mediating leptin signaling contributes to proliferation and migration of cancer cells. PLoS One. 2016;11:e0166172. doi: 10.1371/journal.pone.0166172. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 207.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–6761. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg. 2004;188:560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 209.Ghasemi A, Hashemy SI, Aghaei M, Panjehpour M. RhoA/ROCK pathway mediates leptin-induced uPA expression to promote cell invasion in ovarian cancer cells. Cell Signal. 2017;32:104–114. doi: 10.1016/j.cellsig.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 210.Ghasemi A, Isaac Hashemy S, Aghaei M, Panjehpour M. Leptin induces matrix metalloproteinase 7 expression to promote ovarian cancer cell invasion by activating ERK and JNK pathways. J Cell Biochem. 2017 doi: 10.1002/jcb.26396. (Online Sep 8) [DOI] [PubMed] [Google Scholar]
- 211.Huang Y, Jin Q, Su M, Ji F, Wang N, Zhong C, et al. Leptin promotes the migration and invasion of breast cancer cells by upregulating ACAT2. Cell Oncol (Dordr) 2017 doi: 10.1007/s13402-017-0342-8. (Online Aug 2) [DOI] [PubMed] [Google Scholar]
- 212.Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–428. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martín R, Cordova C, Gutiérrez B, Hernández M, Nieto ML. A dangerous liaison: Leptin and sPLA2-IIA join forces to induce proliferation and migration of astrocytoma cells. PLoS One. 2017;12:e0170675. doi: 10.1371/journal.pone.0170675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mendonsa AM, Chalfant MC, Gorden LD, VanSaun MN. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One. 2015;10:e0126686. doi: 10.1371/journal.pone.0126686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mishra AK, Parish CR, Wong ML, Licinio J, Blackburn AC. Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS One. 2017;12:e0178454. doi: 10.1371/journal.pone.0178454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Noda T, Kikugawa T, Tanji N, Miura N, Asai S, Higashiyama S, et al. Long term exposure to leptin enhances the growth of prostate cancer cells. Int J Oncol. 2015;46:1535–1542. doi: 10.3892/ijo.2015.2845. [DOI] [PubMed] [Google Scholar]
- 217.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sobrinho Santos EM, Guimarães TA, Santos HO, Cangussu LMB, de Jesus SF, Fraga CAC, et al. Leptin acts on neoplastic behavior and expression levels of genes related to hypoxia, angiogenesis, and invasiveness in oral squamous cell carcinoma. Tumor Biol. 2017;39:1010428317699130. doi: 10.1177/1010428317699130. [DOI] [PubMed] [Google Scholar]
- 219.Wang L, Cao H, Pang X, Li K, Dang W, Tang H, et al. The effect of leptin and its mechanisms on the migration and invasion of human breast cancer MCF-7 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (Chinese Journal of Cellular and Molecular Immunology) 2013;29:1272–1276. [PubMed] [Google Scholar]
- 220.Wei L, Li K, Pang X, Guo B, Su M, Huang Y, et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res. 2016;35:166. doi: 10.1186/s13046-016-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yeh WL, Lu DY, Lee MJ, Fu WM. Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia. 2009;57:454–464. doi: 10.1002/glia.20773. [DOI] [PubMed] [Google Scholar]
- 222.Yuan HJ, Sun KW, Yu K. Leptin promotes the proliferation and migration of human breast cancer through the extracellular-signal regulated kinase pathway. Mol Med Rep. 2014;9:350–354. doi: 10.3892/mmr.2013.1786. [DOI] [PubMed] [Google Scholar]
- 223.Rabold K, Netea MG, Adema GJ, Netea-Maier RT. Cellular metabolism of tumor-associated macrophages - functional impact and consequences. FEBS Lett. 2017;591:3022–3041. doi: 10.1002/1873-3468.12771. [DOI] [PubMed] [Google Scholar]
- 224.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8:30576–30586. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol. 2017;147:181–187. doi: 10.1016/j.ygyno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 226.Sousa S, Määttä J. The role of tumour-associated macrophages in bone metastasis. J Bone Oncol. 2016;5:135–138. doi: 10.1016/j.jbo.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39:1588–1596. doi: 10.1007/s12272-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 228.Braune J, Weyer U, Hobusch C, Mauer J, Brüning JC, Bechmann II, et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J Immunol. 2017;198:2927–2934. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- 229.Jung JI, Cho HJ, Jung YJ, Kwon SH, Her S, Choi SS, et al. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: roles of adipocytes and M2-macrophages. Int J Cancer. 2015;136:258–270. doi: 10.1002/ijc.28983. [DOI] [PubMed] [Google Scholar]
- 230.Acedo SC, Gambero S, Cunha FG, Lorand-Metze I, Gambero A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell Dev Biol Anim. 2013;49:473–478. doi: 10.1007/s11626-013-9629-x. [DOI] [PubMed] [Google Scholar]
- 231.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Clawson GA, Matters GL, Xin P, McGovern C, Wafula E, dePamphilis C, et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0184451. doi: 10.1371/journal.pone.0184451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ding J, Jin W, Chen C, Shao Z, Wu J. Tumor associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS One. 2012;7:e41942. doi: 10.1371/journal.pone.0041942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pawlina W. Histology. 7. Wolters Kluwer; Philadelphia: 2016. [Google Scholar]
- 235.Chapnik N, Solomon G, Genzer Y, Miskin R, Gertler A, Froy O. A superactive leptin antagonist alters metabolism and locomotion in high-leptin mice. J Endocrinol. 2013;217:283–290. doi: 10.1530/JOE-13-0033. [DOI] [PubMed] [Google Scholar]
- 236.Macht VA, Vazquez M, Petyak CE, Grillo CA, Kaigler K, Enos RT, et al. Leptin resistance elicits depressive-like behaviors in rats. Brain Behav Immun. 2017;60:151–160. doi: 10.1016/j.bbi.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 237.Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:S3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheung WW, Ding W, Gunta SS, Gu Y, Tabakman R, Klapper LN, et al. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J Am Soc Nephrol. 2014;25:119–128. doi: 10.1681/ASN.2013040432. [DOI] [PMC free article] [PubMed] [Google Scholar]