Abstract
The aim of the present study was to estimate the frequency of under- and over-diagnosis as well as overtreatment and their impact on the financial burden of inhaled drugs for stable chronic obstructive pulmonary disease (COPD). We examined 3200 subjects (65.5% males) of the general population (>40 year old, current or former smokers, and asthma patients were excluded) during a 3-year period. All participants gave detailed medical history, underwent spirometry, and their current and past inhaled medications were registered through the national electronic prescription system. We diagnosed 342 subjects (10.7%) with COPD of whom 180 (52.6%) had no prior medical diagnosis. Overdiagnosis was the case for 306 subjects (9.6%) of whom 35.1% were treated with inhaled drugs during the last year. We calculated that 55.4% of the current cost for inhaled drugs is wasted to overtreatment and overdiagnosis. If there was adherence to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines both for the diagnosis and treatment it would be a net profit of 36,059€ annually, which would be increased to 116,017€ if we had excluded underdiagnosed patients. Under- and over-diagnosis of COPD as well as non-adherence to GOLD guidelines for treatment are common problems in the primary care setting that increase significantly the economic burden of inhaled medications.
Keywords: COPD, primary care, underdiagnosis, overdiagnosis, overtreatment, medication cost
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease, which is characterized by not fully reversible airflow limitation, has major extrapulmonary manifestations while exacerbations of the disease and comorbidities contribute to its severity.1 Even though underdiagnosis of COPD is a common finding among studies2,3—it is estimated to be about 50%—the economic burden of the disease continued to increase during the last decades.4,5 Factors that mainly contribute to the direct economic cost of COPD are as follows: hospital admissions due to exacerbation (more than 50–60% of the total direct cost),6,7 medication cost,8,9 and long-term oxygen therapy. Indirect cost due to productivity losses, absenteeism from work, early retirement/disability pensions, tax revenues lost, and missed work of the carers of the patients is also an important burden of COPD.4,10
Smoking cessation was the only intervention that consistently proved to change the natural history of the disease.11 Pharmacological therapy has been proved to be a cost-effective intervention as it reduces symptoms and exacerbations rate and improves quality of life and lung function.12
Diagnosis of COPD is not always consistent with Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines in everyday clinical practice as symptoms of the disease are nonspecific and spirometry is underused, especially in the primary care setting.13 Underdiagnosis leads to delay in the establishment of proper treatment that might increase symptoms and exacerbation rates but on the other hand overdiagnosis is also a common problem in the primary care setting and in this case financial burden is unintentionally increased as well as adverse events.14
The aim of the present study was to estimate the frequency of under- and over-diagnosis as well as overtreatment (non-adherence to GOLD guidelines) and their impact on the current financial burden of inhaled drugs for stable COPD.
Methods
Participants in the present cross-sectional observational study were subjects of the general population, >40 year old, current and former smokers (at least 10 pack-years) in the prefecture of Thessaloniki, Greece. They were invited to participate via advertisement posters in collaboration with a network of general practitioners (GPs) during the time period 1 January 2012 to 31 January 2015. Only the inclusion criteria were printed on the posters (age and smoking status) and there was no reference about respiratory symptoms or inhaled medication.
Exclusion criteria were a previous medical diagnosis of bronchial asthma or other chronic pulmonary diseases (e.g. bronchiectasis, lung cancer, tuberculosis, interstitial lung disease). We recorded their medical history (previous medical diagnosis of COPD recorded in the health-care booklet, modified Medical Research Council (mMRC) scale for dyspnea during the last 4 weeks, exacerbations during the last year, and comorbidities). A group of experienced board-certified pulmonologists performed and analyzed spirometries (MIR SpiroLab II, Roma Italy, CE0476, before and 15-30 minutes after 400 μg of salboutamol) for all participants. Medical Ethics Committee of ‘G. Papanikolaou’ Hospital approved study protocol and all subjects signed informed consent about their participation in the present study.
COPD patients (post-bronchodilation forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.7) were categorized based on obstruction severity (stage I: FEV1 >80% pred., II: 80 ≤ FEV1 > 50% pred., III: 50 ≤FEV1 >30% pred., and IV: <30% pred.) or GOLD-proposed groups according to exacerbations history during the last year, FEV1, and symptoms (groups A, B, C, and D).1
We defined as overdiagnosis the case of subjects with normal spirometry (pre- and post-FEV1/FVC > 0.7, FVC and FEV1 >80% of predicted values) or restriction (pre- and post-FEV1/FVC > 0.7, FVC and FEV1 <80% of predicted values) who had a previous medical diagnosis of COPD recorded in the health-care booklet and had been prescribed inhaled drugs for at least 12 consecutive months during the last 5 years. None of them had a previous lung function examination suggestive for asthma (e.g. bronchidilation test, metacholine, or mannitol bronchoconstiction test).
Underdiagnosis was defined as persistent airflow limitation (post-FEV1/FVC <0.7) in patients with respiratory symptoms (cough, dyspnea) who had never had a medical diagnosis of COPD in the past and had not been prescribed inhaled drugs.
We retrieved all prescribed inhaled drugs—via the national electronic platform—during the last 12 months for the patients with correct diagnosis (post-FEV1/FVC < 0.7, previous medical diagnosis of COPD) and those with overdiagnosis.
Then we tried to calculated the ‘ideal’ cost per patient and COPD severity group based on the proposed GOLD treatment options (first and alternative choice),1 the current prices of inhaled drugs in Greece (estimations were based on prices during 2014) and the assumption that the patients were totally adherent to therapy for everyday use. As the drug choices were more than one we had to make the following hypotheses in order to calculate a specific amount as the ideal monthly cost/patient/COPD severity group (according to FEV1 and mMRC dyspnea scale and history of exacerbations during the last year).
Patients in group A were equally divided to three possible prescriptions: 1/3 of them could receive short acting b2 agonists (SABA; salbutamol) or short acting muscarinic antagonists (SAMA; ipratropium bromide), 1/3 long acting b2 agonists (LABA; salmeterol, formoterol, or indacaterol) and 1/3 long acting muscarinic antagonists (LAMA; tiotropium). Mean estimated ideal monthly cost: 23.8 €.
Patients in group B were equally divided to three possible prescriptions: 1/3 of them could receive LAMA (see above), 1/3 LABA (see above), and 1/3 LAMA and LABA (tiotropium + formoterol or indacaterol or salmeterol). Mean estimated ideal monthly cost: 44.82 €.
Patients in group C/D were equally divided to three possible prescriptions: 1/3 of them could receive LAMA and LABA (see above), 1/3 LAMA and LABA/inhaled corticosteroids (ICS; tiotropium + formoterol/budesonide or tiotropium + salmeterol/fluticasone), and 1/3 LABA/ICS (formoterol/budesonide or salmeterol/fluticasone). Mean estimated ideal monthly cost: 72.15 € (supplementary Table).
Results
We examined 3200 subjects (mean age: 60.5 ± 13.4 years, 65.5% males, median pack-years: 25, current smokers: 52.8%, subjects with mMRC ≥2 were 436, 13.6%) of whom 342 (10.7%) had respiratory symptoms and spirometry confirmed diagnosis of COPD according to GOLD. Patients with COPD had FVC: 85.6 ± 21.4% pred., FEV1/FVC: 62.7 ± 6.9%, FEV1: 68.7 ± 18.7% predicted while 226 patients (66%) presented with mMRC ≥ 2. Of them, 180 patients were underdiagnosed (52.6%) while 162 (47.4%) had a correct diagnosis and were receiving inhaled drugs at the time of the study.
COPD patients were categorized based on FEV1 as stage I: 105 (79 underdiagnosed, 75%), stage II: 186 (89 underdiagnosed, 48%), stage III: 45 (10 underdiagnosed, 24%), and stage IV: 6 (two underdiagnosed). We also estimated severity according to combined COPD assessment as proposed by GOLD (spirometry, exacerbation rate, and symptoms). They were divided to group A: 236, B: 55, C: 20, and D: 31, based on mMRC dyspnea scale while using COPD assessment test for evaluating symptoms, we found group A: 156, B: 135, C: 14, and D: 37.
Patients with correct diagnosis were treated with inhaled drugs as shown in Table 1. It is worth noticing that 85% of them were treated with ICS and only 15% were receiving bronchodilators exclusively. Then we divided them into three categories: those who were treated according to GOLD guidelines first or alternative choices (45%), the majority (53.1%) was over treated (ICS for GOLD groups A and B) and only three patients (1.9%) were undertreated. We also found that the mean (±SD) number of inhaler devices/patient/year that had been prescribed was 7.5 ± 2 with a calculated adherence rate of 71 ± 8% ( Table 1 ).
Table 1.
Prescribed inhaled medications during the last 12 months before the present study.
| Current inhaled medication | Patients with correct diagnosis, N (%) | Subjects with overdiagnosis, N (%) |
|---|---|---|
| LABA/ICS | 72 (44.4) | 68 (60.2) |
| LABA/ICS + LAMA | 66 (40,6) | 13 (11.5) |
| LAMA + LABA | 10 (6,2) | 11 (9.7) |
| LAMA | 7 (4.4) | 12 (10.6) |
| LABA | 7 (4,4) | 9 (8) |
LABA: long acting b2 agonists, ICS: inhaled corticosteroids, LAMA: long acting muscarinic antagonists.
Overdiagnosis was the case for 306 (9.6%) subjects of whom 113 (36.9%) were treated with inhaled drugs for the last 12 months (Table 1) while the rest of them had taken drugs in the past (during the last 5 years). Dyspnea score with mMRC ≥ 2 was the case for 210 subjects (68.6%). We should mention that 71.7% of subjects with a wrong diagnosis of COPD were treated with a combination of ICS and long-acting bronchodilators. The calculated adherence rate to treatment for this subgroup was 67 ± 7%.
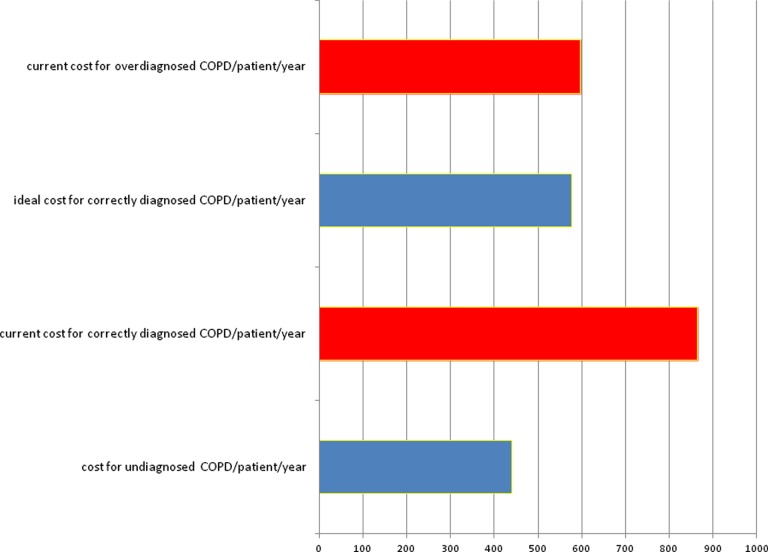
Then we calculated the total as well as the individual annual current (last 12 months) and ideal cost for the study sample (Table 2). If there was adherence to GOLD guidelines both for the diagnosis (rule out all overdiagnosis cases and include underdiagnosed patients) and treatment (based on COPD severity group) it would be a net profit of 35,882€ annually compared to the current cost for the whole study sample. We calculated that 55.4% of the current cost for inhaled drugs due to COPD (209,432€) is wasted to overtreatment (47,735€) and overdiagnosis (68,336€) and could cover the cost of underdiagnosed cases. Additionally, if we take into account only the subjects who were currently treated with inhaled drugs, excluding underdiagnosed patients who nevertheless don’t seek medical advice, and apply GOLD guidelines for correct diagnosis and ideal treatment then the net profit would have been 116,017€ (Table 2, Figure 1).
Table 2.
Total and individual annual current and ideal cost (inhaled drugs) in Euros for the three subgroups.
| Total current cost | Per patient current cost | Ideal cost | Per patient ideal cost | Difference | |
|---|---|---|---|---|---|
| Correct diagnosis (N = 162) | 141,096 | 862 ± 36 | 93,361 | 577 ± 186 | 47,735 |
| 1. GOLD A (N = 26) | 17,807 | 675 ± 141 | 7,425 | 286 | 10,382 |
| 2. GOLD B (N = 97) | 78,235 | 798 ± 153 | 52,170 | 538 | 26,065 |
| 3. GOLD C+D (N = 39) | 45,054 | 1148 ± 178 | 33,766 | 866 | 11,288 |
| Underdiagnosis (N = 180) | 0 | 0 | 80,189 | 441 ± 131 | −80,189 |
| Overdiagnosis (N = 113) | 68,336 | 596 ± 167 | 0 | 0 | 68,336 |
| Total | 209,432 | 173,550 | 35,882 |
The ideal cost per patient has no SD as we calculated a fixed mean monthly cost based on some hypotheses (see text) and 100% adherence (12 months per year).
Figure 1.
Estimated ideal and current costs/patient/year for the three COPD groups (underdiagnosed, correct previous diagnosis, incorrect previous diagnosis). COPD: chronic obstructive pulmonary disease.
Discussion
The main findings of the present study are as followa: (a) more than 50% of the patients with COPD are underdiagnosed, (b) people with respiratory symptoms in the primary care setting are incorrectly diagnosed as COPD patients and receive inhaled drugs, in our study sample they were quite as many as “real” COPD patients, and (c) more than 50% of the current financial burden of the inhaled drugs is wasted to overtreatment and overdiagnosis, which could cover the cost for all underdiagnosed patients.
Diagnosis of COPD is not always accurately based on GOLD guidelines in everyday clinical practice as many patients are characterized as COPD based solely on symptoms without spirometric confirmation.13 The percentage of patients with misdiagnosis is >20% even among patients who are hospitalized for COPD or asthma exacerbation15 while only one in five patients who is admitted to hospital with a COPD exacerbation (based on International Classification of Diseases, Ninth Revision, Clinical Modification) had a documented spirometry in the previous 2 years.16 Additionally, a study in the Netherlands showed that ICS use in the primary care setting has no clear indication in about one-third of cases and 11% of them with normal or near normal spirometry could successfully stop these medications after 1 year of follow-up.17 We have previously showed that overdiagnosis of COPD in Greece is commonly observed in the primary care.14 In the present study, the percentage of overdiagnosis (9.6%) was quite similar with that of correct diagnosis (10.7%). It is interesting to mention that in Greece, GPs are not trained in pulmonary medicine during their residency so they prescribe drugs based on patients’ symptoms, clinical examination, and smoking history while they do not use spirometry in everyday clinical practice. Even if their diagnosis of an obstructive pulmonary disease is correct, GPs usually prescribe LABA/ICS in order to “cover” all stages of COPD, bronchial asthma even the case of asthma–COPD overlap syndrome. As GPs overprescribe LABA/ICS combination drugs they unintentionally increase the cost as it is difficult for them to have an accurate diagnosis for every patient with airways obstruction.
The problem of overtreatment is another aspect that increases the financial burden of the disease as well as adverse events due to inhaled drugs overuse. An observational study in Italy across 49 Pulmonary Units showed that 37.9% of COPD patient were receiving appropriate treatment, in 54.9% there was over-prescription while 66.8% used ICS in combination with long-acting bronchodilators and 15.2% used ICS alone.18 Another real-world study in the United Kingdom based on primary care database came to similar conclusions,19 53.7% of the total COPD population was receiving ICS and 49% of those with GOLD stage 2 and no previous exacerbation were treated with ICS. In a retrospective analysis of records from 41 general practices in London, the percentage of overtreated COPD spirometrically confirmed patients was 25–38%, depend on the GOLD guidelines version that was used, and overuse of ICS was 33.6–38%.20 A recent prospective, randomized study among patients with FEV1 < 50% and at least one exacerbation during the last year proved that the withdrawal of fluticasone propionate did not lead to increased exacerbation rates or dyspnea.21 In the present study, 33.8% of the current cost of COPD (47,735 of 141,096€) could be attributed to overtreatment which was observed in the 53.1% of the patients. It is mainly due to overuse of ICS (85% were receiving ICS) among patients with no clear indication (preserved lung function with no history of frequent exacerbations). It has been already proved that adherence to the current GOLD guidelines was associated with lower costs in subjects diagnosed with moderate to severe COPD.22 Likewise, Improving and Integrating Respiratory Services (IMPRESS) guide in the United Kingdom strongly recommends against overtreatment and unnecessary treatment for mild–moderate COPD (the majority of COPD patients in the present study).23
Finally, we should mention that the proportion of COPD patient who were underdiagnosed in the present study is similar to studies performed in the United States.2,24 Higher probability of underdiagnosis is observed in the early stages of the disease.24 Early diagnosis of this subgroup, using screening programs in the general population and active case findings algorithms, and proper treatment could lead to better outcomes.25 In the present study, the treatment cost of underdiagnosed patients could be covered by eliminating overdiagnosis and overtreatment.
Some limitations of the present study are as follows: (a) the sample is not representative of the general population (there was a high percentage of subjects who were symptomatic or were taking inhaled drugs) so we could not extrapolate the results to national level, (b) we calculated only the cost of inhaled drugs and not the entire financial burden of the disease (oxygen therapy, exacerbations, lost working hours), (c) there is a possibility that some patients in the overdiagnosis group, with normal spirometry who were treated by ICS/LABA, may suffer from adult-onset asthma but even in that case the diagnosis of COPD was wrong, and (d) we compute ideal costs taking into account that patients would be strictly adherent to treatment (12 months per year), which is usually not the case for everyday clinical practice.
Under- and over-diagnosis of COPD as well as nonadherence to the current GOLD guidelines are common problems in the primary care setting that increase significantly the economic burden of inhaled medication. COPD diagnosis should always been based on spirometry while therapy should be based on GOLD staging recommendations and modified based on lung function results, exacerbations’ frequency and patients’ symptoms.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online [appendices/data supplements/etc] are available at http://crd.sagepub.com/supplemental
References
- 1. Global Initiative for the Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (updated 2014), www.goldcopd.org.
- 2. Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveiillance-United States, 1971-2000. MMWR Surveill Summ 2002; 51: 1–16. [PubMed] [Google Scholar]
- 3. Pena VS, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD. Results of the IBERPOC multicentre epidemiological study. Chest 2000; 118: 981–989. [DOI] [PubMed] [Google Scholar]
- 4. Foster TS, Miller JD, Marton JP, et al. Assessment of the economic burden of COPD in the U.S.: a review and synthesis of the literature. COPD 2006; 3: 211–218. [DOI] [PubMed] [Google Scholar]
- 5. Dal Negro R. Optimizing economic outcomes in the management of COPD. Int J Chron Obstruct Pulmon Dis 2008; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menzin J, Boulanger L, Marton J, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in a U.S. medicare population. Respir Med 2008; 102: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 7. Geitona M, Hatzikou M, Steiropoulos P, et al. The cost of COPD exacerbations: a university hospital-based study in Greece. Respir Med 2011; 105: 402–409. [DOI] [PubMed] [Google Scholar]
- 8. Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013; 5: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilleman DE, Dewan N, Malesker M, et al. Pharmacoeconomic evaluation of COPD. Chest 2000; 118: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 10. van Boven JF, Vegter S, van der Molen T, et al. COPD in the working age population: the economic impact on both patients and government. COPD 2013; 10: 629–639. [DOI] [PubMed] [Google Scholar]
- 11. Anthonisen N, Skeans M, Wise R, et al. The effects of smoking cessation intervention on 14.5 year mortality: a randomized clinical trial. Ann Intern Med 2005; 142: 233–239. [DOI] [PubMed] [Google Scholar]
- 12. Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J Chron Obstruct Pulmon Dis 2008; 3: 71–88. [PMC free article] [PubMed] [Google Scholar]
- 13. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD. How do we measure up? Chest 2007; 132: 403–409. [DOI] [PubMed] [Google Scholar]
- 14. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J 2007; 16: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prieto Centurion V, Huang F, Naureckas ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med 2012; 12: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stein BD, Bautista A, Schumock GT, et al. The validity of international classification of diseases, ninth revision, clinical modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest 2012; 141: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lucas AE, Smeenk FW, Smeele IJ, et al. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam Pract 2008; 25: 86–91. [DOI] [PubMed] [Google Scholar]
- 18. Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med 2012; 106: 989–997. [DOI] [PubMed] [Google Scholar]
- 19. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014; 9: 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White P, Thornton H, Pinnock H, et al. Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study. PLoS One 2013; 8(10): e75221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 22. Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for COPD among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis 2012; 7: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. IMPRESS Guide to the relative value of COPD interventions - Executive summary, British Thoracic Society Reports, Vol. 5(2), 2012. [Google Scholar]
- 24. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest 2015; 148(4): 971–985. [DOI] [PubMed] [Google Scholar]
- 25. Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374(9696): 1171–1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.