Abstract
The two-minute walk test (2MWT) is less well validated than the well-known six-minute walk test (6MWT) as a field walking test in patients with chronic obstructive pulmonary disease (COPD). The primary objective of this study was to compare the accuracy of the 2MWT to the 6MWT in detecting exercise-induced oxygen desaturation in patients with severe COPD. Twenty-six patients with COPD (age: 61 ± 10 years, forced expired volume in one second: 37 ± 10%) that were normoxemic at rest performed a 2MWT and a 6MWT under normal ambient conditions on two consecutive days in random order. Oxygen saturation, total walking distance, heart rate, breathing frequency, dyspnea, and leg fatigue were evaluated. Average walking distances were 150 m (95% confidence interval (95% CI): 134–165 m) and 397 m (95% CI: 347–447 m) for the 2MWT and 6MWT, respectively (r = 0.80, p < 0.0001). The difference in minimum oxygen saturation during the 2MWT (83%, 95% CI: 81–86%) and 6MWT (mean 82%, 95% CI: 80–84%) was not statistically different and the data strongly correlated between the groups (r = 0.81, p < 0.0001). Other measurements from the 6MWT, including heart rate, breathing rate, and levels of perceived exertion were also comparable in 2MWT. The 2MWT showed comparable validity in detecting exercise-induced oxygen desaturation in patients with severe COPD compared to the 6MWT.
Keywords: COPD, walk test, six-minute walk test, two-minute walk test, oxygen saturation, pulmonary rehabilitation
Introduction
The six-minute walk test (6MWT) plays an important role in evaluating functional exercise capacity, assessing prognosis, and evaluating response to treatment in patients with chronic obstructive pulmonary disease (COPD).1 The 6MWT is a self-paced test in which patients walk as far as possible under supervision for a duration of 6 minutes. The distance covered in 6 minutes (6MWD) is strongly related to important clinical outcomes. 6MWD is positively associated with peak exercise capacity and quality of life, and negatively associated with risk of disease exacerbation, number of hospitalizations, and mortality.2 Furthermore, results of testing can be used to describe disease severity and estimate prognosis.3 Continuous recording of oxygen saturation during testing is recommended by the American Thoracic Society (ATS) and the European Respiratory Society for patients with COPD.1 The presence of exercise-induced oxygen desaturation strongly correlates to the severity of disease4 and is associated with impaired daily physical activity, decreased lung function, and worse prognosis.4,5
Although the 6MWT has been shown to be a valuable diagnostic tool in rehabilitation of COPD patients, this test may be very exhausting for some patients with severe COPD. Furthermore, a test shorter in duration would likely be an attractive alternative in the clinical and therapeutic setting, where time is often very limited. Therefore a two-minute walk test (2MWT) was first proposed by Butland et al.6 in 1982 and was reported to be a valid test in terms of reproducibility in patients with COPD.7 Nevertheless, currently the 2MWT is rarely applied in the clinical setting and is not well-known compared to the 6MWT. It remains unclear to what extent the 2MWT could be a valuable alternative test modality. Therefore, the primary aim of this study was to evaluate the validity of the 2MWT in detecting exercise-induced oxygen desaturation in patients with COPD, compared to the 6MWT.
Methods
Study design
Consecutive patients admitted to a 3-week inpatient pulmonary rehabilitation program were asked to participate in this randomized crossover study. The trial was approved by the Ethics Committee of the University of Marburg (identification number 30/07) and registered at the clinicaltrials.gov registry (NCT02462343).
Study population
Twenty-six patients were enrolled in this study. For baseline characteristics see Table 1. Inclusion criteria were as follows: confirmed diagnosis of COPD stage III or IV according to the Global Initiative for Chronic Obstructive Lung Disease definition8 and ability to read and understand the German language and study procedures. All participants gave written informed consent before taking part in the study. Exclusion criteria were defined as follows: severe acute COPD exacerbation, severe respiratory failure (resting partial pressure of oxygen in standard ambient conditions of less than 55 mmHg), and inability or unwillingness to understand or comply with the study protocol.
Table 1.
Baseline characteristics.
| Parameters | |
|---|---|
| n | 26 |
| Age (years) | 61.2 ± 10.2 |
| Sex (male/female) | 17/9 |
| BMI (kg/m2) | 24.2 ± 5.1 |
| FEV1, l | 0.98 ± 0.30 |
| FEV1 (% predicted) | 37.4 ± 5.3 |
| RV (% predicted) | 235.9 ± 62.0 |
| TLC (% predicted) | 130.9 ± 22.1 |
| RAW (% predicted) | 220.8 ± 78.0 |
| DLCO (% predicted) | 40.3 ± 15.4 |
| PImax (% predicted) | 57.2 ± 17.6 |
| P0.1 (% predicted) | 267.3 ± 73.2 |
| PaO2 (at rest) | 67.4 ± 7.1 |
| PaCO2 (at rest) | 37.4 ± 5.3 |
BMI: body mass index; FEV1: forced expiratory volume in 1 second; RV: residual volume, TLC: total lung capacity, RAW: airway resistance; DLCO: diffusing capacity factor of the lung for carbon monoxide; PImax: maximal inspiratory pressure, P0.1: negative airway pressure generated during the first 100 millisecond of an occluded inspiration, PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide.
Intervention
All measurements were performed on two consecutive days at the beginning of a pulmonary rehabilitation program at the Schoen Klinik Berchtesgadener Land (Schoenau am Koenigssee, Germany) in a randomized crossover study design. On the first day, each patient was randomly assigned to perform either the 2MWT or the 6MWT. On the second day each patient performed the test that was not performed on the first day (see flowchart in Figure 1). All walk tests were conducted by the same investigator (ST) on the same 30 m track and time of day, following the guidelines of the ATS.9 Reference equations for the 6MWT and 2MWT were used according to Troosters et al.10 and Selman et al.,11 respectively. Patients were instructed to remain on their regular medication schedules.
Figure 1.
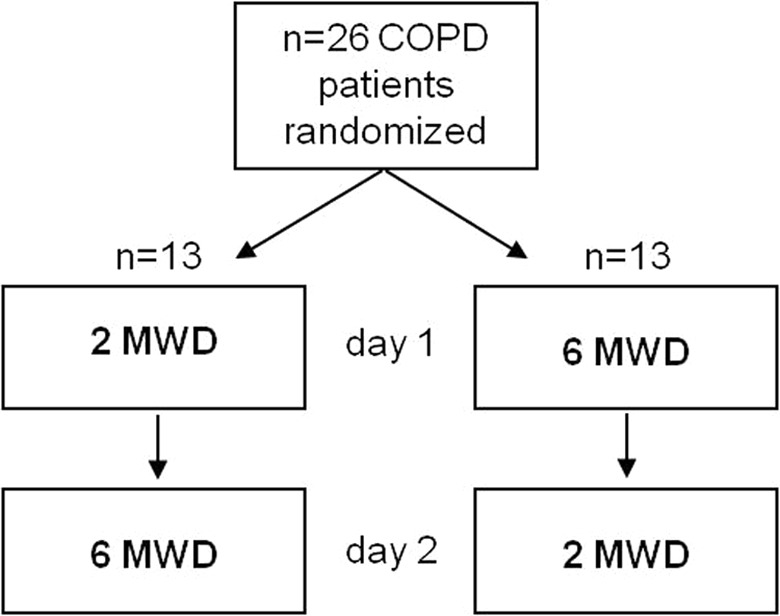
Study flow chart.2MWD: two-minute walk distance, 6MWD: six-minute walk distance.
Hypothesis
Our primary hypothesis was that 2MWT will detect exercise-induced oxygen desaturation as accurately as the 6MWT. We further investigated differences in other standard measures taken during walk tests, including heart rate and ratings of perceived exertion in both the 2MWT and 6MWT.
Outcomes and measures
Patients wore a mobile pulse oximeter (Pulsox-3iA, Konica Minolta, Japan) that continuously recorded heart rate and oxygen saturation. The ApneaLink Oxi™ (ResMed, Munich, Germany) was used to record breathing frequency via a nasal cannula. Baseline values of oxygen saturation, heart rate, breathing frequency, and ratings of perceived exertion on the modified Borg scale (0–10) for dyspnea and leg fatigue were recorded at rest after 10 minutes of sitting. Patients were then instructed to walk as far as possible without running for the duration of the test (2 or 6 minutes). Patients received brief standardized verbal encouragement every minute during testing. There was no further conversation during the tests. The software program DS-5 (Konica Minolta) was used to analyze the pulse oximeter data and to detect the highest heart rate as well as the lowest oxygen saturation that occurred during the test. A device specific software (ResMed, version 6.01) was applied to detect breathing frequencies. Lung function was measured with a Master Screen Body plethysmograph (Jaeger, Wuerzburg, Germany) in accordance with the ATS guidelines.12,13
Blinding and randomization
Blinding
Blinding of group assignments was not possible for the patients or the investigator (ST) who supervised the walk tests due to the crossover design of the study. Data analyses were performed by an investigator who was not involved in any other aspects of the study and was blinded to the group allocation of the patients.
Randomization and allocation concealment
A computer-based random number generation program was used to randomly allocate the sample of patients 1:1 to the two treatment schedules. The allocation assignments were put into sealed envelopes, which were opened and revealed per patient after verifying eligibility and obtaining written informed consent from the patients.
Statistical methods
Data were analyzed for outliers and for normality. A crossover design was used to analyze continuously distributed data to test for significant differences of means, possible period, or carry over effects. In addition, data were tested for equivalence, whereby a +/-10% rule for equivalence was used to define equivalence margins. Equivalence was determined if the ratio between both means was between 90% and 110%. Westlake’s test for equivalence based on confidence intervals test was used. Confidence intervals of 95% (95% CI) were computed for means and for medians – based on Hodges–Lehmann intervals as well as for treatment effects, that is, difference of means. The crossover, period, carry over, as well as the equivalence alpha levels were set to 5%. For non-parametric data, the Wilcoxon signed-rank test was used to detect differences between the groups. Pearson product-moment correlation coefficient was used to analyze correlations between metric variables and Spearman’s rank correlation coefficient for ordinal scaled values. All statistical analyses in this report were performed by use of NCSS (NCSS 10, NCSS, LLC. Kaysville, Utah, USA), SPSS 19 (IBM SPSS Statistics for Windows, Version 19.0., Armonk, New York, USA) and StatXact 10 (Cytel Software 2013, Cambridge, Massachusetts, USA).
Results
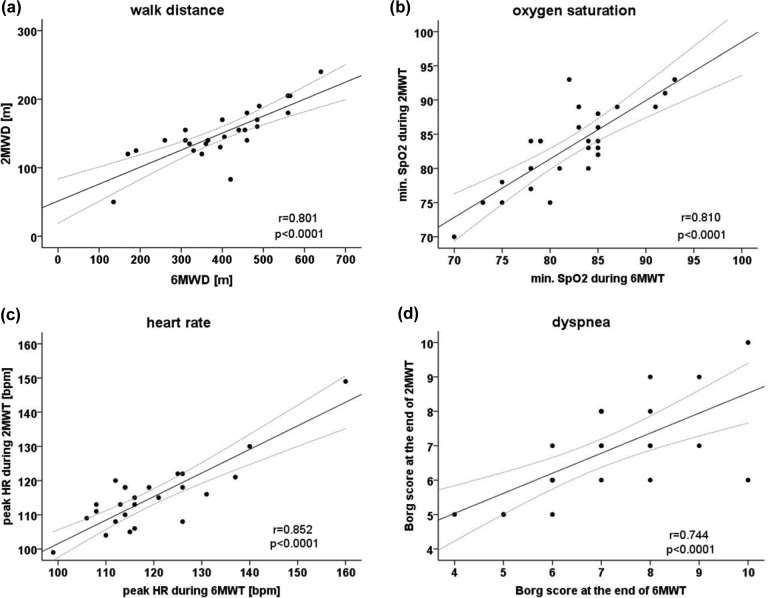
All 26 patients were able to complete the two walking tests on two consecutive days (for baseline characteristics see Table 1). There were no missing values for any of the measured outcome parameters. Patients walked a shorter distance during the 2MWT (150 m [95%CI: 134–165]) than during 6MWT (397 m [95%CI: 347–447]). Four patients needed a break during the 6MWT due to dyspnea compared to one patient during the 2MWT. Mean walking speed during the 2MWT (4.5 km/h [95%CI: 4.0–5.0]) was significantly (p = 0.002) higher than during 6MWT (4.0 km/h [95%CI: 3.5–4.5]). The mean decrease in oxygen saturation during the 2MWT (−10.6%) and the 6MWT (−11.3%) with a treatment effect of −0.7% [95% CI: −2.3 to 0.5] were not found to be significantly different (p = 0.177) or to be equivalent. The mean increase of heart rate during the walking tests was 28.5 bpm at the 2MWT and 29.0 bpm at the 6MWT (treatment effect: 0.5 bpm [95%CI −3.5 to 4.4], p = 0.827). Equivalence of heart rate increase between both walking tests was not proven. Breathing frequency increased similar during both walk tests (2MWT: +7.7 [95%CI: 5.0–9.9] and 6MWT: +7.3 [95%CI: 5.6–9.4], p = 0.52). Neither period nor carry over effects were found for any of the continuous variables (Table 2). As shown in Table 3, these outcome parameters correlated strongly between the 2MWT and 6MWT (walking distance: r = 0.80, minimal oxygen saturation: r = 0.81, and maximal heart rate: r = 0.85; all p < 0.0001).
Table 2.
Cross-over analysis (n = 26). Data are presented as mean (95% confidence interval).
| 2MWT | 6MWT | Between-group differences | p | Equivalent at the 5.0% sign. level? | |
|---|---|---|---|---|---|
| Walking distance (m) | 149.7 (134.1 to 165.3) | 396.9 (346.5 to 447.4) | 247.2 (209.8 to 286.1) | <0.0001 | NO |
| Walking distance (% predicted) | 79.5a (71.8 to 87.2) | 61.0b (53.8 to 68.1) | −18.5 (−24.5 to −12.5) | <0.0001 | NO |
| Heart rate before walking test (bpm) | 85.9 (81.1 to 90.8) | 89.7 (85.0 to 94.2) | 3.8 (−0.61 to 8.8) | 0.085 | YES |
| Peak heart rate during walking test (bpm) | 114.4 (110.2 to 118.7) | 118.7 (113.4 to 123.9) | 4.3 (1.7 to 7.4) | 0.002 | YES |
| Δ heart rate (bpm) | 28.5 (23.9 to 33.2) | 29.0 (24.0 to 34.0) | 0.5 (−3.5 to 4.4) | 0.83 | NO |
| Oxygen saturation before walking test (%) | 93.8 (92.8 to 94.7) | 93.3 (92.4 to 94.3) | −0.5 (−1.3 to 0.7) | 0.54 | YES |
| Lowest oxygen saturation during walking test (%) | 83.2 (80.8 to 85.5) | 82.0 (79.8 to 84.3) | −1.2 (−2.6 to 0.1) | 0.072 | YES |
| Δ oxygen saturation (%) | −10.6 (−12.6 to −8.6) | −11.3 (−13.3 to −9.2) | −0.7 (−2.3 to 0.5) | 0.177 | NO |
| Breathing frequency before walking test (n) | 17.7 (15.9 to 20.0) | 19.6 (17.9 to 20.7) | 1.9 (0.3 to 3.6) | 0.023 | NO |
| Highest breathing frequency during walking test (n) | 25.4 (23.0 to 27.8) | 26.9 (25.0 to 28.7) | 1.5 (−0.1 to 3.1) | 0.063 | YES |
| Δ breathing frequency (n) | 7.7 (5.0 to 9.9) | 7.3 (5.6 to 9.4) | −0.4 (−3.0 to 1.6) | 0.52 | NO |
| Dyspnea before walking test (Borg scale)c | 3.5 (3.0 to 5.0) | 4.0 (2.0 to 5.0) | 0.5 (−0.5 to 1.0) | 0.63 | –d |
| Dyspnea at the end of walking test (Borg scale)c | 6.5 (5.8 to 8.0) | 7.0 (6.0 to 8.0) | 0.5 (−1.0 to 1.5) | 0.62 | –d |
| Δ dyspnea (Borg scale)c | 3.0 (2.0 to 5.0) | 3.0 (2.0 to 4.0) | 0.0 (−0.5 to 1.0) | 1.0 | –d |
| Leg fatigue before walking test (Borg scale)c | 3.0 (1.8 to 5.0) | 3.0 (1.8 to 4.3) | 0.0 (−1.0 to 1.0) | 0.75 | –d |
| Leg fatigue at the end of walking test (Borg scale)c | 4.5 (3.5 to 5.4) | 5.0 (2.0 to 6.3) | 0.5 (−1.0 to 1.0) | 0.97 | –d |
| Δ leg fatigue (Borg scale)c | 1.0 (0.0 to 2.3) | 1.5 (0.0 to 2.0) | 0.5 (−1.5 to 1.0) | 0.97 | –d |
2MWT: two-minute walk test; 6MWT: two-minute walk test; bpm: beats per minute.
aReference equation according to Selman et al.
bReference equation according to Troosters et al.
cData presented as median and 25th to 75th quartile.
dEquivalence analysis was not possible for nonmetric data.
Table 3.
Correlations between two- and six-minute walk test parameters (n = 26).
| r | p | |
|---|---|---|
| Walk distance | 0.80a | <0.0001 |
| Minimum oxygen saturation during test | 0.81a | <0.0001 |
| Maximum heart rate during test | 0.85a | <0.0001 |
| Highest breathing frequency during test | 0.75a | <0.0001 |
| Dyspnea at the end of the test (Borg) | 0.74b | <0.0001 |
| Leg fatigue at the end of the test (Borg) | 0.87b | <0.0001 |
aPearson product-moment correlation coefficient.
bSpearman’s rank correlation coefficient.
Rating of perceived dyspnea at the end of both walking tests was comparable (2MWT: median 6.5 [IQR: 5.8–8.0] and 6MWT: median 7.0 [IQR: 6.0–8.0], p = 0.62). Rating of perceived leg fatigue at the end of walking tests was not significantly different (p = 0.97) during the 2MWT (median: 4.5 [IQR: 3.5–5.4]) and the 6MWT (median: 5.0 [IQR: 2.0– 6.3]). Dyspnea and leg fatigue showed strong correlations (Figure 2) between the 2MWT and 6MWT (dyspnea: r = 0.74 and leg fatigue: r = 0.87; both p < 0.0001).
Figure 2.
Correlations between the 2MWD and 6MWD for (a) walking distance, (b) minimal oxygen saturation (SpO2) during the tests, (c) peak heart rate during the tests, and (d) dyspnea at the end of walking tests in patients with severe COPD. Dashed lines represent the margins for the 95%CI. 2MWD: two-minute walk distance, 6MWD: six-minute walk distance; COPD: chronic obstructive pulmonary disease; 95% CI: 95% confidence interval.
No adverse events related to the walk tests occurred during the study.
Discussion
Main findings
In the current study, we found a high correlation (r = 0.801) between the 2MWD and 6MWD in estimating peak exercise capacity, which is in agreement with previous findings.6,7 However, a shorter test duration could offer practical advantages over the 6MWT, especially in highly symptomatic patients with COPD. The 2MWT has already been used in several different clinical populations, especially in severely diseased and or functionally limited populations. For example, in patients with heart failure, the 2MWT has been found to be feasible for patients presenting a severe motor impairment and who therefore cannot walk for a more extended period of time.14 The test was sensitive to changes in exercise capacity after cardiac surgery and showed moderate correlation with measures of physical functioning in this population.15 The 2MWT was also found to be a feasible measure of functional capacity, which was better tolerated than the 6MWT in older persons in geriatric rehabilitation.16 In a study by Eiser et al.,17 the 2MWT was performed a total of nine times in three consecutive days in 57 COPD patients (forced expired volume in one second: 35 ± 13% predicted). In this study, it was found that the test reliability (95%) as well as the repeatability (intra-subject variation: 5%) were excellent. Furthermore, the 2MWT has been shown to be sensitive to changes after bronchodilator therapy.17
This is in accordance with a former finding that patients usually choose a stable gait speed throughout self-paced walk tests.18 Perceived exertion at the end of walk tests and leg fatigue was not significantly different. This suggests that although the 2MWT is considerably shorter, the perceived difficulty is similar. This might be due to the faster walking speed during the 2MWT, compared to the 6MWT. The peak heart rate measured during the two walk tests was significantly higher during the 6MWT (between-group difference: 4.3 bpm). However, this finding is related to the higher baseline heart rate before the 6MWT since the increase of heart rate during testing was not significantly different between the tests (between-group difference: 0.5 bpm). In our study, we also measured the patients’ breathing frequency as a possible outcome parameter in walk tests. The breathing rate can be assessed quite easily with portable spirometers and might represent a valuable parameter reflecting the ventilatory burden of patients with COPD during exertion. However, breathing frequency is a rarely documented parameter in the literature and in studies performing spiroergometry during walk tests.19,20
Oxygen desaturation
More importantly, the decline in oxygen saturation was very similar during the 2MWT and the 6MWT (see Table 2). It seems that the short duration of a 2MWT is sufficient to induce a similar oxygen desaturation under room air conditions in patients with severe COPD as the 6MWT. In general, walking tests are more sensitive in detecting oxygen desaturation than cardiopulmonary exercise testing on a bicycle.21 Between the 2MWT and the 6MWT there was not only a high correlation in minimal oxygen saturation but also a high congruence in detecting oxygen desaturation. In 22 of 26 patients (85%), oxygen saturation dropped below 90% during the 6MWT. Of these 22 patients, 21 subjects (81%) also reached below 90% during the 2MWT, with the one exception reaching 91% during the 2MWT. Very severe oxygen desaturation levels (<85%) were detected in the same 13 patients during both the 6MWT and the 2MWT. These results suggest that the 2MWT seems to be sufficient for the detection of clinically relevant oxygen desaturation.
Safety
In a recently published study by Roberts et al.22 adverse events that occurred during 6MWT were analyzed in a large cohort of 1136 patients with moderate to severe COPD. It was found that 2.2% of the investigated subjects had an adverse event during the 6MWT. Most common adverse events were dizziness, chest tightness, chest pain, and palpitations. No significant morbidity or mortality was recorded. Although the time of 6MWT event occurrence was not reported, it is likely that a 2MWT would reduce the chance of test-related events based on the much shorter test duration. Although the current study was not designed to evaluate safety, we did not observe any adverse events during or related to six- or two-minute walk testing.
Implications for daily practice
Beyond these suggested aspects of walk testing related to safety, there may be other advantages to the 2MWT compared to the 6MWT. In patients with severe COPD, it would be advantageous to use walk tests of shorter duration if they yield similar results. The 2MWT may also be repeated more often than the 6MWT to either validate an intervention intermediately or to adjust the optimal supplemental oxygen flow rate during exertion. The 2MWT may also be used to determine intensity for a walking training program. However, further studies are needed to investigate if the 2MWT is as effective as the 6MWT in deriving adequate training intensities based on walking speeds during testing.23 In clinical practice, a 2MWT could be a time-saving test for the busy clinical routine assessment.
Study limitations
Our study also has some limitations that should be considered. Firstly, we only investigated a small sample size. Larger and adequately powered studies should further investigate the potential of the 2MWT. Secondly, we only investigated patients with severe COPD. The short duration of the 2MWT may not stress the cardiopulmonary function adequately in patients with mild COPD and the ceiling effect may further limit the evaluation of the effectiveness of an intervention.24 However, patients with severe COPD are more likely to benefit from a shorter walk test duration. Because of its short duration, the magnitude of improvement following any intervention will be small and perhaps difficult to interpret. Further studies should be performed to investigate the minimal important difference for the 2MWT like it has been done in the 6MWT.25,26
Conclusion
The 2MWT and 6MWT show high grades of congruence and correlation in evaluating exercise-induced oxygen desaturation, an important marker of disease status in patients with severe COPD. Other measurements from the 6MWT, including heart rate, breathing rate, and levels of perceived exertion were also comparable in 2MWT in patients with severe COPD. The 2MWT is practical, simple, and was well tolerated by these patients. Therefore, the 2MWD may be of higher relevance than previously expected.
Footnotes
Authors’ Contribution: Study concept and design was done by KK and ST; acquisition of data was done by ST; analysis and interpretation of data was done by WH and RG; drafting of the article was done by RG; all authors revised the article critically for important intellectual content; and RG had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 2. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1447–1478. [DOI] [PubMed] [Google Scholar]
- 3. Casanova C, Cote CG, Marin JM, et al. The 6-min walking distance: long-term follow up in patients with COPD. Eur Respir J 2007; 29(3): 535–540. [DOI] [PubMed] [Google Scholar]
- 4. Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008; 134(4): 746–752. [DOI] [PubMed] [Google Scholar]
- 5. van Gestel AJ, Clarenbach CF, Stowhas AC, et al. Prevalence and prediction of exercise-induced oxygen desaturation in patients with chronic obstructive pulmonary disease. Respiration 2012; 84(5): 353–359. [DOI] [PubMed] [Google Scholar]
- 6. Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982; 284(6329): 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung AS, Chan KK, Sykes K, et al. Reliability, validity, and responsiveness of a 2-min walk test to assess exercise capacity of COPD patients. Chest 2006; 130(1): 119–125. [DOI] [PubMed] [Google Scholar]
- 8. GOLD. Global Strategy of the diagnosis, management, and prevention of chronic obstructive pulmonary disease, www.goldcopd.org (2013, accessed 3 May 2013).
- 9. ATS-statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 10. Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J 1999; 14(2): 270–274. [DOI] [PubMed] [Google Scholar]
- 11. Selman JP, de Camargo AA, Santos J, et al. Reference equation for the 2-minute walk test in adults and the elderly. Respir Care 2014; 59(4): 525–530. [DOI] [PubMed] [Google Scholar]
- 12. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26(4): 720–735. [DOI] [PubMed] [Google Scholar]
- 13. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6): 532–555. [DOI] [PubMed] [Google Scholar]
- 14. Casillas JM, Hannequin A, Besson D, et al. Walking tests during the exercise training: specific use for the cardiac rehabilitation. Ann Phys Rehabil Med 2013; 56(7–8): 561–575. [DOI] [PubMed] [Google Scholar]
- 15. Brooks D, Parsons J, Tran D, et al. The two-minute walk test as a measure of functional capacity in cardiac surgery patients. Arch Phys Med Rehabil 2004; 85(9): 1525–1530. [DOI] [PubMed] [Google Scholar]
- 16. Brooks D, Davis AM, Naglie G. The feasibility of six-minute and two-minute walk tests in in-patient geriatric rehabilitation. Can J Aging 2007; 26(2): 159–162. [DOI] [PubMed] [Google Scholar]
- 17. Eiser N, Willsher D, Dore CJ. Reliability, repeatability and sensitivity to change of externally and self-paced walking tests in COPD patients. Respir Med 2003; 97(4): 407–414. [DOI] [PubMed] [Google Scholar]
- 18. DePew ZS, Karpman C, Novotny PJ, et al. Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir Care 2013; 58(12): 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J 2002; 20(3): 564–569. [DOI] [PubMed] [Google Scholar]
- 20. van Gestel AJ, Baty F, Rausch-Osthof AK, et al. Cardiopulmonary and gas-exchange responses during the six-minute walk test in patients with chronic obstructive pulmonary disease. Respiration 2014; 88(4): 307–314. [DOI] [PubMed] [Google Scholar]
- 21. Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123(5): 1401–1407. [DOI] [PubMed] [Google Scholar]
- 22. Roberts MM, Cho JG, Sandoz JS, et al. Oxygen desaturation and adverse events during 6-min walk testing in patients with COPD. Respirology 2015; 20(3): 419–425. [DOI] [PubMed] [Google Scholar]
- 23. Zainuldin R, Mackey MG, Alison JA. Prescription of walking exercise intensity from the 6-minute walk test in people with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2015; 35(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 24. Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6 MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol 2005; 43(1): 36–39. [DOI] [PubMed] [Google Scholar]
- 25. Puhan MA, Mador MJ, Held U, et al. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008; 32(3): 637–643. [DOI] [PubMed] [Google Scholar]
- 26. Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91(2): 221–225. [DOI] [PubMed] [Google Scholar]