Abstract
Mouthpiece ventilation (MPV) allows patients with neuromuscular disease to receive daytime support from a portable ventilator, which they can disconnect at will, for example, for speaking, eating, swallowing, and coughing. However, MPV carries a risk of underventilation. Our purpose here was to evaluate the effectiveness of daytime MPV under real-life conditions. Eight wheelchair-bound patients who used MPV underwent daytime polygraphy at home with recordings of airflow, mouthpiece pressure, thoracic and abdominal movements, peripheral capillary oxygen saturation (SpO2), and transcutaneous partial pressure of carbon dioxide (PtcCO2). Times and durations of tasks and activities were recorded. The Apnea–Hypopnea Index (AHI) was computed. Patient–ventilator disconnections ≥3 minutes and episodes of hypoventilation defined as PtcCO2>45 mmHg were counted. Patient–ventilator asynchrony events were analyzed. The AHI was >5 hour−1 in two patients. Another patient experienced unexplained 3% drops in arterial oxygen saturations at a frequency of 70 hour−1. Patient–ventilator disconnections ≥3 minutes occurred in seven of eight patients and were consistently associated with decreases in SpO2 and ≥5-mmHg increases in PtcCO2; PtcCO2 rose above 45 mmHg in two patients during these disconnections. The most common type of patient–ventilator asynchrony was ineffective effort. This study confirms that MPV can be effective as long as the patient remains connected to the mouthpiece. However, transient arterial oxygen desaturation and hypercapnia due to disconnection from the ventilator may occur, without inducing unpleasant sensations in the patients. Therefore, an external warning system based on a minimal acceptable value of minute ventilation would probably be useful.
Keywords: Neuromuscular disease, noninvasive mechanical ventilation, mouthpiece, follow up
Introduction
Numerous neuromuscular diseases are characterized by a relentless decline in respiratory muscle performance, resulting in progressive respiratory failure, initially during sleep and subsequently also during the day. Noninvasive ventilation (NIV) is a proven first-line treatment for respiratory failure in patients with neuromuscular disease.1,2 NIV at night normalizes nocturnal gas exchange and, initially, daytime gas exchange also.3 However, as the respiratory muscles become increasingly weak over time, daytime ventilatory failure may occur, requiring NIV also during the day. Daytime ventilation can be delivered either with the interface used at night or via a mouthpiece, a method known as sip ventilation.4,5 The main advantage of mouthpiece ventilation (MPV) is that the patient can connect and disconnect from the ventilator at will, depending on social activities and respiratory sensations. Thus, compared to other interfaces, a mouthpiece interferes less with speaking, eating, swallowing, and coughing. In addition, the patient’s appearance is improved, no headgear is needed, and there is no feeling of claustrophobia or risk of skin damage. However, the patients may unknowingly underventilate themselves. In a recent survey6 of 30 patients who used MPV more than 20 hour/24 hour, only 11 (including 5 under around-the-clock NIV) confirmed that the number of cycles per minute (assisted and controlled) was consistently greater than or equal to the backup rate. Moreover, 22 patients reported frequently disconnecting from the ventilator for longer than 3 minutes during the periods of mechanical ventilation.
These data prompted us to perform daytime polygraphy at home (under real-life conditions) in eight MPV users with neuromuscular disease to determine whether respiratory events such as apnea/hypopnea and hypoventilation occurred during MPV. In addition, we analyzed patient–ventilator asynchrony events.
Methods
This prospective single-center observational study was approved by the appropriate ethics committee (CPP IDF XI Saint-Germain-en-Laye; approval number: 11090). All patients gave their written informed consent before study inclusion.
Patients
We studied eight adults with neuromuscular disease who were in stable clinical condition and used daytime NIV via a mouthpiece while on their electric wheelchair sat home. From October 1, 2012, to June 30, 2013, outpatients receiving support from a home respiratory-care provider were considered for inclusion. Exclusion criteria were guardianship, pregnancy, recent acute respiratory event, need for oxygen therapy, and unwillingness to participate.
Measurements
Daytime polygraphy (SOMNOscreen, SomnoMEDICS, Randersacker, Germany) was performed at home in all patients. Each patient transferred to his or her wheelchair before being connected to the recording device. The recording period included a meal and ended in mid- to late afternoon depending on the duration of wheelchair use. The following parameters were recorded: airflow using a pneumotachograph (Hamilton Medical, Rhäzüns, Switzerland) situated between the mouthpiece and the Y piece of the ventilatory circuit, mouthpiece pressure using a differential pressure transducer (SOMNOscreen), thoracic and abdominal movements using respiratory inductive plethysmography (SOMNOscreen), capillary oxygen saturation (SpO2) using pulse oximetry (SOMNOscreen), and transcutaneous partial pressure of carbon dioxide (PtcCO2) via a monitor (StandAloneDigitalMonitoring System; SenTech AG; Therwil, Switzerland) connected to the SOMNOscreen device and synchronized with all the recorded channels. Times and durations of tasks and activities (e.g. meals, leisure activities, and work) and whether MPV use was continued during these periods were recorded.
Respiratory parameters
Gas exchange
The percentages of recording time spent with PtcCO2>45 mmHg and SpO2<90% were calculated.
Patient–ventilator interaction
Effective breaths were categorized as disconnected cycles when the patient was disconnected from the mouthpiece and connected cycles, which included assisted cycles (identified by a slight drop in pressure and gentle rise in inspiratory flow immediately before the cycle) and controlled cycles. Each cycle type was quantified as the percentage of total effective breaths.7 A central apnea–hypopnea event was defined as a ≥50% decrease in thoracoabdominal movement signal intensity compared to that during effective breaths, lasting 10 seconds or longer and followed by a ≥3% SpO2 decrease.8 All patient–ventilator disconnections lasting ≥3 minutes, detected as absence of a mouthpiece pressure increase during insufflation, were counted, irrespective of effects on blood gas levels.
As previously described,9 the main patterns of patient–ventilator asynchrony (defined as a mismatch between the patient’s inspiratory effort and triggering of the ventilator7) were detected by visual analysis of airflow and airway pressure recordings and of the thoracoabdominal movement signal. Ineffective triggering was defined as an airway pressure drop coinciding with an inspiratory movement and/or a positive deflection in expiratory flow not followed by a ventilator cycle.10–12 Double triggering was defined as two consecutive ventilator cycles separated by an expiratory time of less than half the mean inspiratory time.9 Auto triggering was defined as at least three consecutive ventilator pressurizations at a frequency >40 minute−1 that were not triggered by the patient.9,10 We determined the numbers of asynchrony events per hour of recording time.9
Statistical analysis
Data were described as median and interquartile range.
Results
Patients
Table 1 lists the patient characteristics during the last admission. All patients used ventilation for more than 14 hours day−1 via a mouthpiece and nasal mask. For all patients, daytime MPV was indicated in case of diurnal hypercapnia (defined as PaCO2 > 45 mmHg) associated with clinical signs or symptoms of respiratory failure, despite efficient nocturnal NIV. Table 2 reports the ventilator settings and blood gas levels during MPV. Blood gas levels were not available for two patients (patient numbers 1 and 8), because they had declined hospital admissions for the past 3 years. Six patients used a volumetric mode (Eole ventilator, Resmed, Bella Vista, Australia: patient numbers 1, 2, and 5; Legendair ventilator, Covidien, Dublin, Ireland: patient 8;or PB 560 ventilator, Covidien, patient numbers 5 and 6), whereas two used a barometric mode (Legendair ventilator in patient numbers 3 and 7). The ventilator settings were similar during daytime as during nighttime, except for the tidal volume (VT) adjustment (or the minimal targeted VT when a pressure support with a targeted volume was used), which was slightly increased in patient numbers 2, 5, 7, and 8 (the increase of VT was respectively of 100, 50, 160, and 130 mL) and the backup rate was reduced in patient number 5 from 22 to 20 cycles minute−1. Settings were adjusted to avoid the triggering of low-pressure alarms.13 VT settings were also a compromise between the patient’s tolerance to inflation and the possibility to lengthen the disconnection duration from the ventilator by a deeper breathing.
Table 1.
Characteristics of the eight patients.
| Patient number | Diagnosis | Age/sex (years) | BMI (kg cm−2) | VC (%)a | PImax (cmH2O) | PEmax (cmH2O) | Duration of mouthpiece use (months) | Mechanical ventilation (hours day−1) | MPV (hours day−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Polio | 59/M | 30.0 | 24 | NA | NA | 21 | 23.5 | 13.5 |
| 2 | DMD | 41/M | 13.2 | 19 | 4 | 4 | 190 | 24 | 12 |
| 3 | DMD | 38/M | 23.5 | 8 | 10 | 9 | 54 | 24 | 12 |
| 4 | Adhal | 48/M | 10.4 | 14 | 11 | 9 | 155 | 24 | 13 |
| 5 | DMD | 20/M | 18.4 | 22 | NA | NA | 65 | 17 | 9 |
| 6 | DMD | 21/M | 16.5 | 14 | 18 | 6 | 36 | 14 | 6 |
| 7 | DMD | 22/M | 14.7 | 9 | 9 | 7 | 84 | 23.5 | 14.5 |
| 8 | DMD | 19/M | 24.2 | 6 | NA | NA | 13 | 20 | 10 |
BMI: body mass index; VC: vital capacity; PImax: maximal inspiratory pressure; PEmax: maximal expiratory pressure; MPV: mouthpiece ventilation; DMD: Duchenne muscular dystrophy; Polio: post-poliomyelitis syndrome; Adhal: primary adhalinopathy; NA: not available.
aVC is measured using a spirometer and expressed in % of the predicted value.
Table 2.
Ventilator parameters and blood gas values during noninvasive ventilation via a mouthpiece.
| Patient numbers | Mode of MV | Backup rate (cycles minute−1) | PEEP (cmH2O) | Ti (seconds) | VT (mL kg−1) | IPAP (cm H2O) | Arterial pH | PaO2 (mmHg) | PaCO2 (mmHg) | Bicarbonates (mmol L−1) | Base excess (mmol L−1) | SpO2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VC | 18 | 0 | 1.11 | 11.9 | / | NA | NA | NA | NA | NA | NA |
| 2 | VAC | 18 | 0 | 1.11 | 14.1 | / | 7.46 | 105 | 35 | 24.5 | 1.4 | 99.9 |
| 3 | PSV | 15 | 0 | / | 8.8 | 20 | 7.38 | 104 | 38 | 22.3 | −2.3 | 98.4 |
| 4 | VAC | 17 | 0 | 1.20 | 25.0 | / | 7.51 | 103 | 28 | 21.8 | 0.3 | 98.8 |
| 5 | VAC | 20 | 0 | 1.20 | 14.0 | / | 7.53 | / | 20 | 22.2 | −0.7 | 97.4 |
| 6 | VAC | 17 | 0 | 1.20 | 12.9 | / | 7.43 | 61 | 41 | 26.6 | 2.6 | 93.9 |
| 7 | PSV | 14 | 4 | / | 15.0 | 16 | 7.36 | 95 | 46 | 25.6 | 0.1 | 97.7 |
| 8 | VAC | 22 | 4 | 0.90 | 7.9 | / | NA | NA | NA | NA | NA | NA |
MV: mechanical ventilation; VC: volume-control mode; VAC: volume assist-control mode; PSV: pressure-support ventilation; PEEP: positive end-expiratory pressure; Ti: inspiratory time; VT: tidal volume; IPAP: inspiratory positive airway pressure; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; SpO2: arterial oxygen saturation; NA: not available.
Polygraphy results
Apnea–Hypopnea Index
The Apnea–Hypopnea Index (AHI) was >5 hour−1 in two patients (patient numbers 7 and 8: 6.6 hour−1 and 5.1 hour−1, respectively; Table 3). Patient number 1, whose AHI was normal, experienced unexplained 3% drops in SpO2 at a frequency of 70 hour−1. He had post-poliomyelitis syndrome, his body mass index was 30 kg m−2, and his respiratory cycles were predominantly controlled (80%) with a relatively low insufflated volume (11.9 mL kg−1) and a backup rate of 18 minute−1. These three patients with oxygen desaturations were the only patients with SpO2 drops to less than 90%. None of them had PtcCO2 above 45 mmHg. The percentages of non-assisted respiratory cycles were 20, 56, and 47 in patient numbers 1, 7, and 8, respectively (see Table 3 for the comparison with the other patients).
Table 3.
Polygraphy results.
| Patient numbetr | AHI (hour−1) | SpO2 <90% (% time) | PtCO2 min (mmHg) | PtCO2 max (mmHg) | PtCO2 >45 mmHg (% time) | Unassisted cycles (% of cycles) | Assisted cycles (% of cycles) | Controlled cycles (% of cycles) | Double triggering (% of cycles) | Auto Triggering (% of cycles) | Ineffective efforts (% of cycles) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0 | 2 | 29 | 36 | 0 | 20 | 80 | 0 | 0.2 | 0.0 | 10.8 |
| 2 | 1.6 | 0 | 31 | 44 | 0 | 1 | 0 | 99 | 1.5 | 0.2 | 104.9 |
| 3 | 0.2 | 0 | 35 | 51 | 30 | 9 | 0 | 91 | 0.1 | 2.0 | 3.6 |
| 4 | 0.0 | 0 | 25 | 41 | 0 | 72 | 9 | 19 | 6.2 | 0.6 | 0.0 |
| 5 | 4.2 | 0 | 31 | 46 | <1 | 58 | 42 | 0 | 0 | 0.0 | 0.7 |
| 6 | 0.2 | 0 | 36 | 44 | 0 | 51 | 49 | 0 | 7.7 | 1.3 | 17.9 |
| 7 | 6.6 | <1 | 31 | 44 | 0 | 56 | 24 | 29 | 0.0 | 0.0 | 1.0 |
| 8 | 5.1 | <1 | 28 | 41 | 0 | 47 | 14 | 39 | 0.0 | 0.0 | 0.0 |
AHI: Apnea–Hypopnea Index; SpO2: arterial oxygen saturation; PtCO2: transcutaneous carbon dioxide pressure.
Long disconnections
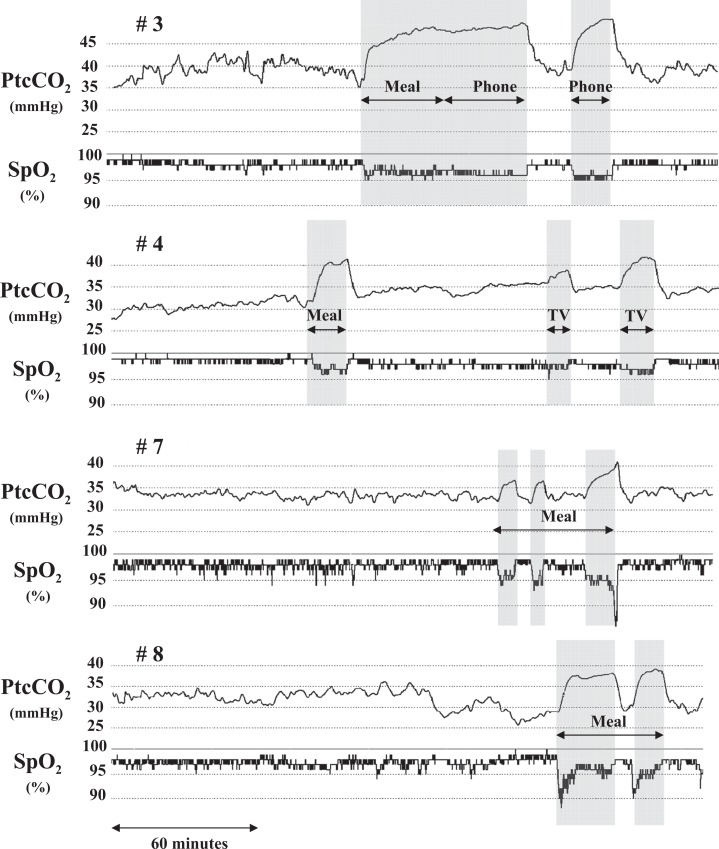
Disconnections from the mouthpiece lasting ≥3 minutes occurred in all patients except patient number 1. However, patient number 2 switched from MPV to nasal ventilation for 90 minutes for lunch and for 30 minutes while watching television; he was thus never disconnected from the ventilator for ≥3 minutes. Disconnections from the ventilator were excluded from the AHI analysis. They were consistently associated with at least 5 mmHg PtcCO2 increases and at least 2% SpO2 decreases. Figure 1 shows four examples of PtcCO2 and SpO2 recordings (patient numbers 3, 4, 7, and 8). Patient numbers 3, 4, 5, and 6 stopped their mechanical ventilation completely during the meal, for 35, 20, 30, and 60 minutes, respectively. Patient number 7 ate the meal without ventilation during three periods of 6, 5, and 13 minutes, respectively, separated by 6 and 14 minutes of mechanical ventilation. Patient number 8 ate without ventilation during two periods of 27 and 12 minutes, respectively, separated by 7 minutes of mechanical ventilation. In addition, patient numbers3 and 4 also had periods of disconnection from MPV outside the mealtime.
Figure 1.
PtcCO2 and SpO2 tracings of patients numbers 3, 4, 7, and 8 who disconnected from the ventilator during the meal. Patient number 7 ate the meal in two parts and patient number 8 in three parts, with connection to the ventilator during the intervening rest periods. Patient numbers 3 and 4 also disconnected from the ventilator during activities other than eating, namely, for phone communications (patient number 3) and for a coffee break and watching television in patient number 4. Disconnections are shaded in gray. PtcCO2: transcutaneous partial pressure of carbon dioxide; SpO2: peripheral capillary oxygen saturation.
Hypoventilation events
PtcCO2 rose above 45 mmHg during MPV in patient numbers 3 and 5 (to peaks of 50.6 mmHg and 45.5 mmHg, respectively). The duration of hypoventilation was 30% and <1% of the recording time in these patients, respectively. Thus, only patient number 3 experienced periods of severe hypoventilation, although his lowest PtcCO2 value was 35.0 mmHg during NIV and his mean PtcCO2 value was 39.6 mmHg when counting both NIV periods and disconnections. These periods corresponded to long periods of total disconnection described above. During these periods, the inductive plethysmography signal amplitude was generally 80% lower than during MPV and, therefore, very few respiratory cycles were counted as unassisted cycles. However, although PtcCO2 increased markedly, the decrease in SpO2 remained moderate (Figure 1, patient # 3).
Patient–ventilator interactions
As reported in Table 3, unassisted spontaneous cycles occurred; they accounted for 40% of all cycles in four patients (patient numbers 5, 6, 7, and 8) and less than 20% of all cycles in the other four patients (patient numbers 1, 2, 3, and 4). Vital capacity did not seem higher in the group with a substantial proportion of unassisted spontaneous cycles (11.5% [8.2–16.0] vs. 16.5% [12.5–20.2] in the group with few unassisted cycles; Table 1). Both patients whose AHI was >5 hour−1 (patient numbers 7 and 8) and the patient with severe hypoventilation (patient number 3) were in the group with more than 40% of unassisted cycles. Double triggering and auto triggering were rare (Table 3), whereas ineffective efforts occurred for more than 10% of all cycles in patient numbers1, 2, and 6 (Table 3).
Patient perceptions and subjective symptoms
Although perceptions varied across patients, most patients were satisfied with MPV (Table 4). Five patients preferred the mouthpiece to the nasal mask, two had no preference, and one preferred the nasal mask. Most patients felt that MPV was efficient and comfortable. Of the eight patients, five made little or no use of the inspiratory trigger and the remaining three found this trigger easy to use. Three patients reported symptoms suggestive of hypercapnia, such as headaches, but these did not occur during the study afternoon. Patient number 2 reported headaches and felt that MPV was insufficiently effective but had normal polygraphy findings.
Table 4.
Patient impressions, frequency of using, and symptoms.a
| Patient numbers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Impressions | ||||||||
| Satisfaction of NIV with mouthpiece | 9 | 5 | 8 | 9 | 5 | 9 | 10 | 9 |
| Satisfaction of mouthpiece’s support | 4 | 2 | / | 9 | 9 | 5 | 9 | 9 |
| Difficulty to connect/disconnect to mouthpiece | 0 | 8 | 0 | 9 | 3 | 5 | 1 | 1 |
| Tolerance of mouthpiece | 8 | 8 | 5 | 9 | 5 | 5 | 9 | 9 |
| Comfort of mouthpiece | 7 | 7 | 5 | 9 | 5 | 8 | 8 | 9 |
| Efficacy of NIV with mouthpiece | 10 | 4 | 9 | 10 | 5 | 9 | 10 | 10 |
| Preference to mouthpiece rather than nasal mask | 0 | 5 | 9 | 10 | 5 | 9 | 8 | 10 |
| Inspiratory trigger | ||||||||
| Frequency of using | 0 | 5 | 1 | 8 | 5 | 2 | 1 | 1 |
| Easiness of using | / | 5 | 9 | 9 | 5 | 9 | 9 | 9 |
| Patient adaptation to it | / | 5 | 9 | 9 | 5 | 9 | 5 | 5 |
| Frequency of expiration through the mouthpiece | 10 | 5 | 9 | 5 | 5 | 2 | 5 | 5 |
| Symptoms | ||||||||
| Headaches | N | Y | N | N | N | Y | N | Y |
| Upon awakening | N | Y | N | N | N | N | N | Y |
| During the afternoon | N | N | N | N | N | N | N | N |
| After a long time of mouthpiece use | N | N | N | N | N | N | N | N |
| At any time | N | N | N | N | N | Y | N | N |
| Asthenia | N | N | N | N | N | N | N | N |
| Drowsiness | N | N | Y | N | N | Y | N | N |
NIV: noninvasive ventilation.
aFrequency of using is measured using visual analog scales: from 0 (not) to 10 (very) and symptoms: N = no; Y = yes.
Discussion
In our study, six of the eight patients had disconnections from the ventilator ≥3 minutes, four only while eating, and two also at other times. These disconnections were consistently associated with ≥5 mmHg PtcCO2 increases and ≥2% SpO2 decreases. Furthermore, three patients experienced episodes of oxygen desaturation with SpO2 values <90%, although none had PtcCO2 values >45 mmHg. A single patient experienced PtcCO2 elevation for substantial periods (>30% of the recording time); the concomitant decrease in SpO2 was small. Only one patient preferred the nasal mask over the mouthpiece. Although the long-term effectiveness of daytime MPV has been evaluated in several studies of patients with neuromuscular diseases who required continuous ventilatory support,4,14 this is the first investigation of the effectiveness of MPV under real-life conditions.
During the day, using a mouthpiece allows patients to connect to, or disconnect from, the ventilator at will, particularly when they are seated. Thus, patients can remove the mouthpiece and breathe unassisted while talking and eating. A major concern, however, is that excessively frequent or long disconnections might induce apnea/hypopnea or hypoventilation episodes with alterations in blood gas levels. The risk of such events depends on the patient’s ability to breathe efficiently while off the ventilator and on whether the patient perceives air hunger in the event of hypoxia or hypercapnia and is thus warned that reconnection to the ventilator is mandatory.
Interestingly, a single patient switched from MPV to nasal ventilation during the meal. The other other patients discontinued ventilation while eating. Among them, two ate the meal in two or three parts, resting and reconnecting to the ventilator in the intervals. Disconnections were consistently associated with PtcCO2 increases. Furthermore, hypercapnia occurred in two patients, very briefly in one and for 30% of the recording time in the other. Nasal ventilation during MPV disconnection for meals may avoid long periods of ventilator disconnection and hypercapnia. Terzi et al.15 reported that relieving the urgency to breathe in patients with respiratory failure using nasal mechanical ventilation also improved breathing–swallowing synchronization and swallowing performance to avoid inadequate ventilator insufflation, thus preventing aspiration during swallowing, Terzi et al. used a prototype equipped with an off switch that could be activated at will by the patient to interrupt air delivery by the ventilator.
We documented rare but severe and somewhat prolonged hypercapnic episodes as well as respiratory events associated with arterial oxygen desaturation. Interestingly, no clinical complications occurred before or after the monitoring periods. Furthermore, most of the patients with abnormal events during monitoring reported not usually experiencing headaches, asthenia, or drowsiness during the day. The only exception was patient number 3 who had prolonged periods of daytime hypercapnia and reported frequent daytime drowsiness. However, the hypercapnic episodes in this patient occurred during activities such as physiotherapy, eating, and phone conversations, which did not coincide with the usual time pattern of drowsiness.
All types of cycles (unassisted, assisted, and controlled) were observed, supporting the usefulness of maintaining an assisted mode with a backup rate. The most common type of asynchrony was ineffective effort, also suggesting a need for improved trigger sensitivity. Recently introduced MPV software allowing insufflation to be triggered only by the positioning of the patient’s lips seems to hold promise.16,17
Few patients tolerated prolonged disconnections without developing hypercapnia. One of them was patient number 8 who was disconnected from the ventilator for 27 minutes during the meal and had no hypercapnia, although his vital capacity was only 6% of the predicted value. The rest provided by the mechanical ventilation probably allowed the respiratory muscles to achieve sufficient ventilation for short periods.
Two patients used a pressure support mode. Although both experienced abnormal events, these showed no clear relationship with the mode of mechanical ventilation. Thus, our data do not contradict previous evidence that both volume-controlled mode and pressure-controlled mode can be used during MPV.6,13
A single patient experienced episodes of arterial oxygen desaturation without detectable disconnection from the ventilator. As mentioned above, we did not have blood gas values for this patient, who also had a high body mass index (30 kg m−2). The most likely explanation to the desaturations is baseline hypoxia combined with an insufficient VT delivered by the ventilator, given the obesity.
The main limit of our study is that our tested population used sip ventilation for at least 1 year before being included in the study, which may not be representative of all sip users but of a subgroup of successful long-term sip ventilation users. Further larger studies would be necessary to appreciate fully the possible adverse consequences of ineffective mechanical ventilation
In conclusion, this pilot study involving daytime polygraphy with PtCO2 recording under real-life conditions confirms that MPV can be efficient as long as the patient remains connected to the mouthpiece. However, disconnection from the ventilator may result in transient arterial oxygen desaturation and hypercapnia, which do not always induce discomfort in the patient. The potential long-term consequences of these events are unknown, and there are no published evidence-based guidelines about MPV. Nevertheless, our findings support the usefulness of educating patients about behaviors that can induce hypercapnia or oxygen desaturation and of developing an external warning system based on the minimal minute ventilation in order to prevent blood gas abnormalities. They also support that the recording of SpO2 and PtCO2 should be sufficient for detecting abnormal breathing during the sip ventilation.
Acknowledgment
We thank Dr Bruyelle Sophie, Mr Rolde Philippe and Mr David Michel for their help in the patients’ inclusion and management.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Goldberg A, Leger P, Hill N, et al. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116: 521–534. [DOI] [PubMed] [Google Scholar]
- 2. Finder JD, Birnkrant D, Carl J, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respirat Crit Care Med 2004; 170: 456–465. [DOI] [PubMed] [Google Scholar]
- 3. Robert D, Willig TN, Leger P, et al. Long-term nasal ventilation in neuromuscular disorders: report of a consensus conference. Eur Respir J 1993; 6: 599–606. [PubMed] [Google Scholar]
- 4. Toussaint M, Steens M, Wasteels G, et al. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J 2006; 28: 549–555. [DOI] [PubMed] [Google Scholar]
- 5. Bach JR, Alba AS, Saporito LR. Intermittent positive pressure ventilation via the mouth as an alternative to tracheostomy for 257 ventilator users. Chest 1993; 103: 174–182. [DOI] [PubMed] [Google Scholar]
- 6. Khirani S, Ramirez A, Delord V, et al. Evaluation of ventilators for mouthpiece ventilation in neuromuscular disease. Respir Care 2014; 59: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 7. Rabec C, Rodenstein D, Leger P, et al. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax 2011; 66: 170–178. [DOI] [PubMed] [Google Scholar]
- 8. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006; 32: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 10. Schwab RJ, Schnader JS. Ventilator autocycling due to an endotracheal tube cuff leak. Chest 1991; 100: 1172–1173. [DOI] [PubMed] [Google Scholar]
- 11. Meyer TJ, Pressman MR, Benditt J, et al. Air leaking through the mouth during nocturnal nasal ventilation: effect on sleep quality. Sleep 1997; 20: 561–569. [DOI] [PubMed] [Google Scholar]
- 12. Bach JR, Robert D, Leger P, et al. Sleep fragmentation in kyphoscoliotic individuals with alveolar hypoventilation treated by NIPPV. Chest 1995; 107: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 13. Boitano LJ, Benditt JO. An evaluation of home volume ventilators that support open-circuit mouthpiece ventilation. Respir Care 2005; 50: 1457–1461. [PubMed] [Google Scholar]
- 14. Dean S, Bach JR. The use of noninvasive respiratory muscle aids in the management of patients with progressive neuromuscular diseases. Respir Care Clin North Am 1996; 2: 223–240. [PubMed] [Google Scholar]
- 15. Terzi N, Normand H, Dumanowski E, et al. Noninvasive ventilation and breathing-swallowing interplay in chronic obstructive pulmonary disease*. Crit Care Med 2014; 42: 565–573. [DOI] [PubMed] [Google Scholar]
- 16. Benditt JO, Boitano LJ. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med 2013; 187: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 17. Hess DR. The growing role of noninvasive ventilation in patients requiring prolonged mechanical ventilation. Respir Care 2012; 57: 900–918; discussion 18–20. [DOI] [PubMed] [Google Scholar]