Abstract
The aim was to undertake a cost-utility analysis of a self-management programme of activity, coping and education (SPACE) for chronic obstructive pulmonary disease (COPD). The analysis was conducted alongside a six-month randomized controlled trial in 30 primary care settings. The economic analysis used data from 184 patients with confirmed diagnosis of COPD, forced expiratory volume in one second/forced vital capacity ratio <0.7 and with grade 2–5 on the Medical Research Council dyspnoea scale. Participants received either a self-management programme consisting of an education manual (SPACE for COPD) and consultation or usual care. Six-month costs were estimated from the National Health Service and Personal Social Services perspective and quality-adjusted life years (QALYs) were calculated based on patient responses at baseline and six months.
The mean difference in costs between usual care and SPACE FOR COPD programme was −£27.18 (95% confidence interval (CI); −£122.59 to £68.25) while mean difference in QALYs was −0.10 (95% CI; −0.17 to −0.02). The results suggest that the intervention is more costly and more effective than usual care. The probability of the intervention being cost-effective was 97% at a threshold of £20,000/QALY gained. We conclude that the SPACE FOR COPD programme is cost-effective compared to usual care.
Keywords: COPD, cost-effectiveness analysis, cost-utility analysis, RCT, self-management, education
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of disability and mortality with the death of more than 3 million people in 2012, which is equal to 6% of all deaths globally that year.1 As well as being recognized to contribute to the substantive deterioration of health-related quality of life (HRQoL) for many patients,2 COPD places a heavy burden on the healthcare system, patients and society overall.3–5
Non-pharmacological-based treatments such as self-management interventions are acknowledged as important strategies to support patients with long-term conditions6 such as COPD.7 Although there is no one definition of self-management, the broad approach is to teach individuals the skills needed to cope with the disease, ability to recognize and respond to symptoms proactively and adopt a healthy lifestyle as well as manage social interactions.8 The clinical effectiveness of self-management educational programmes has already been established for a range of chronic illnesses including COPD.9–12 The most recent Cochrane systematic literature review on self-management showed that it improved HRQoL in patients with COPD compared with usual care.2 They also showed a reduction in all-cause and COPD-related hospital admissions in those participating in the self-management interventions. The authors of this review, however, claim that they were unable to come to any definitive conclusions due to the heterogeneity of the studies, in terms of both the study populations, follow-up times, outcome measured, methodology used and the different specification of the ‘self-management intervention’2.
To date only a few studies have attempted to evaluate the cost-effectiveness of self-management programmes for people with COPD. Some studies reported only the programme costs.9,13,14 The evidence suggests that self-management programmes are cost-effective in improving patient outcomes and reducing cost.10,15,16 These studies have taken different methodological approaches and differ on the primary outcome considered.17
A randomized controlled trial was carried out to determine the short-term (baseline to 6 weeks) and medium-term (6 weeks to 6 months) effectiveness of a Self-management Programme of Activity, Coping and Education (SPACE) for COPD (SPACE FOR COPD) on patient outcomes, compared with usual care alone. Full clinical results of this trial are reported elsewhere.18 In brief, at 6 weeks, there were significant differences between groups in Chronic Respiratory Questionnaire-Self-Reported (CRQ-SR) dyspnoea, fatigue and emotion scores, exercise performance, anxiety and disease knowledge. However, there was no between-group difference in change of the primary outcome measure (CRQ-SR dyspnoea) at 6 months. However, exercise performance, anxiety and self-reported smoking status were significantly different between groups, in favour of the intervention.
The aim of this study was therefore to undertake a cost-utility analysis to examine the cost-effectiveness of SPACE FOR COPD versus usual care at 6 months.
Methods
Study design
This was a single-centre, investigator-blinded, randomized controlled trial conducted with 6 months follow-up, which took place between December 2009 and April 2012. One hundred and eighty four participants were randomized to either SPACE FOR COPD (n = 89) or to usual care (n = 95). Practices screened patient registers to identify eligible candidates. To be included, participants were required to: (1) have a diagnosis of COPD confirmed by spirometry, with a forced expiratory volume in one second/forced vital capacity ratio <0.7; (2) be grade 2–5 on the Medical Research Council dyspnoea scale19; and (3) have been clinically stable for 4 weeks. Individuals were excluded if they; (1) were unable to undertake an exercise regime due to neurological, musculoskeletal or cognitive co-morbidities; (2) unable to read English to the reading age of an 8-year-old; and (3) had completed pulmonary rehabilitation within the previous 12 months.
SPACE for COPD
Participants randomized to SPACE FOR COPD were introduced to the programme by a physiotherapist during a 30–45 minute consultation using principles of Motivational Interviewing. This comprehensive programme has been described elsewhere18,20 and is structured around the SPACE FOR COPD manual. Briefly, the manual contains educational material on a wealth of topics and a home exercise programme. Acquisition of skills is promoted through goal-setting strategies, coping planning and case studies. The manual advises on training progression and includes an action plan for exacerbation management.20
Participants’ needs were discussed and goal-setting strategies were introduced at initial consultation. It was anticipated that participants would work through the manual in approximately 6 weeks; participants received two telephone contacts at 2 and 4 weeks into the programme from the physiotherapist, with the aim of reinforcing skills and providing encouragement to progress. There was no further contact between the physiotherapist and participant after the telephone contact at 4 weeks.
Usual care
All study participants continued to receive usual care for their COPD management. Within primary care, all participants were managed under a general practitioner (GP) and practice team. No participants received pulmonary rehabilitation during the study period.
Health outcomes
Participants were asked to describe their HRQoL at baseline, 6 weeks and 6 months post-randomization using the EuroQol EQ-5D-3 L instrument (the Netherlands).21 The EQ-5D is the generic, multi-attribute, preference-based measure preferred by National Institute for Health and Care Excellence (NICE)22 for broader cost-effectiveness comparative purposes. The EQ-5D consists of two principal measurement components. The first is a descriptive system, which defines HRQoL in five dimensions: ‘mobility’, ‘self- care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’. A total of 243 health states are generated by the EQ-5D descriptive system. For the purposes of this study, the York A1 tariff was applied to each set of responses to the descriptive system to generate an EQ-5D utility score.23 Resulting utility scores range from scores −0.59 to 1.0, with ‘0’ representing death and ‘1’ representing full health. Utility values <0 indicate health states worse than death. The second measurement component of the EQ-5D, the vertical visual analogue scale (VAS) ranging from 100 (best imaginable health state) to 0 (worst imaginable health state), was also included.
Collection and valuation of resource-use cost data
Data on the resource items and services used for the intervention and the control arm were collected over the 6-month time horizon. Data on consumable use (SPACE FOR COPD manual), introduction to SPACE FOR COPD and telephone contact duration were recorded prospectively in research/study records. Healthcare data were obtained through three primary sources. First, the hospital records were investigated to capture all resources relating to the participants’ use of health and social care services over the 6-month study period. Second, GP records were also analysed to capture the use of primary healthcare and social care services. Thirdly, patients were asked at their follow-up research appointment to detail any healthcare contacts. The following data were captured: the dates, duration and reasons for healthcare contacts (classified as respiratory or non-respiratory related), the healthcare professional involved, mode of delivery (i.e. clinic, home visit or telephone contact) for all appointments, investigations, admissions, emergency department visits and referrals. Information was obtained from these three data sources (hospital, GP and patient) and triangulated to give the most likely, overall healthcare resource use. Medication use was obtained from prescription and medication records from GP sources. Type, dose and frequency of medications prescribed were recorded (including oxygen therapy) and classified as respiratory or non-respiratory medications.
The unit cost for resources used for the implementation of SPACE FOR COPD was mainly obtained from the SPACE FOR COPD study records, apart from the unit costs of physiotherapists’ time obtained from the Personal Social Services Research Unit (PSSRU) ‘Unit costs of health and social care 2013–’ cost compendium.24 Unit costs for hospital and community-based health and social care services were derived from National Health Service (NHS) Reference costs25 and the PSSRU24 for the economic evaluation. The medication collected included respiratory antibiotics and steroids. The drug prescription costs were obtained from the British National Formulary.26
Economic Analysis
The incremental cost-effectiveness of SPACE FOR COPD compared with usual care is based on EQ-5D data, as this is the utility measure currently recommended by NICE for the evaluation of cost-effectiveness of interventions used or services delivered in the public and non-public sectors.22 The study was conducted from a NHS and Personal Social Services perspective including COPD-related and all-cause healthcare costs.
Our analysis used a regression approach to better reflect the nature of the data.27 The distribution of costs are highly skewed, so that ordinary least square (OLS) assumptions of normality might not be appropriate; hence a Generalized Linear Model was fitted for costs using a Gamma distribution and identity link function. Quality-adjusted life years (QALYs) were estimated using an OLS regression with baseline utility (EQ-5D) as a covariate to adjust for differences between groups. This method of adjusting for baseline utility differences is more efficient than estimation of QALYs using the ‘change from baseline’ method. Results were based on 1000 bootstrap samples, which was sufficient to provide stable estimates of costs and effects.28
Results were presented as incremental cost-effectiveness ratio (ICER) statistics, ‘the cost per QALY’. This is the estimated difference in mean costs between the SPACE FOR COPD and usual care arms (the incremental cost), divided by the difference in mean QALYs between the two arms (incremental effect). The ICERs can be compared against the benchmark thresholds for cost-effectiveness in the NHS context of £20,000 to £30,000 per QALY gained, as applied by NICE.29 If the ICER is below £20,000 per QALY, this suggests that the intervention is a cost-effective alternative to usual care, above £30,000 per QALY this suggests that the intervention is not cost-effective and in between these figures, the result is indeterminate. We also present the results using Incremental Net Benefit (INB) statistics, calculated by multiplying the incremental effects by an assumed monetary value of a QALY (the ‘cost-effectiveness threshold’) and subtracting the incremental cost. We calculate INB statistics based on the two cost per QALY thresholds of £20,000 and £30,000 per QALY. A positive INB suggests that the intervention is cost-effective compared with usual care at the defined threshold.
Uncertainty over the cost-effectiveness of the intervention is reflected in an estimated probability that the INB is positive. If this figure is greater than 0.5, it indicates that the intervention is more likely to be cost-effective than not.
Results
Complete economic data regarding costs and QALYS over the 6-month study period were available for all patients. Mean EQ-5D utility scores for SPACE FOR COPD and usual care at baseline and 6 months and the mean QALYs gained are presented in Table 1. Estimated by the simple area-under-the-curve method, mean QALYs attained over the trial period based on EQ-5D utility measure were slightly lower and statistically significant for the usual care group than for SPACE FOR COPD group: −0.10 (−0.17 to −0.02). For comparison with the utility measure, we also show results based on the EQ-5D VAS (−3.05 (−8.25 to 2.14)), which provides us with a value for the participant’s self-rated health at the time of survey completion on a scale of 0–100 (Table 2).
Table 1.
Mean utilities derived from the EQ-5D, and the associated mean QALYs.a
| Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | SPACE | Usual care | SPACE | ||
| Baseline | 90 | 87 | 0.63 (0.03) | 0.70 (0.02) | −0.07 (−0.14 to 0.00) |
| 6 months | 81 | 69 | 0.62 (0.03) | 0.71 (0.03) | −0.08 (−0.16 to −0.01) |
| Change 0–6 months | 78 | 68 | 0.00 (0.02) | −0.01 (0.02) | 0.00 (−0.06 to 0.06) |
| QALYs | 78 | 68 | 0.61 (0.03) | 0.71 (0.03) | −0.10 (−0.17 to −0.02) |
QALY: quality-adjusted life year; CI: confidence interval; SPACE: self-management programme of activity, coping and education.
aQALYs, estimated by the area-under-the-curve for individual patients.
Table 2.
Mean utilities derived from the VAS scores and the associated mean QALYs.a
| Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | SPACE | Usual care | SPACE | ||
| Baseline | 88 | 82 | 64.3 (1.71) | 66.9 (1.87) | −2.20 (−7.19 to 2.80) |
| 6 months | 81 | 68 | 65.0 (2.02) | 66.2 (2.26) | −1.16 (−7.14 to 4.82) |
| Change 0-6 months | 76 | 65 | 0.18 (1.96) | −2.68 (1.82) | 2.86 (−2.49 to 8.22) |
| QALYs | 76 | 65 | 64.58 (1.75) | 67.6 (1.98) | −3.05 (−8.25 to 2.14) |
QALY: quality-adjusted life year; VAS: visual analogue scale; SPACE: self-management programme of activity, coping and education; CI: confidence interval.
aQALYs, estimated by the area-under-the-curve for individual patients.
Information about the use of NHS services was obtained by GP and hospital records at 6 months. Reported estimates of healthcare use including prescription and diagnostic tests over 0–6 months are given in Table 3. These resource quantities were multiplied by the relevant unit costs (Table 4) to provide estimates of the mean costs per patient from 0 to 6 months (Table 5). Differences between the groups in the costs of healthcare use over these periods were modest, with wide confidence intervals (CIs). A summary of all included costs over the trial period is given in Table 5. This shows a difference, though non-significant, between the groups. The mean cost for the SPACE FOR COPD group was £181.39 compared with £160.65 for the usual care group: a difference of £20.74 (−£51.77 to £10.29). Overall, taking account of costs for the intervention, for prescribed medications and for other NHS services, the estimated between-group difference in costs is £27.18 (−£122.59 to £68.25), indicating reduced cost in favour of the usual care group.
Table 3.
Health care utilization over the study period.
| Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | |||
|---|---|---|---|---|---|
| Type of care | Usual care | SPACE | Usual care | SPACE | |
| GP, surgery visit | 90 | 85 | 0.96 (0.15) | 0.76 (0.13) | 0.19 (−0.20 to 0.58) |
| GP, home visit | 90 | 85 | 0.08 (0.04) | 0.01 (0.01) | 0.07 (−0.02 to 0.15) |
| GP, phone | 90 | 85 | 0.21 (0.06) | 0.26 (0.09) | −0.05 (−0.26 to 0.16) |
| Community physiotherapist, hour | 90 | 85 | 0.06 (0.02) | 0.08 (0.03) | −0.03 (−0.10 to 0.05) |
| Social worker, hour | 90 | 85 | 0.01 (0.01) | 0 (0) | 0.01 (−0.01 to 0.03) |
| District nurse, hour | 89 | 85 | 0.63 (0.09) | 0.53 (0.08) | 0.10 (−0.15 to 0.35) |
| District nurse, phone | 90 | 85 | 0.06 (0.03) | 0.02 (0.02) | 0.03 (−0.03 to 0.10) |
| District nurse, home visit | 90 | 84 | 0.37 (0.34) | 0.01 (0.01) | 0.35 (−0.35 to 1.06) |
| Clinical decision clinic | 95 | 86 | 0.04 (0.02) | 0.02 (0.02) | 0.02 (−0.03 to 0.07) |
| Inpatient stay, episode | 95 | 86 | 0.01 (0.01) | 0.01 (0.01) | 0 (−0.03 to 0.03) |
| Respiratory clinic, visit | 95 | 86 | 0.08 (0.03) | 0.22 (0.07) | −0.14 (−0.29 to 0.01) |
| Hospital nurse | 95 | 86 | 0 (0) | 0.02 (0.02) | −0.02 (−0.05 to 0.01) |
| Emergency department, visit | 95 | 86 | 0.01 (0.01) | 0.03 (0.02) | −0.02 (−0.07 to 0.02) |
| Physiotherapist department, visit | 95 | 86 | 0.01 (0.01) | 0.02 (0.02) | −0.01 (−0.05 to 0.02) |
| X-rays, test | 95 | 86 | 0.17 (0.05) | 0.20 (0.06) | −0.03 (−0.18 to 0.12) |
| CT scan, test | 95 | 86 | 0.01 (0.01) | 0.10 (0.04) | −0.09 (−0.17 to 0.01) |
| Blood tests, test | 94 | 86 | 1.71 (0.40) | 2.10 (0.40) | −0.39 (−1.50 to 0.72) |
| Spirometry test, test | 95 | 86 | 0.07 (0.03) | 0.10 (0.03) | −0.03 (−0.11 to 0.05) |
| Lung function test, test | 95 | 86 | 0.02 (0.01) | 0.05 (0.02) | −0.03 (−0.08 to 0.030 |
| Sputum test, test | 95 | 86 | 0.11 (0.05) | 0.20 (0.08) | −0.09 (−0.28 to 0.09) |
| Electrocardiogram monitoring, test | 95 | 86 | 0.09 (0.03) | 0.15 (0.05) | −0.06 (−0.17 to 0.05) |
| Urine test, test | 95 | 86 | 0.35 (0.08) | 0.28 (0.06) | 0.07 (−0.13 to 0.26) |
SPACE: self-management programme of activity, coping and education; CI: confidence interval; GP: general practitioner.
Table 4.
Unit costs of healthcare resources used.a
| Healthcare resource | Unit cost | Details | Source of unit cost |
|---|---|---|---|
| Community care | PSSRU | ||
| GP surgery consultation | 45.00 | Per session | PSSRU |
| GP home visit | 292.00 | Per home visit | PSSRU |
| GP phone call | 27.00 | Per hour phone call | PSSRU |
| Physiotherapist | 34.00 | Per hour of consultation | PSSRU |
| Social worker | 57.00 | Per hour of consultation | PSSRU |
| District nurse surgery consultation | 48.00 | Per hour of consultation | PSSRU |
| District nurse home visit | 70.00 | Per hour of home visit | PSSRU |
| District nurse phone call | 58.00 | Per hour phone call | PSSRU |
| Hospital Services | |||
| Clinical decision unit | 40.00 | Per session | Ref Cost |
| Inpatient | 478.00 | Per inpatient day | Ref Cost |
| Respiratory clinic | 154.00 | Per session | Ref Cost |
| Nurse | 75.00 | Per session | Ref Cost |
| Emergency Department | 117.00 | Per session | Ref Cost |
| Physiotherapy | 42.00 | Per session | Ref Cost |
| X-rays | 5.00 | Per test | assumption |
| CT scan | 92.00 | Per test | Ref Cost |
| Blood tests | 3.00 | Per test | Ref Cost |
| Spirometry tests | 167.00 | Per test | Ref Cost |
| Lung function test | 167.00 | Per test | Ref Cost |
| Sputum test | 7.00 | Per test | Ref Cost |
| ECGs | 53.00 | Per test | Ref Cost |
| Urine test | 4.00 | Per test | Ref Cost |
Table 5.
Mean NHS costs (£) of healthcare resource use over the 6-month study period.
| Type of care | Number of patients | Mean cost (SE) | Mean treatment difference (95% CI) | ||
|---|---|---|---|---|---|
| Usual care | SPACE | Usual care | SPACE | ||
| Intervention (therapy deliver, consumables and sessions attended | 95 | 89 | 160.65 (108.69) | 181.39 (104.37) | −20.74 (−51.77 to 10.29) |
| NHS services | |||||
| GP, surgery visit | 90 | 85 | 43 (6.59) | 34.41 (5.87) | 8.59 (30.10 to 47.57) |
| GP, home visit | 90 | 85 | 22.71 (11.51) | 3.44 (3.43) | 19.28 (−5.02 to 43.58) |
| GP, phone | 90 | 85 | 5.7 (1.62) | 6.99 (2.40) | −1.29 (−6.95 to 4.37) |
| Community Physiotherapist, hour | 90 | 85 | 1.89 (0.83) | 2.8 (1.02) | −0.91 (−3.49 to 1.67) |
| Social worker, hour | 90 | 85 | 0.63 (0.63) | 0 (0) | 0.63 (−0.65 to 1.92) |
| District nurse, hour | 89 | 85 | 30.20 (4.43) | 25.41 (3.99) | 4.79 (−7.01 to 16.59) |
| District nurse, phone | 90 | 85 | 3.22 (1.68) | 1.36 (0.96) | 1.86 (−2.02 to 5.73) |
| District nurse, home visit | 90 | 84 | 25.67 (24.12) | 0.83 (0.83) | 24.83 (−24.48 to 74.15) |
| Clinical Decision Clinic | 95 | 86 | 1.68 (0.83) | 0.93 (0.65) | 0.75 (−1.35 to 2.87) |
| Inpatient stay, episode | 95 | 86 | 5.03 (5.03) | 5.56 (5.56) | −0.53 (−15.28 to 14.23) |
| Respiratory clinic, visit | 95 | 86 | 12.97 (4.98) | 34.02 (10.92) | −21.05 (−44.02 to 1.91) |
| Hospital nurse | 95 | 86 | 0 (0) | 1.74 (1.23) | −1.74 (−4.05 to 0.56) |
| Emergency department, visit | 95 | 86 | 1.23 (1.23) | 4.08 (2.33) | −2.85 (−7.94 to 2.22) |
| Physiotherapist department, visit | 95 | 86 | 0.44 (0.44) | 0.98 (0.69) | −0.53 (−2.12 to 1.05) |
| X-rays, test | 95 | 86 | 0.84 (0.24) | 0.99 (0.30) | −0.15 (−0.90 to 0.61) |
| CT scan, test | 95 | 86 | 0.97 (0.97) | 9.63 (3.74) | −8.66 (−15.96 to −1.37) |
| Blood tests, test | 94 | 86 | 5.14 (1.19) | 6.31 (1.18) | −1.18 (−4.50 to 2.15) |
| Spirometry test, test | 95 | 86 | 12.31 (4.50) | 17.48 (5.54) | −5.17 (−19.15 to 8.81) |
| Lung function test, test | 95 | 86 | 3.51 (2.48) | 7.77 (3.81) | −4.25 (−13.10 to 4.56) |
| Sputum test, test | 95 | 86 | 0.74 (0.32) | 1.39 (0.59) | −0.65 (−1.94 to 0.64) |
| Electrocardiogram monitoring, test | 95 | 86 | 5.02 (1.79) | 8.01 (2.40) | −2.99 (−8.83 to 2.85) |
| Urine test, test | 95 | 86 | 1.39 (0.31) | 1.12 (0.24) | 0.27 (−0.51 to 1.05) |
| All NHS services | 88 | 84 | 178.24 (33.6) | 166.42 (25.21) | 11.82 (−71.69 to 95.33) |
| Prescriptions | |||||
| Respiratory | 95 | 89 | 59.95 (5.26) | 77.07 (9.05) | −17.11 (−37.45 to 3.22) |
| Antibiotics | 95 | 89 | 1.42 (0.22) | 1.14 (0.31) | 0.27 (−0.48 to 1.02) |
| Steroids | 90 | 85 | 0.91 (0.19) | 1.07 (0.39) | −0.16 (−0.99 to 0.68) |
| All prescriptions | 90 | 85 | 62.28 (5.34) | 79.28 (9.25) | −17.00 (−37.74 to 3.74) |
| Total cost | 88 | 84 | 409.54 (351.72) | 436.72 (275.72) | −27.18 (−122.59 to 68.25) |
NHS: National Health Service; SPACE: self-management programme of activity, coping and education; CI: confidence interval; GP: general practitioner.
The results of the incremental cost-effectiveness analysis of the SPACE FOR COPD group compared with usual care are presented in Table 6. The estimated mean healthcare cost with the intervention was approximately £30 higher than the mean cost under usual care, but there was a wide CI around this estimate (−£80.19 to £134.54). The estimated difference in mean QALYs accrued over the 6-month period was approximately 0.10 greater in the intervention group than in the usual care group, with a CI of 0.02–0.17. These results suggest that the SPACE FOR COPD costs around £280.00 more per additional QALY gained compared with the usual care control.
Table 6.
Cost effectiveness results.
| GLM regression | ||||
|---|---|---|---|---|
| Mean treatment difference | 95% CI | p Value | ||
| Total NHS cost (£) | 27.18 | (−80.19 to 134.54) | 0.620 | |
| QALYs | 0.097 | (0.02 to 0.17) | 0.012 | |
| ICER (£ per QALY) | 280.39 | (−12,581 to 13,142.04) | 0.966 | |
| NMB (£) | 20K | 1879.92 | ||
| 30K | 2845.9 | |||
| 40K | 3811.9 | |||
GLM: generalized linear model; CI: confidence interval; NHS: National Health Service; QALY: quality-adjusted life year; ICER: incremental cost effectiveness ratio; NMB: net monetary benefit.
Uncertainty surrounding the estimated costs and effects is represented on the cost-effectiveness plane (Figure 1). The joint density of incremental costs and effects straddles both east quadrants of the cost-effectiveness plane, with the majority of the points lying in the north-east quadrant. This indicates that there is some degree of uncertainty surrounding both the presence and the magnitude of cost-savings and effectiveness. This uncertainty is also shown in the cost-effectiveness acceptability curve (CEAC) in Figure 2. The CEAC illustrates the probability of SPACE FOR COPD being more cost-effective than usual care at different thresholds of decision makers’ willingness to pay for a QALY. There is a 97% chance that the intervention is cost-effective at a threshold of £20,000 per QALY and 99% chance at a threshold of £30,000.
Figure 1.
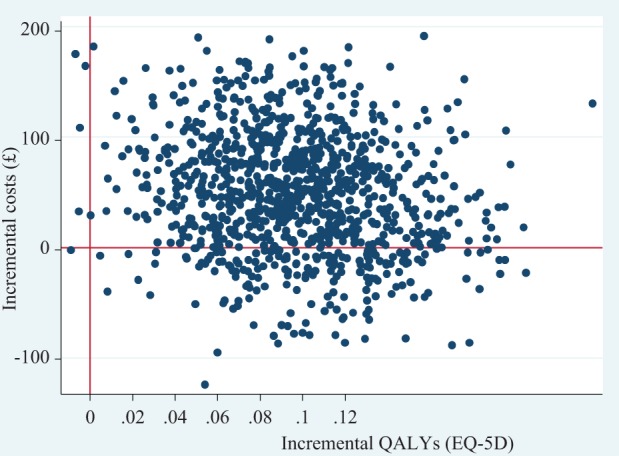
Cost effectiveness plane, bootstrap samples using GLM. GLM: generalized linear model.
Figure 2.
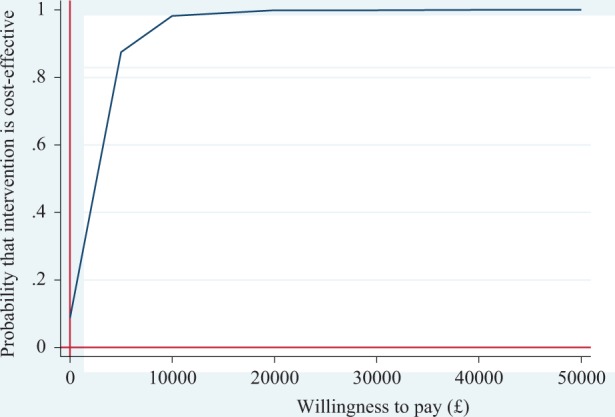
Cost effectiveness acceptability curve.
Discussion
The aim of this study was to perform an economic evaluation of a SAFE in patients with COPD. The intervention showed to have a statistically significant benefit to patients’ health gain (0.10 QALYs), compared to usual care. Also, it showed that there was no statistically significant difference in cost for SPACE FOR COPD compared to usual care. Analysis of the costs and effects of the self-management programme over the 6-month trial period indicates that this is likely to be a cost-effective use of NHS resources. At £280.39 per QALY gained, the estimated ICER is well within NICES’s cost-effective thresholds of £20,000.
The results of this study indicate that the self-management programme slightly increased the use of respiratory clinic visits, emergency department visit and community physiotherapist visits, but reduced, on average, the use of district nurse visits and GP visits. Our results differ from previous findings,15 which demonstrated that a pharmacy-led education and self-management programme reduced hospital days by 60%, emergency visits by 48% and unscheduled GP visits by 48% while another study of a comprehensive intervention9 reported 42% reduction in hospital days, 35% reduction in accident and emergency visits and 59% reduction in unscheduled GP visits. This difference may be due to the fact that as patients are better at self-managing their condition, they may actually use healthcare resources more because they identify when there is a problem so want help/advice to overcome it, that is, they may be better able to identify an exacerbation, so will seek to take their exacerbation medication quicker than someone who isn’t able to identify this. Also, if they are aware of the reasons for taking their medication/inhalers, then they may be more likely to actually take them as prescribed, which may increase medication costs, and so on. The study by Bourbeau et al.9 differed from this one because their intervention was far more intense than SPACE FOR COPD and also they recruited patients with advanced COPD and at least one previous hospitalisation, whereas, we recruited from primary care where our participants’ number of hospital visits was actually rather low and the patient population was relatively mild compared to the more traditional secondary care population in other studies13 that recruited in secondary care and post exacerbation reported much higher number of hospital visits. Perhaps this indicates that there are differences between the patient populations in these studies that could significantly impact on healthcare resource use. Furthermore, in different healthcare systems what constitutes ‘usual care’ may be quite different, so what people are ‘used to’ or expect from care may also affect their use of resources.
In contrast with previous studies that were unable to document any benefit in terms of QALYs between treatment groups,13,16 we did find significant differences on HRQoL and QALYs, so we can conclude that SPACE FOR COPD has a beneficial effect on patient well-being. Furthermore, our results are in line with other secondary clinical outcomes that have showed a significant change in favour of the SPACE FOR COPD intervention at 6 weeks (i.e. CRQ-SR-dyspnoea dimension) and 6 months (i.e. CRQ-SR-emotional dimension).18
To date there are only four studies, we are aware of, that analysed the cost-effectiveness of self-management in patients with COPD.10,13,15,30 Gallefoss’s study10 concluded that patient education reduced costs and improved outcomes. Similarly, the cost-effectiveness analysis conducted by Effing et al.30 showed that a self-treatment strategy was cost saving and resulted in lower probabilities for hospital admissions and healthcare contacts. Also, more recently Kdour et al.15 proved that a pharmacist-led self-management education programme was cost saving and improved HRQoL (0.065 QALYs gain). On the other hand, Monninkhof’s study13 showed that a self-management programme costs twice as much as usual care without providing any measurable beneficial effects. The latter may partly be explained by the fact that they have used a far more expensive intervention in a relative short-time horizon that might have not allowed patients’ benefits to become well pronounced. Comparison of the studies by Gallefoss10 and Effing30 with our study is difficult because they have used other outcome measured rather than QALYs, thus preventing comparisons across these different programmes of care.22 Our study differs from the above since SPACE FOR COPD is the first brief, light-touch self-management intervention to show a beneficial effect in terms of symptom burden, exercise performance, anxiety18 and HRQoL (QALYs), with limited use of healthcare professional support.
Conclusion
Combining our cost-effectiveness results presented here, with the clinical analysis published elsewhere,18 we conclude that SPACE FOR COPD has resulted in significant clinical improvements (exercise performance, disease knowledge and anxiety) and a significant HRQoL gain (in terms of QALYs) during a 6-month period compared to usual care, at a cost increase of £27.18 per patient. Furthermore, SPACE FOR COPD is likely to be cost-effective for the NHS over 6 months at the £20, 000 threshold.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Institute for Health Research (NIHR), Research for Patient Benefit Programme (grant PB-PG-0808-17146) and Leicestershire, Northamptonshire and Rutland Collaboration for Leadership in Applied Health Research and Care, and took place at University Hospitals of Leicester National Health Service (NHS) Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. This study is registered as www.controlled-trials.com with identifier number ISRCTN35501175.
References
- 1. The World Health Organisation. Available at: http://www.who.int/respiratory/copd/en/ (2012, accessed 22 July 2015).
- 2. Zwerink M, Brusse-Keizer M, van der Valk PDLPM, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (3): CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest 2000; 117(2 suppl): 1S–4S. [DOI] [PubMed] [Google Scholar]
- 4. Strassels S, Sullivan D, Smith DH. Characterization of the incidence and cost of COPD in the US [abstract]. Eur Respir J 1996; 9(suppl 23): 421s. [Google Scholar]
- 5. Wouters EF. Economic analysis of the confronting COPD survey: overview of results. Respir Med 2003; 97(suppl C): S3–S14. [DOI] [PubMed] [Google Scholar]
- 6. Davies MJ, Heller S, Skinner TCM, et al. ; Diabetes education and self management for ongoing and newly diagnosed collaborative. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008; 336(7642): 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Effing T, Monninkhof EEM, van der Valk PP, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007; (4): CD002990. [DOI] [PubMed] [Google Scholar]
- 8. Kaptein Ad A, Fischer MJ, Scharloo M. Self-management in patients with COPD: theoretical context, content, outcome, and integration into clinical care. Int J Chron Obstruct Pulmon Dis 2014; 9: 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Int Med 2003; 163(5): 585–591. [DOI] [PubMed] [Google Scholar]
- 10. Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med 1999; 160(6): 2000–2005. [DOI] [PubMed] [Google Scholar]
- 11. Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2010 Update. Available at: http://www.goldcopd.com (2010, accessed 13 July 2015).
- 12. National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update). NICE clinical guideline 101. London: National Institute for Health and Clinical Excellence (NHS Evidence accredited), 2010. [Google Scholar]
- 13. Monninkhof E, van der Valk P, van der Palen J, et al. Effects of a comprehensive self-management program in patients with chronic obstructive pulmonary disease. Eur Respir J 2003; 22: 815–820. [DOI] [PubMed] [Google Scholar]
- 14. Toy EL, Gallagher KF, Stanley EL, et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7(3): 214–228. [DOI] [PubMed] [Google Scholar]
- 15. Kdour MR, Kidney JC, Smyth BM, et al. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009; 68(4): 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoogendoorn M, van Wetering CR, Schols AM, et al. Is interdisciplinary community-based COPD management (INTERCOM) cost-effective? Eur Respir J 2010; 35(1): 79–87. [DOI] [PubMed] [Google Scholar]
- 17. Disler RT, Inglis SC, Davidson PM. Non-pharmacological management interventions for COPD: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2013; (2): CD008427. [Google Scholar]
- 18. Mitchell KE, Johnson-Warrington V, Apps LD, et al. A self-management programme for COPD: a randomised controlled trial. Eur Respir J 2014; 44: 1538–1547. [DOI] [PubMed] [Google Scholar]
- 19. Fletcher CM, Elmes PC, Fairbairn AS, et al. Significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2(5147): 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apps LD, Mitchell KE, Harrison SL, et al. The development and pilot testing of the self-management programme of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD). Int J COPD 2013; 8: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooks R. EuroQol: the current state of play. Health Policy 1996; 37(1): 53–72. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Clinical Excellence. The social care guidance manual. London: NICE, 2013. [PubMed] [Google Scholar]
- 23. Dolan P, Gudex C, Kind P, et al. A social tariff for EuroQol: results from a UK general population survey (Discussion paper 138). York: Centre for Health Economics, University of York, 1995. [Google Scholar]
- 24. Curtis L. Unit costs of health and social care 2013: personal social services research unit. Kent: University of Kent, 2013. [Google Scholar]
- 25. Department of Health. Reference costs 2012. England:Department of Health, 2013. [Google Scholar]
- 26. British National Formulary. British National Formulary 63. UK: Pharmaceutical Press, 2012. [Google Scholar]
- 27. Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002; 11(5): 415–430. [DOI] [PubMed] [Google Scholar]
- 28. Glick HA, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. Oxford: Oxford University Press, 2007. [Google Scholar]
- 29. Earnshaw J, Lewis G. NICE guide to the methods of technology appraisal: pharmaceutical industry perspective. Pharmacoeconomics 2008; 26: 725–727. [DOI] [PubMed] [Google Scholar]
- 30. Effing T, Kerstjens H, van der Valk P, et al. Cost-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009; 64(11): 956–962. [DOI] [PubMed] [Google Scholar]


