Abstract
Early detection and treatment of lung disease in patients with rheumatoid arthritis (RA) may ameliorate disease progression. The objectives of this study were to investigate the frequency of asymptomatic lung abnormalities in early RA patients and the potential association of positive RA blood reactive biomolecules with lung involvement. A prospective observational study was performed in a cohort of patients with early RA (joint symptoms < 2 years) without respiratory symptoms, who were included in a screening program for lung disease with a baseline chest radiograph (CR) and complete pulmonary function tests (PFTs). In those patients with lung abnormalities on the CR or PFTs, a high-resolution chest computed tomography scan (HRCT) was performed. We included 40 patients (30 women). Altered PFTs were detected in 18 (45%) of these patients. These cases had a diffusion lung transfer capacity of carbon monoxide (DLCO) of <80% of predicted, without a significant reduction in the forced vital capacity. The HRCT detected abnormalities in 11 of the 18 patients. Diffuse bronchiectasis was the main finding. An inverse correlation between the anti-citrullinated peptide antibody (ACPA) levels and DLCO was found. Asymptomatic lung disease is present in up to 45% of early RA patients and can be determined by PFTs and ACPA levels.
Keywords: Arthritis, rheumatoid, autoantibodies, lung diseases, multidetector computed tomography, respiratory function tests
Introduction
Rheumatoid arthritis (RA) affects all the anatomic structures of the lung. Airway disease is common, with up to 30% incidence of bronchiectasis on high-resolution computerized tomography scan (HRCT) in some studies. However, clinically significant disease is much less frequent.1 Small airway disease that manifests as bronchiolitis can also be present. Interstitial lung disease (ILD) is the most common manifestation of rheumatoid lung disease and is associated with high morbidity and mortality. For example, in a population-based survey, the median survival was only 2.6 years after ILD diagnosis. ILD represented 13% of the overall mortality of RA.2 The risk of death is three times higher for RA patients with ILD than for those without.2
Clinically significant ILD related to RA (RA-ILD) is observed in 8%10% of RA patients.2–5However, depending on the diagnostic method used (radiography, pulmonary function test (PFT), HRCT, bronchoalveolar lavage, or autopsy) a much larger proportion of patients exhibit subclinical findings.2,6–10Although ILD is more common in RA patients who were older at the time of disease onset and in male patients, the natural history and pathogenic determinants of RA-ILD are not well defined. However, the relationship between the presence of ILD and the severity of the rheumatologic disease remains controversial.11 The only risk factor that has been identified and can therefore be prevented is smoking. Importantly, it has been suggested that while smoking, the HLA-DRB1 “shared epitope” and anti-citrullinated peptide antibodies (ACPAs) interact and increase the risk of RA-ILD.9,12 In support of this, smoking has been associated with higher levels of pulmonary peptidyl arginine deiminase-2 (PAD2), an enzyme that catalyzes the posttranslational modification of arginine to citrulline.13 Higher levels of citrullination were observed in the cells obtained by bronchoalveolar lavage from smokers than in those obtained from nonsmokers.14 In another study,15 citrullinated proteins were detected in the lung tissue of patients with both RA and idiopathic ILD. Despite this circumstantial evidence, it remains unclear whether citrullination of lung proteins represents a mechanistic link between respiratory exposure and autoimmunity in RA. An association between ACPA and ILD in RA is still unknown.
In most cases, the lung disease occurs in the first 5 years after RA onset. A diagnosis of lung disease is preceded by an asymptomatic period of variable duration, during which functional or radiographic alterations can already be observed.2 Early detection of such alterations could change the course of the lung disease by avoiding risk factors (such as smoking or environmental exposures), preventing infections, or choosing RA drugs that are less harmful for the lung. Nonetheless, the prevalence of asymptomatic preclinical lung disease in individuals with RA is unknown. The majority of studies on lung involvement in RA have included patients with long-standing RA. Only a few studies have included patients with early RA (duration of symptoms less than 2 years) and clinical pulmonary involvement.,16–19 To the best of our knowledge, none has focused on patients who do not present with respiratory symptoms. It is also not yet known whether the new intensive treatment strategies for RA result in a reduction of the prevalence of lung disease involvement (which seems to be related to RA severity), which as has been demonstrated with other extra-articular manifestations, such as vasculitis.
The objectives of the present study were to: (1) investigate the frequency of asymptomatic preclinical lung disease in early RA patients (duration of symptoms less than two years) to determine how early the lungs are affected in the disease and establish whether systematically screening asymptomatic patients for this complication is clinically advantageous and (2) study the potential association of ACPA positivity with RA-associated lung disease.
Materials and methods
Patient recruitment and selection criteria
A total of 40 patients with early diagnosis of RA between January 2011 and December 2012 were included in this prospective study. All the subjects attended an early arthritis outpatient clinic in the Rheumatology Department of the Hospital Universitari de Bellvitge, a 1000-bed tertiary care teaching institution in Barcelona, Spain. The inclusion criteria were as follows: (1) age >18 years; (2) diagnosis of RA according to the 1987 American College of Rheumatology classification criteria20; (3) duration of joint symptoms >6 weeks and ≤24 months; (4) previous treatment with nonsteroidal anti-inflammatory drugs, small doses or corticosteroids (≤7.5 mg/day of prednisone or equivalent) or disease-modifying anti-rheumatic drugs (i.e., methotrexate or leflunomide) for no more than 6 months; and (5) absence of respiratory symptoms (patients were asked about dyspnea, cough, or wheezing). Subjects were excluded if they had other collagen vascular disorders, chronic disorders involving the lung prior to the rheumatologic disease (cardiac or lung disease) diagnosis, or a history of exposure to fibrogenic substances.
In accordance with the guidelines of our institutional ethics committee, formal approval for this study was not required. Informed consent was obtained for every participating patient.
Clinical assessment
Baseline data were collected by the same rheumatologist and included age, sex, duration of joint symptoms, smoking habits, acute phase reactants at the time of the study (erythrocyte sedimentation rate and C-reactive protein (CRP)), serological status for rheumatoid factor and ACPA, swollen and tender joint count in 28 joints, DAS28-CRP score, Health Assessment Questionnaire (HAQ), presence of rheumatoid nodules, and evidence of erosion (as established by radiographs of hands, wrists, and feet). ACPA levels (immunoglobulin G) were measured using a commercially available second-generation enzyme-linked immunosorbent assay kit: EliATM CCP Assay on an ImmunoCAP250 instrument (Phadia, Germany).
For every participant, a baseline chest radiograph (CR) and PFTs, including spirometry and assessment of diffusing lung capacity for carbon monoxide (DLCO) were performed in accordance with the Spanish Society of Respiratory Medicine and Thoracic Surgery guidelines.21 In those patients with lung abnormalities in the CR, that is, any alteration in spirometry or impaired diffusion (defined as <80% of the predicted value for forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and DLCO, respectively), an HRCT of the chest was obtained to confirm the presence of lung disease and determine the radiographic pattern. HRCT features of ILD included ground glass opacities, septal lines, reticulation, honeycombing, traction bronchiectasis, and pulmonary nodules. Every radiologic test was reviewed by an expert lung disease radiologist.
Statistical analysis
Continuous data are reported as the mean ± standard deviation when a normal distribution was observed and as the median and range when the assumption of normality was not justified. Categorical variables are reported as percentages. Comparisons were made using the Student’s t-test for independent continuous variables or the Mann–Whitney U test when the assumption of normality was not justified. To analyze categorical data, we performed a χ2 test or Fisher’s exact test when the expected values were less than five.
Possible correlations between the DLCO values and demographic, clinical, laboratory, and treatment variables were explored. Correlations were demonstrated using a linear regression. Significance was tested using Pearson’s or Spearman’s test when the assumption of normality was not justified. A linear regression multivariate model was used to adjust the correlation between DLCO values and ACPA levels for tobacco exposure. Data were analyzed with the SPSS 5.0 statistical package. The values of p < 0.05 were considered statistically significant.
Results
The main characteristics of the study cohort are summarized in Table 1. The mean age of the 40 patients (30 women) at the time of the study was 47 ± 12 years (range: 19–74). The median disease duration was 12 months. Fifteen patients (41.7%) had exposure to tobacco smoking. None reported respiratory symptoms at the time of inclusion in the study.
Table 1.
Clinical characteristics of the study cohort.
| Clinical characteristics | |
|---|---|
| Age (years), median (range) | 47 (18–74) |
| Time from RA diagnosis (months), median (range) | 12 (1–23) |
| Gender, n (%) | |
| Male | 10 (25%) |
| Female | 30 (75%) |
| Treatment for RA, n (%) | |
| Leflunomide | 10 (25%) |
| Methotrexate | 27 (67.5%) |
| FVC (%), mean (SD) | 110(16.23) |
| DLCO(%), mean (SD) | 85.18 (20.29) |
| Smoking, n (%) | 15 (41.7%) |
RA: rheumatoid arthritis; DLCO: diffusion lung transfer capacity of carbon monoxide; FVC: forced vital capacity; SD: standard deviation.
PFT abnormalities
A total of 18 patients (45%) presented with alterations in the PFTs. All cases had a DLCO <80% of predicted, without any significant reduction in the FVC values (Figures 1 and 2). Only one patient presented with an FEV1 lower than 80% of the predicted value. The mean value (SD) of the DLCO in this subgroup of patients was 68% (9.74). There were no differences in the PFT results according to the age of the patients. Confounding factors, such as smoking history and methotrexate treatment, were analyzed. While lower DLCO values were found in previous or current smokers, no differences were observed between the patients with exposure to methotrexate and those without (Table 2).
Figure 1.

Distribution of DLCO values.
Figure 2.
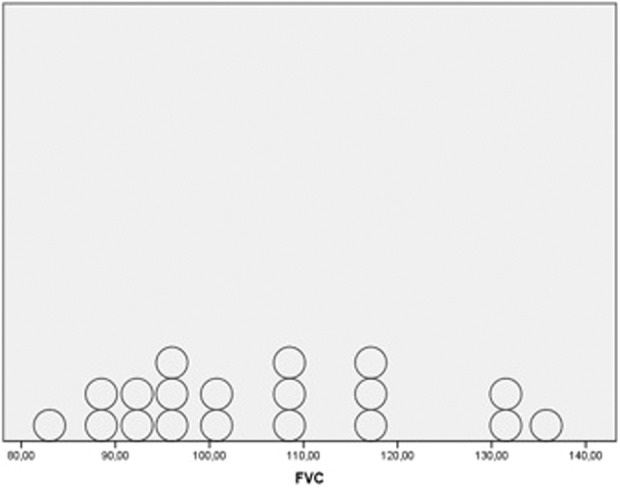
Distribution of FVC values.
Table 2.
ACPA and DLCO values according to tobacco and methotrexate exposures.
| Smokersa | Nonsmokers | Student’s t-test | p | |
|---|---|---|---|---|
| ACPA | 608.67 IU/mL (930.69 IU/mL) | 421.57 IU/mL (581.40 IU/mL) | −0.73 | 0.470 |
| DLCO | 76.35% (16.86%) | 91.7% (20.45%) | −15.34 (12.32) | 0.016 |
| MTX | No MTX | Student’s t-test | p | |
| DLCO | 86.66% (17.50%) | 82.71% (24.60%) | 3.95 (13.53) | 0.56 |
ACPA: anti-citrullinated peptide antibodies; DLCO: diffusion lung transfer capacity of carbon monoxide, MTX: methotrexate.
aResults are expressed as mean (standard deviation).
Imaging studies
Five patients presented with pulmonary findings in the CR defined as subpleural reticular infiltrates, alveolar bilateral infiltrates, unilateral pleural effusion, and alterations suggesting emphysema (in two patients). An HRCT was performed on 19 patients. Abnormalities were detected in 11 patients. Of these patients, one presented with nonspecific nodules and ground glass opacities with traction bronchiectasis that were suggestive of nonspecific interstitial pneumonia (NSIP) and another presented with a radiographic pattern of organizing pneumonia (OP) with ground glass opacities and focal alveolar consolidations. Other radiologic abnormalities found in other patients were emphysema, nonspecific pulmonary nodules, nonspecific air trapping or cylindric bronchiectasis, and meeting Fleischner criteria22(Table 3). In our series, bronchiectasis was the predominant radiologic finding, with 8 of 11 patients presenting diffuse cylindric bronchiectasis in the absence of fibrosis (Figure 3). In two of these cases, bronchiectasis was associated with radiologic findings consistent with ILD (NSIP and OP patterns). The patient with bilateral alveolar infiltrates on the CR presented with an OP pattern on the HRCT. One of the two patients with alterations suggesting emphysema on the CR presented with an NSIP pattern. The other patient presented with emphysema on the HRCT. The patient with pleural effusion on the CR also presented with diffuse bronchiectasis, emphysema, and nonspecific nodules on the HRCT.
Table 3.
HRCT findings.
| HRCT findings | |
|---|---|
| Patient 1 | Nonspecific air trapping |
| Patient 2 | Diffuse cylindric bronchiectasis |
| Patient 3 | Nonspecific air trapping |
| Patient 4 | Diffuse cylindric bronchiectasis |
| Patient 5 | Diffuse cylindric bronchiectasis |
| Patient 6 | Centrolobular emphysema |
| Patient 7 | Diffuse cylindric bronchiectasis and nonspecific air trapping |
| Patient 8 | Centrolobular emphysema in upper lobes, nonspecific nodules, ground glass opacities, traction cylindric bronchiectasis. Suggestive of NSIP |
| Patient 9 | Diffuse cylindric bronchiectasis, centrolobar emphysema, and nonspecific nodules |
| Patient 10 | Diffuse cylindric bronchiectasis |
| Patient 11 | Diffuse cylindric bronchiectasis, centrolobar emphysema, nonspecific nodules, gorund glass opacities, and focal alveolar consolidations. Suggestive of OP |
HRCT: high-resolution computed tomography; NSIP: nonspecific interstitial pneumonia; OP: organizing pneumonia.
Figure 3.
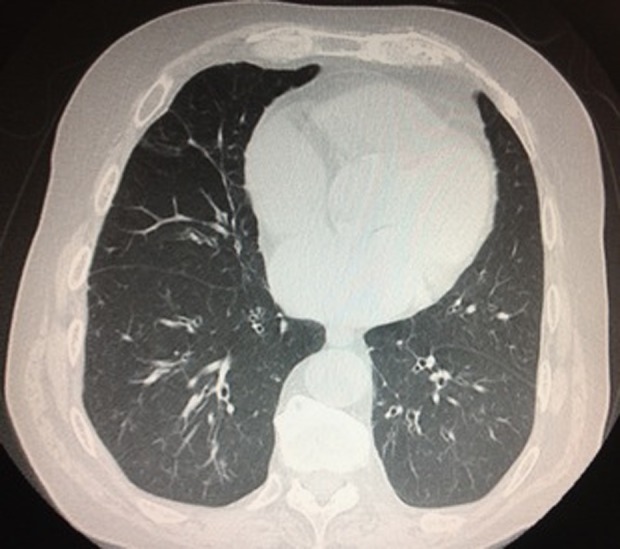
HRCT imaging in a patient with diffuse cylindric bronchiectasis in the absence of fibrosis.
In eight patients with abnormal DLCO values (<80%), the HRCT was normal. This alteration could not be attributed to the presence of anemia in any of these cases.
Correlations between baseline DLCO values and demographic, clinical, laboratory, and treatment variables
A significant inverse correlation between the ACPA levels and baseline DLCO was found in the entire cohort (−0.45, p = 0.004). This correlation was also found in the subgroup of patients with a low DLCO (−0.62, p = 0.008). This correlation was present even after adjusting for tobacco exposure (p = 0.1). No significant association was found between tobacco smoking and the ACPA serum levels. We also observed a significant association between the severity of disease activity as measured by DAS28-CRP and baseline DLCO values. The correlation coefficients are shown in Tables 2 and 4.
Table 4.
Correlation between DLCO values and clinical and laboratory parameters.
| Median values | Pearson correlation coefficients with DLCO (p value) | |
|---|---|---|
| ESR | 29.25 mm | −0.15 (0.370) |
| CRP | 16.46 mg/L | −0.31 (0.053) |
| ACPA | 542.77 IU/mL | −0.45 (0.004) |
| RF | 86.60 IU/mL | −0.01 (0.977) |
| DAS28-ESR | 5.73 | −0.29 (0.072) |
| DAS28-CRP | 5.23 | −0.42 (0.007) |
ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; ACPA: anti-citrullinated peptide antibody; RF: rheumatoid factor; DAS: disease activity score; DLCO: diffusion lung transfer capacity of carbon monoxide.
Discussion
The results of the present study show that the patients with recent onset RA and no respiratory symptoms frequently present with PFT abnormalities. Furthermore, the correlation between physiological lung abnormalities and rheumatoid activity parameters indicates a possible association between lung involvement and severity of disease activity. This suggests that some biological parameters could help to identify patients with a higher risk of lung involvement.
The majority of previous studies regarding RA-associated lung disease included patients with long-standing articular disease. Previous studies of early RA show a prevalence of physiological lung abnormalities similar to that presented here (33%–40%).16–19 This indicates that there is a subclinical period of lung involvement in RA disease and that when respiratory symptoms appear clinically, the pulmonary disease is already advanced. Therefore, including a pulmonary function assessment could be useful for all RA patients from the initial diagnosis, regardless their clinical respiratory symptoms.
Abnormalities in the HRCT were observed in 58% of the patients who underwent this diagnostic procedure, all of whom had a low DLCO. Contrary to other studies, an HRCT was not evaluated in patients with a normal DLCO, because we considered that this procedure was not justified in the absence of clinical manifestations and functional lung impairment. In our sample, the main finding was diffuse cylindric bronchiectasis, in contrast to other previously reported,16,17,19 in which the main findings were septal thickening, ground glass opacities, and air trapping. We should mention that there is no agreement on the HRCT findings between the different series reported, which could mean that in the early stage of rheumatologic disease, the radiological abnormalities are variable and nonspecific.
A recent meta-analysis23 showed that serum ACPA positivity is associated with a higher risk for RA-related pulmonary disease in patients with different time of evolution of RA. In early RA, Wilsher et al.17 found a correlation between lower values of DLCO and higher serum levels of ACPA. Our findings in the present study support this association. The mechanism of increased risk for associated lung disease in the presence of higher serum levels of ACPA remains unclear. Several theories involving smoking, the HLA-DRB1 shared epitope, and ACPA as mediators of pulmonary tissue damage have been proposed.9,12,24,25
In our study, lower values of DLCO and the unique case of decreased FEV1 were observed in patients with exposure to tobacco. The four patients with emphysema on HRCT were current or former smokers. No significant relationship was found between exposure to tobacco and ACPA levels. Nevertheless, the ACPA levels were higher in smokers. This trend could have reached statistical significance if the number of patients had been higher. The ACPA levels were higher in the patients with a low DLCO even after adjusting for tobacco exposure.
Habib et al.19 demonstrated a correlation between clinically significant lung impairment and the severity of articular disease as measured by DAS28-erythrocyte sedimentation rate. In other studies, no correlations were found between lung impairment and rheumatologic disease activity.17,26 To our knowledge, DAS28-CRP has not been tested in recent onset RA. In the present study, a significant correlation between the severity of the articular disease measured by DAS28-CRP and the levels of DLCO was observed, which leads us to think that the articular inflammatory process and lung disease of these patients could share common pathophysiological processes as previously reported.27
One limiting factor is the total number of patients included, although this number is similar to that in previous studies. Furthermore, the majority of patients were undergoing treatment with prednisone or methotrexate, drugs that could interfere in PFT results, although no differences in DLCO were found after adjusting for treatment with methotrexate. Smoking can be a confusing factor because exposure to tobacco smoking could trigger lung damage in predisposed individuals, such as RA patients. Despite these factors, the present results show that lung disease may be present from the beginning of the rheumatic process, without presenting respiratory symptoms, mainly in cases with higher ACPA serum levels. DLCO, a noninvasive procedure, is a good method for identifying this lung disease.
Identifying lung disease in the early stages of RA is important because it is the second most common cause of death in RA patients. The detection of subclinical lung function impairment could help lower the incidence of clinically significant lung disease in the future by implementing smoking advice, preventing respiratory infections or selecting those drugs for RA that are less harmful to the lung in these patients.
Despite the fact that more than one-third of the patients with recent onset RA have functional alterations compatible with lung disease, until the present study, the existing data did not show sufficient evidence to recommend lung disease screening using a PFT in this group of patients. Nevertheless, the prevalence of lung disease in these patients is remarkable, and its future significance is yet to be determined. These aspects should be the focus of future longitudinal studies that would help to elucidate the natural evolution of these alterations and provide a better understanding of the natural history of the disease.
Footnotes
Authors’ Note: Authors MMM and JNG share senior authorship.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Roche-Intermune, SEPAR-EPID futuro Scholarship for Research (grant number 6000€).
References
- 1. Remy-Jardin M, Remy J, Cortet B, et al. Lung changes in rheumatoid arthritis: CT findings. Radiology 1994; 193: 375–382. [DOI] [PubMed] [Google Scholar]
- 2. Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62(6): 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheum 2010; 49: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 4. Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheum 2007; 46: 350–357. [DOI] [PubMed] [Google Scholar]
- 5. Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Res Crit Care Med 2011; 183: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim E, Collard H, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease, the relevance of histopathologic and radiographic pattern. Chest 2009; 136: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amital A, Shitrit D, Adir Y. The lung in rheumatoid arthritis. Presse Med 2011; 40: e53–e70. [DOI] [PubMed] [Google Scholar]
- 8. Dawson JK, Fewins HE, Desmond J, et al. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 2001; 56: 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giles JT, Danoff SK, Sokolove J, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis 2014; 73(8): 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonagh J, Greaves M, Wright AR, et al. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Br J Rheumatol 1994; 33: 118–122. [DOI] [PubMed] [Google Scholar]
- 11. Kim SK, Park SH, Shin IH, et al. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol 2008; 35(6): 995–1001. [PubMed] [Google Scholar]
- 12. Ruiz-Esquide V, Gomara MJ, Peinado VI, et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? Clin Rheumatol 2012; 31: 1047–1050. [DOI] [PubMed] [Google Scholar]
- 13. Vossenaar ER, Zendman AJ, van Venrooij WJ, et al. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays 2003; 25: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 14. Makrygiannakis D, Hermansson M, Ulfgren AK, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Rheum 2008; 67: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 15. Bongartz T, Cantaert T, Atkins SR, et al. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheum 2007; 46: 70–75. [DOI] [PubMed] [Google Scholar]
- 16. Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Arch Intern Med 2008; 168: 159–166.18227362 [Google Scholar]
- 17. Wilsher M, Voight L, Milne D, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir Med 2012; 106: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 18. Mori S, Cho I, Koga Y, et al. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol 2008; 35(8): 1513–1521. [PubMed] [Google Scholar]
- 19. Habib HM, Eisa AA, Arafat WR, et al. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol 2011; 30(2): 217–221. [DOI] [PubMed] [Google Scholar]
- 20. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31(3): 315–324. [DOI] [PubMed] [Google Scholar]
- 21. Maestu LP. Manual SEPAR de Procedimientos 3: Evaluación de la función pulmonar. Spain: Luzán 5, S.A. de Ediciones, 2002. [Google Scholar]
- 22. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology 2008; 246(3): 697–722. [DOI] [PubMed] [Google Scholar]
- 23. Zhu J, Zhou Y, Chen X, et al. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol 2014; 41(7): 1282–1289. [DOI] [PubMed] [Google Scholar]
- 24. Shelef MA, Bennin DA, Mosher DF, et al. Citrullination of fibronectin modulates sinovial fibroblast behavior. Arthritis Res Ther 2012; 14: R240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willis VC, Demoruelle MK, Derber LA, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013; 65: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pappas DA, Giles JT, Connors G, et al. Respiratory symptoms and disease characteristics as predictors of pulmonary function abnormalities in patients with rheumatoid arthritis: an observational cohort study. Arthritis Res Ther 2010; 12: R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perry E, Kelly C, Eggleton P, et al. The lung in ACPA-positive rheumatoid arthritis: an initiating site of injury? Rheum 2014; 53: 1940–1950. [DOI] [PubMed] [Google Scholar]


