Abstract
To identify patients hospitalized for an acute exacerbation of chronic obstructive pulmonary disease (COPD) who have a poor prognosis and might benefit from proactive palliative care, a set of indicators had been developed from the literature. A patient is considered eligible for proactive palliative care when meeting ≥2 criteria of the proposed set of 11 indicators. In order to develop a doctor-friendly and patient-convenient tool, our primary objective was to examine whether these indicators are documented consistently in the medical records. Besides, percentage of patients with a poor prognosis and prognostic value were explored. We conducted a retrospective medical record review of 33 patients. Five indicators; non-invasive ventilation (NIV), comorbidity, body mass index (BMI), previous admissions for acute exacerbation COPD and age were always documented. Three indicators; hypoxaemia and/or hypercapnia, professional home care and actual forced expiratory volume1% (FEV1%) were documented in more than half of the records, whereas the clinical COPD questionnaire (CCQ), medical research council dyspnoea (MRC dyspnoea) and the surprise question were never registered. Besides, 78.8% of the patients met ≥2 criteria and there was a significant association between meeting ≥2 criteria and mortality within 1 year (one-sided Fisher’s exact test, p = 0.04). The set of indicators for proactive palliative care in patients with COPD appeared to be user-friendly and feasible.
Keywords: Chronic obstructive pulmonary disease, exacerbation, indicators, proactive palliative care, prognosis
Background
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease with a high prevalence1 and the third leading cause of death worldwide.2 The symptoms that occur in the end-stage of COPD are as severe as or even worse than in the final stage of lung cancer.3,4 However, patients with COPD are less likely than those with cancer to receive palliative care.5,6 This may be due to the fact that historically the initial focus of palliative care has been on oncology7 but also to the different disease trajectory.8 The unpredictable disease course of COPD can virtually paralyse lung specialists and prevent them from starting palliative care.9 But, since patients with advanced COPD experience similar palliative care needs as patients with advanced cancer,10 they probably also benefit from palliative care.
There is evidence that, for patients with cancer, provision of palliative care improved outcomes in the domain of pain and symptom control and reduced hospital admissions.11 Besides, early integration of palliative care improved quality of life, reduced depressive symptoms and even prolonged survival of patients with non-small-cell lung cancer.12,13 Recently, it has been demonstrated that early integration of palliative care improved breathlessness mastery for patients with diseases other than cancer, including COPD.14 Further research specifically aimed at COPD is needed.15 For this reason, a prospective study has been set-up to examine the identification of patients with COPD with poor prognosis and implementation of proactive palliative care.16
Identification of patients with COPD for proactive palliative care is a challenge. In stable COPD, population models of survival do exist but they are of limited value to predict survival for individual patients.17 Therefore, a patient-centred approach to palliative care has been proposed not based on prognosis but on palliative needs of the patient.18 Although good palliative care indicators tools have been developed, such as the supportive and palliative care indicators tool , they are not specifically intended for patients with COPD, do not use criteria for the indicators and/or do not use a clear moment to be applied.18,19 Since COPD has a gradual decline that is punctuated by acute severe exacerbations, any one of which may be fatal,8,20 a hospitalization for an acute exacerbation COPD (AECOPD) might be such a clear moment to identify a need for palliative care.21,22 Not only is the patient during hospitalization literally available to the lung specialist, the patient is probably also more willing to address palliative issues. Several studies focused on the identification of predictive factors associated with poor prognosis for patients hospitalized for an AECOPD.23 One of the conclusions in a recent review on this topic was that post-discharge mortality reflects the severity of COPD as well as concomitant specific comorbidities, while functional limitation and poor health-related quality of life influence the frequency of readmissions.23 In accordance with these findings and based on existing literature,23–27 we developed a concept set of indicators for lung specialists to identify patients hospitalized for an AECOPD who have a poor prognosis. Poor prognosis was defined as having a hospital readmission for an AECOPD within 8 weeks or mortality within 1 year. We hypothesized that meeting ≥2 criteria of this set of indicators could be reason to start proactive palliative care.28,29 Since palliative care needs increase during the course of COPD, the prediction of poor prognosis will be used to ensure not to miss out patients with COPD hospitalized for an acute exacerbation who are in need of proactive palliative care. The final tool therefore should have a high sensitivity (near 100) and a high as possible specificity.
The use of this set of indicators was examined in this retrospective pilot study to get an indication of its applicability in our prospective study. Since we want to develop a doctor-friendly and patient-convenient tool that is easy to implement, our primary objective was to examine whether these indicators are documented consistently in the medical records of patients hospitalized for an AECOPD. Secondary objectives were to get an indication of the percentage of patients hospitalized for an AECOPD that meet ≥2 criteria of the set of indicators and the prognostic value of meeting ≥2 criteria of the set of indicators regarding readmission within 8 weeks and/or mortality within 1 year.
Methods
Study design
A retrospective medical record review was conducted in October 2013.
Study population
All patients who were admitted to the Radboud University Medical Centre in Nijmegen, the Netherlands, with the clinical diagnosis of COPD in the period January 2012 until March 2012 were considered. This period was chosen to ensure 1 year follow-up data of each patient. A clinical diagnosis COPD was defined as chronic airway obstruction as determined by spirometry prior to hospitalization with a forced expiratory volume1/forced vital capacity (FEV1)/FVC ratio <70%. Next, the medical records of only those patients hospitalized for an AECOPD were selected. An AECOPD was defined as an acute worsening of the patient’s condition from the stable state, which is sustained and may warrant the patient to seek additional treatment.30 Excluded from the analysis were medical records of patients who were not hospitalized and patients who came to the pulmonary rehabilitation centre of the Radboud University Medical Centre for current pulmonary rehabilitation.
This study was approved by the Medical Ethics Committee (CMO) of the Radboud University Nijmegen Medical Centre (METC registration no. 2013/449). Informed consent was not obtained, since we only used data from an existing clinical database, which was anonymized and de-identified prior to analysis.
Data collection
Data from the medical records were obtained from the electronic patient record . We searched for the following variables: (1) demographic variables namely age, gender, marital status, condition of living and place of living; (2) COPD-related variables namely Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage and smoking history; (3) variables needed to explore the documentation of the set of indicators for proactive palliative care (see Table 1) and (4) date of first readmission for AECOPD and date of death if appropriate.
Table 1.
Variables needed to explore the documentation of the set of indicators.
| Indicator | Variable looked for in medical records | |
|---|---|---|
| 1. | Hypoxaemia at discharge. | PaO2 (kPa), oxygen administration, and O2 saturationat admission and discharge |
| Hypercapnia at discharge | PaCO2 (kPa) at admission and discharge | |
| 2. | NIV | Noted as NIV or NPPV |
| 3. | Professional home care | Existence of professional home care after admission |
| 4. | Surprise question | Surprise question answered with ‘yes’ or ‘no’ |
| 5. | Severe comorbidity | Existence of non-curable malignancy Existence of cor pulmonale Existence of proven CHF Existence of diabetes mellitus with neuropathy Renal failure, clearance (GFR: in ml/min) |
| 6. | CCQ | CCQ total, day version (0–6) |
| 7. | MRC dyspnoea | MRC dyspnoea (1–5) |
| 8. | FEV1 | FEV1% predicted |
| 9. | BMI | BMI (kg/m2), or length (m) and weight (kg) |
| Weight change | Weight loss in last 6 months (%) | |
| 10. | Previous hospital admissions | Previous hospital admissions AECOPD in last 2 years |
| 11. | Age | Birth date and admission date |
PaO2: partial pressure of oxygen in arterial blood; kPa: kilopascal; PaCO2: partial pressure of carbon dioxide in arterial blood; NIV: non-invasive ventilation; NPPV: non-invasive positive pressure ventilation; CHF: chronic heart failure; GFR: glomerular filtration rate; CCQ: clinical COPD questionnaire; MRC dyspnoea: medical research council dyspnoea questionnaire; FEV1: forced expiratory volume in 1 second; BMI: body mass index; AECOPD: acute exacerbation chronic obstructive pulmonary disease; O2: oxygen.
To explore the documentation of hypoxaemia and/or hypercapnia at discharge, two methods were used, a strict and a clinical method. In the strict method, only measurements documented at discharge were used, whereas in the clinical method also measurements documented at admission were considered. Hypoxaemia was defined as having oxygen administration, or partial pressure of oxygen <8 kPa (without oxygen administration) or oxygen saturation ≤90% (without oxygen administration).31 Hypercapnia was defined as having a partial pressure of carbon dioxide >6 kPa.
Statistical analysis
Derived variables were calculated to see whether ≥2 criteria of the set of indicators for proactive palliative care (see Table 2) were met. In order to decide if the criteria of the indicator hypoxaemia and/or hypercapnia at discharge were met, the documentation according to the strict method was used.
Table 2.
Set of indicators with criteria for proactive palliative care.
| A patient hospitalized for AECOPD is eligible for proactive palliative care when meeting ≥2 criteria of the following set of indicators: | |
|---|---|
| 1. | Hypoxaemia and/or hypercapnia at discharge |
| 2. | Treatment of the exacerbation with non-invasive ventilation (NIV) |
| 3. | Patient needs professional home care service for personal care after discharge |
| 4. | Negative answer to the surprise question: ‘Would I (as lung specialist) be surprised if this patient would have a subsequent readmission for AECOPD within 8 weeks and/or would die in the next year? |
| 5. | The diagnosis of a severe comorbidity such as: Non-curable malignancy or Cor pulmonale (proven or non proven) or Proven Chronic Heart Failure (CHF) or Diabetes mellitus with neuropathy or Renal failure, clearance < 40 (GFR: in ml/min) |
| 6. | CCQ total, day version ≥ 3 |
| 7. | MRC dyspnoea = 5 |
| 8. | FEV1 (measured before AECOPD) <30% of predicted |
| 9. | BMI < 21 or unplanned weight loss (>10% weight loss in last 6 months or > 5% in last month) |
| 10. | Previous hospital admissions for AECOPD (last 2 years > 2 and/or last year > 1) |
| 11. | Age > 70 years |
AECOPD: acute exacerbation chronic obstructive pulmonary disease; GFR = glomerular filtration rate; CCQ: clinical COPD questionnaire; MRC dyspnoea:medical research council dyspnoea questionnaire; FEV1 = forced expiratory volume in 1 second; BMI: body mass index.
The statistical program SPSS version 20 was used to analyse the data. Non-continuous variables were reported as frequencies. Normally distributed continuous variables were reported as mean + standard deviation (SD) and non-normally distributed continuous variables were reported as median (interquartile range). In order to get an indication of the prognostic power of meeting ≥2 criteria of the set of indicators, the differences between study groups in baseline characteristics and clinical outcomes were assessed and tested for statistical significance with the use of one-sided Fisher’s exact tests for categorical variables and independent sample t tests for continuous variables. Differences were considered significant with a p value of <0.05. If appropriate, the sensitivity and the specificity for death within 1 year and unexpected hospital admission within 8 weeks were explored.
Results
Study population
The medical records of 149 patients with the clinical diagnosis of COPD were considered. Excluded from the analysis were 116 medical records of patients who were not hospitalized or who were included in a clinical pulmonary rehabilitation program. Finally, the medical records of 33 patients hospitalized for an AECOPD were examined. The characteristics of the study population are presented in Table 3.
Table 3.
Characteristics of the study population.
| Study population (N = 33) | |
|---|---|
| Mean (+SD) | |
| Age | 72 (+10.4) |
| Duration of hospitalization | 11 (+7.6) |
| Pack years | 33.1 (+18.7) |
| N (%) | |
| Sex | |
| Male | 19 (58) |
| Female | 14 (42) |
| Marital status | |
| Married | 18 (55) |
| Unmarried | 3 (9) |
| Divorced | 3 (9) |
| Widow | 8 (24) |
| Unknown | 1 (3) |
| Condition of living | |
| Non single | 22 (67) |
| Single | 11 (33) |
| Place of living | |
| Home | 29 (88) |
| Residential home | 0 (0) |
| Nursing home | 4 (12) |
| GOLD stage | |
| I | 0 (0) |
| II | 11 (33) |
| III | 7 (21) |
| IV | 14 (43) |
| Unknown | 1 (3) |
| Smoking at admission | |
| Yes | 9 (27) |
| No | 24 (73) |
SD: standard deviation.
Documentation of the set of indicators
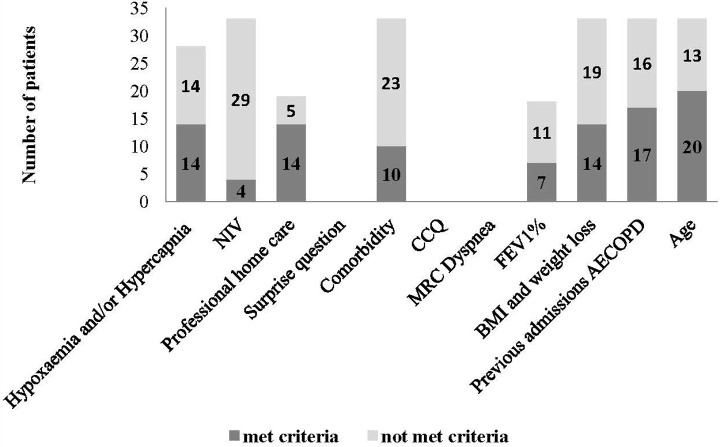
Of the 11 indicators for proactive palliative care, 5 were always documented in the medical records (see Figure 1). These were non-invasive ventilation (NIV), comorbidity, body mass index (BMI), previous admissions for AECOPD and age. According to the strict method, hypoxaemia, hypercapnia and hypoxaemia and/or hypercapnia at discharge were documented, respectively, in 85%, 36% and 85% of the cases. For the clinical method, these numbers were, respectively, 100%, 97% and 100%. Having professional home care and actual FEV1% were recorded half of the time (57.5% and 54.5%, respectively). The surprise question, clinical COPD questionnaire (CCQ) and medical research council (MRC) dyspnoea were never documented (0%).
Figure 1.
Documentation of each indicator in the medical records and the number of patients that met or did not met the criterion of each indicator for proactive palliative care.
Meeting ≥2 criteria of the set of indicators
Of the 33 patients hospitalized for AECOPD, 26 (78.8%) patients met ≥2 criteria of the set of indicators for proactive palliative care. The number of patients who met or did not meet the criterion of each documented indicator is presented in Figure 1.
Exploration of the prognostic value of the set of indicators
Deceased versus non-deceased
There was a significant association between meeting the criteria for proactive palliative care and whether or not the patient died (one-sided Fisher’s exact test, p = 0.04). All patients who died within 1 year after admission (n = 11) met the criteria of the set of indicators for proactive palliative care (see Table 4) meaning that the sensitivity of the set of indicators in predicting death within 1 year was 100%. From the 22 patients who survived, 7 patients did not meet the criteria of the set of indicators for proactive palliative care meaning that the specificity of the set of indicators in predicting death in 1 year was 31.8%. Deceased patients met significantly more indicators (M = 4.27, SE = 0.24) than patients who survived (M = 2.41, SE = 0.36), t(31) = −3.47, p = 0.002+, r = 0.53.
Table 4.
Contingency table showing the number of patients deceased within 1 year in patients who meet or do not meet criteria for palliative care.
| ≥2 indicators | <2 indicators | Total | |
|---|---|---|---|
| Deceased | 11 (42.3%) | 0 (0%) | 11 (33.3%) |
| Non deceased | 15 (57.7%) | 7 (100%) | 22 (66.7%) |
| Total | 26 (100%) | 7 (100%) | 33 (100%) |
Readmission versus no readmission within 8 weeks
There was no association between meeting criteria for palliative care and whether or not the patient had a readmission for an AECOPD within 8 weeks (one-sided Fisher’s exact test, p = 0.718). The contingency table is presented in Table 5.
Table 5.
Contingency table showing the number of patients with a readmission for an AECOPD within 8 weeks in patients who meet or do not meet criteria for palliative care.
| ≥2 indicators | <2 indicators | Total | |
|---|---|---|---|
| Readmission | 4 (15.4%) | 1 (14.3%) | 5 (15.2%) |
| No readmission | 22 (84.6%) | 6 (85.7%) | 28 (84.8%) |
| Total | 26 (100%) | 7 (100%) | 33 (100%) |
Discussion
This study explored the use of a set indicators to get an indication of its applicability in a prospective study. This set of indicators has been developed from the literature to identify those patients hospitalized for an AECOPD who have a poor prognosis and might benefit from proactive palliative care.
Documentation of indicators
Five of the eleven indicators were documented consistently in the medical records, three in more than half of the records and three not at all. The consistent documentation of age and BMI was as expected, since this is basic patient information written down every time a patient visits the hospital. Previous admissions for AECOPD, NIV and comorbidity were also always documented when appropriate. The presence or absence of hypoxaemia and/or hypercapnia at discharge was documented in 85% of the cases. At admission, the necessary measurements of arterial blood gas (ABG) were consistently documented. At discharge, these measurements were sometimes missing (15% for hypoxaemia and 64% for hypercapnia). It is recommended to have ABG documented before hospital discharge for all patients with an AECOPD complicated by respiratory failure.32 Taking into account this recommendation, the necessary ABG measurements of only one patient, who was admitted being hypercapnic, was not documented at discharge (3%). Hence, no ABG documented before hospital discharge in most cases meant that the patient was not hypoxaemic or hypercapnic at admission. Professional home care and actual FEV1% were documented half of the time. A possible explanation of these missing documentations is for professional home care that this is not documented if the patient is independent of such care and for actual FEV1% that if unknown at admission, it cannot be assessed in an unstable phase such as an AECOPD and therefore is not documented. An answer to the surprise question was never documented. Although widely known by palliative care specialists, no validation studies on the use of the surprise question to identify patients with COPD who can benefit from a palliative care approach have been published, which explains its absence in the medical record. Besides, up to now, the surprise question has mainly been used in research. Also, the CCQ and MRC dyspnoea were never documented. This confirms the finding that these disease-specific tools are mainly used in clinical trials or when selecting patients for treatment but less often in usual care.18
Most indicators are fairly consistently documented in the medical records. This suggests that lung specialists already use these variables in clinical practice. With respect to these variables, no extra measurements seem to be necessary to identify patients with COPD with poor prognosis. Only an answer to the surprise question, the CCQ and MRC dyspnoea are variables never documented. For our prospective study, this implies that these variables should be specifically asked for and measured. The surprise question, the CCQ and MRC dyspnoea are short, easy to use questionnaires. We decided to include them into the concept set of indicators since they provide valuable information about the view of the lung specialist, the severity of the dyspnoea and the health status of the patient. The measurement of health status (CCQ) is important since functional limitation and poor health-related quality of life are recognized to influence the frequency of readmissions.23 Beside anxiety, part of the mental state domain of the CCQ, is associated with mortality and may be a variable that influences early readmission rates.25 Whether the surprise question, the CCQ and MRC dyspnoea are also included into the final version of the set of indicators will depend on the outcome of the prospective study.
Meeting ≥2 criteria
In this pilot study, 78.8% of the patients hospitalized for an AECOPD met ≥2 criteria of the set of indicators. This suggests that 3 out of 4 patients hospitalized for an AECOPD could have a poor prognosis and may have an indication for proactive palliative care. Although it seems to be a high number, it is in line with the recent tendency of reducing demand for unscheduled hospital admissions by admitting the more physically ill patients with an AECOPD and avoiding admissions for psychosocial reasons by optimizing care at home.33
Prognostic exploration
Finally, we explored the prognostic value of meeting ≥2 criteria of the set of indicators for proactive palliative care. Meeting ≥2 criteria of the set of indicators was associated with risk of mortality within 1 year. The sensitivity of the set of indicators proved to be 100% in predicting mortality in this small sample. None of the patients who met less than two criteria of the set of indicators died within 1 year. The specificity of the set of indicators in predicting mortality proved to be 31.8%. So, meeting ≥2 criteria of the set of indicators did not always necessarily mean that the patient died within 1 year. However, in order to use this set of indicators in a prospective study to investigate the potential benefit of proactive palliative care, the risk of missing people in need of this care should be as minimal as possible. Of all the patients who were hospitalized for an AECOPD, 33.3% died within 1 year stressing the risk for this specific population. This number is in line with the risk of death presented in a recent review on predictors of mortality in hospitalized patients with an AECOPD.27 According to this review, the risk of death was 3.6% for short-term mortality (not more than 90 days after exacerbation) and 31.0% for long-term mortality (between 90 days and 2 years after exacerbation). Meeting ≥2 criteria of the set of indicators was not associated with a risk of readmission for an AECOPD within 8 weeks. There is a considerable variability in exacerbation susceptibility between patients with COPD.34 A history of COPD exacerbations showed to be the best predictor of future events independent of the severity of airflow limitation.35 It has been assumed that some patients with a distinct and stable COPD phenotype are at high risk of recurrent exacerbations.35 However, it seems that our set of indicators did not select this COPD phenotype and therefore was not able to make a distinction between patients with infrequent and frequent exacerbations. Nevertheless, in this small sample, the majority of patients with early readmission seem to be selected by our criteria.
Strengths and limitations
This is the first time that the use of a set of indicators for proactive palliative care in COPD was examined in order to be used in a prospective study. Still, some considerations, concerning the external validity and the statistical power, need to be addressed. This pilot study was performed in an academic hospital. Mortality rates have shown to be higher in academic hospitals than in general hospitals.36 We therefore expect that in general hospitals, the percentage of patients hospitalized for an AECOPD who have a poor prognosis will be less and as a consequence the percentage of those patients who die within 1 year will be less too. Furthermore, in this pilot study, a small sample size was used which may have resulted in less statistical power to detect an effect. However, this pilot study was performed to get an indication of the applicability in practice of a set of indicators in advance of a prospective study. The results of such a study will prove the actual characteristics and prognostic value of the set of indicators for proactive palliative care. Thereafter, it will be decided which indicators have to be included into the final tool.
Conclusions
In a prospective study, it seems feasible to use a set of indicators for proactive palliative care in patients with COPD. The three indicators that were not documented, being an answer to the surprise question, the CCQ and MRC dyspnoea, are tools mainly used for clinical trials and less in usual care. They will specifically be requested in the prospective trial. Three out of four patients hospitalized for an AECOPD had a poor prognosis according to our set of indicators. Besides, meeting ≥2 criteria of the set of indicators was associated with risk of mortality within 1 year. The sensitivity of the set of indicators in predicting mortality within 1 year was 100% and the specificity was 31.8%. For a prospective study, this suggests that the risk of missing patients with COPD in need of proactive palliative care is small. Which indicators will be included into the final tool will depend on the outcomes of the prospective study.
Acknowledgement
The authors would like to thank Renske Nielen for her valuable contribution to the acquisition of data.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was financially supported by a grant of the Netherlands Organization for Health Research and Development-ZonMw, The Hague. Project no.: 80-82100-98-080.
References
- 1. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741–750. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000; 55: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31: 58–69. [DOI] [PubMed] [Google Scholar]
- 5. Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc 2000; 48: S146–S153. [DOI] [PubMed] [Google Scholar]
- 6. Beernaert K, Cohen J, Deliens L, et al. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respir Med 2013; 107: 1731–1739. [DOI] [PubMed] [Google Scholar]
- 7. Addington-Hall JHK. Non-cancer patients as an underserved group In: Cohen JDL. (ed.) A public health perspective on end of life care. Oxford: Oxford University Press, 2012, pp. 151–159. [Google Scholar]
- 8. Murray SA, Kendall M, Boyd K, et al. Illness trajectories and palliative care. BMJ 2005; 330: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stewart S, McMurray JJ. Palliative care for heart failure. BMJ 2002; 325: 915–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bausewein C, Booth S, Gysels M, et al. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med 2010; 13: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 11. Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J 2010; 16: 423–435. [DOI] [PubMed] [Google Scholar]
- 12. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New Engl J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 13. Pirl WF, Greer JA, Traeger L, et al. Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol 2012; 30: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2: 979–987. [DOI] [PubMed] [Google Scholar]
- 15. Rocker GM, Sinuff T, Horton R, et al. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med 2007; 10: 783–797. [DOI] [PubMed] [Google Scholar]
- 16. Duenk RG, Heijdra Y, Verhagen SC, et al. PROLONG: a cluster controlled trial to examine identification of patients with COPD with poor prognosis and implementation of proactive palliative care. BMC Pulm Med 2014; 14: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coventry PA, Grande GE, Richards DA, et al. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: a systematic review. Age Ageing 2005; 34: 218–227. [DOI] [PubMed] [Google Scholar]
- 18. Boyd K, Murray SA. Recognising and managing key transitions in end of life care. BMJ 2010; 341: c4863. [DOI] [PubMed] [Google Scholar]
- 19. Highet G, Crawford D, Murray SA, et al. Development and evaluation of the supportive and palliative care indicators tool (SPICT): a mixed-methods study. BMJ Support palliat Care 2014; 4: 285–290. [DOI] [PubMed] [Google Scholar]
- 20. Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA 2003; 289: 2387–2392. [DOI] [PubMed] [Google Scholar]
- 21. Reinke LF, Engelberg RA, Shannon SE, et al. Transitions regarding palliative and end-of-life care in severe chronic obstructive pulmonary disease or advanced cancer: themes identified by patients, families, and clinicians. J Palliat Med 2008; 11: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel K, Janssen DJ, Curtis JR. Advance care planning in COPD. Respirology 2012; 17: 72–78. [DOI] [PubMed] [Google Scholar]
- 23. Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM 2010; 103: 817–829. [DOI] [PubMed] [Google Scholar]
- 24. Murray SA, Pinnock H, Sheikh A. Palliative care for people with COPD: we need to meet the challenge. Prim Care Respir J 2006; 15: 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 155–161. [DOI] [PubMed] [Google Scholar]
- 26. Kocks JW, Asijee GM, Tsiligianni IG, et al. Functional status measurement in COPD: a review of available methods and their feasibility in primary care. Prim Care Respir J 2011; 20: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013; 10: 81–89. [DOI] [PubMed] [Google Scholar]
- 28. Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J 2008; 32: 796–803. [DOI] [PubMed] [Google Scholar]
- 29. Carlucci A, Guerrieri A, Nava S. Palliative care in COPD patients: is it only an end-of-life issue? Eur Respir Rev 2012; 21: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003; 41: 46s–53s. [DOI] [PubMed] [Google Scholar]
- 31. Fraanje WLGP, Knobbe K, Van Putten AM, et al. Farmacotherapeutische richtlijn Geneesmiddelen en zuurstof in spoedeisende situaties. Huisarts Wet 2012; 50(5): 210–220. [Google Scholar]
- 32. National Institute for Health and Clinical Excellenc. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. London: National Clinical Guideline Centre – Acute and Chronic Conditions, 2010. [Google Scholar]
- 33. Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med 2011; 11: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 35. Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res 2013; 14: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heijink R, Koolman X, Pieter D, et al. Measuring and explaining mortality in Dutch hospitals; the hospital standardized mortality rate between 2003 and 2005. BMC Health Serv Res 2008; 8: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]