Abstract
Sarcopenia and frailty are geriatric syndromes characterized by multisystem decline, which are related to and reflected by markers of skeletal muscle dysfunction. In older people, sarcopenia and frailty have been used for risk stratification, to predict adverse outcomes and to prompt intervention aimed at preventing decline in those at greatest risk. In this review, we examine sarcopenia and frailty in the context of chronic respiratory disease, providing an overview of the common assessments tools and studies to date in the field. We contrast assessments of sarcopenia, which consider muscle mass and function, with assessments of frailty, which often additionally consider social, cognitive and psychological domains. Frailty is emerging as an important syndrome in respiratory disease, being strongly associated with poor outcome. We also unpick the relationship between sarcopenia, frailty and skeletal muscle dysfunction in chronic respiratory disease and reveal these as interlinked but distinct clinical phenotypes. Suggested areas for future work include the application of sarcopenia and frailty models to restrictive diseases and population-based samples, prospective prognostic assessments of sarcopenia and frailty in relation to common multidimensional indices, plus the investigation of exercise, nutritional and pharmacological strategies to prevent or treat sarcopenia and frailty in chronic respiratory disease.
Keywords: COPD, exercise, frail, respiratory disease, rehabilitation, sarcopenia
Introduction
Skeletal muscle dysfunction is a well-recognized manifestation of chronic respiratory disease.1,2 Among people with chronic obstructive pulmonary disease (COPD), for example, common changes in the muscular system include quadriceps weakness,3 atrophy4 and a fibre type shift,5 each of which offers prognostic information independent of lung function.6–8 One mechanism through which skeletal muscle dysfunction may contribute to poor outcome is by precipitating so-called ‘geriatric syndromes’ – age-related multifactorial health conditions9 – most notably sarcopenia and frailty.
Sarcopenia describes the loss of skeletal muscle and associated decline in physical function,10 a diagnosis of which under current international consensus requires a marker of low muscle mass and reduced muscular/physical performance.11 Frailty overlaps with sarcopenia, though describes a broader syndrome characterized by vulnerability and a heightened state of risk following minor stressor events.12 Skeletal muscle dysfunction is often considered within common diagnostic criteria for frailty, via muscle weakness and a positive weight loss history that is often the product of muscle wasting.12,13 As well as reflecting skeletal muscle dysfunction, both syndromes consider wider impacts of disease, from within and beyond the lungs, which influence morbidity and mortality.14 The presence of sarcopenia or frailty can therefore be considered a ‘vital sign’ and provides prognostic information further to that offered by markers of skeletal muscle dysfunction alone.
In older people, sarcopenia and frailty have proved to be useful tools for risk stratification, prognostication and to direct interventions aimed at preventing functional decline towards those carrying the greatest risk. Both are consistently associated with increased risk of incident disability, falls, hospitalization and mortality.12,15–20 Early intervention with exercise or nutrition can help reduce this risk, and both syndromes can be effectively managed, in some cases reversed, thus benefitting older people and their families plus reducing dependence on health and social care services. These syndromes have only recently been applied to groups with chronic respiratory disease. However, early findings have sparked interest in the field, particularly those relating to frailty that appears highly prevalent,16 a strong predictor of poor outcome,21 and provides important information for care planning, for example, in relation to lung transplant listing.19
In this review, we consider sarcopenia and frailty syndromes in the context of chronic respiratory disease. We provide an overview of the common approaches and assessment of these syndromes from gerontology, summarize studies examining sarcopenia and frailty in people with chronic respiratory disease and explore the relationships between these syndromes and markers of skeletal muscle weakness. Finally, we propose potential areas for future research.
Identification of literature
Studies were identified through electronic searches of Medline, EMBASE and CINAHL for articles published from January 1966 to May 2016, using key search terms based on ‘sarcopenia’ (muscle, sarco*, wasting), ‘frailty’ (frail*, geriatric) and ‘respiratory disease’ (COPD, fibrosis, lung disease, pulm* disease, respir*), modified according to the specific vocabulary of each database. Reference and citation lists of all identified articles were hand-searched, and authors in the topic area were contacted to identify additional studies. We limited the review to studies defining sarcopenia as a syndrome, in line with an international consensus definition, and excluded studies where sarcopenia was defined on the basis of low muscularity or low fat-free mass alone (see the study by Schols et al.22 for a recent review).
Sarcopenia and frailty as syndromes
Sarcopenia
Sarcopenia is a common condition with reported prevalence of 5–13% in those aged 60–70 years and as high as 50% for those aged 80 or above.23 In older people, sarcopenia has been associated with a number of adverse outcomes including physical disability, poor quality of life, dependency in activities of daily living (ADL) and excess mortality.24,25 The term is originally derived from the Greek words ‘sarx’ and ‘penia’ literally meaning ‘loss of flesh’, and classically sarcopenia has been defined as the ‘involuntary loss of muscle mass that occurs with advancing age’.26,27 However, multiple genetic, lifestyle and environmental factors (e.g. smoking, physical inactivity, poor diet) have been shown to contribute and hasten the development of sarcopenia, irrespective of age.28,29 With the exact aetiology of sarcopenia unknown, and knowledge of how these multiple factors interact lacking, a concrete definition of sarcopenia for use across clinical and research settings has been elusive.
More recently, there has been a move to understanding sarcopenia as a clinical ‘geriatric syndrome’ rather than simply as an age-related disease. A geriatric syndrome is a term used to describe common conditions, occurring as a result of impairments across multiple physiological systems, which ultimately lead to vulnerability, poor reserve and significant morbidity and mortality.9 Geriatric syndromes do not fit typical patterns of disease but are manifested by a number of frequently observed characteristics.9 Sarcopenia fulfils the definition of a geriatric syndrome on a number of counts. It is without a doubt a common and complex medical condition, with multiple causative factors, and the potential for huge personal and financial cost.23 Sarcopenia is also characterized by progressive and generalized loss of skeletal muscle mass and strength and crosses a number of diseases.24 To reflect this understanding, most consensus criteria require measurable markers of both low muscle mass and low muscle function (strength or performance) to be present for a sarcopenia diagnosis to be given.30 This view is supported by data demonstrating that loss of muscle mass does not always lead to further functional impairment4,31 and the relative lack of cut-points for weakness that relate to functional status.3
Frailty
Frailty is a broader syndrome than sarcopenia that encompasses physical, social, cognitive and psychological domains. Frailty also develops as a result of multisystem age-related decline, which results in a gradual reduction in physiological reserve and increased vulnerability to sudden changes in health status which can be triggered by minor stressor events, for example, a minor infection.12 The prevalence of frailty has been shown to increase non-linearly with adult age and is present in 10% of those over 65 years and a quarter of those older than 85 years.32 Frailty substantially increases the risk of falls, delirium, disability, institutionalization, and death.33,34 The prevalence of frailty is higher in women than men,35 but the relative mortality risk is lower in women than men.36
Agreeing an operational definition for frailty has also been controversial, and in the current International Classification of Diseases (ICD), frailty is listed simply as a condition of ‘age-related physical disability’ (ICD-10-R54). Like sarcopenia, frailty can be considered a clinical geriatric syndrome; it is common and complex, has multiple causative factors and spans multiple disease states. From a landmark study in older people, Fried et al. demonstrated that a combination of unintentional weight loss, exhaustion, weakened grip strength, slow walking and low physical activity was associated with a mortality rate of 43% at 7 years in those who were frail (defined as having at least three of these characteristics), compared to only 12% among those who were not frail.35 Shortly following this work, Rockwood et al. published on a clinical Frailty Index from the Canadian Study of Health and Aging, which quantified the presence or absence of 92 variables as a ratio.37 The index suggests that frailty is a result of the proportion of deficits or diseases accumulated with age and that this increasing deficit characterizes a person’s health status and determines their risk of future adverse events, including death.37,38 An index of 0.67 (62/92 variables) identified an amount of frailty beyond which further deficit accumulation was not sustainable and death was imminent.39 This model of frailty supports Fried’s concept of a reduced functional reserve but is more explicit in the view that once a critical number of deficits have been amassed, any further insult will result in an adverse event. Here, frailty can also be quantified, and the accumulated vulnerability measured, rather than dichotomized into the presence or absence of frailty as with the phenotypic models.
Contextualizing sarcopenia and frailty as syndromes has helped to develop practical ways to screen, identify and assess those at high risk of adverse outcomes. By assessing contributing factors, clinicians are also able to identify appropriate strategies to reduce risk in a personalized manner, aiming to prevent or delay the occurrence of disability, falls, dependency and even death.
Assessment of sarcopenia and frailty
Sarcopenia
Numerous national and international groups have reached consensus on the definition, assessment and diagnosis of sarcopenia. There is now widespread agreement that sarcopenia should be defined as a combination of low muscle mass and loss of function, indeed a new ICD code (ICD-10-M62.84) recognizes sarcopenia as a separately reportable condition to muscle wasting or weakness alone, and age-related physical disability. Definitions typically include a measure of physical performance related to muscle loss, most often either weak hand grip strength or a slow gait speed (Table 1).15, 51
Table 1.
Assessment domains of common sarcopenia and frailty instruments.
| Domains | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle mass | Physical performance/mobility | Muscle strength | Physical activity | Falls | Exhaustion/fatigue | Weight loss | General health | Physical symptoms | Functional independence | Cognition | Social support | Psychological symptoms/mood | Medication use | |
| Sarcopenia | ||||||||||||||
| Society for Sarcopenia, Cachexia and Wasting Disorders, sarcopenia with limited mobility15 | Appendicular lean mass index (>2 SD below mean) | 6MWT (<400 m) or gait speed (<1.0 m/second) | – | – | – | – | – | – | – | – | – | – | – | – |
| ESPEN40 | Muscle mass (>2 SD below mean) | 4MGS (<0.8 m/second) | – | – | – | – | – | – | – | – | – | – | – | – |
| EWGSOP30 | BIA skeletal muscle index (men <8.5 kg/m2; women <5.75 kg/m2) | 4MGS (<0.8 m/second) | Grip strength (men <30 kg, women <20 kg) | – | – | – | – | – | – | – | – | – | – | – |
| IWGSTask Force41 | Appendicular lean mass index (men <7.23 kg/m2; women <5.67 kg/m2) | 4MGS (<1.0 m/second) | – | – | – | – | – | – | – | – | – | – | – | |
| AWGS42 , 43 | DXA (men 7.0 kg/m2; women 5.4 kg/m2) or BIA (men 7.0 kg/m2; women 5.7 kg/m2) | 6MGS (<0.8 m/second) | Grip strength (men <26 kg; women <18 kg) | – | – | – | – | – | – | – | – | – | – | – |
| FNIH Sarcopenia Project44 | DXA appendicular lean mass/BMI (men 0.512; women 0.789) | 4/6 MGS (<0.8 m/second) | Grip strength (men <26 kg; women <16 kg) | – | – | – | – | – | – | – | – | – | – | – |
| SARC-F45 | – | Assistance walking across a room | Difficulty lifting and carrying 10 lb, difficulty climbing 10 stairs | – | Falls (past year) | – | – | – | – | Transfer from chair or bed | – | – | – | – |
| Frailty | ||||||||||||||
| Fried’s frailty phenotype35 | – | 4MGS | Grip strength | Physical activity level | – | Self- reported exhaustion | Weight loss (>5% in the last year) | – | – | – | – | – | – | – |
| FRAIL Scale46 | – | Ability to walk several hundred yards | Ability to climb a flight of stairs | – | – | Tiredness | Weight loss (>5% in the last 6 months) | Illnesses (>5) | – | – | – | – | – | – |
| CSHA Clinical Frailty Scale38 | – | Mobility | – | Physical activity level | – | Tiredness | – | – | Level of active symptoms | Help with ADLs | Dementia | – | – | – |
| Frailty Index47 (defined according to 70 deficits38) | – | Mobility, gait pattern | – | – | Falls | Tiredness | – | Illnesses | – | Help with ADLs | Memory, cognition | Social support | Mood | – |
| E-Frailty Index48 (defined according to 36 deficits) | – | Mobility, transfers | – | Activity limitation | Falls | – | Weight loss, anorexia | Presence of chronic conditions | Symptoms including dyspnoea, dizziness | Care requirement | Memory, cognition | Social support, house- bound | – | >5 drugs prescribed |
| Tilburg Frailty Indicator49 | – | Walking | Strength in hands | Physical activity level | – | Tiredness (physical) | Weight loss | Physical health | Balance, vision and hearing | Cognition | Social support, relations, living status | Depression, anxiety, coping | ||
| Edmonton Scale50 | – | Timed up and go test | – | – | – | – | Weight loss (yes/no) | General health | – | Help with ADLs, continence | Draw clock-face task | Social support | Sadness, depression | >5 drugs prescribed |
6MWT: 6-minute walk time; ESPEN: European Society for Clinical Nutrition and Metabolism; SD: standard deviation; 4MGS: 4-m gait speed; EWGSOP: European Working Group on Sarcopenia in Older Persons; DXA: dual X-ray absorptiometry; BIA: bioelectrical impedance analysis; IWGS: International Working Group Sarcopenia; ADLs: activities of daily living; AWGS: Asian Working Group for Sarcopenia; FNIH: Foundation for the National Institutes of Health.
Consensus on measurement standards or diagnostic cut-point is still lacking. Regarding assessment of muscle mass, different groups incorporate dual X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA) and/or computational tomography assessment into their diagnostic schemes (Table 1). The ease with which these measures can be applied is variable. While DXA may offer a more accurate assessment of muscle mass than BIA,52 a disadvantage is that DXA is not widely available in clinical practice, particularly within settings where sarcopenia may be particularly relevant (nursing homes or critical care). To highlight this issue, the Foundation for the National Institutes of Health (FNIH) Sarcopenia Group required measures derived from DXA and in doing so had to exclude more than half of their validation data set in whom measures were unavailable.53 , 54 In contrast, the European Working Group on Sarcopenia in Older Persons (EWGSOP) criteria are more pragmatic and accept the use of BIA, a practical measure routinely used in our day-to-day practice,55 but this may overestimate muscle mass, particularly in overweight or obese patients, resulting in a ‘hidden’ population with undiagnosed sarcopenic obesity.
The assessment of physical function commonly includes an objective measure of hand grip strength and/or gait speed, both of which have strong psychometric properties assuming there is sufficient operator training and standard testing procedures to reduce measurement error.56–58 Despite consistency in the type of assessment required, important variation exists in the cut-points used. For example, cut-points for grip strength in women range from 16 kg to 20 kg and gait speed cut-points range from 0.8 m/second to 1.0 m/second across the different tools (Table 1). As a result, prevalence estimates for sarcopenia vary considerably, though where reported EWGSOP and FNIH criteria tend to share the highest levels of agreement.44,59,60
An alternative approach to sarcopenia assessment is seen in the SARC-F, a short questionnaire designed for clinical screening. It considers falls, stair climb and lifting/carrying as functional deficits related to muscle dysfunction but does not consider markers of muscle mass. The SARC-F has been validated against three consensus definitions of sarcopenia from Europe, United States and Asia (European Working Group for Sarcopenia in Older People (EWGSOP), International Working Group for Sarcopenia (IWGS), Asian Working group for Sarcopenia; see Table 1) to predict 4-year physical limitation, walking speed and chair stand45 and could be used to identify patients in whom a more comprehensive assessment is warranted. The assessment of functional deficit in this and other sarcopenia tools underscores the overlap between sarcopenia and frailty. Gait speed and grip strength are utilized in instruments across both syndromes, especially those focusing on physical manifestations of frailty (Table 1).
Frailty
As outlined earlier, two predominant models of frailty have emerged: the phenotype model35 and the cumulative deficit model.38 The phenotype model developed by Fried et al.35 focuses on physical frailty as being distinct from disability and comorbidity. Fried’s model offers an objective measure that categorizes people into three categories: frail, pre-frail and robust. An alternative, but not conflicting, perspective is that frailty is the accumulation of physiological deficits across multiple organ systems.61 Rockwood et al.’s Frailty Index typifies this approach by assessing frailty based on the number of deficits observed, each given equal weighting. There is flexibility in how an index is derived, as long as there are over 40 variables that fulfil specified criteria.61 This approach to frailty assessment is more inclusive than the phenotype model as it considers multiple deficits across physical-, cognitive- and illness-related domains that are assessed through a comprehensive assessment. In contrast to the phenotype model, disability and comorbidity are here seen as integral components of frailty, which some view as a criticism since it is contended that frailty precedes disability.38 Other common instruments such as the Edmonton Frail Scale50 take an even broader view of frailty and include social support within an assessment (Table 1). Sternberg et al. examined the most common domains within frailty instruments and identified the top three as being physical function, mobility and cognition.62
A recent systematic review found a total of 67 different frailty instruments, nine of which were had accumulated over 200 citations.63 Fried’s phenotype was the most widely used and cited, followed by the Frailty Index from Rockwood et al.38 Other common instruments include the Clinical Frailty Scale and the FRAIL scale, the use of which has increased dramatically in the last decade.63,46 Frailty instruments vary widely in terms of the domains assessed, whether objective tests are included, and data sources. For instance, the FRAIL scale uses five self-report questions, whereas the Edmonton Scale50 requires a drug review, tests of cognitive and physical function, plus assessments of ADL dependence, mood and general health. Frailty may be assessed in clinical practice or in research to inform policy.63 Each instrument has its advantages and disadvantages,64 and the choice of an instrument should reflect the context and overall purpose of assessment. In clinical practice, frailty assessment may guide decision-making around an approach to care, decision to undertake an investigation or procedure or signposting to other services. A nurse may consider the FRAIL scale to screen for frailty due to its ease and simplicity, or turn to the more holistic Edmonton Scale, which although more time consuming to complete may help them understand what is causing someone’s frailty to direct input from other services. In research, frailty instruments have mostly been used to predict adverse outcome,63 but their role to determine eligibility for a study or as a target for intervention should not be overlooked. In the case of a physical exercise intervention, Fried’s model is well suited given its focus on physical frailty,20 whereas for more integrated approaches, a global instrument from frailty may be more appropriate.
Sarcopenia and frailty in chronic respiratory disease
There are limited studies examining sarcopenia and frailty in chronic respiratory disease to date and a reliance on the stable disease state, which is important to recognize given that exacerbations and/or hospitalization will hasten deconditioning and likely increase sarcopenia and frailty states.1,2 Only one study has focused on sarcopenia,65 which found a 15% prevalence in people living with stable COPD (Table 2). Of those studies examining frailty prevalence, the overall interpretation is that frailty is increased in the presence of chronic respiratory disease. Only a single retrospective study suggested frailty is not increased in respiratory disease and this concerned patients with very mild disease (mean (standard deviation (SD)) Forced expiratory volume in one second (FEV1) 79.6 (25.2)% predicted).17 Prevalence estimates vary considerably across the studies, ranging from 5% to 65% for frailty and 22% to 64% for pre-frailty (Table 2). This variation is likely due to differences in both the criteria used and populations or settings studied. Frailty prevalence has been associated with a number of factors including physical inactivity, impairment due to breathlessness, poor respiratory function and increasing comorbidity burden.17,20,21 When assessed cross-sectionally, the combination of frailty and these factors has led to poorest outcomes, with evidence of a cumulative adverse effect.17,69
Table 2.
Studies of sarcopenia and frailty syndromes in chronic respiratory disease.
| Reference | Design | Sample | Key measures | Main findings | Implications |
|---|---|---|---|---|---|
| Sarcopenia | |||||
| Jones et al.65 | Cross-sectional case–control | 622 with COPD, 43 sarcopenic patients and 43 propensity score-matched controls pre–post rehabilitation | EWGSOP criteria ISWT QMVC CAT Physical activity by questionnaire and accelerometry |
Overall prevalence of sarcopenia 15%. Prevalence associated with age, breathlessness and disease severity. Sarcopenic patients had reduced exercise capacity, functional performance, physical activity and health status (p < 0.001). Outcomes to pulmonary rehabilitation were similar across patients with sarcopenia and a propensity-matched control group. Following rehabilitation, 12/43 (28%) sarcopenic patients no longer met EWGSOP criteria. |
Sarcopenia is a distinct phenotype from generalized muscle wasting or physical function alone and is associated with a worse functional and health status. Response to pulmonary rehabilitation is not impaired by sarcopenia and can lead to a reversal of the syndrome in some patients. |
| Frailty | |||||
| Akgün et al.66 | Prospective cohort | 7144 (3538 HIV positive) of whom 154 HIV positive with COPD and 182 HIV negative without COPD | 4-item adapted of Fried’s Frailty Phenotype Physical Limitation Scale |
Prevalence of frailty in patients with COPD 59% and 58% in those with HIV positive and negative status, respectively. COPD was associated with increased odds of being frail (p < 0.01) and with physical limitation (p < 0.001). COPD associated with fivefold greater odds of frailty in HIV-positive group and 3.5-fold greater odds in those with HIV-negative status. |
Optimizing COPD management may be important to minimize frailty and maintain physical function for individuals aging with HIV. |
| Valenza et al.67 | Cross-sectional | 212 with COPD (104 stable, 108 acute during exacerbation) and 100 without COPD | Fried Frailty Phenotype Physical activity questionnaire Barthel Index Charlson Index | Prevalence of frailty 63% in acute COPD and 65% in stable COPD. Cut-points to detect frailty using Baecke questionnaire 3.54 and 3.88 for acute and stable COPD. |
Measuring physical activity can help to predict the presence of frailty in acute and stable COPD. Interventions aimed at increasing physical activity may reduce or delay frailty. |
| Park et al.21 | Cross-sectional | 98 chronic bronchitis, 70 COPD, 43 chronic bronchitis and COPD | 9-item criteria of frailty (based on Tilburg Frailty Indicator) Physical activity by accelerometry Basic and instrumental ADLs | Prevalence of frailty 58% and of pre-frailty 22%. Self-reported breathlessness was the strongest predictor for frailty (odds ratio 3.98; 95% CI: 1.79–8.88; p < 0.05). Frail patients had a greater number of disabilities and poorer outcomes including difficulties in ADLs. |
Highlights the importance of recognizing Frailty is highly prevalent in COPD and may have implications for care. Knowledge of frailty determinants can help healthcare providers identify pre-frail patients and provide preventative interventions to delay frailty onset. |
| Mittal et al.16 | Prospective cohort | 120 chronic pulmonary disease (67 COPD) | Fried Frailty Phenotype Physical activity questionnaire 100-foot walk test | Prevalence of frailty 18% and pre-frailty 64%. Frailty was associated with increased number of falls (p = 0.018) and hospitalizations (p = 0.011) in the past year. Gait speed correlated with frailty status (r2 = 0.36, p < 0.001) and decreased as frailty increased (p = 0.001). |
Frailty could help predict falls and frail patients may benefit from a comprehensive geriatric assessment. Gait speed may help screen for frailty in chronic respiratory disease, but is only a single component of frailty. |
| Galizia et al.68 | Cross-sectional with mortality follow-up | 489 with COPD and 799 without COPD | Frailty Staging System Basic ADLs Charlson Index | Prevalence of frailty 49%. With increasing frailty stage, mortality at follow-up (12 years) increased from 54% to 97% in patients with COPD (p < 0.001). |
Clinical frailty stage offers prognostic information on long-term mortality risk in people with COPD. |
| Lahousse et al.17 | Prospective cohort | 402 with COPD and 1740 without COPD | Fried Frailty Phenotype Spirometry Exacerbation history | Prevalence of frailty 5% and pre-frailty 45% Those with COPD more than twice as likely to be frail (odds ratio 2.2; 95% CI: 1.34–3.54; p = 0.002). Prevalence of frailty in COPD associated with breathlessness, airflow limitation and frequent exacerbations. Frailty was an important determinant of mortality in COPD (hazard ratio 4.03; 95% CI: 1.22–13.30, p = 0.022) along with lung function and comorbidity count. |
Increased prevalence of frailty with COPD related to breathlessness and exacerbation frequency. For patients with COPD, frailty appears to offer prognostic information on mortality risk. |
| Vaz Fragoso et al.69 | Prospective cohort | 3578 older persons (262 with COPD) | Fried Frailty Phenotype Spirometry 15-foot walk test | Prevalence of frailty in patients with COPD 10% and pre-frailty 54%. Frailty associated with increased airflow limitation (odds ratio 1.88; 95% CI: 1.15–3.09), and restrictive lung function (odds ratio 3.05; 95% CI: 1.91–4.88). In those without respiratory impairment at baseline, frailty was associated with increased odds of developing respiratory impairment at 4 years (odds ratio 1.42; 95% CI: 1.11–1.82). In those not frail at baseline, those with respiratory impairment had increased odds of developing frailty at 3 years (odds ratio 1.58; 95% CI: 1.17–2.13). Greater mortality in those with frailty and respiratory impairment (2.5-fold increase in those with both compared to neither) during follow-up. |
Frailty and respiratory impairment increase mortality risk, especially when both are present. There may be a bidirectional relationship between frailty and respiratory impairment which could be important for therapy. Strategies targeting frailty- or respiratory-related impairment may extend to both conditions. |
| Singer et al.19 | Prospective cohort | 395 lung transplant candidates | Fried Frailty Phenotype SPPB 6MWD Blood Biomarkers (IL-6, TNFR1, IGF-1, leptin) |
Prevalence of frailty based on Fried Frailty Phenotype 28%. Prevalence was not associated with low skeletal muscle index. Frailty was associated with higher levels of plasma IL-6 and TNFR1, and lower levels of IGF-1 and leptin. Frailty was associated with greater disability and twice the incidence of delisting or death before transplantation (27% vs. 13%, p = 0.077). |
Frailty assessment could provide important morbidity and mortality risk information. Frailty assessment could be used to identify lung transplant candidates at increased risk of post-transplant disability or poorer outcomes. |
| Mittal et al.70 | Prospective cohort | 30 chronic pulmonary disease (23 COPD) | Fried Frailty Phenotype Physical activity questionnaire 100-foot walk test |
Prevalence of frailty 17% and pre-frailty 61%. Patients with frailty had frequent falls and hospitalizations within the last year. Following pulmonary rehabilitation, gait speed was improved (mean 0.88 to 1.02 m/second, p < 0.001) and 3/5 (60%) previously frail patients were no longer met case criteria for frailty. |
Pulmonary rehabilitation may improve frailty specifically through an effect on gait speed in some patients, but this effect is not consistent. |
| Maddocks et al.20 | Prospective cohort | 816 COPD | Fried Frailty Phenotype MRC dyspnoea score Physical activity questionnaire ISWT HADS CRQ CAT |
Prevalence of frailty 26% and pre-frailty 64%. Prevalence of frailty increased with age, GOLD stage, MRC score and comorbidity burden (p < 0.001) Frailty associated with over twice the odds of pulmonary rehabilitation non-completion (odds ratio 2.20; 95% CI: 1.39–3.46; p = 0.001). Patients who were frail had better treatment outcomes following rehabilitation, including better responses in MRC score, exercise capacity, physical activity and health status (p < 0.001). 71/115 (62%) previously frail patients no longer met case criteria for frailty following pulmonary rehabilitation. |
Frailty is an independent predictor for pulmonary rehabilitation non-completion. This highlights the importance of understanding frailty in the management of COPD and should prompt exploration of new ways to support frail patients through rehabilitation. |
ADLs: activities of daily living; CAT: COPD assessment test; COPD: chronic obstructive pulmonary disease; CRQ: chronic respiratory questionnaire; EWGSOP: European Working Group on Sarcopenia in Older People; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HADS: Hospital Anxiety and Depression Scale; IGF-1: insulin-like growth factor 1; IL-6: interleukin 6; ISWT: incremental shuttle walk test; QMVC: quadriceps maximum voluntary contraction; SPPB: short physical performance battery; TNFR1: tumour necrosis factor receptor 1; 6MWD: 6-minute walk distance.
Consistent with the literature in older people, studies demonstrate that frailty is associated with poor outcomes in chronic respiratory disease including increased falls, hospitalizations and greater levels of disability.16,19,65,70 Prospective studies also support frailty as a predictor of mortality; often being frail at least doubles the risk of mortality, which has obvious implications for effective disease management17 , 68 , 69 (Table 2). There are also examples of frailty adversely affecting patients’ odds of receiving disease modifying surgical19 and non-pharmacological20 treatments, which should equally be considered an important adverse outcome.
Pulmonary rehabilitation has been shown to improve outcomes in both sarcopenic and frail patients. Improvements in symptom burden, physical function and overall health status have been demonstrated following a rehabilitation programme, and in some patients, this led to a reversal and declassification of their sarcopenia and frailty status.20,65,70 The change in status partly reflects the working of phenotype models, as patients falling close to one or more cut-points only require a small improvement for their status to be changed. Nonetheless, there is significant overlap between key characteristics of sarcopenia and frailty and common targets of rehabilitation, for example, muscular strength, physical activity and vitality. The presence of sarcopenia does not appear to restrict patients from participating in pulmonary rehabilitation,65 but the impairment associated with frailty does seem to hinder completion of a programme. Of those referred for rehabilitation in one study, being frail doubled a patient’s odds of programme non-completion.20 Although limited to one study, there is some evidence to suggest that the relationship between frailty and chronic respiratory disease could be bidirectional. Vaz Fragoso et al. observed that frailty was associated with increased odds of developing respiratory impairment, and conversely respiratory impairment was associated with increased odds of developing frailty.69 This finding needs to be confirmed and perhaps extended to exacerbations of disease where respiratory impairment can persist71 but could have important implications as strategies targeting one condition may be extend to both.
Another interesting aspect linking frailty and chronic respiratory disease warranting further study is the role of inflammatory biomarkers.19 It is possible that cachectic COPD patients with persistent inflammation could be at particular risk for the development of frailty, and it is therefore important to better understand this potentially treatable biological mechanism.
Relationships between skeletal muscle weakness, sarcopenia and frailty
Two cohort studies arising from the Harefield Hospital Pulmonary Rehabilitation service20,65 provide data to examine the relationships between skeletal muscle weakness, sarcopenia and frailty in more detail (see Table 2). Of 90 participants with COPD who were sarcopenic by EWGSOP criteria, 89% had hand grip weakness, 54% a slow gait speed and 48% both markers of reduced physical performance. An additional 27 participants from this study (4% of the overall sample) had low skeletal muscle index without either marker of reduced physical performance. In this subgroup, there was also no evidence of reduced global function or exercise capacity. This supports the contemporary view of sarcopenia requiring a degree of functional muscular impairment in that adding low physical performance to a sarcopenia diagnosis appears to further differentiate those with and without the syndrome. In a related but larger cohort, 209 participants were found to be frail by Fried’s phenotype criteria. Among this frail group, the majority of patients demonstrated hand grip weakness (80%) and had a slow gait speed (72%).20 These findings endorse the view that muscle dysfunction is an important contributor to sarcopenia and frailty in chronic respiratory disease.
Another way to explore muscle dysfunction in relation to sarcopenia and frailty is to observe upper and lower limb muscle strength according to the presence of these syndromes. Mean (SD) hand grip strength values of 21.5 (7.5) kg and 21.3 (8.2) kg were found among sarcopenic and frail patients from the two studies, respectively, compared with values of 27.6 (10.0) and 33.0 (8.9) kg among other study participants. While these values are in part a product of the diagnosis for sarcopenia or frailty, values for the lower limb (which are note considered in a diagnosis) revealed a similar pattern. Quadriceps maximum voluntary contraction values of 19.8 (7.6) kg and 21.0 (9.0) kg were found among sarcopenic and frail patients, respectively, compared with 27.1 (10.2) kg and 31.0 (10.1) kg among those not sarcopenic or not frail in the two studies. The between-groups differences of about 8–10 kg are likely to be clinically significant but this needs to be confirmed. The ratios of upper to lower limb strength are also noteworthy, with mean hand grip values exceeding those for the quadriceps, which reflect the propensity of muscle dysfunction in COPD towards the lower limbs.1,2
The relationships between sarcopenia, frailty and quadriceps weakness, defined according to healthy predicted values,3 could be further explored in 707 participants with full measurements. A complex interplay exists between quadriceps weakness, sarcopenia and frailty, which appear as overlapping but distinct clinical phenotypes (Figure 1). With the caveat that each phenotype depends on cut-points used (derived from observational studies), quadriceps weakness was the most common phenotype, observed in 57% of patients, followed by frailty, observed in 23%, and sarcopenia, observed in 12%. About two-thirds (64%) of those patients with quadriceps weakness did not exhibit concurrent sarcopenia or frailty, whereas only a minority of patients with frailty (16%) had neither quadriceps weakness nor sarcopenia. Just 3% of patients had all three phenotypes and we hypothesize this group carry the highest risk of adverse outcome (Figure 1).
Figure 1.
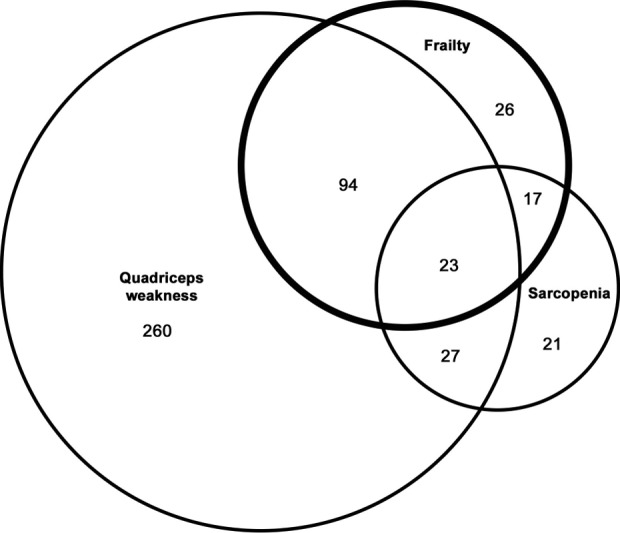
Relationships between frailty, sarcopenia and quadriceps weakness in patients with COPD derived from the study by Maddocks et al.20 Numbers represent patients with each phenotype (n = 707).
Future directions and opportunities
Having reviewed current evidence around sarcopenia and frailty in chronic respiratory disease, future work may include applying models to groups not represented in studies to date, for example, restrictive diseases; comparing the prognostic utility of sarcopenia and frailty models against each other and multidimensional indices; optimizing exercise-based treatments to manage these syndromes; and exploring additional strategies focused on nutrition, lifestyle factors and pharmaceuticals.
The evidence to date is biased towards studies of frailty rather than sarcopenia, phenotypic rather than cumulative deficit models of frailty, COPD rather than other chronic lung diseases and stable rather than acute settings. Applying sarcopenia and frailty models across the range of diseases and settings will be necessary to fully understand these syndromes and their value to the field. Recent studies have assessed constructs closely related to sarcopenia and frailty in the acute setting, for example, localized muscle wasting72 and gait speed,18 and provided a strong basis on which validated models of sarcopenia and frailty are examined. Studies investigating the prognostic utility of sarcopenia and frailty have generally been retrospective and used modified frailty criteria that deviated from validated instruments. Again, new prospective validations based on this work should be undertaken to confirm these initial findings, adhering to the original instruments, and capturing outcomes using robust collection methods. Further, as studies have made use of existing data sets, the adverse outcomes collected are often limited to mortality alone, and the full range of outcomes common to geriatric syndromes has not been exploited. As well as tracking mortality, studies should, where possible, assess incident falls, ADL disability, care home admission and hospitalization. The comparative prognostic value of these syndromes, both in relation to each other and to leading prognostic indices, for example, Age, Dyspnoea, Obstruction (ADO) and body mass index, obstruction, dyspnoea, exercise capacity (BODE), should also be tested if they are to compete as mainstream clinical markers.
Future work should also address how sarcopenia and frailty can be optimally managed within respiratory disease. Exercise-based strategies can be used to reduce the impact of these syndromes on patients and the evidence suggests both sarcopenia and frailty can be reversed not just prevented, a notion supported by the gerontology literature.73 The holistic pulmonary rehabilitation model has proven to be highly effective at improving health status in respiratory disease. Many components of this model target sarcopenia and frailty-related outcomes, for example, falls prevention strategies. The ‘dose’ of rehabilitation delivered through the model also appears sufficient to change sarcopenia and frailty domains, which suggests a reduced risk of adverse events occurring, though this needs to be confirmed. Given the difficulty frail people experience completing a programme, further work is required to understand how better to support frail patients, perhaps via organizational changes, for example, transport schemes or flexible class scheduling,74 or via supplementary training strategies, for example, muscle stimulation.75 The overarching goal would be for more people to access and benefit from a rehabilitation approach.
Additional treatment strategies could include nutritional interventions and review of polypharmacy. Nutritional assessment should be an integral part of holistic disease management but is often overlooked or not given sufficient attention.22 In some patients, malnutrition may be a key driver of the sarcopenia and frailty syndromes and appropriate nutritional support may be paramount to bringing meaningful change. Finally, with increasing multi-morbidity, more patients are prescribed multiple medicines. The introduction of a new drug can represent a stressor and the cumulative side effects and/or drug interactions can contribute directly to frailty.12 Tools to support evidence-based medication reviews and/or appropriate rationing are advocated for the care of older people.15,76,77 Conversely, the advent of medicines directed specifically at muscle78 may change the treatment landscape and offer new prospects in sarcopenia and frailty management in chronic respiratory disease and beyond.
Summary
Sarcopenia and frailty are geriatric syndrome that are related to and reflected by markers of skeletal muscle dysfunction. Numerous instruments have been validated to help assess sarcopenia and frailty, and the choice of one over another depends on the context and primary purpose of assessment. Both sarcopenia and frailty are common in people with chronic respiratory disease, and prevalence is positively associated with increasing age, disease severity, symptoms and comorbidity burden. Frailty assessment can be used to identify patients with chronic respiratory disease at increased risk of falls, hospitalizations and mortality, in whom preventative interventions can be commenced. A complex interplay exists between quadriceps weakness, sarcopenia and frailty, which are overlapping but distinct clinical phenotypes. Suggested areas for future work include studies in the acute setting, the prospective prognostic assessment of sarcopenia and frailty models in relation to each other and to current multidimensional indices, as well as the continued investigation of exercise, nutritional and pharmacological strategies to help prevent or treat sarcopenia and frailty in chronic respiratory disease.
Footnotes
Author Note: The views expressed in this publication are those of the authors and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MM is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for South London. AEB and MM are supported by Cicely Saunders International.
References
- 1. Maltais F, Decramer M, Casaburi R, et al. An official American thoracic society/European respiratory society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189(9): e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donaldson AV, Maddocks M, Martolini D, et al. Muscle function in COPD: a complex interplay. Int J Chron Obstruct Pulmon Dis 2012; 7: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36(1): 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012; 40(5): 1115–1122. [DOI] [PubMed] [Google Scholar]
- 5. Natanek SA, Gosker HR, Slot IG, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve 2013; 48(4): 488–497. [DOI] [PubMed] [Google Scholar]
- 6. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62(2): 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166(6): 809–813. [DOI] [PubMed] [Google Scholar]
- 8. Patel MS, Natanek SA, Stratakos G, et al. Vastus lateralis fiber shift is an independent predictor of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 190(3): 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007; 55(5): 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014; 2(10): 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cruz-Jentoft AJ, Landi F. Sarcopenia. Clin Med (Lond) 2014; 14(2): 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381(9868): 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calvani R, Marini F, Cesari M, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J cachexia Sarcopenia Muscle 2015; 6(4): 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanfleteren LE, Spruit MA, Wouters EF, et al. Management of chronic obstructive pulmonary disease beyond the lungs. The Lancet Respiratory medicine. 2016; 4(11): 911–924. [DOI] [PubMed] [Google Scholar]
- 15. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Directors Assoc 2011; 12(6): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mittal N, Raj R, Islam EA, et al. The frequency of frailty in ambulatory patients with chronic lung diseases. J Prim Care Commun Health 2016; 7(1): 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lahousse L, Ziere G, Verlinden VJ, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci 2015; 71(5): 689–695. [DOI] [PubMed] [Google Scholar]
- 18. Kon SS, Jones SE, Schofield SJ, et al. Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax 2015; 70(12): 1131–1137. [DOI] [PubMed] [Google Scholar]
- 19. Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med 2015; 192(11): 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; 71(11): 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SK, Richardson CR, Holleman RG, et al. Frailty in people with COPD, using the national health and nutrition evaluation survey dataset (2003-2006). Heart Lung 2013; 42(3): 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European respiratory society statement. Eur Respir J 2014; 44(6): 1504–1520. [DOI] [PubMed] [Google Scholar]
- 23. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J cachexia Sarcopenia Muscle 2010; 1(2): 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007; 55(5): 769–774. [DOI] [PubMed] [Google Scholar]
- 25. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006; 61(10): 1059–1064. [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997; 127(5 Suppl): 990S–991S. [DOI] [PubMed] [Google Scholar]
- 27. Argiles JM, Muscaritoli M. The three faces of sarcopenia. J Am Med Dir Assoc 2016; 17(6): 471–472. [DOI] [PubMed] [Google Scholar]
- 28. Timiras PS. Disuse and aging: same problem, different outcomes. J Gravit Physiol 1994; 1(1): P5–P7. [PubMed] [Google Scholar]
- 29. Lee JS, Auyeung TW, Kwok T, et al. Associated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology 2007; 53(6): 404–410. [DOI] [PubMed] [Google Scholar]
- 30. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39(4): 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med 2010; 104(6): 849–857. [DOI] [PubMed] [Google Scholar]
- 32. Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60(8): 1487–1492. [DOI] [PubMed] [Google Scholar]
- 33. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010; 58(4): 681–687. [DOI] [PubMed] [Google Scholar]
- 34. Eeles EM, White SV, O’Mahony SM, et al. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012; 41(3): 412–416. [DOI] [PubMed] [Google Scholar]
- 35. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3): M146–M156. [DOI] [PubMed] [Google Scholar]
- 36. Theou O, Brothers TD, Pena FG, et al. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc 2014; 62(5): 901–906. [DOI] [PubMed] [Google Scholar]
- 37. Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci 2004; 59(6): M627–M632. [DOI] [PubMed] [Google Scholar]
- 38. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173(5): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127(5): 494–496. [DOI] [PubMed] [Google Scholar]
- 40. Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010; 29(2): 154–159. [DOI] [PubMed] [Google Scholar]
- 41. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12(4): 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee ES, Park HM. Prevalence of Sarcopenia in Healthy Korean Elderly Women. J Bone Metab 2015; 22(4): 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15(2): 95–101. [DOI] [PubMed] [Google Scholar]
- 44. Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014; 69(5): 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc 2014; 15(9): 630–634. [DOI] [PubMed] [Google Scholar]
- 46. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16(7): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitnitski AB, Graham JE, Mogilner AJ, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45(3): 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gobbens RJ, van Assen MA, Luijkx KG, et al. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11(5): 344–355. [DOI] [PubMed] [Google Scholar]
- 50. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35(5): 526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147(8): 755–763. [DOI] [PubMed] [Google Scholar]
- 52. Steiner M, Barton R, Singh S, et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002; 19(4): 626–631. [DOI] [PubMed] [Google Scholar]
- 53. Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014; 69(5): 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maddocks M, Jones SE, Kon SS, et al. Sarcopenia definitions: where to draw the line? Response to Scarlata et al. Thorax 2015; 70(7): 694. [DOI] [PubMed] [Google Scholar]
- 55. Maddocks M, Kon SS, Jones SE, et al. Bioelectrical impedance phase angle relates to function, disease severity and prognosis in stable chronic obstructive pulmonary disease. Clin Nutr 2015; 34(6): 1245–1250. [DOI] [PubMed] [Google Scholar]
- 56. Kon SS, Canavan JL, Nolan CM, et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J 2014; 43(5): 1298–1305. [DOI] [PubMed] [Google Scholar]
- 57. Kon SS, Patel MS, Canavan JL, et al. Reliability and validity of 4-metre gait speed in COPD. Eur Respir J 2013; 42(2): 333–340. [DOI] [PubMed] [Google Scholar]
- 58. Norman K, Stobaus N, Gonzalez MC, et al. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011; 30(2): 135–142. [DOI] [PubMed] [Google Scholar]
- 59. Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol 2015; 61: 31–37. [DOI] [PubMed] [Google Scholar]
- 60. Lee WJ, Liu LK, Peng LN, et al. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc 2013; 14(7): 528.e1–7. [DOI] [PubMed] [Google Scholar]
- 61. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 62. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011; 59(11): 2129–2138. [DOI] [PubMed] [Google Scholar]
- 63. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011; 10(1): 104–114. [DOI] [PubMed] [Google Scholar]
- 65. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015; 70(3): 213–218. [DOI] [PubMed] [Google Scholar]
- 66. Akgün KM, Tate JP, Oursler KK, et al. Association of chronic obstructive pulmonary disease (COPD) with frailty measurements in HIV-infected and uninfected Veterans. AIDS 2016; 30(14): 2185–2193. [DOI] [PubMed] [Google Scholar]
- 67. Valenza MC, Torres-Sanchez I, Cabrera-Martos I, et al. Physical activity as a predictor of absence of frailty in subjects with stable COPD and COPD exacerbation. Respir Care 2016; 61(2): 212–219. [DOI] [PubMed] [Google Scholar]
- 68. Galizia G, Cacciatore F, Testa G, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res 2011; 23(2): 118–125. [DOI] [PubMed] [Google Scholar]
- 69. Vaz Fragoso CA, Enright PL, McAvay G, et al. Frailty and respiratory impairment in older persons. Am J Med 2012; 125(1): 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mittal N Raj R, Islam E pulmonary rehabilitation improves frailty and gait speed in some ambulatory patients with chronic lung diseases. SWRCCC 2015; 3(12): 2–10. [Google Scholar]
- 71. Donaldson GC, Law M, Kowlessar B, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192(8): 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Greening NJ, Harvey-Dunstan TC, Chaplin EJ, et al. Bedside assessment of quadriceps muscle using ultrasound following admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med 2015; 192(7): 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011; 2011: 569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vogiatzis I, Rochester CL, Spruit MA, et al. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J 2016; 47(5): 1336–1341. [DOI] [PubMed] [Google Scholar]
- 75. Maddocks M, Nolan CM, Man WD, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4(1): 27–36. [DOI] [PubMed] [Google Scholar]
- 76. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14(6): 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014; 43(6): 744–747. [DOI] [PubMed] [Google Scholar]
- 78. Steiner MC, Roubenoff R, Tal-Singer R, et al. Prospects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: a translational review. Thorax 2012; 67(12): 1102–1109. [DOI] [PubMed] [Google Scholar]


