Abstract
Fatigue is a common manifestation of sarcoidosis, often persisting without evidence of disease activity. First-line therapies for sarcoidosis have limited effect on fatigue. This review aimed to assess the treatment options targeting sarcoidosis-associated fatigue. Medline and Web of Science were searched in November 2015; the bibliographies of these papers, and relevant review papers, were also searched. Studies were included if they reported on the efficacy of interventions (both pharmacological and non-pharmacological) on fatigue scores in sarcoidosis patients. Eight studies were identified that fulfilled the inclusion criteria. These studies evaluated six different interventions (infliximab, adalimumab, ARA 290, methylphenidate, armodafinil and exercise programmes). There is evidence to support a treatment effect of anti-tumour necrosis factor (TNF)-αtherapies (adalimumab and infliximab) and neurostimulants (methylphenidate and armodafinil), but within five of the studies, the risk of bias was high within most domains and the remaining three studies included only small numbers of participants and were short in duration. Trial evidence for treating fatigue as a manifestation of sarcoidosis is limited and requires further investigation. Anti-TNF-α therapies may be beneficial in patients with organ-threatening disease. Neurostimulants have some trial evidence supporting improvements in fatigue but further investigation is needed before they can be recommended.
Keywords: Sarcoidosis, fatigue, fatigue syndrome, chronic, antibodies, monoclonal, exercise therapy, central nervous system stimulants
Introduction
Fatigue is a common problem in patients with sarcoidosis. Its prevalence varies between studies, from 50%1 to over 80% of patients,2 with a significant adverse impact on quality of life.3 The aetiology is poorly understood and likely to be multifactorial, encompassing active inflammation, cytokine release, depression, sleep disturbance and/or small-fibre neuropathy.4 Furthermore, systemic treatments used to treat sarcoidosis can themselves cause fatigue, including corticosteroids.5,6 Management of this symptom can be challenging for physicians. Chronic fatigue in stable sarcoidosis patients was described as long ago as 1993, with management suggested to include ‘unremitting sympathy’7; despite being identified as a feature of sarcoidosis for so long, it remains a poorly understood and often forgotten problem.
Identifying and treating any underlying and reversible causes of fatigue should be the first priority for any physician faced with a sarcoidosis patient with significant fatigue, which can include including sleep disordered breathing8,9 and periodic limb-movement syndromes.9 Once these have been excluded, strategies to improve fatigue should be considered. The evidence available for treatments of fatigue in sarcoidosis patients is limited. Few trials have specifically investigated treating fatigue, although measurement of fatigue is recommended as an outcome measure for trials involving sarcoidosis patients by the World Association of Sarcoidosis and Other Granulomatous Diseases.10
Various potential management strategies for sarcoidosis fatigue have been suggested previously, including both pharmacological and non-pharmacological interventions. Tumour necrosis factor (TNF)-α is released by alveolar macrophages and is involved in the initial pathogenesis of sarcoidosis11 as well as influencing prognosis.12 Anti-TNF-α therapies have been investigated as a treatment for refractory sarcoidosis; among these trials, measurements of fatigue pre- and post-treatment have been taken. ARA 290,13 a novel peptide modelled on erythropoietin that has anti-inflammatory and tissue protective activities, has also been investigated for its potential effects on symptoms of small-fibre neuropathy, including cognitive failure and fatigue.14
The use of neurostimulants has shown promising results in treating other causes of fatigue. Methylphenidate and its d-isomer dexmethylphenidate act by inhibiting dopamine and noradrenaline transporters, elevating dopamine and noradrenaline levels within the brain.15 Methylphenidate is known for its use in attention-deficit hyperactivity disorder (ADHD)16 but has been trialled for fatigue in other situations (including chemotherapy,17 post-radiotherapy,18 human immunodeficiency virus,19 and Parkinson’s disease20) with some benefit. Modafinil and its enantiomer armodafinil are neurostimulants that are used for promoting wakefulness in narcolepsy. They have a complex profile of neurochemical effects which are different to those of amphetamines.21
Finally, physical activity programmes, including pulmonary rehabilitation in chronic obstructive pulmonary disease (COPD) patients, have been shown to have wide reaching benefits, including improving dyspnoea scores, health-related quality of life, anxiety and depression.22 The use of pulmonary rehabilitation in patients with interstitial lung diseases is recommended by guidelines,23,24 with evidence of benefit in this patient group similar to that seen in COPD patients.25 Recent trials have investigated the use of such programmes in patients with sarcoidosis and their impact on fatigue.
The objective of this systematic review was to examine the evidence for treatments or management strategies of fatigue in sarcoidosis patients who are experiencing significant fatigue, as well as evaluating the quality of the evidence that presently exists, and to present the results of a qualitative analysis of the available data.
Methods
Publication search
This systematic review was registered with the international prospective register of systematic reviews (PROSPERO, registration number CRD42015030079) and was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.26 The protocol can be accessed on the PROSPERO database using the registration number. The electronic databases Medline (using PubMed) and Web of Science were searched using the following search strategy: ‘sarcoid’ OR ‘sarcoidosis’ OR sarcoidosis [MeSH Terms] OR Sarcoid* (truncation) AND ‘fatigue’ OR ‘chronic fatigue’ OR ‘chronic fatigue syndrome’ OR fatigue [MeSH Terms] OR fatigue syndrome, chronic [MeSH Terms] AND ‘treatment’ OR ‘management’ OR ‘clinical trial’.
Bibliographies of appropriate papers, including review articles, were undertaken to identify relevant additional sources. The search included all trials published to the end of November 2015. The title and abstract of all papers identified were reviewed for relevance, with irrelevant studies and reports excluded. Remaining papers were reviewed in full.
Selection criteria
Papers that were considered suitable for inclusion were any studies that evaluated the effect of an intervention or management strategy (pharmacological or non-pharmacological) on fatigue in sarcoidosis patients. All trial designs (case series, case–control, crossover and parallel-arm randomized controlled trials (RCT)) were included in the qualitative synthesis with the exception of single case reports, and so trials where no comparator group or comparator intervention were included. This ensured the broadest collection of evidence. The studies must have (1) evaluated sarcoidosis patients exclusively, or if the trials included other diseases then the sarcoidosis cohort had to be evaluated separately in the results, (2) evaluated the efficacy of the intervention on an assessment/measurement score of fatigue, (3) reported quantitative results for reduction in fatigue score between pre- and pot-intervention results and (4) be presented in full-text form and in English.
Data collection
Data extraction was performed independently by the two authors (CA and AMW) using a checklist of data to extract. In addition, the Cochrane Collaboration’s tool for assessing risk of bias was also used to assess the methodological quality of each trial and identify possible sources of bias at a study level. The following data was collected: Main author’s last name, year of publication, study design, number of participants, severity of sarcoidosis in participants by chest X-ray staging (if given), intervention (including dose), duration of intervention, measurement score of fatigue used, change in fatigue outcome, number of participants reaching minimum clinically important difference on outcome score (if given), number of participants completing the intervention and adverse events reported within the trial. The summary measure of interest was mean improvement of fatigue score, however, a meta-analysis of data was not possible due to the heterogeneity of interventions and study designs included. Therefore, a narrative review of the data is presented.
Results
Search results and characteristics of eligible trials
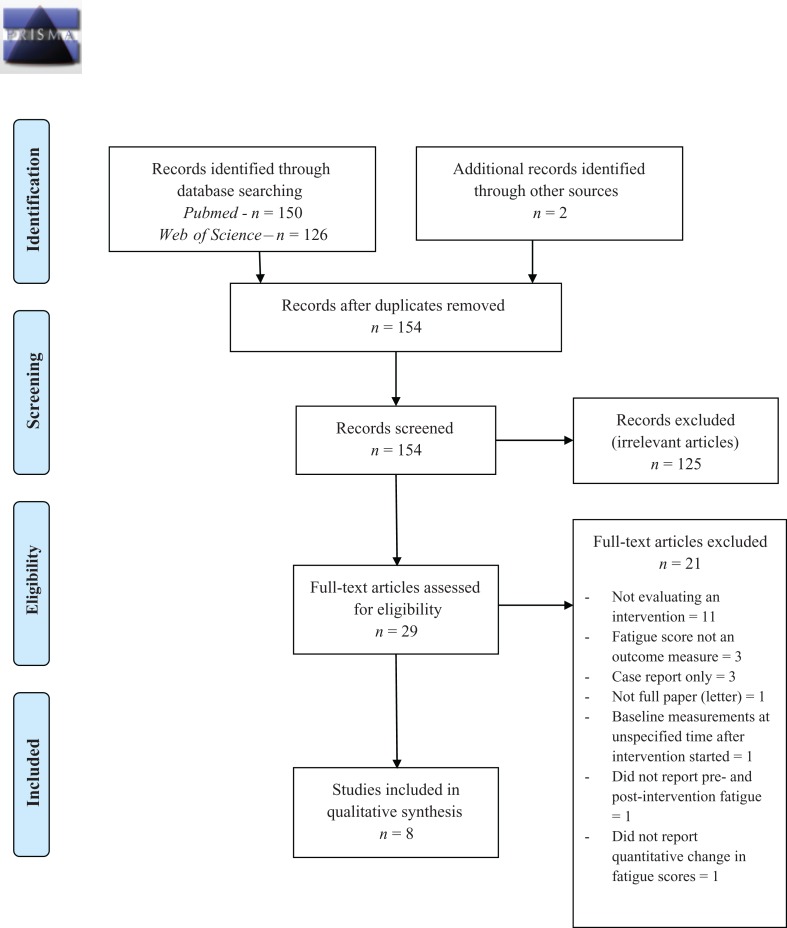
The search strategy identified 150 records through Medline and 126 records through Web of Science. Two further papers of interest were identified through bibliography searches. Titles and abstracts were reviewed for relevance, with studies excluded if they did not include patients with sarcoidosis, were evaluating a potential intervention, or were review articles or case reports. A short list of 29 studies was identified, of which eight met the criteria for inclusion. The flow diagram (PRISMA 2009) of screening articles is shown in Figure 1. Only three RCTs (either parallel arm or crossover) were identified, the remaining articles consisted of two case–control studies, three case series and one retrospective case review. Three papers evaluated formal physical activity programmes, two evaluated systemic treatment with TNF-α (TNF-α) inhibitors, two evaluated symptom-targeted therapy with neurostimulants (dexmethylphenidate and armodafinil) and one trial looked at a novel molecule aimed at treating small-fibre neuropathy. All but one of the trials used the Fatigue Assessment Scale (FAS), a 10-point questionnaire with a maximum score of 50 points. This scale has been validated in sarcoidosis patients,27 with a well-defined cut-off score for fatigue of >21 points27 and minimal clinically important differences (MCID) of four points or 10% reduction.28 A total of 185 patients were included across all the trials. The details of the included studies are shown in Table 1 (study information, safety and efficacy) and Table 2 (risks of bias within studies). In addition, three papers that did not meet the inclusion criteria but provided useful information are discussed.
Figure 1.
PRISMA 2009 flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Table 1.
Overview of trials in sarcoidosis patients including change in fatigue as an outcome measure.
| Author (year) | Lower et al. (2007)29 | Erckens et al. (2011)30 | Heij et al. (2012)13 | Van Rijswijk et al. (2013)31 | Lower et al. (2013)32 | Strookappe et al. (2015)33 | Marcellis et al. (2015)34 | Strookappe et al. (2015)35 |
|---|---|---|---|---|---|---|---|---|
| Study design | Crossover RCT | Case series | RCT | Retrospective case review | Crossover RCT | Case series | Case series | Retrospective, observational |
| Intervention | Dexmethylphenidate | Adalimumab | ARA 290 | Infliximab | Armodafinil | Physical activity programme | Physical activity programme | Physical activity programme |
| Dose | Up to 10 mg twice daily | 40 mg S/C weekly | 2 mg IV weekly | 5 mg/kg IV at 0, 2, 6, 10, 14 and 18 weeks | Up to 250 mg twice daily | 12-week physical activity programme | 13-week physical activity programme | 12-week physical activity programme |
| Comparator | Placebo | None | Placebo | None | Placebo | None | None | 41 patients who chose not to participate in programme |
| Duration | 8 weeks per arm | 12 months | 4 weeks | 18 weeks | 8 weeks per arm | 12 weeks | 13 weeks | 12 weeks |
| No. participants | 10 | 26 | 22 (10 placebo) | 27b | 15 | 12 | 24 | 49 |
| % Male | 20% | 36.6% | 50% | 60% | 33.3% | 91.7% | 75% | 57.1% |
| Age (mean ± SD) | 52 (range 39–74) | 51 ± 15 | 48.1 ± 2.7 | 48.9 ± 10.1 | 54 (range 35–62) | 53.2 ± 11.7 | 49.4 ± 10.5 | 47.6 ± 11.3 |
| Disease stage (0/I/II/III/IV) | Not stated | 16/4/4/2/0 | Not stated | 5/7/14/5/14 | 3/5/2/2/3 | 0/0/0/0/12 | 0–I = 29.2%; I–III = 66.6%; IV = 4.2% | 4/11/22/0/4 |
| Measurement of fatigue | FAS and FACIT-F | FAS | FAS | ‘Fatigue severity’ within ‘checklist individual strength’ | FAS and FACIT-F | FAS | FAS | FAS |
| Intervention: Pre- and Post-fatigue scores (mean ± SD unless stated) | FAS (range): Pre: 32.5 (9–44); Post: 27.5 (23–43); FACIT-F (range): Pre: 19.5 (13–48); Post: 29.5 (5–50) | Pre: 31.1 ± 11.1; Post: 28.9 ± 10.0 | Pre: 37.9 ± 2.6; Post: 33.3 ± 2.8 | Pre: 49.4 ± 9.2; Change: −5.3 ± 8.5 | FAS (range): Pre: 37 (14–43); Change: −4.5 (−20, 5); FACIT-F (range): Pre: 23 (10–47) Change: +9(−12, 26) | Pre: 28.5 ± 5.4; Post: 27.7 ± 5.7; (6 of the 12 patients had FAS >21 at baseline) | Pre: 29.7 ± 7.7; Post: 27.0 ± 7.3 (in 18 patients completing course) | Pre: 29.8 ± 8.1; Post: 25.6 ± 7.5 |
| Comparator: Pre- and Post-fatigue scores (mean ± SD unless stated) | Average scores in placebo arma: FAS: 33.6 ± 4.43; FACIT-F: 24.3 ± 5.41 | N/A | Pre: 33.6 ± 2.3; Post: 29.8 ± 3.3 | N/A | FAS (range): Pre: 37 (14–43); Change: +3.5 (−9, 14); FACIT-F (range): Pre: 23 (10–47); Change: −5 (−17, 11) | N/A | N/A | Pre: 30.3 ± 9.0; Post: 28.6 ± 9.0 |
| Statistical difference versus comparator | FAS: p = 0.0295; FACIT-F: p = 0.0040 (between groups) | p < 0.01 compared with baseline | Non-significant between groups | p = 0.003 compared with baseline | FAS: p = 0.0295; FACIT-F: p = 0.0040 (between groups) | Non-significant compared with baseline | p = 0.003 compared with baseline | Within group (intervention): p = 0.009 Within group (placebo): p = 0.408 Groups not directly compared |
| Number of participants with clinically significant improvement | Not stated | Not clear; 14/21 less fatigued but doesn’t state MCID used. | Not stated | Not stated | 64% treated with armodafinil improved FAS by 4 points (MCID); only 7% in placebo | 66% of patients with FAS >21 at baseline had improvement in FAS, not specified if MCID used | 6 (3%) improve FAS by 4 points (MCID); 9 (50%) improved FAS by 10% | 74.4% of intervention group and 48.5% of comparator group reduced FAS by 4 points (MCID). |
| Dropouts/side effects | No withdrawals. Four patients required lower afternoon dose | No withdrawals. one severe injection site reaction | No drop outs | Three patients discontinued within six infusions: (1) allergic reaction, (2) progression of dyspnoea and (3) hepatitis (due to methotrexate) | One withdrawal due to severe anxiety | No withdrawals | Six patients withdrew; 3 = problems other than sarcoid; 2 = health insurance problems; 1 = No reason | Not stated |
| Evidence of treatment effect in fatigue | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes |
RCT: randomized control trial; FAS: Fatigue Assessment Scale; FACIT-F: Functional Assessment of Chronic Illness Therapy – Fatigue; MCID: minimal clinically important difference.
aGroup values at the end of the placebo phase not given, data only presented as average values across placebo phase.
bForty-eight patients included in study but quality of life scores (including fatigue) only available in 27.
Table 2.
Risks of bias within trials in sarcoidosis patients reporting fatigue as an outcome measure.
| Author (year) | Lower et al. (2007)29 | Erckens et al. (2011)30 | Heij et al. (2012)13 | van Rijswijk et al. (2013)31 | Lower et al. (2013)32 | Strookappe et al. (2015)33 | Marcellis et al. (2015)34 | Strookappe et al. (2015)35 |
|---|---|---|---|---|---|---|---|---|
| Sequence generation | Low risk: Random sequence computer-generated | High risk: No randomization (not RCT) | Low risk: Computer generated randomization code | High risk: No randomization (not RCT) | Unclear: No statement on randomization procedure | High risk: No randomization (not RCT) | High risk: No randomization (not RCT) | High risk: No randomization (not RCT) |
| Allocation concealment | Low risk: Pharmacy-controlled allocation | High risk: No concealment, all patients receive drug | Low risk: Pharmacy-controlled allocation | High risk: No concealment, all patients received drug | Unclear: No statement on allocation procedure | High risk: No concealment, all patients on programme | High risk: No concealment, all patients on programme | High risk: No concealment, all patients on programme |
| Blinding of participants, personnel and outcome assessors | Low risk: Double-blind with low risk of breaking blinding | High risk: No blinding | Low risk: Only allocating pharmacist unblinded | High risk: No blinding | Low risk: Double-blind with low risk of breaking blinding | High risk: No blinding | High risk: No blinding | High risk: No blinding, including assessors of physical function pre- and post. |
| Incomplete outcome data | Unclear: No description of missing data points or handling of missing data | Unclear: No description of missing data points or handling of missing data | Low risk: Missing data compensated for by taking forward last value | Unclear: No description of missing data points or handling of missing data | Unclear: No description of missing data points or handling of missing data | Unclear: No description of missing data points or handling of missing data | Unclear: No description of missing data points or handling of missing data | Unclear: No description of missing data points or handling of missing data |
| Selective outcome reporting | Low: All outcomes reported | Unclear: No protocol available | Unclear: No protocol available | Unclear: No protocol available | Low: All outcomes reported | Unclear: No protocol available | Unclear: No protocol available | Unclear: No protocol available |
| Other sources of bias | No other clear causes of bias identified; crossover ensures groups balanced, patients are own controls. Small sample. | Study design (case series) limits conclusions – no comparator group to eliminate placebo effect. | Baseline imbalance in FAS and health status score (SF36) between groups – significantly lower fatigue scores in placebo arm. | Retrospective review – data collected pre- and post-intervention but high risk of bias from retrospective nature | No other clear causes of bias identified; crossover ensures groups balanced, patients are own controls. Small sample. | Not an RCT, also participants enrolling on programme would self-select as motivated people? generalizable | Not an RCT, also participants enrolling on programme would self-select as motivated people? generalizable | Patients choosing the intervention would be a self-selecting cohort; controls not randomized but refused intervention |
| Overall risk of bias | Low – Well designed crossover trial, though only small sample | High – Study design (case series) means no blinding, randomization or comparator. | Low – Well designed RCT but not powered to look at change in fatigue. | High – Design (retrospective case series) has no blinding, randomization or comparator. | Unclear – Issues with description of randomisation allocation and concealment mean study at risk of bias | High – Study design (case series) means no blinding, randomisation or comparator. | High – Study design (case series) means no blinding, randomisation or comparator. | High – Self-selecting intervention group, high risk of bias given control group declined intervention |
RCT: randomized controlled trial.
Systemic therapy: Anti-TNF-α treatment and anti-inflammatories
Three trials evaluated interventions which have systemic or disease-modifying effects. Two investigated anti-TNF-α drugs (adalimumab30 and infliximab31) and one investigated ARA 290.13 Only one trial, investigating ARA 290,13 was a blinded, RCT; neither of the studies investigating anti-TNF-α therapies were of a randomized design.
Erckens and colleagues30 followed 26 sarcoidosis patients with refractory uveitis who had been commenced on Adalimumab (40 mg subcutaneously once weekly) over a 12-month treatment period. All patients had previously received prednisolone and methotrexate. Fatigue was measured using the FAS, with 21 patients (80.7%) having a baseline FAS score >21 points. After the 12-month treatment period, there was a mean reduction in FAS score of 2.2 points and an improvement in FAS score in 14 of the 21 patients with a baseline FAS score >21 points; although it is not stated what constituted an improvement in the FAS and whether these patients met the MCID for the FAS score. The risk of bias is high with this trial design, as there is no comparator group to determine if this is a placebo effect or natural progression of the disease, however, there is a suggestion that fatigue is improved by anti-TNF-α therapy.
In another study from the Netherlands, Van Rijswijk and colleagues31 retrospectively reviewed 48 sarcoidosis cases who required treatment with Infliximab (5 mg/kg intravenously at 0, 2, 6, 10, 14 and 18 weeks). All patients had previously received immunosuppression; 30 had received prednisolone and methotrexate, 12 received prednisolone only and 1 received methotrexate only. Data involving quality of life measures, including fatigue scores (within the ‘Checklist of Individual Strength’ questionnaire), had been collected pretreatment in the most recent 27 cases. The fatigue score improved by a mean of 5.3 points from a baseline of 49.4 points (p = 0.003), although it is not clear if this is a clinically important improvement. Furthermore, as this was a retrospective review, and without a comparator or placebo group, it is not possible to definitively attribute the change in fatigue scores to the intervention and does not conclude the benefits of TNF inhibition in treating fatigue.
Heij and colleagues13 undertook a small double-blind, RCT investigating the safety and efficacy of ARA 290 for symptoms of small-fibre neuropathy in sarcoidosis patients. Only 12 patients were included in the active treatment arm. Of these patients, four were receiving steroids and one received a systemic anti-inflammatory drug. Participants were excluded if they received an anti-TNF drug within 6 months of the trial. Fatigue was measured using the FAS score, though this was a secondary outcome. The baseline groups were imbalanced with regard to fatigue (mean FAS 37.9 in the ARA 290 group, 33.6 in the placebo group), but despite the higher levels of fatigue in the treatment arm at baseline, there was an identical reduction in FAS scores over the 4-week trial period in both arms. There was no evidence that ARA 290 improved fatigue scores, but the trial was not powered for this outcome and was very short. It did show improvement in its primary outcome (the small-fibre neuropathy screening list) as well as being well tolerated; if a larger and longer trial investigating the drug were to be performed, measuring change in fatigue would be important to ensure that a treatment effect was not missed in this trial.
Neurostimulants
Two RCTs, both using a crossover design, investigated the use of neurostimulants for treating fatigue in sarcoidosis. One trial investigated the use of armodafinil32 and one trial investigated dexmethylphenidate.29 In each trial, the change in fatigue was the primary outcome being measured.
Lower and colleagues29 treated 10 patients with sarcoidosis-related fatigue with dexmethylphenidate and assessed its response by measuring fatigue using the FAS score. Participants received up to 10 mg twice daily of dexmethylphenidate or matched placebo and were investigated weekly for 8 weeks per arm (treatment and placebo). All patients were receiving at least one systemic agent for their sarcoidosis. Treatment effect in the intervention arm was seen after 5 weeks of therapy, with a mean reduction of 5 points in the FAS score after 8 weeks of treatment. The number of patients meeting the MCID was not reported. The drug was well tolerated, with no withdrawals. The small scale of the trial means that the results should be interpreted with caution but suggests clinical benefit.
In a crossover trial by Lower and colleagues,32 15 patients with stable sarcoidosis received up to 250 mg of armodafinil daily, all of whom underwent polysomnography and multiple sleep latency testing pre- and post-intervention. All participants had received systemic treatment for sarcoidosis (prednisolone, methotrexate, leflunomide, hydroxychloroquine, azathioprine or anti-TNF therapy) and nine were receiving continuous positive airway pressure therapy. There was a mean reduction of 4.5 points in the FAS score (the primary outcome) and nine patients (64%) exhibited a reduction of 4 points or more. Participants receiving armodafinil did have a prolonged sleep onset latency compared with placebo which was statistically significant, although it is not clear if this was a clinically significant difference as no participants discontinued the medication due to insomnia. The article did not report randomization or allocation procedures, although it was double-blind and appeared an appropriate design for the study’s aims. The small number of patients and short period of the trial suggest that further evidence is needed to confirm the efficacy of armodafinil in this setting.
Non-pharmacological treatment strategies
Three recent papers have investigated the effect of structured physical training programmes on fatigue in sarcoidosis. Marcellis and colleagues34 undertook an observational case-series study of 24 sarcoidosis patients in the Netherlands suffering with fatigue and/or impaired exercise tolerance. The intervention consisted of both upper- and lower extremity peripheral muscle resistance training, with progressively increasing resistance through the training period, and endurance training, consisting of either treadmill or walking or cycling on an ergometer. Eighteen patients completed the entire training regime, with six not completing the programme. A statistically significant improvement was observed in FAS scores at the completion of the intervention: mean baseline and post-exercise FAS scores of 29.7 and 27.0 points, respectively. Of the 18 participants who completed the exercise programme, 6 patients (33.3%) had a reduction of 4 points in their FAS score; when using the alternative MCID of a 10% reduction, 9 patients (50%) met this criteria. This study had no comparator group, it did not state how many patients were approached or screened for inclusion, and patients entering the trial and completing the exercise programme are likely to have been motivated to undertake such an intervention. Although the results from this study suggest that the intervention is beneficial for sarcoidosis-associated fatigue, it is unlikely to be beneficial in all patients.
Strookappe and colleagues35 investigated physical activity programmes in a similar cohort of Dutch sarcoidosis sufferers in a retrospective observational study. From an initial cohort of 147 sarcoidosis patients who had undergone physical performance assessment, 49 patients undertook a 12-week supervised exercise programme. This was similar to that described by Marcellis and colleagues, with the peripheral muscles strengthening individualized for each patient. Twenty-one (42.9%) of the group were receiving steroids. A comparison was made with 41 sarcoidosis patients who had chosen not to undertake the programme but undertook identical physical assessments. Following reassessment at the end of the 12-week period there was a statistically significant within-group improvement in FAS scores in those who received physical therapy (29.8 pre, 25.6 post, p = 0.009) whereas the comparator group had a non-significant reduction (pre 30.3, post 28.6, p = 0.408). In the exercise programme group, 74.4% of patients had an improvement in their FAS score of four points or more, although 48.5% of the comparator group showed the same reduction despite not receiving any intervention. The within-group results suggest that physical training may be beneficial for fatigue, though the results should be interpreted with caution. As with the other trials investigating exercise programmes on fatigue, participants within the trial would have self-selected as a group keen to undertake the intervention. Even given this source of bias, patients who chose not to receive the intervention reported a clinically significant improvement in their fatigue. Finally, although the authors state that blinding was not possible in this trial, it would be possible to blind assessors to patient groups when assessing physical and psychological parameters pre- and post-intervention; whether this occurred was not stated in the paper.
Another study from Strookappe and colleagues33 investigated the physical training in patients with end-stage sarcoidosis-related pulmonary fibrosis, alongside a cohort with idiopathic pulmonary fibrosis. Twelve patients with stage IV sarcoidosis participated in a 12-week exercise programme, similar to those described in the previous trials.34,35 Patients were recruited from the same centre and during the same period as those included in another study by the same authors,35 though it is not specified whether these patients were also included in the results from the earlier study. Despite this, the study was included as it reported the results of a subgroup of patients with end-stage pulmonary sarcoidosis, which were not reported separately in the other paper. FAS score was measured pre- and post-intervention. Baseline mean FAS score was 25.1 in the sarcoidosis group, with only six patients (50%) having clinically significant fatigue. Following the programme, four of the six patients reported improved fatigue, although the paper does not state whether participants that improved met the MCID for the FAS score. This study did not have a non-intervention group, and only a small number of participants with fatigue at baseline were included. The same source of biases existed ass described with the other two studies investigating this intervention, making it difficult to be confident of the effect of physical training programmes for sarcoidosis patients suffering fatigue.
Excluded studies of interest
Two studies investigating anti-TNF-α therapy in large cohorts were not included in the systematic review; the first had no measurement of pretreatment fatigue scores14 and the second failed to describe a quantitative change in fatigue.36 One article describing methylphenidate use for sarcoidosis-associated fatigue was excluded because it was not a full article and did not describe a baseline fatigue score. Furthermore, one cross-sectional study has suggested that the antimalarial hydroxychloroquine may have benefits on fatigue.
One study investigating cognitive failure and sarcoidosis-associated fatigue in 343 patients was excluded as baseline assessment of fatigue occurred after patients had already been established on treatment.14 The study was a 6-month cross-sectional assessment of patients who had already received various therapies, including TNF inhibition in 42 patients. The results showed an improvement in FAS scores in the anti-TNF α therapy group over 6 months (baseline FAS 32.8+/−7.31, 6-month change −4.90+/−5.57) when compared with patients on no treatment (baseline FAS 28.6+/−7.94, 6-month change 0.44+/−5.13), or on corticosteroids with or without antimetabolite (methotrexate) therapy (baseline FAS 28.2+/−7.81, 6-month change +1.19+/−4.87). The patients in the anti-TNF-α therapy group had higher fatigue scores on their initial questionnaires compared with the other groups, and, after 6 months, there was no difference in fatigue scores between patients receiving other forms of therapy. However, without baseline characteristics in each group before commencing treatment, it is not possible to directly compare the results of the groups or establish the effect of treatment on fatigue scores.
A further study investigating TNF-inhibitor treatment in sarcoidosis patients (adalimumab or infliximab) recorded pretreatment fatigue levels in 111 patients was excluded because no numerical data for change in fatigue measure (FAS) were included.36 All patients had received prednisolone and methotrexate before receiving TNF blockade and had evidence of ongoing disease activity despite treatment. Of the 111 patients included, 100 (90.1%) reported a FAS score >21 (mean baseline FAS 33.0) and 59 reported severe fatigue (FAS score >34). After 12 months of therapy, 60 patients who were fatigued at baseline had improvement in their fatigue score; unfortunately, the definition of improvement and the scale of change in the FAS score required to be classified as a responder are not stated, therefore it is not possible to evaluate whether the intervention was clinically effective from these results.
Methylphenidate was used in a series of five patients with severe sarcoidosis-associated fatigue that was described in a research letter from Wagner and colleagues.37 Five patients received 10 mg twice daily of methylphenidate. There was no formal measure of fatigue severity at baseline, but the paper describes a statistically significant reduction on the Symptoms of Fatigue scale after 1 month. There were positive reports from four of the five patients, with two reporting that they felt as if their lives were ‘back to normal’. The five patients continued on methylphenidate long term; at 2 years, all five of the patients remained on methylphenidate and reported continuing improvement in fatigue, although no formal fatigue scoring was performed. The authors concluded that further studies in larger groups of patients are required, though at the time of writing, only the two small crossover studies of armodafinil and dexmethylphenidate have been undertaken.
Although no papers directly investigated the use of chloroquine or hydroxychloroquine for fatigue, a possible effect of the drug on fatigue scores was noted in a cross-sectional study comparing two cohorts of sarcoidosis patients.2 This article was not included in this systematic review, as it did not report change in fatigue scores pre- and post-treatment, but the authors noted that patients receiving hydroxychloroquine (n = 22) had lower fatigue scores than patients on other agents in the absence of any other differences in disease activity or severity. The lack of pre- and post-treatment fatigue scores, as well as the small number of patients receiving the agent, mean that conclusions about the effectiveness of hydroxychloroquine for treating fatigue cannot be directly drawn from these results.
Discussion
The evidence base for treating fatigue in sarcoidosis remains weak. Only eight trials were identified, all of which have been presented here. Of these studies, all were either small or were of poor quality study design which led to the possibility of inherent biases affecting the results. This makes it difficult to draw strong conclusions about the benefits of each therapy.
In patients with clinically significant fatigue with evidence of disease activity despite use of first line immunosuppressants, anti-TNF-α therapy may be indicated. In the absence of active, organ-threatening disease, the risks and potential side-effects of these drugs make them difficult to recommend for treating fatigue alone.
Physical exercise programmes appear to lead to improvements in fatigue scores, but in the one trial that had a comparison group35 almost half of the controls demonstrated clinically significant improvements in fatigue without any intervention. The patients who did enrol on the exercise programmes were likely to have been motivated to undertake this and therefore most likely to benefit. Nevertheless, improvements were seen in physical measures beyond fatigue and so in patients with physical limitation and fatigue who express an interest in undertaking physical therapy a structured exercise programme may provide benefits.
The management of fatigue in patients with quiescent disease is often a clinical challenge, especially given the potential side effects of disease-modifying medications. Neurostimulants such as modafinil or methylphenidate may be appropriate in these cases. The two trials investigating these interventions were well designed, but only included a small number of patients. Long-term use of these medications has been safe in other conditions (ADHD) but the trials investigating their use in sarcoidosis have been very short. Further evidence to investigate the longer term safety and efficacy of these medications is required.
Chloroquine and hydroxychloroquine are considered effective for treating cutaneous sarcoidosis.38 Its use in patients with sarcoidosis-associated fatigue who require corticosteroid therapy has been suggested in a previous review.39 The possible effectiveness of treating fatigue with these agents is interesting, but evidence from trials investigating pre- and post-intervention fatigue scores is needed before stronger recommendation can be made for its use.
Future research considerations
The main limitation of the evidence base for managing fatigue is the lack of trials of sufficient sample size or duration to make firm recommendations for managing patients with fatigue in clinical practice. Much of the data available are from observational studies or studies of less than 30 patients. Any future trials investigating therapies for treating sarcoidosis should include fatigue as an outcome measure given the frequency and significance of fatigue in sarcoidosis cohorts.
In patients with quiescent disease, where fatigue is the primary symptom driving treatment decisions, more randomized placebo-controlled clinical trials are required. The need to eliminate any placebo effect is important; in one of the trials included in this review,13 almost identical changes in fatigue from baseline were seen in both intervention and placebo arms. Designing these trials appropriately to inform clinical decision making is therefore the primary concern. The randomized trials that have already been performed have been very short, either 4- or 8-week duration. Clinical use of agents such as neurostimulants would likely be over many months and future trials should therefore assess change in fatigue scores over a much longer period of time than previously seen, at least 6 months.
There are limitations to this review. The review included only English language papers, although no papers in other languages were found in the search strategy. Although efforts were made to contact authors regarding missing data or unclear elements of trial design, there remains gaps in the data presented here. Furthermore, the existing data is limited; the studies included involved only a small number of participants or followed a methodology that would include intrinsic bias.
Conclusions
The available data for treating sarcoidosis-associated fatigue is limited. Anti TNF-α therapies appear to improve fatigue but all data come from observational trials without placebo arms. The neurostimulants dexmethylphenidate and armodafinil both appeared to improve fatigue scores compared with placebo but the trials were very small and short. Given the frequency that fatigue occurs in sarcoidosis, and the importance of this symptom for patients, larger and longer trials are necessary to help inform management decisions. The increasing awareness of fatigue as a problematic manifestation of sarcoidosis will hopefully ensure that any future trials investigating interventions for sarcoidosis will include measures of fatigue.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is independent research supported by the National Institute for Health Research (NIHR Doctoral Research Fellowship, Dr Chris Atkins, DRF-2015-08-190). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
References
- 1. Michielsen HJ, Drent M, Peros-Golubicic T, et al. Fatigue is associated with quality of life in sarcoidosis patients. Chest 2006; 130(4): 989–994. [DOI] [PubMed] [Google Scholar]
- 2. de Kleijn WPE, Elfferich MDP, De Vries J, et al. Fatigue in sarcoidosis: American versus dutch patients. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26(2):92–97. [PubMed] [Google Scholar]
- 3. Drent M, Marcellis R, Lenssen A, et al. Association between physical functions and quality of life in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31(2): 117–128. [PubMed] [Google Scholar]
- 4. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012; 40(1): 255–263. [DOI] [PubMed] [Google Scholar]
- 5. Judson MA, Chaudhry H, Louis A, et al. The effect of corticosteroids on quality of life in a sarcoidosis clinic: the results of a propensity analysis. Respir Med 2015; 109(4): 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleischer M, Hinz A, Braehler E, et al. Factors associated with fatigue in sarcoidosis. Respir Care 2014; 59(7): 1086–1094. [DOI] [PubMed] [Google Scholar]
- 7. James DG. Complications of sarcoidosis. Chronic fatigue syndrome. Sarcoidosis 1993; 10(1): 1–3. [PubMed] [Google Scholar]
- 8. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest 2015; 148(4): 1105–1114. [DOI] [PubMed] [Google Scholar]
- 9. Verbraecken J, Hoitsma E, van der Grinten CPM, et al. Sleep disturbances associated with periodic leg movements in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21(2): 137–146. [DOI] [PubMed] [Google Scholar]
- 10. Baughman RP, Drent M, Culver DA, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2012; 29(2): 90–98. [PubMed] [Google Scholar]
- 11. Fehrenbach H, Zissel G, Goldmann T, et al. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur Respir J 2003; 21(3): 421–428. [DOI] [PubMed] [Google Scholar]
- 12. Ziegenhagen MW, Benner UK, Zissel G, et al. Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2 R are prognostic markers. Am J Respir Crit Care Med 1997; 156(5): 1586–1592. [DOI] [PubMed] [Google Scholar]
- 13. Heij L, Niesters M, Swartjes M, et al. Safety and efficacy of ARA 290 in Sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study. Mol Med 2012; 18(11): 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elfferich MD, Nelemans PJ, Ponds RW, et al. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration 2010; 80(3): 212–219. [DOI] [PubMed] [Google Scholar]
- 15. Schmeichel BE, Berridge CW. Neurocircuitry underlying the preferential sensitivity of prefrontal catecholamines to low-dose psychostimulants. Neuropsychopharmacol 2013; 38(6): 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storebo OJ, Ramstad E, Krogh HB, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev 2015; 11: CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage 2009; 38(5): 650–662. [DOI] [PubMed] [Google Scholar]
- 18. Butler JM, Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007; 69(5): 1496–1501. [DOI] [PubMed] [Google Scholar]
- 19. Breitbart W, Rosenfeld B, Kaim M, et al. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med 2001; 161(3): 411–420. [DOI] [PubMed] [Google Scholar]
- 20. Mendonca DA, Menezes K, Jog MS. Methylphenidate improves fatigue scores in Parkinson disease: a randomized controlled trial. Mov Disorder 2007; 22(14): 2070–2076. [DOI] [PubMed] [Google Scholar]
- 21. Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 2008; 33(7): 1477–1502. [DOI] [PubMed] [Google Scholar]
- 22. Nici L, Donner C, Wouters E, et al. American thoracic society/European respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173(12): 1390–1413. [DOI] [PubMed] [Google Scholar]
- 23. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British thoracic society in collaboration with the thoracic society of Australia and New Zealand and the Irish thoracic society. Thorax 2008; 63 Suppl 5: v1–v58. [DOI] [PubMed] [Google Scholar]
- 24. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183(6): 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188(8): e13–e64. [DOI] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Vries J, Michielsen H, Van Heck GL, et al. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br J Health Psychol 2004; 9(Pt 3): 279–291. [DOI] [PubMed] [Google Scholar]
- 28. de Kleijn WPE, De Vries J, Wijnen PAHM, et al. Minimal (clinically) important differences for the fatigue assessment scale in sarcoidosis. Respir Med 2011; 105(9): 1388–1395. [DOI] [PubMed] [Google Scholar]
- 29. Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest 2008; 133(5): 1189–1195. [DOI] [PubMed] [Google Scholar]
- 30. Erckens RJ, Mostard RLM, Wijnen PAHM, et al. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol 2012; 250(5): 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Rijswijk HNAJ, Vorselaars ADM, Ruven HJT, et al. Changes in disease activity, lung function and quality of life in patients with refractory sarcoidosis after anti-TNF treatment. Expert Opin Orphan Drugs 2013; 1(6): 437–443. [Google Scholar]
- 32. Lower EE, Malhotra A, Surdulescu V, et al. Armodafinil for sarcoidosis-associated fatigue: a double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage 2013; 45(2): 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strookappe B, Elfferich M, Swigris J, et al. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: a pilot study. Sarcoidosis Vasc Diffuse Lung Dis 2015; 32(1): 43–52. [PubMed] [Google Scholar]
- 34. Marcellis RGJ, van der Veeke MAF, Mesters I, et al. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis 2015; 32(1): 53–62. [PubMed] [Google Scholar]
- 35. Strookappe B, Swigris J, De Vries J, et al. Benefits of physical training in sarcoidosis. Lung 2015; 193(5): 701–708. [DOI] [PubMed] [Google Scholar]
- 36. Wijnen PA, Cremers JP, Nelemans PJ, et al. Association of the TNF-alpha G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J 2014; 43(6): 1730–1739. [DOI] [PubMed] [Google Scholar]
- 37. Wagner MT, Marion SD, Judson MA. The effects of fatigue and treatment with methylphenidate on sustained attention in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005; 22(3): 235. [PubMed] [Google Scholar]
- 38. Beegle SH, Barba K, Gobunsuy R, et al. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7: 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eklund A, du Bois RM. Approaches to the treatment of some of the troublesome manifestations of sarcoidosis. J Int Medi 2014;275(4): 335–349. [DOI] [PubMed] [Google Scholar]