Abstract
Epidemiological studies have identified a trend in the development of depressive and anxiety disorders following a diagnosis of chronic obstructive pulmonary disease (COPD). However, the relationship between COPD and subsequent bipolar disorder remains unclear. From January 1, 2000, we identified adult patients with COPD from the Taiwan National Health Insurance Research Database. A nationwide population-based study was conducted; 46,778 COPD patients and 46,778 age-, sex-, and comorbidity-matched subjects between 2000 and 2011 were enrolled. The two cohorts were followed up till December 31, 2011 and observed for occurrence of bipolar disorder. We observed the COPD and comparison cohorts for 263,020 and 267,895 person-years, respectively, from 2000 to 2011. The incidence rate for bipolar disorder was 1.6/1000 person-years in the COPD cohort and 1.2/1000 person-years in the comparison cohort (p < 0.001). After multivariate adjustment, the hazard ratio (HR) for subsequent bipolar disorder among the COPD patients was 1.42 (95% confidence interval [CI], 1.22–1.64; p < 0.001). In the COPD patients, short-acting beta-agonists (SABAs) was associated with a significantly increased risk of bipolar disorder development (HR = 1.83, 95% CI = 1.25–2.69, p = 0.002). Other COPD medications were not associated with the risk of bipolar disorder development. The study results indicate that COPD may be an independent risk factor for the development of bipolar disorder. The regular use of SABAs might increase the risk of bipolar disorder in COPD patients.
Keywords: Chronic obstructive pulmonary disease, bipolar disorder, obstructive airway disease, bronchodilator
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide and is associated with disability and impaired quality of life.1 During the past 20 years, the number of deaths from COPD has markedly increased. COPD reduces quality of life by reducing the ability to work, limiting normal physical exertion, and affecting social activities. The incidence of COPD is projected to increase in several regions of the world, which continues to be an important cause of morbidity, mortality, and health-care costs worldwide.2 The pathophysiology of COPD has not been fully determined. However, COPD has been established as a condition that leads to lung abnormalities and also exerts systemic effects, including skeletal muscle dysfunction, cardiovascular disease, osteoporosis, diabetes, and psychiatric disorders. Systemic inflammation might play a major role in the pathogenesis of these systemic effects.3
Epidemiological studies have identified a strong association between COPD and comorbid psychiatric disorders including anxiety and depression.4 In COPD patients, anxiety5 and depression6 are associated with poor prognosis and often associated with younger age, female gender, smoking, cough, lower forced expiratory volume in 1 s, and more respiratory symptoms. The Global Initiative for Chronic Obstructive Lung Disease guidelines7 recommend that newly diagnosed COPD patients should be assessed for depression and anxiety, and anxiety, depression, and social problems should be discussed. In epidemiological studies, the association between COPD and the subsequent bipolar risk has been seldom reported. Most of the studies focused on the negative influences of bipolar disorder on the prognosis of COPD. Therefore, previous researches have provided abundant information on the topics including the prevalence, comorbidities, and mortality of patients with bipolar disorder in the COPD population.8–10
Over the past few years, inflammation has been revisited as an important etiologic factor of bipolar disorder.11 Although few studies could describe the clear mechanisms on the relationship between systemic inflammation and the development of bipolar disorder at present, evidence implies that systemic inflammation, especially those chronic or persistent one, might play crucial roles in the pathophysiology of bipolar disorder.12 One national cohort study have reported that COPD is more common in patients with bipolar disorder than in patients without the condition.10 However, the relationship between COPD and subsequent bipolar disorder remains unclear and there was no large-scale study addressed this issue. Therefore, we hypothesized that COPD increases the risk of bipolar disorder development. To evaluate this hypothesis, we designed a nationwide population-based study to evaluate the risk of bipolar disorder in patients with COPD.
Materials and methods
Database
Taiwan’s National Health Insurance (NHI) program, initiated by the government in 1995, provides comprehensive health care to almost all Taiwanese citizens, with a coverage rate of >99% of the entire population.13 The National Health Research Institutes (NHRIs) of Taiwan manages multiple NHI databases, which include basic demographic information and detailed orders related to ambulatory and inpatient care. The NHRI publicly releases the NHI databases for research purposes. In the databases, diseases are coded based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), 2001 Edition. The NHRI created a research database that includes a random sample of 1,000,000 subjects from the registry of all NHI enrollees in 2000. Personal information that could identify any individual patient is encrypted in the database. This study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital. Written consent was not obtained from study patients because the NHI dataset consists of deidentified secondary data for research purposes, and the IRB of Taipei Veterans General Hospital issued a formal written waiver for the need to obtain consent.
Study design and population
In this retrospective cohort study utilizing a nationwide database, patients aged 20 years or older with newly diagnosed COPD (ICD-9-CM codes 491.xx, 492.xx, 494.xx, and 496.xx) between January 1, 2000 and December 31, 2011 were enrolled. The COPD patients who were enrolled should be prescribed at least one bronchodilator during the follow-up period. Patients with a history of bipolar disorder prior to the enrollment date were excluded. A-code is a disease classification system developed especially for filing medical claims that was mainly used for ambulatory care before 2000 in Taiwan. A-code is a much simplified version of ICD-9-CM codes. To maintain the consistence between different claims records and to truly reflect the distribution of different diseases, the NHI program has switched the disease coding to the ICD-9-CM codes since 2000. In our study, A-code (A-code: A212) was mainly used to exclude the patients who were diagnosed with mood disorders, including both bipolar and depressive disorders, between 1996 and 2000.
Potential confounders
To analyze the effects of COPD on the outcome of bipolar disorder, we controlled for age, sex, and comorbidities as potential confounders. To adjust for the severity of comorbidities, the Charlson comorbidity index was calculated and used as a variable. According to the study design, chronic lung disease was exempted from the scoring system.
Comparison group
For each patient with newly diagnosed COPD, one subject matched for age, sex, and Charlson comorbidity index was randomly selected using the same index date. Comparison subjects were selected using incidence density sampling by computer programming.14 Subjects with a history of bipolar disorder prior to enrollment were excluded.
Outcome measures
The primary outcome of interest was the occurrence of bipolar disorder (ICD-9-CM codes 296.0X, 296.1X, 296.4X, 296.5X, 296.6X, 296.7X, 296.80, or 296.89) after the initial diagnosis of COPD. Patients with bipolar disorder who were prescribed with at least 1 month medication for bipolar disorder were included. All enrollees were followed from the index date until the first diagnosis of bipolar disorder, date of death, withdrawal from the NHI program, or December 31, 2011.
Exposure to COPD medications
Information on COPD medications was extracted to analyze their effects on bipolar disorder development in patients with COPD. During the follow-up period, patients who received ≥28 days of prescriptions for each COPD medication were categorized into the COPD medication groups. The five groups of COPD medication were short-acting beta 2-agonists (SABA: albuterol, fenoterol, terbutaline, and procaterol), long-acting beta2-agonists (LABA: salmeterol and formoterol), short-acting muscarinic antagonists (SAMA: ipratropium bromide), long-acting muscarinic antagonists (LAMA: tiotropium bromide), and inhaled corticosteroids (budesonide, fluticasone, and beclomethasone).
Statistical analysis
Data extraction and computation were performed using the Perl programming language (Version 5.12.2; Perl Foundation, Walnut, CA, USA). Microsoft SQL Server 2012 (Microsoft Corporation, Redmond, WA, USA) was used for data linkage, processing, and sampling. All statistical analyses were performed using IBM SPSS statistical software (version 19.0 for Windows; IBM Corp., New York, NY, USA). All data are expressed as mean ± standard deviation or n (%) unless otherwise stated. Comparisons between groups were performed using Student’s t test for continuous variables and Pearson’s chi-square test or Fisher’s exact test, as appropriate, for categorical variables. A log-rank test was used to compare the cumulative incidence curves of bipolar disorder between groups. Cox proportional hazards models were used to evaluate the association between COPD and bipolar disorder. Time-dependent Cox proportional hazards regression analysis was used to determine the effects of COPD medications on bipolar disorder development during the follow-up period. Risk factors with p < 0.1 in the univariate model were entered into the multivariable analysis. A p value < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study population
In this study, we evaluated 46,778 COPD patients and 46,778 subjects without COPD, who were matched 1:1 according to age, sex, and comorbidities. The COPD patients were predominantly male (n = 25,813, 55.2%) and their median age was 63 years (interquartile range, 50–74 years). From 2000 to 2011, we observed the COPD and comparison cohorts for 263,020.39 and 267,895.97 person-years, respectively (Figure 1). The median follow-up period for the COPD and comparison cohorts was 5.50 (interquartile range, 2.44–8.75) and 5.64 (interquartile range, 2.59–8.82) years, respectively. The median age at diagnosis was 63 years (interquartile range, 50–74). As shown in Table 1, the basic characteristics and selected comorbidities were similar between the two groups.
Figure 1.
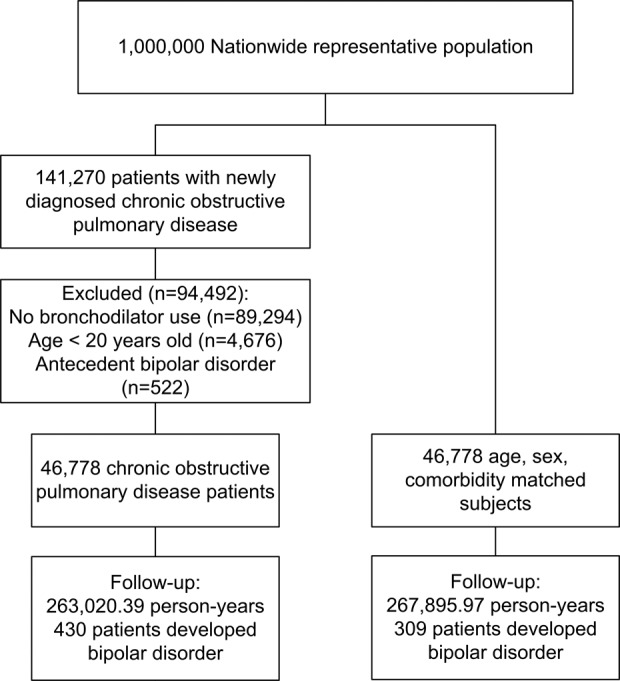
Flow diagram summarizing the process of enrollment and follow-up.
Table 1.
Basic characteristics of patients with COPD and the matched cohort.
| Patients with COPD (n = 46,778) | Matched cohort (n = 46,778) | p value | |||
|---|---|---|---|---|---|
| Characteristics | n | % | n | % | |
| Median age, years (IQR) | 63 (50–74) | 63 (50–74) | 1.000 | ||
| Median follow-up, years (IQR) | 5.50 (2.44–8.75) | 5.64 (2.59–8.82) | <0.001 | ||
| Age, years | |||||
| ≥65 | 24,556 | 52.5 | 24,556 | 52.5 | 1.000 |
| <65 | 22,222 | 47.5 | 22,222 | 47.5 | |
| Sex | |||||
| Male | 25,813 | 55.2 | 25,813 | 55.2 | 1.000 |
| Female | 20,965 | 44.8 | 20,965 | 44.8 | |
| Median Charlson comorbidity index (IQR)a | 2 (1–4) | 2 (1–4) | 1.000 | ||
| 0–1 | 20,925 | 44.7 | 20,925 | 44.7 | 1.000 |
| 2–3 | 14,047 | 30.1 | 14,047 | 30.1 | |
| ≥4 | 11,806 | 25.2 | 11,806 | 25.2 | |
| Charlson comorbiditiesa | |||||
| AIDS | 46 | 0.1 | 46 | 0.1 | 1.000 |
| Cancer | 5522 | 11.8 | 5522 | 11.8 | 1.000 |
| Cerebrovascular disease | 11,444 | 24.5 | 11,443 | 24.5 | 0.994 |
| Congestive heart failure | 6590 | 14.1 | 6587 | 14.1 | 0.978 |
| Dementia | 2495 | 5.3 | 2496 | 5.3 | 0.988 |
| Diabetes with end-organ damage | 3557 | 7.6 | 3557 | 7.6 | 1.000 |
| Diabetes without end-organ damage | 12,640 | 27.0 | 12,641 | 27.0 | 0.994 |
| Metastatic cancer | 760 | 1.6 | 760 | 1.6 | 1.000 |
| Myocardial infarction | 1688 | 3.6 | 1688 | 3.6 | 1.000 |
| Peptic ulcer disease | 22,842 | 48.8 | 22,845 | 48.8 | 0.984 |
| Peripheral vascular disease | 1986 | 4.2 | 1984 | 4.2 | 0.974 |
| Hemiplegia or paraplegia | 1678 | 3.6 | 1678 | 3.6 | 1.000 |
| Kidney disease | 5952 | 12.7 | 5954 | 12.7 | 0.984 |
| Connective tissue disease | 2858 | 6.1 | 2858 | 6.1 | 1.000 |
| Severe liver disease | 397 | 0.8 | 397 | 0.8 | 1.000 |
IQR: interquartile range; AIDS: acquired immunodeficiency syndrome; COPD: chronic obstructive pulmonary disease.
aChronic lung disease was exempted from scoring.
Predictors of the risk of bipolar disorder
During the follow-up period, 430 (0.92%) patients with COPD and 309 (0.66%) comparison subjects developed bipolar disorder. The log-rank test indicated a higher cumulative incidence of bipolar disorder in the COPD group than in the comparison group during the follow-up period (p < 0.001; Figure 2), suggesting that COPD patients are associated with increased risk of subsequent bipolar disorder in comparison with non-COPD subjects. After adjusting for patient age, sex, and comorbidities, the hazard ratio (HR) for the development of bipolar disorder in the COPD patients was 1.42 (95% confidence interval [CI], 1.22–1.64; p < 0.001), suggesting that COPD is an independent risk factor for bipolar disorder. In order to avoid the possible reverse causality caused by undiagnosed bipolar disorder and the possibility of surveillance bias, which are frequently seen while using the registered health insurance data, we conducted a sensitivity analysis by excluding the first year of follow-up duration after the date of COPD diagnosis. After excluding the patients with the development of bipolar disorder among both cohorts within the first year of follow-up period (84 patients in the COPD cohort and 61 patients in the comparison cohort were excluded), the adjusted HR for the development of bipolar disorder among both cohorts was 1.42 (95% CI 1.21–1.67; p < 0.001).
Figure 2.
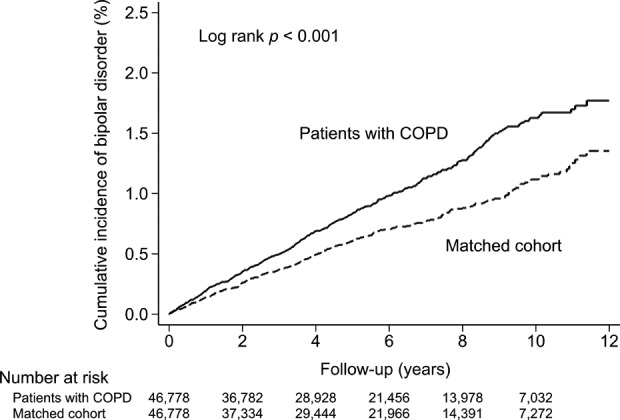
Cumulative incidence of bipolar disorder in patients with COPD and the matched cohort. COPD: chronic obstructive pulmonary disease.
Predictors of the risk of bipolar disorder in patients with COPD
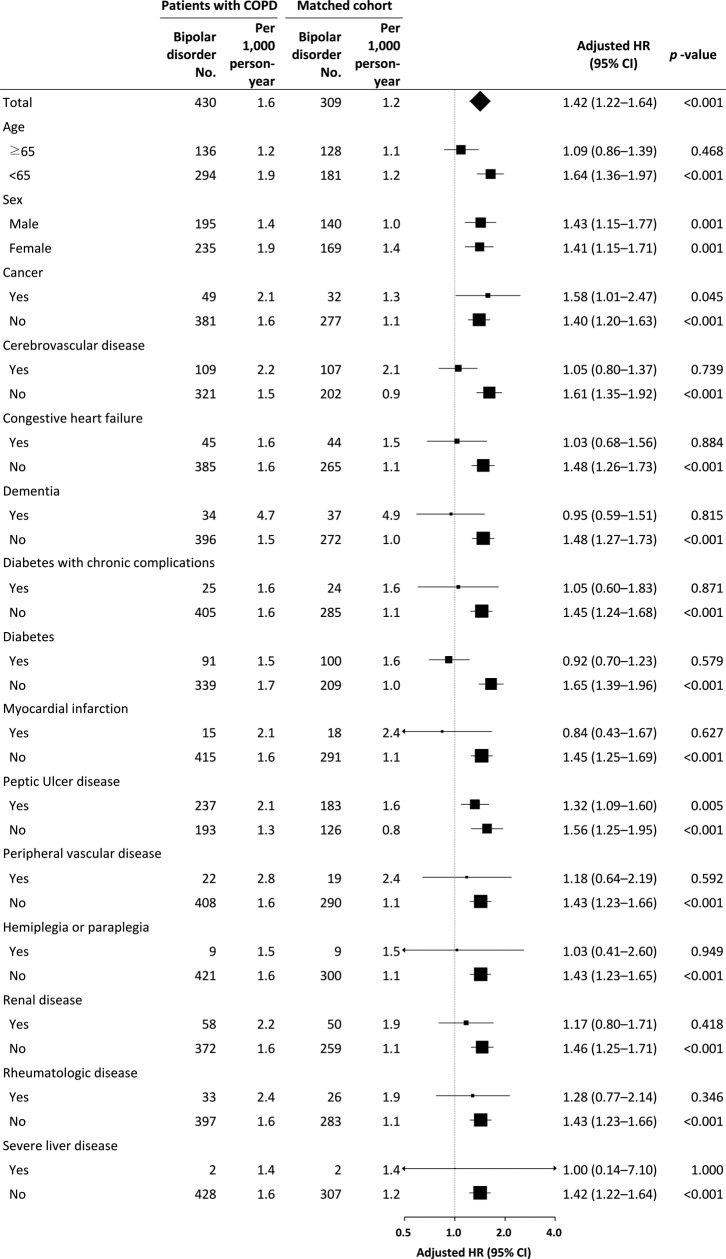
We identified the following independent risk factors for new-onset bipolar disorder in the COPD cohort: age < 65 years (HR, 2.01; 95% CI, 1.61–2.51; p < 0.001), female sex (HR, 1.30; 95% CI, 1.08–1.58; p = 0.007), cerebrovascular disease (HR, 1.35; 95% CI, 1.06–1.72; p = 0.015), dementia (HR, 3.11; 95% CI, 2.12–4.57; p < 0.001), and peptic ulcer disease (HR, 1.48; 95% CI, 1.22–1.80; p < 0.001; Table 2). Figure 3 shows the subgroup analyses. We identified an incidence rate of bipolar disorder of 1.6/1000 person-years in the COPD group and 1.2/1000 person-years in the comparison group (p < 0.001). Compared with the matched subjects, the incidence of bipolar disorder was significantly higher in the male or female or aged <65 years COPD patients. The HRs of the COPD patients were all significantly higher in their counterparts devoid of comorbidities, suggesting the independent role of COPD on bipolar disorder development.
Table 2.
Risk factors for bipolar disorder in patients with COPD.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age < 65 years | 1.56 (1.27–1.91) | <0.001 | 2.01 (1.61–2.51) | <0.001 |
| Female sex | 1.40 (1.16–1.70) | <0.001 | 1.30 (1.08–1.58) | 0.007 |
| Charlson comorbidities | ||||
| AIDS | 0.00 (0.00–a) | 0.968 | ||
| Cancer | 1.30 (0.97–1.76) | 0.081 | 1.24 (0.92–1.68) | 0.160 |
| Cerebrovascular disease | 1.46 (1.17–1.81) | 0.001 | 1.35 (1.06–1.72) | 0.015 |
| Congestive heart failure | 0.96 (0.71–1.31) | 0.810 | ||
| Dementia | 2.94 (2.07–4.19) | <0.001 | 3.11 (2.12–4.57) | <0.001 |
| Diabetes with end-organ damage | 0.99 (0.66–1.48) | 0.955 | ||
| Diabetes without end-organ damage | 0.90 (0.72–1.14) | 0.383 | ||
| Metastatic cancer | 1.77 (0.79–3.97) | 0.165 | ||
| Myocardial infarction | 1.25 (0.75–2.10) | 0.393 | ||
| Peptic ulcer disease | 1.57 (1.29–1.89) | <0.001 | 1.48 (1.22–1.80) | <0.001 |
| Peripheral vascular disease | 1.73 (1.12–2.66) | 0.013 | 1.53 (0.99–2.36) | 0.057 |
| Hemiplegia or paraplegia | 0.92 (0.48–1.79) | 0.815 | ||
| Kidney disease | 1.40 (1.06–1.85) | 0.018 | 1.25 (0.94–1.66) | 0.129 |
| Connective tissue disease | 1.47 (1.03–2.10) | 0.034 | 1.27 (0.88–1.81) | 0.199 |
| Severe liver disease | 0.86 (0.21–3.44) | 0.829 | ||
HR: hazard ratio; CI: confidence interval; AIDS: acquired immunodeficiency syndrome; COPD: chronic obstructive pulmonary disease.
aDo not converge.
Figure 3.
Multivariable stratified analysis for the association between COPD and bipolar disorder. COPD: chronic obstructive pulmonary disease.
We adjusted for the effects of COPD medications in a time-dependent Cox proportional hazards model after adjusting for potential confounding factors, including age, sex, and comorbidities. We analyzed five groups of COPD medication: SABA, LABA, SAMA, LAMA, and inhaled corticosteroids. The time-dependent Cox proportional hazards regression analysis indicated that the use of SABA is associated with significantly increased risk of bipolar disorder development (HR = 1.83, 95% CI = 1.25–2.69, p = 0.002). LABA, SAMA, LAMA, and inhaled corticosteroids were not associated with a risk of bipolar disorder development (Table 3).
Table 3.
The effect of COPD medications on bipolar disorder development among COPD patients.
| Univariate analysis | Multivariate analysisa | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| COPD medicationsb | ||||
| SABA | 1.84 (1.25–2.70) | 0.002 | 1.83 (1.25–2.69) | 0.002 |
| LABA | 0.87 (0.71–1.06) | 0.167 | ||
| SAMA | 0.94 (0.72–1.22) | 0.626 | ||
| LAMA | 0.73 (0.36–1.48) | 0.386 | ||
| Inhaled corticosteroid | 0.92 (0.74–1.14) | 0.435 | ||
HR: hazard ratio; CI: confidence interval; SABA: short-acting beta2-agonist; LABA: long-acting beta2-agonist; SAMA: short-acting muscarinic antagonist; LAMA: long-acting muscarinic antagonists; COPD: chronic obstructive pulmonary disease..
aAdjusted for variables listed in Table 2 multivariate analysis.
bCOPD medications were analyzed as a time-dependent covariate in the Cox regression model.
Discussion
The study results indicate an increased incidence of subsequent bipolar disorder in COPD patients. The COPD patients were associated with a 42% increased incidence of bipolar disorder compared with the comparison group. These results support the hypothesis that COPD may be a risk factor for bipolar disorder. According to our research, the present study is the largest population-based cohort study to investigate the risk of bipolar disorder in patients with COPD.
Previous studies have identified strong associations between COPD and psychiatric disorders such as depressive and anxiety disorders. However, few studies have evaluated bipolar disorders in patients with COPD. Kunik et al.4 conducted a cross-sectional study in which 1334 patients with diagnoses of chronic breathing disorders were analyzed. Of the patients with COPD, 65% had been diagnosed with an anxiety or depressive disorder and 31% were receiving treatment for the psychiatric disorder. In other studies, untreated or undertreated anxiety and depression negatively affected medical compliance, increased the frequency of hospital admissions, and prolonged hospitalization in patients with COPD.5,6 A recent study reported that a lack of treatment for psychiatric disorders leads to poor quality of life and increases mortality.7 Although the effects of psychiatric disorders on COPD patients have been recognized by clinicians and investigators, no previous large-scale study has investigated the association between COPD and bipolar disorder.
Systemic inflammation and chronic hypoxia are common complications of COPD are well-established possible mechanisms that underlie the pathophysiology of COPD.3 Explanations for increased risk of bipolar disorder in patients with COPD may be inflammatory processes and hypoxic damage. Increased circulation levels of inflammatory cytokines in stable COPD or COPD exacerbations result in systemic inflammation.15 Circulating cytokines in the plasma can impair the function of the blood-brain barrier,16 indicating that peripheral inflammation is associated with the upregulation of central nervous system (CNS) inflammation. COPD might increase the risk of CNS abnormalities, including mood disturbance, by increasing systemic inflammation. Studies have demonstrated that chronic inflammation plays a vital role in the pathophysiology of common psychiatric disorders,17 including bipolar disorder.18 Chronic systemic inflammation might provide an explanation for the association between COPD and bipolar disorder. Studies have also identified that intermittent hypoxia from obstructive sleep apnea can cause CNS oxidative stress and inflammation.19 Chronic hypoxia in COPD can promote the progression of brain dysfunction.20 Two large multicenter trials,21,22 conducted in the 1980s, revealed a significant impairment of motor and executive functioning, learning, and memory in COPD patients with mild hypoxia. In hypoxic COPD patients, chronic hypoxia increases blood viscosity, leading to arterial cerebral hypoperfusion23 and hypoxic damage to neurotransmitter regulation.24 Aerobic exercise and regular use of home oxygen could thus improve central nervous system function in hypoxic COPD patients.25,26 We hypothesize that chronic hypoxia in COPD patients can lead to bipolar disorder development through hypoxia-induced inflammatory processes.19
Other possible explanations for the increased risk of bipolar disorder in patients with COPD are sleep disturbance and stress. Sleep apnea and nocturnal desaturation are common in COPD and positively correlate with the severity of COPD.27 Sleep is a powerful regulator of immune function and immune responses. Prolonged sleep loss can induce systemic inflammatory responses.28 In COPD patients, stress is directly related to illness and disabilities. Stress has also been associated with the development of bipolar disorder.29
In this study, we analyzed the effects of COPD medications, including SABA, LABA, SAMA, LAMA, and inhaled corticosteroids, on bipolar disorder development. We observed that SABA are associated with a significantly increased risk of bipolar disorder development (HR = 1.83, p = 0.002), whereas LABA, SAMA, LAMA, and inhaled corticosteroids are not associated with a risk of bipolar disorder development. Previous studies have indicated that numerous cytokines circulating in the peripheral plasma might impair blood-brain barrier function,16 suggesting that peripheral inflammation is associated with the upregulation of central nervous system inflammation.30 The finding is acceptable with regard to the potential anti-inflammatory effects of COPD medications. An increasing body of experimental and clinical evidence31–33 supports that LABA, SAMA, LAMA, and inhaled corticosteroids exert clinically relevant anti-inflammatory effects. By contrast, SABA, when used regularly, increase airway inflammation and bronchial hyperresponsiveness and reduce lung function.34 The present study results indicate that the regular use of SABA might represent a risk factor for bipolar disorder. Another possible explanation is that acute exacerbation of COPD might be triggered by bipolar disorder.35 The explanation provides an association between SABA and bipolar disorder. However, SAMA was also used for acute exacerbations of COPD but did not appear to affect bipolar disorder development in this study.
The major strength of this study is its nationwide population-based study design. Because all pulmonary and psychiatric practices are covered in Taiwan’s insurance system, we were able to trace all cases of newly diagnosed COPD and bipolar disorder during the study period. Participation in the NHI is mandatory, and all Taiwanese residents can use medical care with a low copayment; therefore, the follow-up was completed with minimal referral bias. In this study, the diagnosis of COPD required clinical assessments and a prescription including at least one bronchodilator. Bipolar disorder is categorized as a catastrophic illness in Taiwan’s insurance system. A patient with a certificate of catastrophic illness is not required to pay related medical costs and clinical data must be provided for peer review. Therefore, the diagnoses of COPD and bipolar disorder are considered reliable and exhaustive.
This study has several limitations that must be mentioned. First, we identified patients with COPDusing a diagnostic code in a database, which introduces the possibility of misclassification because of coding errors or misdiagnosis. To reduce misclassification bias, we did not include COPD patients who did not use bronchodilators. However, any misclassification bias would be toward the null hypothesis, leading to the underestimation of the actual effects of COPD. Second, we did not include several potential risk factors, including obesity, smoking, and a family history of bipolar disorder, in the analyses because these data were unavailable. Further research is required to confirm the results and determine the underlying pathogenesis.
Conclusion
This population-based study demonstrated that patients with COPD are associated with an increased risk of bipolar disorder. Additional studies are required to elucidate the pathogenic mechanisms underlying the association between COPD and bipolar disorder.
Footnotes
Author contribution: Vincent Yi-Fong Su and Li-Yu Hu contributed equally to this manuscript.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28: 523–532. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 1997; 349: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 3. Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest 2011; 139: 165–173. [DOI] [PubMed] [Google Scholar]
- 4. Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005; 127: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 5. Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax 2010; 65: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007; 167: 60–67. [DOI] [PubMed] [Google Scholar]
- 7. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 8. Beyer J, Kuchibhatla M, Gersing K, et al. Medical comorbidity in a bipolar outpatient clinical population. Neuropsychopharmacology 2005; 30: 401–404. [DOI] [PubMed] [Google Scholar]
- 9. Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord 2004; 6: 368–373. [DOI] [PubMed] [Google Scholar]
- 10. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psych 2013; 70: 931–939. [DOI] [PubMed] [Google Scholar]
- 11. Rosenblat JD, Cha DS, Mansur RB, et al. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014; 53: 23–34. [DOI] [PubMed] [Google Scholar]
- 12. Stertz L, Magalhaes PV,, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry 2013; 26: 19–26. [DOI] [PubMed] [Google Scholar]
- 13. Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beaumont JJ, Steenland K, Minton A, et al. A computer program for incidence density sampling of controls in case-control studies nested within occupational cohort studies. Am J Epidemiol 1989; 129: 212–219. [DOI] [PubMed] [Google Scholar]
- 15. Wouters EF, Groenewegen KH, Dentener MA, et al. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc 2007; 4: 626–634. [DOI] [PubMed] [Google Scholar]
- 16. Abbott NJ, Ronnback L,, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 17. Kivimaki M, Shipley MJ, Batty GD, et al. Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psych 2014; 19: 149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein BI, Kemp DE, Soczynska JK, et al. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psych 2009; 70: 1078–1090. [DOI] [PubMed] [Google Scholar]
- 19. Yang Q, Wang Y, Feng J, et al. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat 2013; 9: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grant I, Heaton RK, McSweeney AJ, et al. Brain dysfunction in COPD. Chest 1980; 77: 308–309. [DOI] [PubMed] [Google Scholar]
- 21. Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142: 1470–1476. [PubMed] [Google Scholar]
- 22. Prigatano GP, Parsons O, Wright E, et al. Neuropsychological test performance in mildly hypoxemic patients with chronic obstructive pulmonary disease. J Consult Clin Psychol 1983; 51: 108–116. [DOI] [PubMed] [Google Scholar]
- 23. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995; 152: S77–S121. [PubMed] [Google Scholar]
- 24. Dustman RE, Ruhling RO, Russell EM, et al. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging 1984; 5: 35–42. [DOI] [PubMed] [Google Scholar]
- 25. Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 2010; 5: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emery CF, Honn VJ, Frid DJ, et al. Acute effects of exercise on cognition in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1624–1627. [DOI] [PubMed] [Google Scholar]
- 27. Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999; 14: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 28. Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 2009; 51: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Etain B, Henry C, Bellivier F, et al. Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord 2008; 10: 867–876. [DOI] [PubMed] [Google Scholar]
- 30. Lampa J, Westman M, Kadetoff D, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci USA 2012; 109: 12728–12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Remington TL, Digiovine B. Long-acting beta-agonists: anti-inflammatory properties and synergy with corticosteroids in asthma. Curr Opin Pulm Med 2005; 11: 74–78. [DOI] [PubMed] [Google Scholar]
- 32. Zhang W, Fievez L, Cheu E, et al. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur J Pharmacol 2010; 628: 171–178. [DOI] [PubMed] [Google Scholar]
- 33. Perng DW, Tao CW, Su KC, et al. Anti-inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPD. Eur Respir J 2009; 33: 778–784. [DOI] [PubMed] [Google Scholar]
- 34. Aldridge RE, Hancox RJ, Robin Taylor D, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med 2000; 161: 1459–1464. [DOI] [PubMed] [Google Scholar]
- 35. Laurin C, Labrecque M, Dupuis G, et al. Chronic obstructive pulmonary disease patients with psychiatric disorders are at greater risk of exacerbations. Psychosom Med 2009; 71: 667–674. [DOI] [PubMed] [Google Scholar]