Abstract
This study investigated the validity and reliability of fixed strain gauge measurements of isometric quadriceps force in patients with chronic obstructive pulmonary disease (COPD). A total cohort of 138 patients with COPD were assessed. To determine validity, maximal volitional quadriceps force was evaluated during isometric maximal voluntary contraction (MVC) manoeuvre via a fixed strain gauge dynamometer and compared to (a) potentiated non-volitional quadriceps force obtained via magnetic stimulation of the femoral nerve (twitch (Tw); n = 92) and (b) volitional computerized dynamometry (Biodex; n = 46) and analysed via correlation coefficients. Test–retest and absolute reliability were determined via calculations of intra-class correlation coefficients (ICCs), smallest real differences (SRDs) and standard errors of measurement (SEMs). For this, MVC recordings in each device were performed across two test sessions separated by a period of 7 days (n = 46). Strain gauge measures of MVC demonstrated very large correlation with Tw and Biodex results (r = 0.86 and 0.88, respectively, both p < 0.0001). ICC, SEM and SRD were numerically comparable between strain gauge and Biodex devices (ICC = 0.96 vs. 0.93; SEM = 8.50 vs. 10.54 N·m and SRD = 23.59 vs. 29.22 N·m, respectively). The results support that strain gauge measures of quadriceps force are valid and reliable in patients with COPD.
Keywords: Isometric force measurement, muscle testing, rehabilitation, assessment, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with systemic manifestations and comorbidities that impact functional capacity, health-related quality of life and prognosis.1 Peripheral muscle weakness, particularly of the large quadriceps muscles, is highly prevalent in patients with COPD2,3 and is an important target of comprehensive disease management due to its vital role in activities of daily living, its contribution to exercise intolerance,4 known dysfunction compared to healthy controls5–7 and remediable nature.8 Addressing this dysfunction is a key aim of the exercise training component of pulmonary rehabilitation.3,9 Interestingly, little is known about the psychometric properties of strength measures in an elderly population in general and in patients with COPD in particular. Since the latter have altered structural and metabolic properties of their skeletal muscles,6 it seems important to specifically validate techniques to assess muscle strength in this population.
Measurement of peripheral muscle force is typically simple and feasible for most patients with COPD. Common manoeuvres used to measure volitional muscle force include isometric, isotonic or isodynamic maximum voluntary contractions (MVCs)10 or dynamic one-repetition maximum contraction (1RM).11 Common equipment used for this purpose includes handheld dynamometry, seated strain gauge12 or computerized dynamometry.13 The choice of technique usually depends upon the desired level of accuracy and clinical indication(s). All tests suffer from potential error related to central fatigue, poor motivation or variability induced by the assessor. Non-volitional assessment of muscle force is performed via electrical or magnetic stimulation of a peripheral motor nerve to derive a measure of muscle twitch (Tw) force. While excellent correlations have been demonstrated between Tw with MVC force in healthy controls14 and patients with COPD,15 such measures are not routinely performed in clinical practice due to the high equipment costs and necessity for examiner skill. They remain, however, a reference method in research settings to answer specific physiologic questions.
In patients with COPD, quadriceps MVC manoeuvres are frequently performed via isometric contraction.16 Isometric MVCs consist of maximal contractions conducted against a resistance at a fixed joint angle.7 They are easily implemented into clinical practice and provide reliable and reproducible measures of muscle force.6 Measurement of isometric quadriceps force is often performed via commercially available computerized dynamometers; however, despite its reputation as a ‘reference method’18 for volitional muscle force testing, its use in clinical practice is impeded by the high equipment costs and large space requirements. The fixed strain gauge offers simple and fast user applicability at considerably lower cost than computerized dynamometry and was recommended as a ‘low implementation cost’ technique to measure isometric force in the recent american thoracic society (ATS)/european respiratory society (ERS) statement on limb muscle dysfunction in COPD.6 A review by Robles and colleagues16 highlighted its increasing use in COPD research but cited a lack of COPD-specific reliability data as an important area for future research. This knowledge gap underpins the relevance of the present research.
The primary aim of this study was therefore to determine the validity (how well an instrument measures what it purports to measure),19 test–retest reliability (the magnitude of the error in observed measurements of the inherent variability between subjects)20 and agreement (how close two measurements from the same subject are)20 of fixed strain gauge measures of quadriceps muscle force in patients with COPD. The secondary aims were to determine the presence of (1) test fatigue (defined by a decreased repeated force measurement during a single visit), (2) a learning effect (defined by an increased muscle force measurement during the second visit compared to the first visit, with 7 days in between), and (3) any true absolute difference between quadriceps force measurements obtained from the strain gauge and Biodex devices across both visits.
Methods
Test procedures
Data from a sample of convenience of 138 individuals participating in the previous21–23 or current (NCT02113748) clinical trials at UZ Gasthuisberg, Leuven (Belgium) were included in this combined retrospective/prospective study. All studies were approved by the ethics committee of University Hospital Leuven, and a written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Inclusion criteria comprised diagnosis of COPD according to global initiative for chronic obstructive lung disease (GOLD) recommendations,1 age ≥40 years and smoking history ≥10 pack-years. Patients were ineligible for inclusion if they had a primary respiratory disease other than COPD (e.g. asthma) documented in their medical record, impairment of normal biomechanical movement (e.g. significant coexisting orthopaedic, neurological or other condition) or significant cognitive impairment, as judged by study investigators.
In one cohort of 92 patients, peak volitional contractile quadriceps force was assessed during an isometric MVC manoeuvre via a fixed strain gauge dynamometer with signal analogue force transducer (546QD; CDS Milan, Italy) and amplifier (Biopac MP150; Biopac Systems, Goleta, California, USA). Peak volitional force was compared with non-volitional Tw force obtained via magnetic stimulation of the femoral nerve at 100% power output of a Magstim stimulator (Magstim Co Ltd, Whitland, UK) 3 seconds post-MVC (in the passive, relaxed state). Maximality of the non-volitional contraction was ensured by increasing the power output of the magnetic stimulator and ensuring that the Tw force did not further increase between 90% and 100% of the power output (supramaximal stimulation). These measurements were performed during a single visit, with patients seated in a semi-reclined chair that provided 90° knee flexion and 120° hip flexion to optimize the stimulation of the femoral nerve, in accordance with previously published data.21 Isometric quadriceps MVCs were sustained for 3 seconds and repeated a total of five times, with 30-second rest intervals between contractions. These data were retrospectively collected from patients’ records in the aforementioned studies.
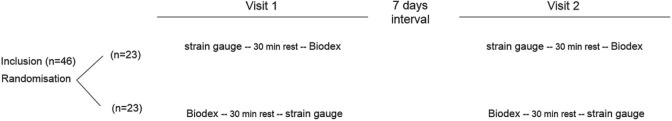
An independent, second cohort of 46 patients was prospectively assigned to undergo repeated assessments conducted over two visits, separated by 1 week. In this group, measures of peak isometric quadriceps force and torque were obtained from both the fixed strain gauge and a computerized dynamometer (Biodex system 4 pro – Enraf Nonius; Delft, the Netherlands) with a minimum of 30 minutes rest between test procedures. Device sequence (strain gauge/Biodex or Biodex/strain gauge) was determined via random allocation and kept constant across visits (Figure 1).
Figure 1.
Overview of data collection design for validity and reliability study (n = 46).
While measures of peak isometric quadriceps force were yielded from both methods, slight differences existed between the test procedures. In accordance with conventional procedures, MVCs for the Biodex were performed over four manoeuvres of 6 seconds duration with 20-second rest intervals and a knee position of 60° flexion. Quadriceps force expressed as absolute and percentage of predicted normal values.24 Strain gauge measures were obtained over five MVC manoeuvres of 5 seconds duration with 30-second rest intervals. As this cohort did not need to perform non-volitional (Magstim) procedures, both the hip and knee joints were positioned in 90° flexion (conventional test position for strain gauge). In order to compare data between the strain gauge (expressed as force, in Newtons) and Biodex (expressed as torque, Newton metres [N·m]) in this cohort, leg length was measured from the middle of the fibula head (axis of rotation) to the top of malleolus (fixed point where the force was applied) and strain gauge torque measures calculated using the formula (N·m = leg length [m] × Newtons).
All data measurements were recorded after one practice trial on each device, and all patients received maximal encouragement by the investigator during MVC manoeuvres, including provision of visual feedback on a computer screen. Final test results were not disclosed to patients until completion of the last test procedure. All test procedures were conducted by the same assessor for each patient, and the assessment was standardized to the right leg. The highest (peak) value of three reproducible manoeuvres from five attempts (allowing no more than 5% variance) was used for analysis.
All participants underwent detailed lung function and functional exercise capacity (6-minute walk test) assessments according to ERS standards25,26 for purposes of characterization.
Analysis
Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Data are presented as mean ± SD. Statistical significance was denoted by p < 0.05 for all statistical tests.
Validity was investigated via two methods: inspection of the relationship between peak volitional strain gauge quadriceps force (Newton [N]) and Tw (N), in the cohort of 92 patients, and Biodex measures (N·m), in the cohort of 46 patients. Pearson correlation coefficients were calculated, with r values in the range of 0.0–0.1 considered trivial, 0.1–0.3 small, 0.3–0.5 moderate, 0.5–0.7 large, 0.7–0.9 very large and 0.9–1.0 extremely large.27
Strain gauge and Biodex test–retest reliability (n = 46) across the two clinical visits were determined via calculation of intra-class correlation coefficients (ICCs) using the formula ICC = S 2 B/(S 2 B + S 2 W), where S 2 B and S 2 W represent the between-subject variance (S 2 B) and the within-subject variance (S 2 W). ICC values were interpreted as <0.4 poor, 0.4–0.75 fair to good and >0.75 excellent.28 Absolute reliability was evaluated by the standard error of measurement (SEM), calculated as SEM = Sx × √(1−ICC), where Sx is the standard deviation of the baseline measurement. The smallest real difference (SRD), indicating a 95% confidence interval around the SEM measurement, was calculated from the formula SRD = 1.96 × √2 × SEM.29 The percentage was calculated as SRD% = (SRD/mean) × 100.
Test–retest agreement of volitional torque measures for both devices during the two clinic visits (n = 46) was also investigated via Bland–Altman plots (mean difference vs. average of the two visits) for each device (separately) using GraphPad Prism 5.0 and mean difference and limits of agreement reported. Repeatability was reported via the coefficient of repeatability and its precision, as described by Bland et al.30
The secondary aims (n = 46) were addressed via linear mixed models with quadriceps torque as the dependent variable. Class variables were order of measurement (first or second assessment, indicative of ‘fatigue’), visit (first or second, indicative of ‘learning’), device (strain gauge or Biodex, indicative of absolute difference between both devices) and patient identification. An interaction factor (device × visit) was included to investigate any differences in learning effects attributable to device.
Results
Baseline characteristics of both cohorts from the study are presented in Table 1. One patient from the first cohort and three from the second did not perform the 6-minute walk test (6MWT). In two patients, one force measurement on one of the devices was missing, so these data were not included in the test–retest analyses, resulting in n = 45 and n = 44 for strain gauge and Biodex, respectively.
Table 1.
Participants’ characteristics.a
| Validity cohort | Validity and reliability cohort | |
|---|---|---|
| n | 92 | 46 |
| Gender (%men) | 67 | 78 |
| Age (years) | 65 ± 8 | 67 ± 6 |
| BMI (kg/m2) | 25 ± 6 | 26 ± 4 |
| FEV1/FVC | 0.41 ± 0.12 | 0.50 ± 0.14 |
| FEV1 (%pred) | 45 ± 15 | 65 ± 21 |
| 6MWT (m) | 426 ± 129 | 515 ± 136 |
| 6MWT (%pred) | 66 ± 19 | 81 ± 19 |
| Quadriceps force (%pred)b | 80 ± 35 | 89 ± 20 |
BMI: body mass index; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; 6MWT: 6-minute walk test.
aData are presented as mean ± SD. One patient from the first cohort and three from the second did not perform 6MWT. One patient from the second cohort did not perform muscle force assessment during visit 1 due to leg pain after completion of the 6MWT.
bPercentage of predicted was calculated for quadriceps force measured by Biodex.
Validity
A very large correlation was evident between strain gauge measures of peak quadriceps force and non-volitional Tw force (r = 0.86, p < 0.001; Figure 2), independent of gender (n = 92). In the cohort of 46 patients, a very large correlation was also evident between MVC recorded from strain gauge (torque calculated from the original force measures) and MVC from Biodex (r = 0.88, p < 0.0001), as assessed during the first visit.
Figure 2.
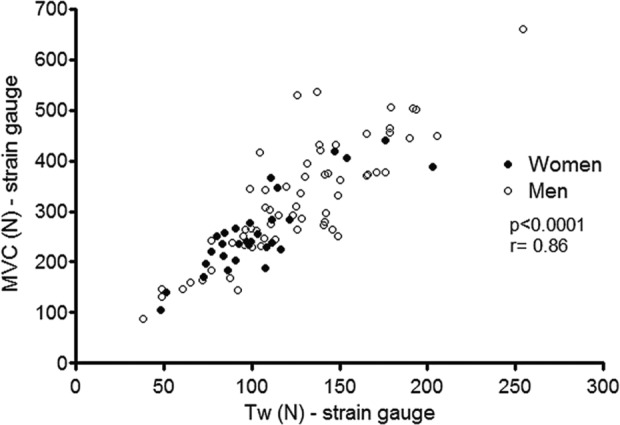
Correlation between non-volitional and maximal voluntary quadriceps force measured by the strain gauge (n = 92).
Test–retest and absolute reliability
A summary of peak volitional quadriceps force measures and reliability estimates (ICC, SEM, SRD, SRD%) obtained during the two visits for both devices is presented in Table 2.
Table 2.
Quadriceps force and reliability estimates obtained from visits 1 and 2 for strain gauge and Biodex (based on the cohort of 46 patients).
| Strain gauge | Biodex | |
|---|---|---|
| Quadriceps torque V1, N·m (mean ± SD) | 130.82 ± 42.48 | 132.54 ± 39.83 |
| Quadriceps torque V2, N·m (mean ± SD) | 135.30 ± 42.87 | 130.92 ± 40.78 |
| ICC | 0.96 | 0.93 |
| SEM, N·m | 8.50 | 10.54 |
| SRD, N·m | 23.56 | 29.22 |
| SRD, % | 18.01 | 22.05 |
V1: first visit for measurements; N·m: Newton meter; V2: second visit for measurements; ICC: intra-class correlation coefficient; SEM: standard error of measurement; SRD: smallest real difference; %: percentage.
Test–retest Bland–Altman analyses for strain gauge (Figure 3) and Biodex (Figure 4) revealed good mean agreement and narrow limits of agreement across the two visits. For the strain gauge, mean difference was 3.74 N·m and limits of agreement −17.68 N·m to 25.15 N·m. For Biodex, mean difference was −1.67 N·m and limits of agreement −31.74 N·m to 28.41 N·m. The coefficients of repeatability were ±21.42 and ±30.07 N·m for strain gauge and Biodex, respectively.
Figure 3.
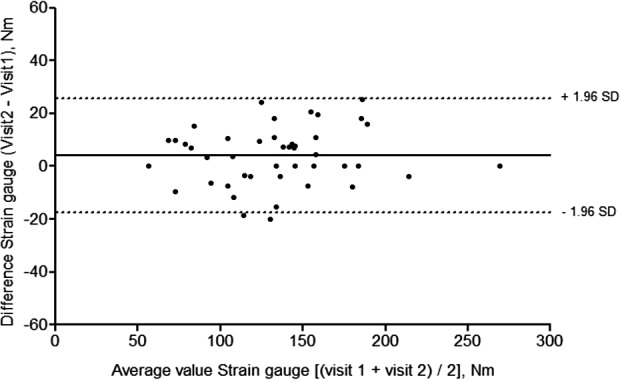
Bland–Altman plot of test–retest agreement across visits 1 and 2, strain gauge (n = 44).
Figure 4.
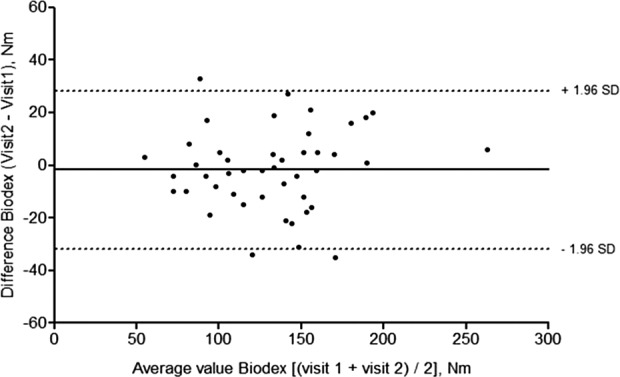
Bland–Altman plot of test–retest agreement across visits 1 and 2, Biodex (n = 45).
Further explorations of test performance (secondary study aims)
Mean muscle force significantly decreased (−6%) from the first to second test of each visit (136 ± 40 N·m vs. 128 ± 40 N·m, respectively; p < 0.001). No differences existed between mean muscle force measurements at visits 1 and 2 (132 ± 40 N·m vs. 133 ± 40 N·m, respectively; p = 0.53), and no learning effects were detected for either device (interaction device × visit, p = 0.18). Overall mean muscle force measures did not differ between strain gauge and Biodex (133 ± 40 N·m and 132 ± 40 N·m, respectively; p = 0.64).
Discussion
The present study findings are novel and relevant in supporting the validity of the strain gauge to measure MVCs in patients with COPD. It also indicates that measurements obtained with this device are at least as reliable and reproducible as those obtained via computerized dynamometry, considered the ‘gold standard’ for MVC measures.18 As such, these data strongly support the recommendations of the ATS/ERS regarding assessment of quadriceps force using the strain gauge in patients with COPD.6 This is important because robust computerized dynamometers, while commonly used to assess isometric force in COPD research,17,31 are not easily available within the clinical environment. A recent international survey reported the evaluation of lower limb muscle force, upper limb force, lung function and body composition (pooled response option), occurs in only 20% of pulmonary rehabilitation programs,32 potentially due to limitations such as the availability of appropriate equipment. Our findings add to this scant literature to support the use of a strain gauge as a simple but equally robust measure of quadriceps muscle force in patients with COPD.
A strength of the present study was validation of the strain gauge against both volitional and non-volitional quadriceps contractions. The very large relations between these measures in our data (p < 0.0001, r 2 = 0.76) are in line with those observed by Polkey et al.14 who reported on the use of magnetic femoral nerve stimulation in healthy subjects and those with suspected muscle weakness (p < 0.0001, r 2 = 0.83). Validation of the strain gauge against the Biodex system enabled comparison with gold standard dynamometry for assessment of muscle function. The very large correlation between isometric MVC measures from the strain gauge and Biodex reinforces the validity of this technique.
To the best of our knowledge, test–retest reliability of the fixed strain gauge has not been previously reported, nor has direct comparison been made between the Biodex system 4 pro used in isometric mode. Test–retest reliability of the strain gauge was confirmed in our study through the verified ICC, SEM and SRD estimates. The results slightly favoured the strain gauge over Biodex (lower SEM and SRD values); however, the very small magnitude of difference is of questionable clinical relevance. These outcomes demonstrate high precision of the measurement29 to discriminate small differences upon measurement.33 No pattern of systematic over- or underestimation was observed for the strain gauge in the Bland–Altman plot and dispersion around the mean was less than the Biodex. Taken in consideration with the small mean differences of each device (3.74 and −1.67 N·m for strain gauge and Biodex, respectively) and the acceptable repeatability coefficients (± 21.42 for strain gauge and ± 30.07 for Biodex), we feel this supports the strain gauge as an adequate method for assessing quadriceps force compared to Biodex. Our test–retest reliability estimates for the Biodex system compare favourably with those of other studies performed in patients with COPD,34 healthy subjects35,36 and people with late effects of polio,37 strengthening the external validity of our findings. While the SRD for both devices was relatively large in our study, this appears consistent with the findings from Flansbjer and Lexell37 derived from Biodex measures of isometric extension of knee extension (SRD% = 17.8 in the less affected limb).
We described slight differences in the testing protocols between the two devices across the different patient cohorts, attributed primarily to positioning of the hip and knee joints. The increased hip extension with the strain gauge was necessary in order to provide effective femoral nerve stimulation with the magnetic stimulator, and the decreased knee flexion with the Biodex used in accordance with previous research in the COPD patient group that allows the comparison with predicted values.24 Position variation may have influenced the generation of torque due to changes in neural activation, muscle fibres length–tension relationship (with 60° typically considered ‘ideal’), and/or the complex force transmission through the knee joint.38,39 Early data from Knapik et al.40 indicated that isometric peak torque of the leg extensors was greater at 60° than 90° flexion, and Hahn41 more recently verified that the isometric multi-joint leg extension torque-generating capacity also differs according to knee angle in young healthy men. Data from Krishnan and Williams39 and Herzog et al.,42 however, contradict this, showing an absence of difference in isometric torque generated at either 60° or 90° knee flexion. In consideration of this information, and the very comparable data pertaining to absolute force and ICCs from both devices in present study, we do not suspect knee position to have adversely affected the findings of our data. Interestingly, the detection of fatigue in our sample suggests that 30 minutes may not be enough for complete muscle recovery. This fact did not, however, prevent clear analysis of results regarding the validity and reliability of the strain gauge as the randomized device order enabled device-specific analysis to proceed with confidence.
Future research may be indicated to develop predictive normal values for strain gauge measures of quadriceps strength. Early research in this area43 suggests normal values for adults’ MVC are approximately 75% of the body weight. While body weight is an important factor, a minimum age and gender should also be taken into account. These aspects were considered in the prediction formula later described by Seymour et al.44 However, the need for fat-free mass measurement limits its applicability in clinical practice. An updated, robust but simple estimate would be of importance to the future clinical implementation of this technique – an integral outcome of the present research.
In summary, this study provides evidence that the fixed strain gauge method to measure quadriceps muscle strength, as proposed in the consensus statement of the ATS and ERS,6 is valid and reliable for the measurement of isometric quadriceps force in patients with COPD.
Acknowledgements
The authors would like to thank the staff of the Pulmonary Function Department at Gasthuisberg University Hospital (Leuven, Belgium) and the clinical trial unit for their help with the clinical assessments of patients enrolled in this study.
Footnotes
Author contribution: Fernanda Machado Rodrigues and Heleen Demeyer have contributed equally. Thierry Troosters and Christian Osadnik supervised equally.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Flemish Research Foundation (FWO #G.0871.13) and PROactive IMI-JU.115011 FMMR and CAC are funded by The National Council for Scientific and Technological Development (CNPq), Brazil (249579/2013-8 and 202425/2011-8, respectively). CO was the recipient of a long-term European Respiratory Society Fellowship (LTRF 2014 – 3132). CB was a doctoral fellow of Research Foundation Flanders at the time of data collection. HD is the recipient of a joint ERS/SEPAR long-term fellowship (LTRF 2015).
References
- 1. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4): 347–365. [DOI] [PubMed] [Google Scholar]
- 2. Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med 2012; 106(1): 102–108. [DOI] [PubMed] [Google Scholar]
- 3. Vogiatzis I, Simoes DC, Stratakos G, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J 2010; 36(2): 301–310. [DOI] [PubMed] [Google Scholar]
- 4. Zanotti E, Felicetti G, Maini M, et al. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003; 124(1): 292–296. [DOI] [PubMed] [Google Scholar]
- 5. Man WD, Hopkinson NS, Harraf F, et al. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax 2005; 60(9): 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189(9): e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyberg A, Saey D, Maltais F. Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015; 12(9): 1269–1277. [DOI] [PubMed] [Google Scholar]
- 8. Troosters T. Endurance versus strength training in chronic obstructive pulmonary disease: 2) Resistance training. Chron Respir Dis 2004; 1(1): 40–41. [DOI] [PubMed] [Google Scholar]
- 9. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188(8): e13–e64. [DOI] [PubMed] [Google Scholar]
- 10. O‘Shea SD, Taylor NF, Paratz JD. Measuring muscle strength for people with chronic obstructive pulmonary disease: retest reliability of hand-held dynamometry. Arch Phys Med Rehabil 2007; 88(1): 32–36. [DOI] [PubMed] [Google Scholar]
- 11. Covey MK, Collins EG, Reynertson SI, et al. Resistance training as a preconditioning strategy for enhancing aerobic exercise training outcomes in COPD. Respir Med 2014; 108(8): 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rausch-Osthoff AK, Kohler M, Sievi NA, et al. Association between peripheral muscle strength, exercise performance, and physical activity in daily life in patients with Chronic Obstructive Pulmonary Disease. Multidiscip Respir Med 2014; 9(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(10): 1072–1077. [DOI] [PubMed] [Google Scholar]
- 14. Polkey MI, Kyroussis D, Hamnegard CH, et al. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve 1996; 19(5): 549–555. [DOI] [PubMed] [Google Scholar]
- 15. Troosters T, Janssens W, Decramer M. Managing skeletal muscle dysfunction in COPD. Eur Respir Monogr 2013; 59: 164–173. [Google Scholar]
- 16. Robles PG, Mathur S, Janaudis-Fereira T, et al. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev 2011; 31(1): 11–24. [DOI] [PubMed] [Google Scholar]
- 17. Vilaro J, Rabinovich R, Gonzalez-deSuso JM, et al. Clinical assessment of peripheral muscle function in patients with chronic obstructive pulmonary disease. Am J Phys Med Rehabil 2009; 88(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 18. Martin HJ, Yule V, Syddall HE, et al. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology 2006; 52(3): 154–159. [DOI] [PubMed] [Google Scholar]
- 19. Dawson B, Trapp RG. Basic and clinical biostatistics. McGraw-Hill: 4th ed 2004. [Google Scholar]
- 20. Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 2008; 31(4): 466–475. [DOI] [PubMed] [Google Scholar]
- 21. Burtin C, Saey D, Saglam M, et al. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J; 40(2): 338–344. [DOI] [PubMed] [Google Scholar]
- 22. Camillo CA, Burtin C, Hornikx M, et al. Physiological responses during downhill walking: A new exercise modality for subjects with chronic obstructive pulmonary disease? Chron Respir Dis 2015; 12(2): 155–164. [DOI] [PubMed] [Google Scholar]
- 23. Gimeno-Santos E, Raste Y, Demeyer H, et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J 2015; 46(4): 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153(3): 976–980. [DOI] [PubMed] [Google Scholar]
- 25. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–338. [DOI] [PubMed] [Google Scholar]
- 26. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 27. Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 2009; 41(1): 3–13. [DOI] [PubMed] [Google Scholar]
- 28. Rosner BA. Fundamentals of biostatistics. 6th ed Belmont: Thomson Higher Education, 2006. [Google Scholar]
- 29. Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005; 19(1): 231–240. [DOI] [PubMed] [Google Scholar]
- 30. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1(8476): 307–310. [PubMed] [Google Scholar]
- 31. Spruit MA, Gosselink R, Troosters T, et al. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest 2005; 128(5): 3183–3190. [DOI] [PubMed] [Google Scholar]
- 32. Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014; 43(5): 1326–1337. [DOI] [PubMed] [Google Scholar]
- 33. Beckerman H, Roebroeck ME, Lankhorst GJ, et al. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res 2001; 10(7): 571–578. [DOI] [PubMed] [Google Scholar]
- 34. Mathur S, Makrides L, Hernandez P. Test-retest reliability of isometric and isokinetic torque in patients with chronic obstructive pulmonary disease. Physiother Can 2014; 56: 94–101. [Google Scholar]
- 35. de Araujo Ribeiro Alvares JB, Rodrigues R, de Azevedo FR, et al. Inter-machine reliability of the Biodex and Cybex isokinetic dynamometers for knee flexor/extensor isometric, concentric and eccentric tests. Phys Ther Sport 2015; 16(1): 59–65. [DOI] [PubMed] [Google Scholar]
- 36. Maffiuletti NA, Bizzini M, Desbrosses K, et al. Reliability of knee extension and flexion measurements using the Con-Trex isokinetic dynamometer. Clin Physiol Funct Imaging 2007; 27(6): 346–353. [DOI] [PubMed] [Google Scholar]
- 37. Flansbjer UB, Lexell J. Reliability of knee extensor and flexor muscle strength measurements in persons with late effects of polio. J Rehabil Med 2010; 42(6): 588–592. [DOI] [PubMed] [Google Scholar]
- 38. Becker R, Awiszus F. Physiological alterations of maximal voluntary quadriceps activation by changes of knee joint angle. Muscle Nerve 2001; 24(5): 667–672. [DOI] [PubMed] [Google Scholar]
- 39. Krishnan C, Williams GN. Effect of knee joint angle on side-to-side strength ratios. J Strength Cond Res 2014; 28(10): 2981–2987. [DOI] [PubMed] [Google Scholar]
- 40. Knapik JJ, Wright JE, Mawdsley RH, et al. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther 1983; 63(6): 938–947. [DOI] [PubMed] [Google Scholar]
- 41. Hahn D. Lower extremity extension force and electromyography properties as a function of knee angle and their relation to joint torques: implications for strength diagnostics. J Strength Cond Res 2011; 25(6): 1622–1631. [DOI] [PubMed] [Google Scholar]
- 42. Herzog W, Hasler E, Abrahamse SK. A comparison of knee extensor strength curves obtained theoretically and experimentally. Med Sci Sports Exerc 1991; 23(1): 108–114. [PubMed] [Google Scholar]
- 43. Edwards RH, Young A, Hosking GP, et al. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 1977; 52(3): 283–290. [DOI] [PubMed] [Google Scholar]
- 44. Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36(1): 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]