Abstract
Background
Multiple sclerosis (MS) is a presumed autoimmune disease caused by genetic and environmental factors. It is hypothesized that environmental exposures (such as air and water quality) trigger the innate immune response thereby activating a pro-inflammatory cascade.
Objective
To examine potential environmental factors in pediatric MS using geographic information systems (GIS).
Methods
Pediatric MS cases and healthy controls were identified as part of an ongoing multicenter case-control study. Subjects’ geographic locations were mapped by county centroid to compare to an Environmental Quality Index (EQI). The EQI examines 5 individual environmental components (air, land, water, social, built factors). A composite EQI score and individual scores were compared between cases and controls, stratified by median proximity to enrollment centers (residence < 20 or ≥20 miles from the recruiting center), using logistic regression.
Results
Of the 287 MS cases and 445 controls, 46% and 49% respectively live in areas where the total EQI is the highest (worst environmental quality). Total EQI was not significantly associated with the odds for MS (p = 0.90 < 20 miles from center; p = 0.43 ≥ 20 miles); however, worsening air quality significantly impacted the odds for MS in those living near a referral center (OR = 2.83; 95%CI 1.5, 5.4) and those who reside ≥20 miles from a referral center (OR = 1.61; 95%CI 1.2, 2.3).
Conclusion
Among environmental factors, air quality may contribute to the odds of developing MS in a pediatric population. Future studies will examine specific air constituents and other location-based air exposures and explore potential mechanisms for immune activation by these exposures.
1. Introduction
Multiple sclerosis (MS) is a presumed autoimmune disease for which susceptibility is determined by the interplay between genetic and environmental factors, resulting in brain and spinal cord inflammation. While several host-based exposures have been associated with MS risk (i.e. remote Epstein-Barr virus exposure, vitamin D levels), few studies have examined physical environmental factors. Air pollution is one of the few physical factors that has been related to MS risk (Gregory et al., 2008; Heydarpour et al., 2014; Angelici et al., 2016; Oikonen et al., 2016). Adults living in areas with higher air particulates were more likely to have MS (Gregory et al., 2008; Heydarpour et al., 2014), and those with MS were more likely to have a higher relapse rate (Angelici et al., 2016; Oikonen et al., 2016). Despite these findings, the environmental impact of other relevant sources such as land or water pollution near a residence have not been explored in adults. None of these relationships have been examined in a pediatric population with MS.
The exploration of air, land, water and other exposures in children with MS offers an advantage over adult studies with respect to temporality of exposure and consistency of data. Relating environmental quality exposures to health risk in adults is challenging given the types, routes, and quantities of exposures. Environmental impact is also difficult to assess in adults as people tend to change locations following life events such as graduations, marriage, having children, and changing jobs. These relationships could be further modified by social or community factors which may dictate how much time a person spends in their living environments. Thus, children are less likely than young adults to have changed residence or to have roommates, providing more consistency with regard to their environmental exposures.
To explore physical environmental factors, we utilized the Environmental Quality Index created by the EPA and partnered institutions as a comprehensive measure of environmental quality for comparison to health measures (Messer et al., 2014). The EQI is based on five domains which could contribute to health: air, water, land, built environment, and sociodemographic factors. Using data from the U.S Network of Pediatric Multiple Sclerosis Centers, a geographic analysis compared the EQI measures by county of residence between pediatric MS patients and healthy controls in a hypothesis-generating study to examine the contribution of environmental quality on the odds for pediatric MS.
2. Materials and methods
2.1. Study participants
Pediatric MS subjects (first clinical attack before 18 years) and healthy controls were identified as part of an ongoing, multicenter case-control study examining environmental and genetic risk factors in pediatric-onset disease. Sixteen pediatric MS centers in the United States enrolled study participants. Selection of the centers and confirmation of diagnosis has been previously described (Casper et al., 2014). Participants were recruited between November 2011 and June 2016. Cases included clinically isolated syndrome at risk to have MS (at least 2 silent T2-bright lesions on imaging) or MS diagnosis according to the McDonald 2010 criteria (Polman and Rudick, 2010). Each site also enrolled healthy controls who had no history of MS, demyelinating disease, autoimmune disorder, or chronic neurological condition with major disability. They also could not have an immediate biological family member with MS. Healthy controls were recruited to be similar to MS patients in age, sex, and race. This study was approved by the institutional review boards at the participating sites. Consent and assent (when appropriate) were obtained for each subject.
2.2. Demographic information
Parents of each participant completed a detailed questionnaire about their child’s demographic information and birth history. The questionnaire also asked the parent to provide complete information about the residence where the child has lived the longest. The zip code of this residence and time living at this residence was entered into a database and geocoded using ArcGIS (ESRI). In order to help protect anonymity, the centroid of the county of residence was used as the reference point for location in map presentations.
2.3. Environmental data
The EQI data file was acquired from the US EPA website and includes scores for each environmental quality component (air, water, land, built, and sociodemographic) and a total score combining each of the individual metrics by U.S. county. Creation of the EQI metric has been previously described (Lobdell, 2016). For the current study, we examined the air, water, and land components. Briefly, the air quality index assesses concentrations of 6 criteria air pollutants from the EPA’s Air Quality System and emission estimates from the National Scale Air Toxics Assessment. This includes data on 180 ambient concentrations of Clean Air Act toxic chemicals, most of which have known relationships to chronic health effects. The water index uses data from the national Pollutant Discharge Elimination database, the Watershed Assessment Tracking and Environmental Results database, and estimates of water use or drought situations by county. The land index includes information about pesticide use, agriculture activity, nearness to national priority sites, geochemical data, and radon exposures. Higher scores within the scale indicate poorer environmental quality while more negative values indicate better environmental quality (Messer et al., 2014).
We excluded the built and social components of the EQI as these represent complex constructs tangential to the physical factors of our primary question. The built environment index combines information about trafic fatalities, availability of public transport, and prevalence of low-income housing in the area. Finally, the socioeconomic index utilizes crime reports, and economic data for the county such as population density, spatial distribution, education, and home and neighborhood features. Of note, many of these factors were accounted for in the analyses of the air, water, and land constructs using other available data as described below.
A shapefile was created for ArcGIS utilizing the U.S. Census Bureau TIGER files which contain county level data (U.S. Census Bureau Internet, 2016). These shapefiles were joined to the map with the county location of MS cases and controls for visual comparison. Using the “joins and relates” tool in ArcGIS, a dataset was created which assigned each study participant EQI scores based on their county of residence. Air, water, and land quality component scores were grouped into percentiles for map presentations.
2.4. Statistical analysis
All statistical analyses were performed using Stata Software 14.0 (College Station, TX). Air, water and land EQI scores were compared between cases and controls using logistic regression analyses, examining the odds for disease given the environmental quality components. Analyses were stratified by proximity to the recruiting site as control patients were more likely to be recruited from areas proximal to MS Centers (pediatric MS clinics are tertiary referral centers). The median distance traveled for MS cases and controls was considered as the stratification point. Covariates considered for the final models include population density of the county, enrollment site, median household income for the county of residence, and basic demographics such as age, sex, and race/ethnicity. Population density for the county of residence was determined by dividing the total population by the total square miles of the county. Both population density and median county income were acquired from the U.S. Census data website (www.census.gov). Univariate analysis was conducted to determine which covariates would be included in the final logistic regression models. Any variables with a p-value < 0.25 were assessed for inclusion in the final multivariable models. Stepwise regression and likelihood ratio tests were performed to determine which variables provided the best fitting models.
Poisson regression was used to estimate whether the number of pediatric MS cases varied by county environmental quality. Although the study did not have all incident cases, this method allowed us to approximate potential differences in pediatric MS cases across areas with varying EQI. Only counties with at least one MS case were included in this analysis. EQI scores were grouped into quartiles based on the control group’s exposure to estimate the risk for having MS in counties with worse environmental quality compared to good environmental quality (the lowest quartile). Stata statistical models were used to predict the number of cases within each quartile of environmental exposure based on the fitted Poisson regression models.
3. Results
A total of 287 pediatric MS cases and 445 controls were utilized for this analysis. Both groups were similar with regard to age and gender (Table 1). A majority of participants were white (65% cases, 65% controls) and non-Hispanic (66% cases, 79% controls). MS cases had slightly longer time at their primary residence (p-value = 0.012), but the population density in their counties was not significantly different from controls (p-value = 0.366). Most controls were enrolled from within a 20 mile radius of the recruiting center (71%), while only 26% of cases were enrolled from inside the 20 mile radius.
Table 1.
Demographics and characteristics of study participants.
| Within 20 Miles of Recruiting Center | More than 20 Miles from Recruiting Center | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| MS Cases (N = 76) | Healthy Controls (N = 3 11) | p-value | MS Cases (N = 208) | Healthy Controls (N = 126) | p-value | |
| Age, years | ||||||
| Mean (SD) | 14.7 (3.4) | 13.5 (4.1) | 0.018 | 14.2 (3.3) | 14.4 (3.7) | 0.590 |
| Female, N(%) | 48 (63%) | 178 (57%) | 0.348 | 131 (63%) | 77 (37%) | 0.230 |
| Race, N(%) | ||||||
| American Indian or Alaska Native | 4 (5%) | 3 (1%) | < 0.001 | 4 (2%) | 4 (3%) | 0.227 |
| Asian | 2 (3%) | 16 (5%) | 10 (5%) | 6 (5%) | ||
| Black | 14 (18%) | 70 (23%) | 29 (14%) | 7 (6%) | ||
| White | 42 (55%) | 191 (61%) | 141 (68%) | 97 (77%) | ||
| Mixed Race | 4 (5%) | 23 (7%) | 13 (6%) | 6 (5%) | ||
| Unknown | 10 (13%) | 8 (3%) | 11 (5%) | 6 (5%) | ||
| Ethnicity, N(%) | ||||||
| Hispanic | 28 (37%) | 51 (16%) | < 0.001 | 58 (28%) | 29 (23%) | 0.346 |
| Non-Hispanic | 45 (59%) | 250 (80%) | 143 (69%) | 95 (75%) | ||
| Unknown | 3 (4%) | 10 (3%) | 7 (3%) | 2 (2%) | ||
| Time at Residence, years | ||||||
| Mean (SD) | 5.6 (4.6) | 6.4 (4.7) | 6.6 (4.5) | 6.1 (4.4) | 0.472 | |
| Population Densitya, County of Residence | ||||||
| Mean (SD) | 3043 (4140) | 3877 (5894) | 0.244 | 3374 (10522) | 881 (3209) | 0.010 |
| Disease Duration, years | ||||||
| Mean (Range) | 0.5 (0.7) | 0.5 (0.8) | ||||
Total population count of the county divided by the total land area of the county in square miles.
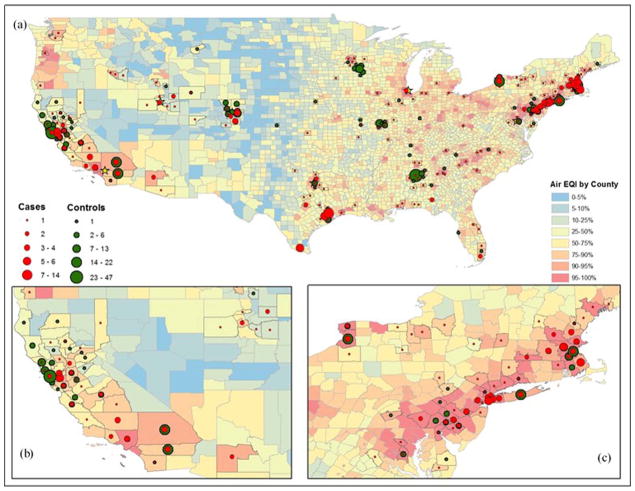
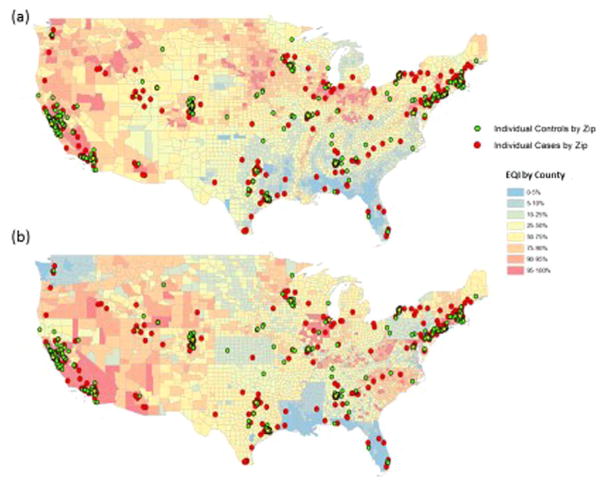
Maps of the United States displaying the number of MS cases and controls within each county by EQI component are shown in Figs. 1 and 2. Red and orange areas reflect the areas with the highest EQI scores (indicating poorer environmental quality) while the blue and green areas reflect areas with the best environmental quality based on EQI percentiles. In general, more urbanized areas had higher air EQI scores than the less densely populated areas, but water and land scores were distributed across the country. Approximately 54% of MS patients and 60% of healthy controls lived in at least the 90th percentile air EQI (worst environmental quality) regions. About 30% of MS cases and controls lived in at least the 90th percentile water EQI regions and about 20% in both groups lived in counties with at least the 90th percentile land EQI regions.
Fig. 1.
Air EQI in relation to pediatric MS and healthy controls, full U.S. (a), west coast (b), and east coast (c).
Fig. 2.
Land (a) and water (b) EQI scores in relation to pediatric MS cases and healthy controls.
Results of the stratified logistic regression analysis are shown in Table 2. Poor air quality was related to an increased odds for pediatric MS in both stratified groups. For those living less than 20 miles from a recruitment center, the odds for MS increased by 4 as the air EQI score worsened (p=0.002). Similarly, for those who live more than 20 miles from the recruitment centers, the odds for MS doubled as air EQI scores worsened (p=0.004). No statistically significant associations were seen between water EQI or land EQI and pediatric MS.
Table 2.
Logistic regression analysis comparing environmental quality (EQI) to the odds for MS, stratified by proximity to recruiting center1.
| Subjects ≤ 20 miles from recruiting center (N = 387) | Subjects > 20 miles from recruiting center (N = 334) | |
|---|---|---|
| EQI Construct | OR (95%CI) | OR (95%CI) |
| Air EQI | 4.35 (1.69, 11.2)2 | 2.17 (1.28, 3.70)2 |
| Water EQI | 1.28 (0.97, 1.68) | 0.75 (0.57, 0.98) |
| Land EQI | 0.66 (0.42,1.03) | 0.78 (0.58, 1.05) |
Models were adjusted for age, sex, ethnicity, referral site, median household income, and population density.
Considered significant at the 0.01 level (Bonferroni Correction = 0.05/3).
Table 3 displays results of the Poisson regression, analyzing the number of MS cases by county in relation to the county’s EQI score. Air EQI again was the only metric that was associated with an increased count of pediatric MS. Although this method only approximated the incidence rate ratio from the MS cases enrolled in the study, the rate of having MS was higher in counties with poor air EQI compared to counties with good air EQI (IRR = 1.49; p-value < 0.001).
Table 3.
Poisson regression results comparing the number of pediatric MS cases by county in relation to EQI scoresa,b.
| EQI Construct | IRR | p-value | Predicted MS Cases | 95%CI |
|---|---|---|---|---|
| Air EQI | ||||
| Reference αbest air EQI) | – | – | 1.18 | 0.36–2.00 |
| 2nd Quartile | 1.29 | 0.51–2.81 | 1.40 | 0.69–2.11 |
| 3rd Quartile | 1.08 | 0.51–2.31 | 1.28 | 0.88–1.67 |
| 4th Quartile (worst air EQI) | 1.53 | 0.75–3.09 | 1.80c | 1.56–2.03 |
| Water EQI | ||||
| Reference | – | – | 1.67 | 1.21–2.13 |
| 2nd Quartile | 0.80 | 0.51–1.25 | 1.33 | 0.86–1.81 |
| 3rd Quartile | 1.03 | 0.72–1.49 | 1.72 | 1.32–2.13 |
| 4th Quartile | 1.01 | 0.73–1.41 | 1.69 | 1.40–1.98 |
| Land EQI | ||||
| Reference | – | – | 1.71 | 1.20–2.24 |
| 2nd Quartile | 0.95 | 0.64–1.42 | 1.63 | 1.23–2.03 |
| 3rd Quartile | 0.87 | 0.57–1.31 | 1.48 | 1.09–1.87 |
| 4th Quartile | 1.01 | 0.71–1.44 | 1.72 | 1.42–2.02 |
Adjusted for population density of the county of residence.
Analysis did not use all incident cases around the U.S. or counties with 0 MS cases.
Considered significant at the 0.05 level.
When analyzed by quartile, a slight trend was observed between the worsening air EQI categories and MS count, although the trend was not significant. Compared to the reference category, counties with the worst air EQI (4th quartile) had 50% more MS cases. The water and land EQI scores did not impact the number of MS cases in the counties as a similar number of MS cases was predicted in the reference categories as the other quartiles.
4. Discussion
Our results suggest that of the physical exposures analyzed in this study, air quality may be an important contributor to pediatric MS. When the number of pediatric MS cases was compared by county, worsening air quality predicted a higher number of MS cases compared to the best air EQI quartile. The other environmental quality components assessed in this study, land and water based metrics, were not associated with an increased odds for MS. These findings provide guidance for future studies, and highlight areas of focus for environmental exposure relationships with MS.
While the EQI captures ecologic data and does not measure individual exposures, our air quality findings in pediatric MS are consistent with other studies relating air pollution and adult MS. Increasedexposure to particulate matter (PM10) has been correlated with both increased risk for MS (Gregory et al., 2008; Heydarpour et al., 2014) and MS relapse (Angelici et al., 2016; Oikonen et al., 2016). Heydarpour et al. found that yearly averages of particulate matter (PM), sulfur dioxide (SO2), and nitrogen dioxide (NO2), were significantly higher in regions where MS patients lived compared to healthy controls (Heydarpour et al., 2014). Acidic gases (SO2 and NO2) along with carbon monoxide (CO) have also been linked with higher odds for MS relapse (acidic gases OR = 6.95, 95%CI 2.75–17.6; CO OR = 3.64, 95%CI 1.22–10.9) (Oikonen et al., 2016). Our results using the comprehensive air EQI score showed similar results. The air EQI score accounted for effects from the six criteria air pollutants including PM, SO2, NO2, and CO. Future studies will examine the individual criteria air pollutants in relation to pediatric MS and assess whether fine particulate matter (PM2.5 compared to larger sized particulate matter, PM10) impacts these relationships. Air pollution is also a known risk factor for other conditions such as asthma, stroke, and cardiovascular disease (Mölter et al., 2015; Gehring et al., 2015; Yorifuji et al., 2014; Zhang et al., 2011; Franklin et al., 2015; Short term exposure to air pollution and stroke, 2016; Maheswaran, 2016). Poor air quality has negative effects on the central nervous system and has been associated with cognitive delays in children (Kristiansson et al., 2015; Guxens et al., 2012; Block and Calderón-garcidueñas, 2010).
Several theories have hypothesized the biological processes behind the association with MS and air pollution. One possible mechanism is through oxidative stress which occurs when there is an imbalance in concentrations of oxidizing agents and anti-oxidant properties in the cells. Through the oxidative process, excess amounts of nitric oxide (NO) can be produced along with inducible NO synthase mRNA (iNOS). Excess amounts of both NO and iNOS have been found in the brain tissue of MS patients, and not in healthy control brain tissue (Bagasra et al., 1995; Calabrese et al., 2003). Other markers of oxidative stress have also been elevated in MS patients compared to healthy controls (Koch et al., 2006; Oliveira et al., 2012). Perhaps repeated exposure to air pollution and related stressors creates a more pro-inflammatory state, thus making a person’s systems quicker to act when a person is exposed to future stressors such as Epstein-Barr virus (Kristiansson et al., 2015).
Additionally, air pollution could contribute to MS directly through inhalation of particulate matter. Animal models have shown that ultra-fine particulate matter can rapidly pass through the blood and cross the blood-brain barrier, possibly aiding in delivery of other toxicants to the brain (Block and Calderón-garcidueñas, 2010; Nemmar et al., 2001). Calderón-Garcidueñas et al. (2008) found that brain tissue of people in highly polluted areas showed increases in several immune cell types (CD68, CD193, and HLA-DR positive cells) as well as blood-brain barrier damage. Finally, exposure to higher levels of pollution increased pro-inflammatory cytokines in animal brain tissue, showing that particulates may also contribute to MS by triggering an immune response (Calderón-Garcidueñas et al., 2008).
We did not find an association between water and land with odds of MS. The water EQI component incorporates data from 9 different sources including items related to direct exposure such as contaminants in drinking water systems and also items related to water availability such as drought and water stress. The land EQI examines 12 different sources which estimate sources of pesticide use, nearness to superfund and toxic release sites, as well as areas of higher radon zones, many of which may vary the EQI score based on rural and urban settings. Although we did not show associations with the water and land components, we may be missing individual level exposures which may be masked due to the comprehensive nature of the EQI score.
Pruning, axonal myelination, and synaptic changes occur throughout childhood and adolescence. Repeated toxic stress can negatively impact neurological and immunological systems, particularly in a developing brain and immature immune system (Kristiansson et al., 2015). An insult through pollution and increased inflammatory factors during these critical phases could create a point of vulnerability (Guxens et al., 2012).
The strengths of our study include the study of cases within a short period of time after disease onset. Leveraging this, there are fewer issues with recall as there was a closer time proximity to exposures. In addition, studying risk factors for pediatric MS offers a unique advantage as patients with very early disease onset may have a higher rate of environmental exposures than those with adult onset. This study utilized a comprehensive measure of environmental quality to help generate hypotheses about physical environmental exposures in relation to MS, which enabled us to identify environmental areas of concern so that specific pollutants and exposures could be examined in more detail in future studies. Stratifying by proximity to referral center enabled comparison of groups which were more similar geographically despite the fact that MS cases were more likely to travel a longer distance to seek medical care from pediatric MS specialists at referral sites compared to healthy controls.
We acknowledge some limitations to the metrics used for this study. First, although the EQI metrics are excellent tools for comparison to health outcomes, they may not capture individual risk factors such as the contribution of particulate matter to MS risk versus individual acidic gases. EQI data is summarized over a 5 year period, potentially masking fluctuations in the environmental measures during time epochs. This was a large dataset for a rare disease, but we were not able to capture pediatric MS cases across the country who did not seek medical care at one of the referral sites. Our data also does not take into account a person who may have moved several times over their childhood; however, we analyzed information at the child’s longest residence to try to capture the time period with the most consistent exposure. Poisson regression was used to estimate the relative risk of pediatric MS with decreasing air quality, and although this measure helped to estimate the potential impact of air, water, and land environmental quality on pediatric MS, it did not include all pediatric MS cases in the country. Therefore, interpretation of the Poisson regression results are limited. Despite these limitations, the EQI metrics used in this study helped illuminate areas for future exploration.
5. Conclusion
Air quality may be an important risk factor to consider with the odds for developing MS; biological processes at play remain to be determined. Future studies will examine specific air pollutant exposure and other location based exposures which may increase the risk for pediatric MS.
Acknowledgments
Dr. Waubant is funded by the NIH (1R01NS071463). Dr. Casper is funded by the National Multiple Sclerosis Society (HC 0165). Dr. Waldman is funded by the NIH (K23NS069806).
Footnotes
Author contributions
Dr. Lavery conceptualized and designed the analysis for this study, drafted the initial manuscript and submitted the final manuscript.
Drs. Waldman, Waubant, contributed to the conception and design of the analysis for this study, participant recruitment, confirmation of diagnoses, and reviewed and edited the manuscript.
Dr. Casper contributed to the conception and design of the analysis for this study and reviewed and edited the manuscript.
Dr. Kaylor assisted with the geographic information system analysis and editing of the manuscript.
Ms. Roalstad contributed to the acquisition of the data, managed the data and supervised data collection at all sites during the study, and reviewed the final manuscript.
Drs. Candee, Rose, Belman, Weinstock-Guttman, Aaen, Tillema, Rodriguez, Ness, Harris, Graves, Krupp, Charvet, Benson, Gorman, Moodley, Rensel, Goyal, Mar, Chitnis, Schreiner, Lotze, Greenberg, Kahn, and Rubin contributed to the recruitment of participants for this study, confirmed diagnoses for participants, and reviewed and edited the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosures
Dr. Mary Rensel has grant funding through Genentech and the National Multiple Sclerosis Society. Dr. Bianca Weinstock-Guttman has received grant support from Biogen Idec, Teva Neuroscience, EMD Serono, Novartis, Genzyme & Sanofi and Genentech, Celgene and Mallinckrodt Pharmaceuticals, Inc. Dr. Jennifer Graves received grants from Biogen, Genentech, S3 group and Race to Erase MS. Dr. Benjamin Greenberg received grant support from Biogen, Acorda, Chugai, Medimmune, Genentech, NIH, Guthy Jackson Charitable Foundation, and PCORI. Dr. Amy Waldman received funding from the NIH-NINDS, Biogen, and foundation support. The remaining authors have no financial disclosures to report.
References
- Angelici L, Piola M, Cavalleri T, Randi G, Cortini F, Bergamaschi R, et al. Effects of particulate matter exposure on multiple sclerosis hospital admission in Lombardy region, Italy. Environ Res. 2016;145:68–73. doi: 10.1016/j.envres.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-garcidueñas L. Air pollution: mechanisms of neuroinflammation & CNS disease. Trends Neurosci. 2010;32(9):506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield Da, Calvani M, et al. Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res. 2003;28(9):1321–1328. doi: 10.1023/a:1024984013069. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinfiammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and youn. Toxicol Pathol. 2008;36(2):289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Casper TC, Rose JW, Roalstad S, Waubant E, Aaen G, Belman A, et al. The US network of pediatric multiple sclerosis centers: development, progress, and next steps. J Child Neurol. 2014 doi: 10.1177/0883073814550656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Arden Pope C. Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40(5):207–238. doi: 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933–942. doi: 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- Gregory AC, Shendell DG, Okosun IS, Gieseker KE. Multiple Sclerosis disease distribution and potential impact of environmental air pollutants in Georgia. Sci Total Environ. 2008;396(1):42–51. doi: 10.1016/j.scitotenv.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Guxens M, Aguilera I, Ballester F, Estarlich M, Fernández-Somoano A, Lertxundi A, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120(1):144–149. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydarpour P, Amini H, Khoshkish S, Seidkhani H, Sahraian MA, Yunesian M. Potential impact of air pollution on multiple sclerosis in Tehran, Iran. Neuroepidemiology. 2014;43(3–4):233–238. doi: 10.1159/000368553. [DOI] [PubMed] [Google Scholar]
- Koch M, Ramsaransing GSM, Arutjunyan AV, Stepanov M, Teelken A, Heersema DJ, et al. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol. 2006;253:483–487. doi: 10.1007/s00415-005-0037-3. [DOI] [PubMed] [Google Scholar]
- Kristiansson M, Sörman K, Tekwe C, Calderón-Garcidueñas L. Urban air pollution, poverty, violence and health – neurological and immunological aspects as mediating factors. Environ Res. 2015;140:511–513. doi: 10.1016/j.envres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Lobdell DT. U.S. Environmental Protection Agency [Internet] Developer Central Data. 2016 Available from: < https://developer.epa.gov/usepa-environmental-quality-index-eqi-and-associated-domain-indices-by-county-for-the-united-states/>.
- Maheswaran R. Air pollution and stroke – an overview of the evidence base. Spat Spatiotemporal Epidemiol. 2016;18:74–81. doi: 10.1016/j.sste.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Messer LC, Jagai JS, Rappazzo KM, Lobdell DT. Construction of an environmental quality index for public health research. Environ Health. 2014;13(1):39. doi: 10.1186/1476-069X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45(3):610–624. doi: 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164(9):1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- Oikonen M, Laaksonen M, Laippala P, Oksaranta O, Lilius EM, Lindgren S, et al. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. 2016;22(1):95–99. doi: 10.1159/000067108. [DOI] [PubMed] [Google Scholar]
- Oliveira SR, Kallaur AP, Simão ANC, Morimoto HK, Lopes J, Panis C, et al. Oxidative stress in multiple sclerosis patients in clinical remission: association with the expanded disability status scale. J Neurol Sci. 2012;321(1–2):49–53. doi: 10.1016/j.jns.2012.07.045. [DOI] [PubMed] [Google Scholar]
- Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(Suppl 3):S8–S15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]
- Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2016:i4851. doi: 10.1136/bmj.i4851. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau [Internet] TIGER Products. 2016 Available from: < https://www.census.gov/geo/maps-data/data/tiger.html>.
- Yorifuji T, Suzuki E, Kashima S. Cardiovascular emergency hospital visits and hourly changes in air pollution. Stroke. 2014;45(5):1264–1268. doi: 10.1161/STROKEAHA.114.005227. [DOI] [PubMed] [Google Scholar]
- Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, et al. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One. 2011;6(6):e20827. doi: 10.1371/journal.pone.0020827. (Federici M., editor) [DOI] [PMC free article] [PubMed] [Google Scholar]