Abstract
Liver cancer is the second leading cause of cancer mortality worldwide, causing more than 700,000 deaths annually. Because of the wide landscape of genomic alterations and limited therapeutic success of targeting tumor cells, a recent focus has been on better understanding and possibly targeting the microenvironment in which liver tumors develop. A unique feature of liver cancer is its close association with liver fibrosis. More than 80% of hepatocellular carcinomas (HCCs) develop in fibrotic or cirrhotic livers, suggesting an important role of liver fibrosis in the premalignant environment (PME) of the liver. Cholangiocarcinoma (CCA), in contrast, is characterized by a strong desmoplasia that typically occurs in response to the tumor, suggesting a key role of cancer-associated fibroblasts (CAFs) and fibrosis in its tumor microenvironment (TME). Here, we discuss the functional contributions of myofibroblasts, CAFs, and fibrosis to the development of HCC and CCA in the hepatic PME and TME, focusing on myofibroblast- and extracellular matrix—associated growth factors, fibrosis-associated immunosuppressive pathways, as well as mechanosensitive signaling cascades that are activated by increased tissue stiffness. Better understanding of the role of myofibroblasts in HCC and CCA development and progression may provide the basis to target these cells for tumor prevention or therapy.
Keywords: Stroma, inflammation, stiffness, mechanosensitive signaling, primary sclerosing cholangitis (PSC), fluke
1. INTRODUCTION
Liver cancer caused 746,000 deaths in 2012, making it the second leading cause of cancer-related death worldwide, surpassed only by lung cancer (1). Approximately 90% of liver cancers are hepatocellular carcinomas (HCCs) and 10% are cholangiocarcinomas (CCAs) or mixed forms of HCC and CCA. There are additional rare forms of liver cancer such as fibrolamellar carcinoma as well as benign liver tumors such as focal nodular hyperplasia and hepatocellular adenoma (2). In the current review, we focus exclusively on HCC and CCA. Incidence and the overall number of deaths due to liver cancer have rapidly increased in the past decades (1). However, these changes may not be uniform, with decreasing numbers in areas with historically high rates of HCC such as China but increasing rates in Western Europe and North America (1). In the United States, the incidence of HCC almost tripled between 1975 and 2011 (1), whereas the incidence of most other solid tumors has remained stable. Similarly, the incidence of primary liver cancer has increased in England and Wales (3–5). Some studies have suggested an even stronger increase of CCA than of HCC in Western countries (5). HCC and CCA have poor prognoses, with a 5-year survival rate of less than 12% for HCC (6) and 10% for CCA (7) due to a combination of late diagnosis and the lack of efficient therapies for advanced stages. There is a pressing need to better understand the biology of HCC and CCA to develop more efficient therapies.
Although the main focus of oncology has been on oncogenic signaling pathways in tumor cells, an important contribution is also made by the environment in which tumors develop. It is widely believed that cells from the tumor microenvironment (TME) contribute to the hallmarks of cancer (8), namely, sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, reprogramming energy metabolism, and evading immune destruction (9). Accordingly, hepatic gene expression in surrounding nontumor tissue rather than in tumors themselves determines survival in patients undergoing potentially curative treatment for HCC (10), suggesting that the environment in which HCC arises exerts a major influence on tumor development and growth. One of the outstanding features of HCC is its strong association with liver fibrosis, with 80–90% of HCCs developing in fibrotic or cirrhotic livers (6). This differs from most other tumors and organs, where fibrosis is not strongly associated with cancer development but may occur as reactive desmoplasia once the tumor has developed. Accordingly, CCA often develops in nonfibrotic livers but subsequently triggers a strong desmoplastic reaction similar to that seen in pancreatic cancer (11). Based on these findings, we propose to differentiate between the premalignant environment (PME), which precedes tumor development and is characterized by chronic hepatic cell death, inflammation, and fibrosis, and is likely to play a key role in the development of HCC but a lesser role in CCA, and the TME, which develops and coevolves in already developed tumors and is likely to play a key role in CCA and HCC.
2. HEPATOCELLULAR CARCINOMA
2.1. Introduction to Hepatocellular Carcinoma
Virtually all HCCs arise in patients with chronic liver diseases such as chronic viral hepatitis, nonalcoholic fatty liver disease (NAFLD), and alcoholic liver disease. In this regard, increased rates of HCC coincided with the peak of hepatitis C virus (HCV) infection that occurred several decades ago, resulting in the development of HCC two to four decades thereafter. Moreover, the increase in adiposity and NAFLD is believed to contribute to increased rates of HCC (12). Most patients sequentially develop hepatitis, fibrosis, cirrhosis, and then HCC. Although different types of liver diseases entail specific risks for the development of HCC, the presence of liver cirrhosis represents a unifying risk factor (6, 13). Approximately one in three patients with compensated liver cirrhosis will develop HCC in their lifetime. Despite this high risk, there are currently no therapeutic interventions that can decrease the risk for HCC development besides the treatment of the underlying disease. However, this is not possible for a significant proportion of patients. Moreover, successful eradication of the underlying disease does not completely eliminate the risk for HCC development, as documented in patients with chronic HCV infection (14, 15). Significant progress has been made in defining the genetic landscape (16) as well as the cellular source of HCC (17). However, there are still big gaps in understanding the contribution of the PME and TME in the development of HCC. Understanding how myofibroblast activation and liver fibrosis are linked to hepatocarcinogenesis will not only provide important clues to the question of how chronic liver disease triggers the development of HCC but may also provide new therapeutic opportunities for the prevention or treatment of HCC.
2.2. The Premalignant Environment of Hepatocellular Carcinoma
The profound changes of hepatic microarchitecture as well as functional alterations of virtually every type of hepatic cell during chronic liver disease provide a fertile environment for the development of cancer (Figure 1). Changes in the hepatic PME affect not only gene expression and the number and turnover of hepatocytes and nonparenchymal cells but also noncellular components, including the extracellular matrix (ECM) and growth factors, which are often bound to ECM (18, 19). Cooperatively, these different components of the PME provide stimuli for the malignant transformation of hepatocytes toward tumor-initiating cells (TICs). Carcinogenesis is driven not only by the presence and/or active secretion of tumor-promoting mediators but also by the loss of tumor-suppressor mechanisms present in the normal liver. Accordingly, loss of cell contact with normal cells, loss of normal ECM, and reduced levels of tumor-suppressive factors such as retinoids are likely to promote the development of cancer in the chronically injured liver. For example, normal fibroblasts, represented in the liver by quiescent hepatic stellate cells (HSCs), and normal epithelia suppress growth via contact inhibition (9, 20). However, the extent to which the loss of a tumor-suppressive microarchitecture contributes to hepatocarcinogenesis remains poorly understood.
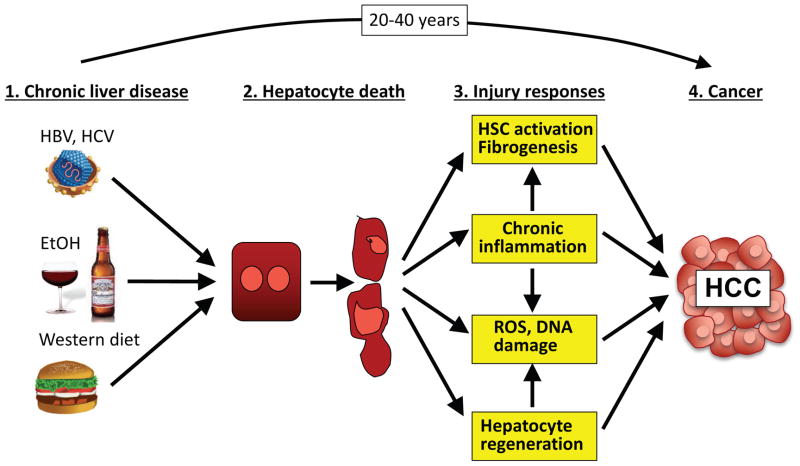
Figure 1. Mechanisms by which the hepatic PME promotes HCC development.
Chronic liver injury, resulting in hepatocyte death, contributes to characteristic features of the PME, including liver fibrosis, hepatocyte regeneration, inflammation, increased generation of ROS, and DNA damage. Together these changes in the PME drive the development of HCC, which typically occurs after chronic injury persists for several decades. Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HSC, hepatic stellate cell; PME, premalignant environment; ROS, reactive oxygen species.
In contrast, the role of tumor-promoting factors that are activated in response to liver injury is much better characterized. Chronic activation of inflammatory signaling pathways by recruited inflammatory cells and increased secretion of inflammatory cytokines result in the generation of reactive oxygen species (ROS). In concert with chronic compensatory hepatocyte regeneration and proliferation-induced mutagenesis, chronic ROS exposure sets the stage for HCC development. Key mechanisms include ROS-mediated DNA damage and genomic instability in hepatocytes (21), as well as inhibitory effects on T lymphocyte-mediated tumor immunosurveillance (22). Accordingly, inhibition of ROS formation by the antioxidants butylated hydroxyanisole or N-acetylcysteine results in a strong suppression of experimental hepatocarcinogenesis (23, 24). In parallel, cells within the hepatic PME, such as inflammatory cells, myofibroblasts, and endothelial cells, produce a vast array of cytokines, growth factors, chemokines, and angiogenesis factors. These mediators may not only contribute to an environment that supports the transformation of hepatocytes but may also promote their survival through the activation of antiapoptotic pathways (25). Additionally, patients with advanced liver disease typically have defective immunity (26), suggesting that immune surveillance within the hepatic PME is reduced, allowing transformed hepatocytes to avoid detection and destruction by the immune system. In experimental models of hepatocarcinogenesis, it is therefore important to reproduce the typical environment in which cancer arises, including the presence of hepatocellular death, injury, and fibrosis (reviewed in 27).
2.2.1. Cell death
Cell death occurs in virtually all types of chronic liver diseases and is the ultimate trigger of liver disease progression, contributing to many characteristic features of the hepatic PME, including chronic inflammation, regeneration, and fibrosis (28). The key role of cell death in driving carcinogenesis has been demonstrated in mouse models and clinical studies. Mice with hepatocyte-specific deletion of the antiapoptotic proteins Mcl-1 and Bcl-xl or the NF-κB activator Nemo display increased hepatocyte apoptosis, leading to the subsequent development of HCC (29–33). Clinically, cell death correlates not only with the development of fibrosis and cirrhosis but also of HCC (28). As such, HBV- and HCV-infected patients with persistent ALT levels >45 U/L have a ten- and seven-fold higher risk, respectively, of developing HCC than patients with persistently normal ALT (34, 35). It is likely that the HCC-promoting effects of cell death are mediated by multiple mechanisms, including the induction of inflammation, fibrosis, and chronic hepatocyte regeneration. Hepatocellular death can be apoptotic, necroptotic, or necrotic. There is evidence that these forms of cell death exist in parallel in patients with chronic liver disease (28). It has been suggested that the form of cell death may dictate the hepatic cell death response and that the release of damage-associated molecular patterns (DAMPs) may exert a key role in the regulation of inflammation, regeneration, and fibrosis in the liver (28). However, the functional contribution of these different forms of cell death and of DAMPs to hepatocarcinogenesis remains unclear. Although apoptosis has been considered a nonreactive form of cell death, there is accumulating evidence that hepatocellular apoptosis can drive both fibrosis and HCC development in patients (28). Likewise, the aforementioned studies in mice with hepatocyte-specific deletion of the antiapoptotic proteins Mcl-1 or Bcl-xl (29–31) suggest a role of apoptosis in driving fibrosis and HCC development. Whether this is due to secondary leakage of apoptotic cells, converting this nonreactive form of cell death into a reactive form, or caused by specific mediators released from apoptotic cells such as apoptotic bodies remains to be determined. Recent in vitro studies also support the concept that apoptosis may cause genotoxic stress in cells that activate caspases and downstream mediators such as DNAse but ultimately do not die (36, 37).
2.2.2. Fibrosis
The presence of fibrosis is the most characteristic feature of the hepatic PME. Of all HCCs, 80–90% develop in fibrotic or cirrhotic livers (6). Although fibrosis is considered a protective response to acute liver injury, it becomes chronic and maladapative when the underlying disease cannot be eradicated and hepatocellular injury persists (18). In the normal liver, ECM constitutes approximately 0.5% of the liver wet weight (38). The most characteristic feature of liver fibrosis is the accumulation of collagens, predominantly type I collagen, resulting in a two- to fivefold increase of total collagen content in the cirrhotic liver (38). Histologically, the deposition of collagen fibrils leads to different degrees and patterns of fibrosis, ranging from mild fibrosis to bridging fibrosis and cirrhosis. Importantly, the changes of hepatic ECM composition go far beyond accumulation of type I collagen. The hepatic ECM consists of 20 genetically distinct types of collagen, noncollagenous glycoproteins such as elastin, laminin, and fibronectin; proteoglycans such as aggrecan, fibromodulin, decorin, biglycan, glypicans, and syndecans; as well as matricellular proteins such as thrombospondins, osteopontin, tenascins, the CCN family of proteins, and connective tissue growth factor (CTGF). Many of these ECM proteins change during liver fibrosis (39).
Importantly, ECM proteins are not inert molecules that merely provide mechanical stability. In addition to their function as structural scaffold elements, they serve a wide array of signaling functions. As such, ECM proteins activate integrins such as integrin αv, which exerts a key role in the activation of transforming growth factor β (TGF-β (40). Discoidin domain receptor (DDR) proteins represent receptors that specifically interact with ECM proteins. As such, DDR2 is a receptor tyrosine kinase that is activated by type I collagen. DDR2 is expressed not only on fibrogenic cells, promoting a positive feedback loop for their activation (41) but also in the epithelial compartment to promote the epithelial-mesenchymal transition (EMT; 42, 43), a key mechanism contributing to the malignant transformation of epithelia. Moreover, ECM proteins, including decorin, biglycan, fibromodulin, and glycosaminoglycans, store growth factors such as HGF, PDGF, TGF-β, CTGF, and VEGF (39). ECM turnover during fibrogenesis and fibrosis resolution results in liberation and activation of growth factor signaling cascades, promoting epithelial proliferation and, most likely, cancer development in chronically injured livers.
On a cellular level, fibrogenesis is mediated by activation of HSCs that transdifferentiate from vitamin A-storing pericyte-like cells to α-SMA-positive, collagen-producing myofibroblasts in response to liver injury. Studies in mice have demonstrated that HSCs contribute 85–95% of the hepatic myofibroblasts in fibrosis triggered by hepatotoxins, biliary injury, or NAFLD (44). Similarly, activation of HSCs is a well-documented feature of human liver disease (45). Although mechanisms of HCC development differ between different types of liver disease, for example, conferring a higher risk in chronic HBV and HCV infection and a lower risk in alcoholic liver disease or NAFLD (6, 13), fibrosis and cirrhosis are considered to be unifying risk factors. Of note, fibrosis precedes the development of HCC, thereby making fibrosis a key component of the hepatic PME. Accordingly, clinical data demonstrate a strong correlation between fibrosis and the development of HCC. Of patients with cirrhosis, 5—30% develop HCC within 5 years (6), and it is estimated that approximately one-third of patients with cirrhosis will eventually develop HCC. Accordingly, a number of studies have suggested an important role of fibrosis, in determining HCC risk or recurrence: HBsAg-positive patients with a high serological fibrosis index (FIB-4) had an up to 15-fold increased risk for future HCC incidence (46). However, it should be pointed out that the FIB-4 index is not a direct measure of fibrosis and is based on age, AST and ALT levels (which are risk factors for HCC development on their own), as well as platelet numbers. Studies in patients with chronic hepatitis B have found that liver stiffness, a noninvasive, indirect measure of liver fibrosis, is positively correlated with HCC risk, increasing the risk of developing HCC by 4- to 13-fold in patients with liver stiffness values of >12.5–13 kPa (47, 48). In another study, no patient with liver stiffness <12 kPa developed HCC within a median observation interval of 21.8 months, whereas 26% of patients (8 of 29) with liver stiffness >12 kPa developed HCC (49). As measurements such as stiffness may reflect not only fibrosis but overall disease stage, they do not causatively link fibrosis to hepatocarcinogenesis and may reflect only a higher risk for HCC development with advanced disease stage. Moreover, with relatively short follow-up periods, it cannot be completely excluded that liver stiffness was increased due to preexisting HCC. In contrast, a 122-gene HSC signature was not associated with the development of HCC in a cohort of patients with chronic hepatitis C and Child-Pugh A stage cirrhosis (50). Several studies have established that the presence of liver fibrosis, as determined by fibrosis staging or the presence of either α-SMA-positive myofibroblasts or activated HSC signatures, is associated with increased recurrence after curative HCC resection (50–53). As recurrences in many of these studies were early and therefore unlikely to represent de novo HCC formation, we discuss these studies in the TME section below. HCC may occur in the absence of cirrhosis, in particular, in patients with nonalcoholic steatohepatitis (12) or subsets of patients with HBV infection (54). However, the majority of these patients still have moderate to advanced fibrosis (51, 55). Of note, all of the above-discussed studies are correlative and do not provide evidence for a causative role of fibrosis in HCC promotion. It is entirely possible that fibrosis and hepatocarcinogenesis are two associated but functionally independent processes. As such, it is conceivable that the presence and degree of liver fibrosis in patients mark disease severity and duration but that other events correlating positively with fibrosis, such as chronic inflammation, regeneration, and associated genotoxicity, are drivers of hepatocarcinogenesis. Although there is a lack of studies providing irrefutable evidence for a functional contribution of fibrosis to hepatocarcinogenesis in animal models and patients, there is accumulating evidence that liver fibrosis and activated HSCs actively contribute to the development of HCC through multiple mechanisms. These include the activation of integrin signaling in hepatocytes surrounded by abnormal ECM, release of growth factors and other tumor-promoting cytokines by HSC, altered presence and activation of ECM-associated proteins (including ECM-associated growth factors), as well as interactions of HSCs with other hepatic cells that could promote tumor growth and/or decrease immunosurveillance. Each of these mechanisms is discussed in detail in the next section.
2.2.3. Inflammation and immunity
Inflammation is an integral part of the hepatic response to injury caused by hepatitis viruses, excess fat, alcohol, and cholestasis and thus a characteristic feature of the hepatic PME. Moreover, there are close functional links between inflammation and fibrosis, with several key players such as macrophages, B cells, and numerous cytokines promoting HSC activation and liver fibrosis (25). Acute inflammation is a beneficial response that serves to restore a healthy liver by eradicating pathogens and promoting regeneration following liver injury. In contrast, chronic inflammation is maladaptive and contributes to the development of liver fibrosis and HCC. A large number of proinflammatory mediators, including IL-1, IL-6, TNF-α, lymphotoxin-β, and IL-17, are upregulated in the chronically injured liver and contribute to the development of HCC (25, 56, 57). Most of these mediators derive from inflammatory cells such as bone marrow (BM)-derived macrophages, neutrophils, B cells, and T cells within the chronically injured liver. Although inflammation and fibrogenesis are functionally linked, it appears that some inflammatory pathways selectively influence carcinogenesis without affecting fibrosis. As such, neutrophils promote the development of HCC (58) but do not appear to exert a role in hepatic fibrogenesis (59, 60). Likewise, ectopic lymphoid structures and cytokines associated with these structures, such as lymphotoxin-β, promote the development of HCC (61) but have no known role in fibrosis. IL-6 is a key contributor to hepatocarcinogenesis (62, 63), whereas its hepatoprotective functions mediate protection from liver fibrosis development (64, 65). Although IL-1 has no or at best a minor role in hepatic fibrogenesis (66, 67), it promotes hepatocarcinogenesis (68). In contrast, inflammatory cell populations such as macrophages have a known role in the promotion of liver fibrosis and liver cancer (19, 56). Likewise, activation of TLR4 by gut-derived lipopolysaccharide (LPS) promotes not only the development of liver fibrosis (69) but also of HCC (70), with HSC-derived cytokines such as epiregulin providing possible links between these two (70). In conclusion, inflammation appears to promote hepatocarcinogenesis through fibrosis-dependent and -independent pathways.
Inflammatory pathways also have a role in immune-mediated antitumor responses. The presence of immune-mediated antitumor responses, as evidenced by immunoediting of tumors, has been demonstrated in many different models and clinical settings (71). However, until recently, it has been unclear whether immunoediting--leading to the elimination of specific tumor antigens but not the clearance of tumors--is proof for efficient antitumor responses or a sign for failed immune responses. Successful clinical application of checkpoint inhibitors not only suggests that this powerful system is an important surveillance system but also provides evidence that it is actively suppressed in later stages of cancer (72). In the liver, the immune system plays an important role in eliminating premalignant or malignant hepatocytes at different stages. In the PME, senescent premalignant hepatocytes are eliminated through a CD4+ T cell-dependent mechanism (73). The elimination of senescent hepatocytes additionally requires the presence of macrophages and is triggered by senescence-induced secretion of inflammatory chemokines and cytokines in hepatocytes (73). In hepatocarcinogenesis, failure of this surveillance system appears to occur at different stages and by different mechanisms, possibly in a disease-specific manner. The abundance of TGF-β, one of the most potent suppressors of antitumor immunity (74), in the fibrotic environment provides a link between fibrosis and suppressed antitumor immunity. As such, activated and proliferating HSCs have been shown to switch monocytes from inflammatory to an immunosuppressive and tumor-promoting M2 phenotype, thus allowing immune evasion by developing tumors (53, 75). In NAFLD, there is a failure of CD4+ T cell-mediated immunosuppression, mediated by linoleic acid-induced mitochondrial ROS generation and subsequent loss of CD4+ T cells, resulting in increased hepatocarcinogenesis (22).
2.3. The Tumor Microenvironment of Hepatocellular Carcinoma
Tumors not only consist of transformed cells but also contain a number of stromal components, including cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs) and other infiltrating immune cells, as well as angiogenic vascular cells (8). These stromal components can influence the growth and behavior of tumor cells and also modulate therapeutic responses. It is believed that tumors hijack resident and recruited cells to support multiple aspects of tumor growth and avoid surveillance by the immune system. Interactions between tumor and TME are bidirectional and the TME coevolves with the tumor. Moreover, different cellular components of the TME closely interact and together form a tumor-promoting microniche.
2.3.1. Cancer-associated fibroblasts
The presence of fibrosis and α-SMA-positive myofibroblasts is not only a key feature of the hepatic PME but also characteristic for the hepatic TME. These CAFs are widely believed to be derived from HSC, but formal fate-tracing studies are lacking. Within the TME, there are profound changes to the ECM, with increased levels of collagens type IV, VI, VII, X, XIV, XV, XVI, and XVIII as well as nidogen 1, decorin, perlecan, and multiple laminin subunits (76). Interestingly, mechanotransduction-induced YAP signals promote the generation of CAFs, suggesting a complex interplay between tumor mechanics and stromal fibroblasts that contributes to the evolution of key components of the TME (77). In addition, specific genomic profiles of HCC appear to influence the activation of the stromal compartment as demonstrated by lower fibrosis in patients with beta-catenin-mutated HCCs (78, 79). There is increasing evidence from clinical studies that the presence of liver fibrosis, α-SMA-positive myofibroblasts, or activated HSC signatures is associated with a poor prognosis after curative HCC resection, suggesting a key role of the fibrotic TME in promoting HCC progression: In patients with curative HCC resection, a high degree of peritumoral myofibroblast infiltration was associated with a 2.6-fold hazard ratio for overall survival and a 3.3-fold hazard ratio for recurrence-free survival (52). Multiple studies have shown poor survival as well as increased metastasis and recurrence following resection in HCC patients with positive HSC gene signatures or high expression of α-SMA (50, 53, 80, 81). Similar findings were made in animal models, where coinjection of activated HSC and HCC cells into mice enhanced subcutaneous or orthotopic tumor growth (53, 80–85). However, these studies lacked the proper hepatic environment in which HCCs develop and progress and may have led to unphysiological interactions between HSC and HCC cells that normally do not occur in hepatocarcinogenesis. Mechanisms through which CAFs promote HCC progression are discussed in the following sections.
2.3.2. Angiogenic vascular cells
HCC displays substantial changes to the vasculature, including arterialization of vessels with abundant smooth muscle cells as well as loss of endothelial fenestration (86). Abnormal blood flow and vascular leakiness are two characteristic functional features that may contribute to hypoxia and necrosis despite the highly angiogenic nature of HCC (86). A number of studies have demonstrated a positive correlation between high levels of serum VEGF and tumor stage, grade, vascular invasion, poor prognosis, and recurrence (86). In addition to VEGF, VEGF homolog placental growth factor (87) and basic fibroblast growth factor (88) promote the growth of HCC. HSCs are in immediate proximity to endothelial cells and form a functional unit with these cells to regulate hepatic blood supply as well as hepatic responses to injury, such as fibrogenesis and regeneration (89). It is likely that the effects of vascular cells on tumors are a pure function of tumor perfusion but that the interactions between endothelial and HSC cells contribute to the typical TME of HCC. As such, activated HSCs promote angiogenesis in vitro and in vivo through VEGF- and angiopoietin-dependent mechanisms in the fibrotic liver and HCC (84, 90, 91), as discussed in more detail in the following section.
2.3.3. Tumor-associated macrophages and myeloid-derived suppressor cells
Macrophages are the most prominent component of leukocyte infiltrate in tumors. TAMs originate from circulating monocytic precursors, which are recruited into the TME by tumor-derived signals such MCP-1 and M-CSF (92, 93). Clinical studies and experimental mouse models strongly suggest a tumor-promoting role of TAMs (94, 95). In HCC, TAM density was associated with large tumor size and poor survival in patients (96–98). TAMs appear to promote hepatocarcinogenesis through multiple mechanisms; in addition to being well established in the promotion of liver fibrosis (66, 69, 99, 100). These multiple mechanisms include several fibrosis-independent mechanisms: (a) TAMs release growth factors, cytokines, chemokines, and matrix metalloproteinases (MMPs), contributing to parenchymal cell transformation, tumor growth, ECM remodeling, angiogenesis, invasion, and metastasis (reviewed in 56); and (b) TAMs suppress antitumor immunity through the recruitment of regulatory T cells (Tregs) and increased expression of inhibitors of tumor-specific T cell immunity, such as PD-L1 (97), galectin 9 (101), IL-10, CCL17, and CCL22 (reviewed in 102).
Myeloid-derived suppressor cells (MDSCs) are a specific population of the myeloid line that potently suppress T cell responses and rapidly accumulate in chronic infection and cancer. MDSCs accumulate in murine models of HCC as well as in patients, preferentially in advanced disease stages (103–105). GM-CSF and CXCL1 produced by HCCs are responsible for the accumulation of MDSCs in mice (103). Of note, HSCs within the TME appear to exert a key role in the differentiation of MDSCs from monocytes (106–109). MDSC numbers correlate with tumor size (110) and ablation of MDSCs restores T cell functions (105), suggesting that immunosuppression by MDSCs contributes to HCC development.
2.4. Mechanisms by Which Myofibroblasts and Fibrosis Promote the Development and Progression of Hepatocellular Carcinoma
As there is a continuous transition from PME to TME during the progression from regenerative nodules to dysplastic foci and HCC, we discuss the mechanisms by which fibrosis-associated myofibroblasts and CAFs in the hepatic PME and TME, respectively, promote HCC in a single section of this review. Currently, there is little evidence that myofibroblasts drive the malignant transformation of hepatocytes. For this reason, we review how myofibroblasts and CAFs support the growth of dysplastic hepatocytes and HCC. Most evidence suggests that this occurs through multiple mechanisms (Table 1) that are mediated not only by the direct effects of myofibroblasts on premalignant hepatocytes or HCCs but also through the interactions of myofibroblasts with many other hepatic cell types (Figures 1 and 2).
Table 1.
Mechanisms by which HSC/CAF promotes hepatocarcinogenesis
| Mediator | Effect on hepatocarcinogenesis | Mechanism | Ref | |
|---|---|---|---|---|
| Quantitatively and quailtatively altered ECM | Laminin 5 | HSC stimulate HCC migration via the production and secretion of Laminin 5 | Activation of the MEK/ERK pathway | (114) |
| Stiffness and integrin β1 | ECM stiffness and integrin β1 expression correlate with pathologic grade and metastasis, and promote proliferation and chemotherapeutic resistance | Increased stiffness promote basal and HGF –stimulated mitogenic signaling through ERK, PKB/Akt, and STAT3 signaling pathway | (117; 131) | |
| DDR2 | Facilitation of HCC cell invasion, migration, EMT as well as in vitro and in vivo growth by DDR2 | Upregulation of MT1-MMP and MMP2 expression in HCC through the ERK2/SNAIL1 pathway. | (122; 123) | |
| Epimorphin | Promotion of invasion and metastasis | Epimorphin promotes HCC invasion and metastasis through FAK/ERK/MMP-9 | (140) | |
| Reduced immune surveillance | HSC inter-acting with T cell and Treg | Co-transplantation of HSC with HCC cells promotes HCC growth and progression | HSC inhibit lymphocyte infiltration, induced apoptosis of infiltrating mononuclear cells, and increased Tregs | (84; 141) |
| HSC interacting with MDSC | Promotion of HCC growth | HSC increase the number of MDSC, mediated by the COX2-PGE2-EP4 pathway | (107; 109) | |
| HSC interacting with monocytes | Protumorigenic effects via enhanced migration and tumor sphere formation | Co-cultured of monocytes with LX-2 cells change monocyte signature form inflammatory to immunosuppressive | (53) | |
| Angiogenesis | VEGF | Induction of tube formation in vitro and angiogenesis in vivo | Hypoxia in HSC induces VEGF via NO. VEGF from HSC increases angiogenesis. | (90; 145) |
| Angiopoietin 1 | Promotion of angiogenesis by angiopoietin 1-producing HSC | TNFα induces the secretion of angiopoietin 1 by activated HSC. Role in HCC unknown. | (91) | |
| Promoting tumor growth, survival, and invasion | TGF-β | Increased tumor formation by co- transplantion of HCC and HSC that depends on TGFβ signaling in HCC | Secretion of TGFβ by HSC activates Smad2/3 β-catenin, promoting HCC growth | (82) |
| HGF | Neutralization of HSC/CAF-secreted HGF inhibits HCC TIC characteristics, proliferation and invasion | HGF, via its receptor c-Met and MAPK/Erk, promotes HCC proliferation, survival, TIC characteristics and invasiveness | (81; 83; 160) | |
| Epiregulin | Reduced HCC in epiregulin-deficient mice | LPS upregulates epiregulin in HSC via NF-κB; epiregulin is a potent hepatomitogen | (70; 80; 158) | |
| Osteopontin | Promotion of HCC migration in vitro and tumor growth and metastasis in vivo. | Low tumor pH induces HSC activation via ERK1/2. Activated HSC secrete osteopontin to promote HCC migration | (155) | |
| Not determined | Promotion of HCC proliferation, migration and invasiveness in vitro and tumor growth in vivo |
|
(85); (80) | |
| Senescence and HCC | SASP (IL-6, IFNγ, IL-4) | HSC senescence, induced by GFAPCre-mediated p53 deletion, reduces HCC formation | HSC senescence leads to SASP, which shifts macrophage polarization to an anti- tumorigenic M1 phenotype | (75) |
| SASP (IL-6, Gro-α, CXCL9) | HSC senescence, induced by DMBA plus HFD, promotes HCC formation | Obesity alters the bacterial microbiota, resulting in increased levels of bacterial metabolite DCA, and subsequent HSC senescence; HSC SASP promotes HCC formation | (164) |
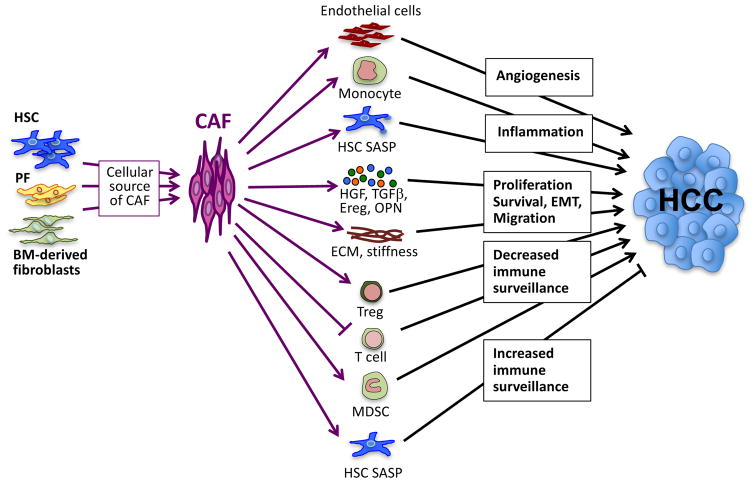
Figure 2. The contribution of CAFs to hepatocarcinogenesis.
CAFs may be derived from different cellular sources, including HSCs, PFs, and BM-derived fibroblasts. CAFs may promote HCC by increasing angiogenesis, inflammation, proliferation, survival, the EMT, and alterations of immune surveillance. These effects are either direct through mediators released from CAFs or indirect via interactions with other cells, including endothelial cells, monocytes, T cells, Tregs, and MDSCs. Abbreviations: BM, bone marrow; CAF, cancer-associated fibroblast; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; Ereg, epiregulin; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; MDSC, myeloid-derived suppressor cell; OPN, osteopontin; PF, portal fibroblast; SASP, senescence-associated secretory phenotype; Tregs, regulatory T cells.
2.4.1. Quantitatively and qualitatively altered extracellular matrix and stiffness promote hepatocarcinogenesis
Besides providing mechanical strength, the ECM regulates multiple signaling cascades through its ability to bind specific receptors, such as integrins, and to bind to growth factors, thereby regulating their distribution, activation, and presentation (111). Through activation of these pathways, ECM proteins exert key roles in regulating growth and differentiation in many settings, including development, stem cell niches, and cancer (111). The above-discussed increases in ECM components such as collagens, laminins, fibronectin, proteoglycans, and glycosaminoglycans result in phenotypic and behavioral changes in epithelial, tumor, and stromal cells (112–114). Surrounding cells sense changes in the ECM with the help of specific transmembrane receptors such as integrins and DDRs that in turn regulate specific signaling pathways within the cell in response to external stimuli. For example, hepatocellular death results in mice with hepatocyte-specific deletion of integrin-linked kinase, suggesting a key role of this pathway in linking the ECM to epithelial cell survival (115). Accordingly, there is an increase in integrin expression within tumors in different mouse models of HCC (76), as well as increased expression of integrin α6 and integrin β1 in HCC samples from patients (116, 117). Moreover, integrin expression correlates with pathologic grade, tumor encapsulation, and outcomes (116, 117). SNPs in integrin αV influence HCC growth in patients (118). Integrin activation triggers signaling cascades such as phosphoinositide 3 kinase (PI3K) and mitogen-activated protein kinase (MAPK), which have key roles in growth, survival, and migration of tumor cells (119, 120). The DDR protein family also has an important role in sensing ECM proteins, including collagens (121). In the liver, DDR2 promotes the development of liver fibrosis through a positive feed-forward loop that increases the proliferation and invasion of HSCs (41). In HCC, DDR2 shows increased expression in tumor tissue (122). High DDR2 expression is correlated with poor prognosis (122). DDR2 overexpression facilitates migration, invasion, and the EMT of HCC by activating ERK2 and stabilizing Snail1, whereas DDR2 silencing in HCC decreases the EMT, Snail1 half-life, and invasion but increases cell death (122, 123).
Enhanced production and reorganization of the ECM leads to a mechanically stiff microenvironment, which has been shown to promote tumor cell proliferation and invasion as well as changes in gene expression and behavior of stromal cells, thereby enhancing tumor progression (113, 124, 125). In cancer, stiffness is not purely a result of ECM production by myofibroblasts but is influenced by both the stromal and the epithelial compartments. As such, tumor cells not only regulate the degree of fibrosis in some cancers (126, 127) but also directly express a number of ECM proteins (128) and enzymes, such as lysl oxidase (124), that regulate cross-linking and matrix stiffness. Moreover, tumors also display increased stiffness due to alterations in tissue architecture and continuing proliferation of tumor cells within a limited volume, affecting both tumor cells and adjacent normal tissue (129). Increases in matrix stiffness that enhance cell contractility have been found sufficient to enhance the transformation of mammary epithelial cells (130). Conversely, a reduction in tissue stiffness by inhibition of collagen cross-linking impedes malignant growth and tumor development in a murine model of breast cancer (124). Hepatoma cells grown on increasingly stiff polyacrylamide gels exhibited increased proliferation and chemotherapeutic resistance, which was mediated by Fak, Erk, PKB/Akt, and Stat3 signaling pathways downstream of integrin β1 activation (131). Recent evidence suggests a key role of receptor-independent mechanosensitive signal transduction pathways. YAP and TAZ, two downstream effectors of the Hippo pathway and powerful regulators of cell differentiation and survival, are activated by stiff ECM (132). The activation of YAP and TAZ is independent of Hippo or known cell surface receptors but requires Rho GTPase activity and tension of the actomyosin cytoskeleton (132). Moreover, increased stiffness also results in activation of beta-catenin signaling during the development of colon cancer (129) and suppression of PTEN expression in breast cancer (133). Of note, the beta-catenin, Hippo, and PTEN pathways are important contributors to hepatocarcinogenesis (16, 134, 135). Further studies are needed to determine the relevance of these receptor-independent mechanosensitive signaling pathways for the development and progression of HCC.
Clinically, elastography provides a measurement of liver stiffness and is a predictor for HCC development (47–49, 136). Matrix stiffness has been directly implicated in aiding tumor development through several mechanisms. Of interest, changes in matrix stiffness modulate the behavior of epithelial cells through the above-discussed activation of oncogenic pathways and are likely to contribute to the transformation and dedifferentiation of hepatocytes. Moreover, changes in ECM and stiffness contribute to the ductular reaction and progenitor expansion in the liver (137), which in turn are linked to hepatocarcinogenesis. Moreover, matrix stiffness also provides a niche for TICs, contributing to their proliferation, resistance to chemotherapy, and dedifferentiation (131). Increased stiffness within tumors also contributes to the activation of CAFs (77), thereby providing a feed-forward loop that amplifies the above-described signals and may contribute to the permanent establishment of a stiff tumor niche. ECM deposition and degradation are highly dynamic processes within the injured liver. Beyond simple removal of ECM, its degradation may also modulate the release of sequestered growth factors, generate bioactive cleavage products, or cleave cell surface receptors (120). In this regard, MMPs and their inhibitors TIMPS (tissue inhibitors of metalloproteinases) are considered key regulators of ECM degradation and regulate cell proliferation, increase hepatocyte growth, and promote tumor progression (reviewed in 120). Moreover, MMPs are required to promote tumor spread within the often highly fibrotic livers in which HCCs arise as they help break down tumor boundaries and promote tumor cell invasion (19). Accordingly, HCC is associated with increased MMP2 expression and higher proteolytic activity (138). Well-differentiated hepatoma cells express MT1-MMP, MMP2, and MMP9, which promote stromal cell invasion (139). HSC-derived epimorphin, an ECM protein that is highly expressed in HSCs in tumor stroma, mediates the invasive potential of cancer cells by upregulating MMP9 expression (140).
2.4.2. Hepatic stellate cells decrease immune surveillance
Several lines of evidence suggest that HSCs reduce tumor immunosurveillance through paracrine signals to surrounding immune cells: (a) Cotransplantation studies demonstrated that HSCs inhibit lymphocyte infiltration in tumors and the spleens of tumor-bearing mice, induce apoptosis of infiltrating mononuclear cells, and increase the number of infiltrating FoxP3-positive immunosuppressive Tregs, resulting in reduced immune surveillance (84, 141). (b) HSCs increase the number of MDSCs in the spleen, BM, and tumor tissues of tumor-bearing mice, which is mediated by the COX2-PGE2-EP4 pathway (107, 109). (c) Moreover, cell-cell contact is also important for the reduced immunosurveillance in the presence of activated HSC Monocytes cocultured with HSC LX-2 cells increased the expression of CD14, CCR2, and CD15 on the cell surface and changed cytokine gene expression from an inflammatory to an immunosuppressive signature, namely, upregulating immunosuppressive cytokines and downregulating inflammatory cytokines (53). Importantly, the interaction between HSCs and monocytes induces protumorigenic and progressive features of HCC cells by enhancing cell migration and tumor sphere formation (53). Activated HSCs can induce the differentation of MDSCs from CD14+ monocytes in a contact-dependent manner, which is abrogated by CD44 blockage (106). In HCC, MDSCs inhibit not only the adaptive but also the innate immune response (142) and are capable of inducing immunosuppressive Tregs (104). TGF-β, which is produced by myofibroblasts as well as other cell types in the PME and TME, is a potent suppressor of antitumor immunity via effects on natural killer cells, dendritic cells, macrophages, neutrophils, CD8+ and CD4+ effector cells and Tregs (74) and may therefore represent a link between CAFs and immunosuppression. In addition to the above-described mechanisms, ECM produced by activated HSCs may also provide a physical barrier that blocks the infiltration of immune cells (143).
2.4.3. Hepatic stellate cells promote tumor angiogenesis
Accelerated tumor growth is accompanied by a hypoxic microenvironment necessitating neovascularization within the tumor. Angiogenesis is a hypoxia-stimulated and growth factor-mediated process in which new vessels are formed from the existing vasculature (144). Activated HSCs produce numerous angiogenic factors such as VEGF (90, 145) and angiopoietin 1 (Angpt1) 1 or Angpt2 (91, 146, 147), which activate their respective receptors on endothelial cells to enhance their functions (143) and promote tumor vascularization and growth. This has been shown in numerous in vitro and in vivo studies using HSC/endothelial cell coculture systems and in tumor cell/HSC coimplantation models (90, 148–150). VEGF is a potent survival factor for endothelial cells that promotes their proliferation and vascular permeability thus supporting the formation of new vasculature. Angpt1 and Angpt2 play a role in vessel stability wherein binding of Ang-1 to its receptor Tie-2 promotes interactions between endothelial cells and pericytes resulting in increased vascular stability. Angpt2, in the presence of VEGF, initiates and augments angiogenesis to promote new vascular growth (reviewed in detail in 151). Overexpression of Angpt1 and Angpt2 was positively correlated with tumor dedifferentiation in HCC (147), and the level of Angpt2 was found to be associated with clinicopathological parameters of HCC patients (152). Moreover, histological staining of Angpt1 and Angpt2 in HCC tissues displayed colocalization with α-SMA-positive HSC, suggesting that these cells actively produce proangiogenic mediators (147).
In addition to active secretion of proangiogenic factors, HSCs may be actively involved in vascular remodeling (143, 153). Three-dimensional spheroid coculture of HSCs with endothelial cells resulted in spontaneous differentiation and organization into a structure with a core of HSCs and a surface of endothelial cells resembling the physiological assembly of blood vessels (153); additionally, coculture of activated HSC with liver sinusoidal endothelial cells triggered the formation of capillary-like sprouts.. These findings suggest that activated HSCs in the TME may participate in neovascularization (153).
2.4.4. Hepatic stellate cell-secreted cytokines may contribute to tumor growth, survival, and invasion
Activated HSCs secrete cytokines and growth factors that can stimulate the growth, proliferation, and migration of cancer cells as well as other nontumor cells within the stroma. As such, HSC culture supernatant contains PDGF-AB, osteopontin, HGF, and TGF-β, which in turn enhance the proliferation and migration of tumor cells in vitro (82, 83, 154, 155). TGF-β is a pleiotropic cytokine with known roles in liver fibrosis and cirrhosis and is therefore a key contributor to the shaping of the hepatic PME. Moreover, TGF-β also contributes to several aspects of liver carcinogenesis leading to tumor progression, and to metastasis via epithelial cell autonomous signals and interdependent stromal-epithelial interactions (156, 157). HSC-derived TGF-β stimulates autocrine TGF-β, signaling in premalignant hepatocytes, and accumulation of β-catenin within the nucleus of transformed hepatocytes in vivo (82). Additionally, TGF-β is a well-known inducer of the EMT that can contribute to the increased motility of cancer cells.
In addition to TGF-β, activated HSCs secrete potent mitogenic factors such as HGF and epiregulin that promote hepatocyte proliferation and tumor growth (70, 157, 158). HGF, which is predominantly expressed in HSC and endothelial cells in the normal liver, has been detected in cirrhotic liver tissue, adenomas, and 80% of HCC cases (159). Neutralization of HSC-secreted HGF inhibits the proliferation and invasion capacity of HepG2, Huh7, Hep3b, and PLC cells in vitro (83, 160). Tumor promotion by HGF is attributed to MAPK/ERK-induced proliferation and survival signals in HCC (reviewed in 161) and activation of a c-Met/FRA1/HEY1 signaling cascade that contributes to HCC invasiveness and chemoresistance (81). Accordingly, an expression signature regulated by the HGF receptor c-Met defines a subgroup of HCC with a poor prognosis and aggressive phenotype (162). Based on these findings, the HGF pathway is currently considered an important therapeutic target in clinical studies with HCC patients (161). Epiregulin is a member of the EGF family of peptide growth hormones and stimulates the proliferation of hepatocytes (158) and HCC cell lines (163). In the chronically injured liver, epiregulin is predominantly expressed by activated HSCs and strongly upregulated by LPS (70). Of note, epiregulin-deficient mice exhibit decreased tumor burden in a model of injury-driven hepatocarcinogenesis, suggesting that epiregulin may represent a link between inflammation, HSCs, and tumor development (70).
2.4.5. Hepatic stellate cell senescence and hepatocellular carcinoma
Two recent studies have implicated HSC senescence in the promotion of hepatocarcinogenesis. In one study, suppression of HSC senescence (achieved by p53 deletion) resulted in M2 macrophage polarization and proliferation of premalignant hepatocytes (75), implying that the senescence-associated secretory phenotype (SASP) of HSCs suppresses HCC. In contrast, a second study found that SASP promoted HCC development in the setting of obesity (164). Although the GFAP promoter has been employed to target HSC, it is not always HSC-specific (also deleting in cholangiocytes). Without confirmation that p53 was indeed efficiently deleted in HSCs but not in other hepatic cell types (75), it is possible that the phenotype in this study may have resulted from combined p53 deletion in non-HSC cell types. Likewise, it is not clear how the high-fat diet employed by Yoshimoto et al. (164) induced HSC activation and HSC senescence, as high-fat diet regimens do not cause HSC activation in genetically unaltered mice unless they are combined with high levels of dietary fructose and/or cholesterol. Further investigations are required to determine whether HSC SASP promotes or restricts hepatocarcinogenesis and whether its role in hepatocarcinogenesis is disease- or model-specific.
3. CHOLANGIOCARCINOMA
3.1 Introduction to Cholangiocarcinoma
CCA is the second-most prevalent primary malignancy in the liver. CCA is a histologically, genetically, and anatomically heterogeneous malignancy that is typically subdivided into perihilar, extrahepatic, and intrahepatic cholangiocarcinoma (iCCA), according to anatomical location (165, 166). Overall, incidence and mortality have increased (167) but appear to be largely due to the increased occurrence of iCCA in the United States, whereas perihilar and extrahepatic CCA have decreased (168–170). There is a strong geographical variation in CCA incidence, reflecting the presence of specific risk factors in some regions (168). The highest incidence of CCA is reported in Southeast Asia, due to chronic infections with liver flukes (171–173). Similar to HCC, the risk for development of CCA is also significantly increased by chronic liver disease, for example, in patients with chronic HBV or HCV infections, but to a much lesser degree than for HCC (174). One notable exception is primary sclerosing cholangitis (PSC), which carries a 20% lifetime risk for the development of CCA (175). Despite these associations, the majority of CCA cases are sporadic without clearly attributable risk factors (7, 176). Recent fate-mapping studies have demonstrated that iCCA may arise not only from the malignant transformation of cholangiocytes (177, 178) but also from the transdifferentiation of hepatocytes (178–180). Transdifferentiation of hepatocytes into cholangiocytes and bipotent progenitor cells is also observed in chronic liver injury (181, 182). Despite the possible shared origin of some cases of CCA with HCC, CCA differs biologically, histologically, and genetically from HCC. The most frequent genomic alterations in CCA are in the P53, KRAS, SMAD4, and IDH1/2 pathways (183–186). With significant differences in genomic alterations between different studies, it appears that genomic profiles are linked to the underlying disease and possibly also geographic region (183–186). Biologically, CCA shares many similarities with pancreatic ductal adenocarcinoma (PDAC), which also displays a similar genetic landscape, with mutations of P53, KRAS, and SMAD4 as well as similar histological features such as the cytokeratin 19-positive tumor compartment surrounded by highly desmoplastic and hypovascularized stroma (176, 187). Prognosis is poor with the majority of CCA patients surviving for less than a year following diagnosis (188). One of the main features of iCCA is the presence of abundant and hypovascularized desmoplastic stroma (187), in which α-SMA+ CAFs are abundant. It is believed that the desmoplastic and hypovascularized nature of CCA contributes to the poor prognosis and therapy resistance of CCA. Although recent in vivo and in vitro studies point toward a pivotal role of α-SMA+ CAFs in iCCA promotion and resistance to chemotherapy (11, 187, 189, 190), we still do not fully understand the biological role and clinical significance of CAFs in iCCA. Of note, there is increasing evidence that CAFs and a fibrotic environment provide both tumor-promoting (127) and tumor-restricting signals (191, 192) in PDAC. Based on the many similarities between PDAC and CCA, it is likely that the contribution of CAFs to the development and growth of CCA is complex and that CAFs and the ECM may exert both tumor-promoting and tumor-restricting roles.
3.2. The Premalignant Environment of Cholangiocarcinoma
Whereas the development of HCC is often a slow and long-term process occurring in the PME of the cirrhotic liver, the development of CCA is more difficult to trace. Recent studies suggest a key role for inflammatory pathways in the development of CCA (193). However, it is not known whether activation of these pathways in the PME drives the development of CCA or occurs predominantly after tumors are established. With many cases of CCA occurring in the absence of an identifiable risk factor (7, 176), it appears that the PME is likely disease-specific. This is different from HCC where cirrhosis represents a common risk factor within the PME. Notable exceptions may be primary sclerosing cholangitis (PSC) and infection with liver flukes. PSC encompasses many changes in the PME that may drive cancer development, including increased cholangiocyte proliferation, inflammation, fibrosis, and in advanced stages, cirrhosis (186, 194, 195). As PSC is rarely diagnosed in early prefibrotic stages and most CCAs develop within the first year of PSC diagnosis, it is difficult to understand the exact nature and role of the PME in PSC. Similar to PSC, infection with liver flukes is accompanied by cholangitis, fibrosis-induced obstruction of ducts, and immune responses to the flukes, as well as secondary bacterial infections that may further exacerbate inflammation and fibrosis (196). However, as opposed to the 20% lifetime risk of CCA development in PSC patients, infections with Clonorchis sinensis (four to six times increased risk; 196) or Opisthorchis viverrini (approximately fivefold increased risk; 197) result in a lower relative risk of developing CCA. Inflammation seems be to an important risk factor in this setting, because high levels of IL-6 (>82.7 pg/ml) confer a >100-fold risk for CCA development in patients with O. viverrini infection (198). This finding and the fact that IL-6 also appears to have a role in the development of liver fibrosis in patients with O. viverrini infections (198) strongly suggest a key role of the PME in the development of liver fluke-associated CCA. Other risk factors for iCCA include liver cirrhosis and hepatitis virus infections, both accompanied by the presence of fibrotic tissue deposition. Although this can be taken as evidence that a fibrotic and inflammatory PME also drive CCA development in this setting, further investigations are needed to understand whether hepatitis virus infection and cirrhosis mediate this risk through specific mechanisms, such as the presence of proliferating cholangiocytes within the ductular reaction or the transdifferentiation of hepatocytes into tumor cells. Understanding the role of the PME in CCA development may facilitate therapeutic approaches to reduce risk for development of CCA beyond treatment of the underlying disease. Due to the lack of efficient therapeutic approaches, this would be particularly relevant for patients with PSC.
3.3. The Tumor Microenvironment of Cholangiocarcinoma
The most characteristic feature of CCA is its highly desmoplastic TME, which contains an abundance of CAFs and the ECM (187). In addition, the stroma contains inflammatory cells, in particular, a high number of macrophages. It is believed that all of these features (Figure 3) contribute to the growth and therapeutic resistance of CCA. This would be similar to a tumor-promoting role of a desmoplastic TME in a number of other tumors, including breast, lung, colon, prostate, and pancreas (199). In support of this concept, patients with “scirrhous” iCCA have a higher proliferation index and lower 1- and 3-year survival (189). This was further confirmed by studies showing that a high number of α-SMA-positive myofibroblasts or high expression of the ECM protein periostin was associated with significantly lower survival in patients (200–202). Another key feature of CCA is the hypovascular nature of its TME (203). Findings from PDAC suggest that its hypovascular TME represents a key barrier preventing the delivery of chemotherapeutic drugs (204). Although CCA’s TME contributes to high resistance to therapy, it may also present a therapeutic opportunity (204, 205).
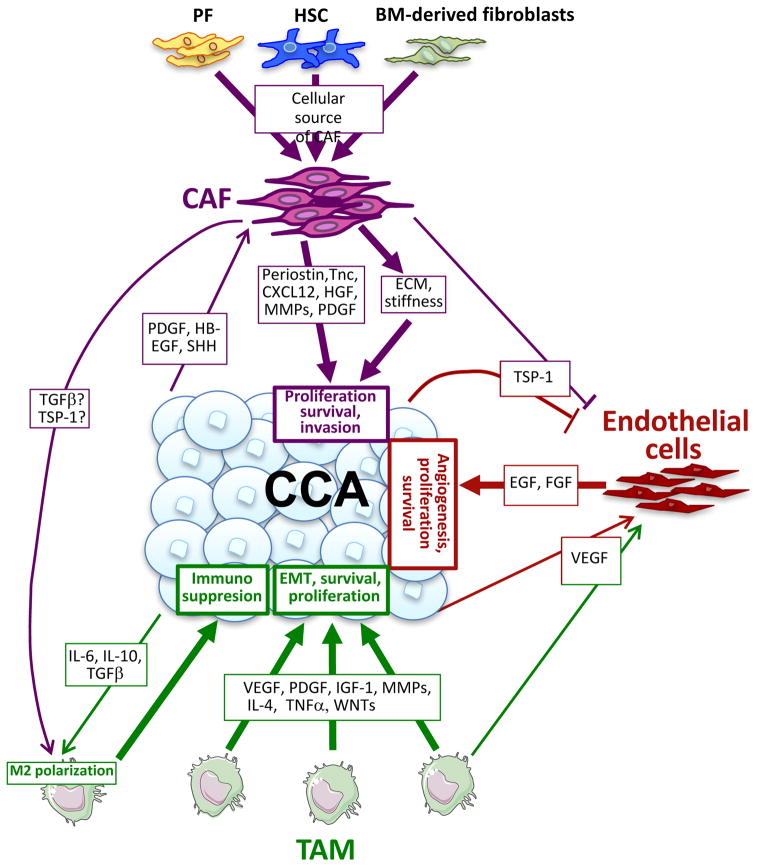
Figure 3. The tumor microenvironment of CCA.
In CCA, CAFs may be derived from different cellular sources, including HSCs, PFs, and BM-derived fibroblasts. CAFs promote direct effects on CCA proliferation, survival, and invasion through secreted growth factors, chemokines, MMPs, and ECM, as well as indirect effects via interactions with macrophages and endothelial cells. Abbreviations: BM, bone marrow; CAF, cancer-associated fibroblast; CCA, cholangiocarcinoma; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; HSC, hepatic stellate cell; PF, portal fibroblast; MMP, matrix metalloproteinase; TAM, tumor-associated macrophage.
3.3.1. Cancer-associated fibroblasts and fibrosis in cholangiocarcinoma
CAFs are one of the most abundant stromal cell types in the TME of CCA. In CCA, CAFs are thought to be phenotypically heterogeneous and possibly from various cellular lineages (11, 187). However, due to the lack of in vivo fate-tracing studies, the cellular source of CAFs in CCA remains a matter of debate. Potential sources of CAFs include HSCs, portal or periductular fibroblasts, BM-derived fibroblasts, and tumor-derived fibroblasts via the EMT. Although the expression of EMT biomarkers such as vimentin, fibronectin, and snail CCA cells supports a pivotal role for EMT in this tumor (206), recent studies have not provided evidence for a transdifferentiation of cholangiocytes into CAFs in CCA (207). Given that HSCs represent a major source of myofibroblasts in multiple models of biliary liver fibrosis (44), it is likely that this cell type also makes a major contribution to the CAF population in CCA. In summary, fate-tracing studies are needed to define the cell source and subtypes of CAFs present in CCA.
CAFs in the TME produce and secrete a large number of ECM proteins, which may also have clinical significance as prognostic markers of CCA. As detailed proteomic studies on the stromal ECM of CCA are still lacking, most of our knowledge is derived from studying which ECM proteins are expressed by CAFs. Periostin, a secreted ECM protein that acts as a ligand for αV integrins and promotes cell survival, invasion, and the EMT in many cancers (208–210), is expressed nearly exclusively in CAFs in both human and rat CCA (202, 211, 212). Periostin expression correlates with shorter survival (202) and increased tumor growth in patients (212). CAFs also express tenascin C (Tnc), an ECM protein with tumor-promoting roles in many cancers (213, 214). Tnc expression correlates with poor prognosis in iCCA patients (215). In addition, CAFs produce growth factors, such as HGF, that typically associate with the ECM. Last but not least, CAFs in iCCA produce metalloproteinases, including MMP-2 and MMP-9, which are implicated in cancer progression and spread via ECM remodeling (216–218).
Clinical evidence supports a crucial role of stromal cells in promoting tumor progression. Patients with strong desmoplasia and increased expression of α-SMA or periostin have shorter survival (200–202). CAFs differ from nontumorigenic liver fibroblasts in their gene expression, which may not only explain how they promote tumor progression but also may also help stratifying patients by CAF-derived mediators such as periostin (202). Likewise, three-dimensional organotypic culture systems have demonstrated enhancement of CCA growth by α-SMA-positive CAFs (211). Based on accumulating evidence for a tumor-promoting role of CAFs, efforts have been made to target CAFs for therapeutic purposes. Navitoclax is a cytotoxic, BH3-mimetic drug, which induces apoptosis in human HSCs but lacks similar effects in quiescent fibroblasts or tumor cells (190). Navitoclax not only decreased expression of α-SMA in tumors but also reduced tumor burden and metastasis while improving survival in a syngeneic, orthotopic rodent model of CCA (190). Likewise, targeting CAFs with TGF-β antagonist 1D11 reduced both fibrosis and CCA development in thioacetamide-treated rats (219). Together, these studies further support the tumor-promoting role of CAFs and indicate that targeting of CAFs may be a promising strategy for CCA prevention or treatment. However, in view of the above-discussed inhibitory role of CAFs in PDAC (191, 192), the lack of good preclinical CCA models, and the rather small-scale clinical studies, further carefully designed functional studies in rodents as well as larger-scale clinical studies are required to firmly establish the role of CAFs in CCA. Without functional studies, one cannot exclude that the presence of fibrosis and α-SMA-positive myofibroblasts is only a marker of more aggressive tumor subtypes without necessarily exerting a tumor-promoting role.
Although the main focus of the field is on how the fibrotic stroma affects CCA, the interactions between stroma and tumor cells are bidirectional, suggesting a key role of the tumor in controlling development of the desmoplastic stroma in CCA. Platelet-derived growth factor (PDGF) is an important mediator of cholangiocyte-fibroblast cross talk, contributing to the recruitment and expansion of CAFs (207). Accordingly, CCA tumor cells are strongly positive for PDGF-A and PDGF-D and weakly express PDGF-B, whereas CAFs show strong expression of PDGFR-B; in contrast to tumor cells, normal cholangiocytes do not secrete high amounts of PDGF-D (207, 220). PDGF-induced CAF migration is mediated by Rho GTPases as well as the c-Jun N-terminal kinase pathway (207). Hypoxia-inducible factor 1α expression is a well-established feature of tumors enriched in desmoplastic stroma, such as iCCA and PDAC (217, 221, 222). Moreover, hypoxia in tumor cells upregulates the expression of periostin, HGF, SDF-1, CXCR4, CTGF, galectin-1, thrombospondin-1, and sphingosine kinase 1 in CAFs in several cancers and has been suggested to exert similar effects in iCCA (11). Sonic Hedgehog (Shh) is highly expressed in iCCA (223) and promotes the survival and proliferation of myofibroblastic HSC (224). Therefore, it is likely that SHH contributes to activation of CAFs by tumor cells in CCA, similar to the role it exerts in PDAC (204, 225). HSCs stimulate CCA cell proliferation, migration, and invasion through aberrant Hedgehog activation (220–222, 224–226), suggesting that Hedgehog signaling may be involved in the bidirectional cross talk between CCA and stroma. Studies in PDAC demonstrate that the spectrum of genomic alterations in the epithelial compartment may dictate the degree of desmoplastic stroma (127). Of note, mutations of Smad4, which are also common in CCA (176), were associated with stronger desmoplasia and reduced survival (127). Clearly, further investigations are needed to better define the matrisome of CCA and understand how CCA triggers the accumulation of desmoplastic stroma.
3.3.2. Other components of the tumor microenvironment in cholangiocarcinoma
In addition to CAFs, the CCA TME contains mainly inflammatory cells, with a prevalence of TAM as well as vascular endothelial cells. In murine models of CCA, the majority of TAMs are derived from bone marrow and not from liver-resident Kupffer cells (227). Several studies have shown a correlation between TAM number, tumor progression, and bad prognosis (228). A recent study highlighted the role of macrophage-derived WNT in promoting CCA growth in a xenograft model (227). Moreover, exposure of human macrophages to conditioned media from CCA cell lines resulted in macrophage polarization toward the protumorigenic M2 phenotype (229). In CCA, M2 TAMs have been suggested to regulate the EMT through the secretion of M2 cytokines, including IL-4, IL-6, IL-10, TGF-β, and TNF-α (230, 231). In addition to inflammation, CCA is characterized by high expression of both VEGF (232) and VEGF receptors (233, 234). Accordingly, increased angiogenesis correlates with lower survival rates (235, 236). Consistent with the known role of phagocytic lineage cells, such as TAMs, in the angiogenic process (95), there is a positive correlation between microvessel density, levels of infiltrating TAMs in tumor vessel areas, and poor prognosis (237, 238). However, interactions among CAF, TAM, and vascular endothelial cells are not well understood and further studies are needed to understand whether CAFs affect the recruitment and function of TAM and vascular endothelial cells in CCA.
3.4. Mechanisms by Which Myofibroblasts and Fibrosis Affect the Development and Progression of Cholangiocarcinoma
Current evidence suggests that CAFS promote the development and progression of CCA through multiple mechanisms, that most likely work hand in hand in a complex multi-cellular signaling network.
3.4.1. Cancer-associated fibroblasts promote the proliferation and survival of cholangiocarcinoma
A main mechanism by which CAFs promote the progression of CCA is through their expression of cytokines and growth factors, which support the proliferation and survival of tumor cells (Table 2). Among the molecules implicated in tumor-stroma cross talk, heparin-binding epidermal growth factor has emerged as a paracrine factor that contributes to intercellular communications between CAF and tumor cells in several cancers, including CCA. Cotransplantation of CCA tumor cells with human liver myofibroblasts increases tumor incidence, size, and metastatic dissemination in vivo, which can be inhibited by EGFR tyrosine kinase inhibitor gefitinib (239). Moreover, coculture experiments demonstrate EGFR activation in tumor cells by myofibroblast-derived EGF, resulting in enhanced migratory and invasive properties in vitro (239). PDGF-BB and PDGFR-β also exert a key role in stroma-tumor cell cross talk in CCA. In addition to the role of PDGF in recruiting CAFs, myofibroblast-derived PDGF-BB promotes hedgehog signaling-dependent survival signals (220). Inhibiting PDGFR-β in co-cultures of HSC cell lines with CCA cells blocks the cytoprotective effects of co-culture and a reduction in tumor size in a rodent model of CCA (220, 240). However, despite these promising studies, erlotinib and imatinib have been relatively disappointing in limited clinical studies in human CCA (241). Periostin is overexpressed in CCA and almost exclusive produced by CAFs in the CCA stroma (202, 242, 243). Periostin interacts with integrin α5β1 or α6β4, resulting in the activation of Akt and FAK signaling pathways (244, 245), contributing to fibrogenesis and desmoplasia, invasive malignant cell growth, chemoresistance, and metastatic colonization (202, 242, 243, 245, 246) Thrombospondin-1 is a matricellular protein expressed by both CAF and tumor cells in iCCA (203, 247). Increased thrombospondin-1 expression correlates with hypovascularity and increased intrahepatic metastasis (248). It appears that thrombospondin-1 promotes CCA invasion by activating the plasminogen/plasmin system and via the upregulation of metalloproteinases (247–250). HGF is expressed in CCA by both CAF and tumor cells (211, 251). Moreover, HGF receptor c-Met is highly expressed in CCA (252), suggesting CAF-mediated promotion of CCA growth through this axis (253). Some effects of HGF-derived CAFs may also be through CXCR4, which is upregulated in CCA in an HGF-dependent manner (211) and promotes CCA migration (254).
Table 2.
Mechanisms by which HSC/CAF promote CCA growth
| Mediator | Effects on tumor | Mechanisms | Ref | |
|---|---|---|---|---|
| Promotion of proliferation and invasion | Periostin | Promotion of tumor cell proliferation and invasion, chemoresistance and metastasis. Contributes to fibrogenesis and desmoplasia. | Interaction with integrins α5β1 orα6β4 and subsequent activation of AKT and FAK, thereby promoting migration. | (202; 211; 212; 242; 245) |
| Thrombospondin-1 | Promotion of CCA invasion and metastasis. Correlates with hypovascularity of iCCA. | Activation of plasminogen/plasmin system and via upregulation of metalloproteinases. | (247; 248) | |
| HGF | Proliferation and possibly invasion in vitro | Activation of HGF receptor c-Met promotes CCA proliferation; HGF pregulation of CXCR4 expression in CCA cells | (211) | |
| Tenascin-c | Tnc expression at the invasive front correlates with poor prognosis. | Not well investigated. Interacts with periostin. Tnc promotes survival and stemness in other tumors. | (215) | |
| CXCL12 | iCCA progression and invasion. | MEK1/2, AKT activation in vitro and via activationof the canonical Wnt signaling pathway in vivo. | (255–257) | |
| MMP2, MMP9 | Promotion of tumor progression and invasion. | ECM proteins degradation | (216; 218) | |
| Promotion of survival | PDGF-BB | Promotion of tumor cell survival. | PDGF-BB protects from TRAIL-induced apoposis through hedgehog-mediated signals. | (220) |
| Modulation of immune response | IL-1β | Enhancement of tumor cell migration and invasion via CXCL5. | IL-1β from HSC induces Cxcl5 in tumor cells, thereby enhancing tumor cells migration and invasion. | (260) |
3.4.2. Cancer-associated fibroblasts modulate inflammation and immune responses in cholangiocarcinoma
Although there is strong evidence for HSCs and CAFs modulating inflammation and immune responses in HCC, their role in regulating theses anti-tumor responses in CCA remains largely unexplored. The best-characterized CAF-derived inflammatory mediator in CCA is CXCL12, also known as SDF-1. CXCL12 expression has been detected in CAFs localized at the invasive front of human iCCA (254). Via its cognate receptor CXCR4, CXCL12 promotes migration and invasion of human CCA cells in vitro through MEK1/2 and Akt pathway activation (255). In vivo, high CXCR4 expression has been associated with iCCA progression and metastasis (255, 256). Accordingly, blockade of CXCL12/CXCR4 signaling inhibits iCCA progression and metastasis via inactivation of the canonical Wnt pathway (255–259). Coculture studies revealed that interactions between cancer cells and CAFs in CCA lead to induction of IL-1β in CAFs, which in turn results in Cxcl5 production by cancer cells (260). Cancer cell-derived Cxcl5 exerts autocrine effects such as enhancement of migration and invasion (260) but may additionally act as a chemoattractant for immune cells. Moreover, several factors produced by CAFs in CCA, such as thrombospondin-1, MMPs, HGF, IGF, and fibroblast activation protein (FAP; 261), are likely to affect CCA growth through immunomodulatory effects. For example, thrombospondin-1 exerts immunosuppressive effects via activation of TGF-β and direct interaction with immune cells (262, 263); MMPs are chemotactic for leukocytes and modulate their proliferation and cytokine release (264, 265); HGF, IGF, and FAP act as CAF-derived immune modulators in other cancers (262, 263). Production of TGF-β, a potent suppressor of antitumor immunity via effects on natural killer cells, dendritic cells, macrophages, neutrophils, CD8+ and CD4+ effector cells, and Tregs (74), by CAFs may also contribute to immunosuppression. Moreover, the abundant stroma in CCA may provide a physical barrier that shields tumors from immune surveillance. In view of the recent clinical successes of checkpoint inhibitor therapies in a wide range of cancers, the role of immunomodulation and the involvement of CAFs in this process clearly need to be investigated in more detail.
4. SUMMARY AND OUTLOOK
Despite a large number of studies that provide evidence for a tumor-promoting role of myofibroblasts/CAFs in the PME and TME of HCC and CCA, we are still lacking definite evidence from carefully designed in vivo studies. PDAC provides a good example in which a genetic model that reproduces many features of human disease has been combined with state-of-the-art approaches to pharmacologically inhibit or genetically deplete myofibroblasts to better understand their contribution to various aspects of the disease (191, 192). Similar in vivo approaches with models in which HCC and CCA arise endogenously and interact with different components of the PME and TME in a physiological manner are needed to study the role of myofibroblasts in HCC and CCA. Coculture and cotransplantation studies have been useful in understanding the relationship between tumor and stroma. However, conclusions from these studies as to the contribution of myofibroblasts to liver cancer development and growth in vivo are limited, as coculture and cotransplantation put these cells into a nonphysiological contact and often employ subcutaneous models (lacking the proper hepatic environment) and immunodeficient mice (lacking adaptive immunity). As a part of studies on myofibroblasts in endogenously arising tumors, it would be important to also understand the source of myofibroblasts in the PME and TME. Although recent studies in multiple models of liver injury have highlighted the dominant role of HSCs as a source of hepatic myofibroblasts (44), the cellular source of myofibrobasts in the TME remains elusive. Detailed knowledge about the origin of myofibroblasts will be important to design studies in which relevant populations can be genetically ablated or pharmacologically targeted and will provide an important step toward the long-term goal of targeting myofibroblasts in the PME or TME for tumor therapy or prevention.
Acknowledgments
This study was supported by grants R01CA190844 and R01CA200597 (both to RFS) and a grant from the Cholangiocarcinoma Foundation (to SA).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors--from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Ladep NG, Khan SA, Crossey MM, Thillainayagam AV, Taylor-Robinson SD, Toledano MB. Incidence and mortality of primary liver cancer in England and Wales: changing patterns and ethnic variations. World J Gastroenterol. 2014;20:1544–53. doi: 10.3748/wjg.v20.i6.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–20. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94:1751–58. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 12.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–91. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–51. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 15.Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, et al. Effects of viral eradication in patients with HCV and cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016;151:130–39. e2. doi: 10.1053/j.gastro.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–39. e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 17.Mu X, Espanol-Suner R, Mederacke I, Affo S, Manco R, et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J Clin Invest. 2015;125:3891–903. doi: 10.1172/JCI77995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–27. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–29. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–57. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XF, Tan X, Zeng G, Misse A, Singh S, et al. Conditional β-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor α/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–65. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–79. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518–23. doi: 10.1038/nm.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caviglia JM, Schwabe RF. Mouse models of liver cancer. Methods Mol Biol. 2015;1267:165–83. doi: 10.1007/978-1-4939-2297-0_8. [DOI] [PubMed] [Google Scholar]
- 28.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–83. e4. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takehara T, Tatsumi T, Suzuki T, Rucker EB, III, Hennighausen L, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–97. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Vick B, Weber A, Urbanik T, Maass T, Teufel A, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–36. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–36. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hikita H, Kodama T, Shimizu S, Li W, Shigekawa M, et al. Bak deficiency inhibits liver carcinogenesis: a causal link between apoptosis and carcinogenesis. J Hepatol. 2012;57:92–100. doi: 10.1016/j.jhep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–32. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240–48. e2. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–93. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, He Y, Li F, Huang Q, Kato TA, et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell. 2015;58:284–96. doi: 10.1016/j.molcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell. 2015;57:860–72. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–19. [PubMed] [Google Scholar]
- 39.Friedman SL. Extracellular matrix. In: Dufour J-F, Clavien P-A, editors. Signaling Pathways in Liver Diseases. Chichester, UK: Wiley; 2015. pp. 85–96. [Google Scholar]
- 40.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–78. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol. 2011;30:243–47. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–87. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suh B, Park S, Shin DW, Yun JM, Yang HK, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology. 2015;61:1261–68. doi: 10.1002/hep.27654. [DOI] [PubMed] [Google Scholar]
- 47.Kim MN, Kim SU, Kim BK, Park JY, Kim do Y, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–59. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 48.Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965–70. doi: 10.1111/j.1872-034X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang HM, Hung CH, Lu SN, Chen CH, Lee CM, et al. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int. 2013;33:756–61. doi: 10.1111/liv.12118. [DOI] [PubMed] [Google Scholar]
- 50.Zhang DY, Goossens N, Guo J, Tsai MC, Chou HI, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65:1754–64. doi: 10.1136/gutjnl-2015-309655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Q, Fiel MI, Blank S, Luan W, Kadri H, et al. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer. 2013;109:573–81. doi: 10.1038/bjc.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131:498–510. doi: 10.1309/AJCP86PPBNGOHNNL. [DOI] [PubMed] [Google Scholar]
- 53.Ji J, Eggert T, Budhu A, Forgues M, Takai A, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62:481–95. doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20:2798–804. doi: 10.1200/JCO.2002.06.102. [DOI] [PubMed] [Google Scholar]
- 55.Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson CL, Jurk D, Fullard N, Banks P, Page A, et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat Commun. 2015;6:6818. doi: 10.1038/ncomms7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moles A, Murphy L, Wilson CL, Chakraborty JB, Fox C, et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J Hepatol. 2014;60:782–91. doi: 10.1016/j.jhep.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saito JM, Bostick MK, Campe CB, Xu J, Maher JJ. Infiltrating neutrophils in bile duct-ligated livers do not promote hepatic fibrosis. Hepatol Res. 2003;25:180–91. doi: 10.1016/s1386-6346(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 61.Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16:1235–44. doi: 10.1038/ni.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–96. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–24. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 64.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–59. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 65.Streetz KL, Wustefeld T, Klein C, Kallen KJ, Tronche F, et al. Lack of gp130 expression in hepatocytes promotes liver injury. Gastroenterology. 2003;125:532–43. doi: 10.1016/s0016-5085(03)00901-6. [DOI] [PubMed] [Google Scholar]
- 66.Pradere JP, Kluwe J, de Minicis S, Jiao JJ, Gwak GY, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–73. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139:323–34. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, et al. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–65. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seki E, de Minicis S, Osterreicher CH, Kluwe J, Osawa Y, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 70.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 72.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 73.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 74.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–60. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai KK, Shang S, Lohia N, Booth GC, Masse DJ, et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLOS Genet. 2011;7:e1002147. doi: 10.1371/journal.pgen.1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in β-catenin gene. Hepatology. 2009;49:821–31. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dal Bello B, Rosa L, Campanini N, Tinelli C, Torello Viera F, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157–66. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 80.Coulouarn C, Corlu A, Glaise D, Guenon I, Thorgeirsson SS, Clement B. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72:2533–42. doi: 10.1158/0008-5472.CAN-11-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–89. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 82.Mikula M, Proell V, Fischer AN, Mikulits W. Activated hepatic stellate cells induce tumor progression of neoplastic hepatocytes in a TGF-β dependent fashion. J Cell Physiol. 2006;209:560–67. doi: 10.1002/jcp.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amann T, Bataille F, Spruss T, Muhlbauer M, Gabele E, et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646–53. doi: 10.1111/j.1349-7006.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao W, Zhang L, Yin Z, Su W, Ren G, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129:2651–61. doi: 10.1002/ijc.25920. [DOI] [PubMed] [Google Scholar]
- 85.Wang ZM, Zhou LY, Liu BB, Jia QA, Dong YY, et al. Rat hepatic stellate cells alter the gene expression profile and promote the growth, migration and invasion of hepatocellular carcinoma cells. Mol Med Rep. 2014;10:1725–33. doi: 10.3892/mmr.2014.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van de Veire S, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–90. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 88.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834–42. doi: 10.1053/jhep.2002.32541. [DOI] [PubMed] [Google Scholar]
- 89.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin N, Chen Z, Lu Y, Li Y, Hu K, Xu R. Role of activated hepatic stellate cells in proliferation and metastasis of hepatocellular carcinoma. Hepatol Res. 2015;45:326–36. doi: 10.1111/hepr.12356. [DOI] [PubMed] [Google Scholar]
- 91.Taura K, de Minicis S, Seki E, Hatano E, Iwaisako K, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 92.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 93.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–66. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 97.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding T, Xu J, Wang F, Shi M, Zhang Y, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40:381–89. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ide M, Kuwamura M, Kotani T, Sawamoto O, Yamate J. Effects of gadolinium chloride (GdCl3) on the appearance of macrophage populations and fibrogenesis in thioacetamide-induced rat hepatic lesions. J Comp Pathol. 2005;133:92–102. doi: 10.1016/j.jcpa.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Wu K, Tao K, Chen L, Zheng Q, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–51. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 102.Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304–12. doi: 10.1002/hep.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–13. doi: 10.1016/j.jhep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 105.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP+CTLA-4+Foxp3+ T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–44. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hochst B, Schildberg FA, Sauerborn P, Gabel YA, Gevensleben H, et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol. 2013;59:528–35. doi: 10.1016/j.jhep.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 107.Zhao W, Zhang L, Xu Y, Zhang Z, Ren G, et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest. 2014;94:182–91. doi: 10.1038/labinvest.2013.139. [DOI] [PubMed] [Google Scholar]
- 108.Resheq YJ, Li KK, Ward ST, Wilhelm A, Garg A, et al. Contact-dependent depletion of hydrogen peroxide by catalase is a novel mechanism of myeloid-derived suppressor cell induction operating in human hepatic stellate cells. J Immunol. 2015;194:2578–86. doi: 10.4049/jimmunol.1401046. [DOI] [PubMed] [Google Scholar]
- 109.Xu Y, Zhao W, Xu J, Li J, Hong Z, et al. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget. 2016;7:8866–78. doi: 10.18632/oncotarget.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, et al. Increase in CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–30. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–19. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rombouts K, Carloni V. The fibrotic microenvironment as a heterogeneity facet of hepatocellular carcinoma. Fibrogenes Tissue Repair. 2013;6:17. doi: 10.1186/1755-1536-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34:834–43. doi: 10.1111/liv.12465. [DOI] [PubMed] [Google Scholar]
- 114.Santamato A, Fransvea E, Dituri F, Caligiuri A, Quaranta M, et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci. 2011;121:159–68. doi: 10.1042/CS20110002. [DOI] [PubMed] [Google Scholar]
- 115.Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, et al. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology. 2007;45:1025–34. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- 116.Begum NA, Mori M, Matsumata T, Takenaka K, Sugimachi K, Barnard GF. Differential display and integrin α6 messenger RNA overexpression in hepatocellular carcinoma. Hepatology. 1995;22:1447–55. doi: 10.1002/hep.1840220518. [DOI] [PubMed] [Google Scholar]
- 117.Zhao G, Cui J, Qin Q, Zhang J, Liu L, et al. Mechanical stiffness of liver tissues in relation to integrin β1 expression may influence the development of hepatic cirrhosis and hepatocellular carcinoma. J Surg Oncol. 2010;102:482–89. doi: 10.1002/jso.21613. [DOI] [PubMed] [Google Scholar]
- 118.Lee SK, Kim MH, Cheong JY, Cho SW, Yang SJ, Kwack K. Integrin αV polymorphisms and haplotypes in a Korean population are associated with susceptibility to chronic hepatitis and hepatocellular carcinoma. Liver Int. 2009;29:187–95. doi: 10.1111/j.1478-3231.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 119.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–20. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 120.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–75. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 122.Xie B, Lin W, Ye J, Wang X, Zhang B, et al. DDR2 facilitates hepatocellular carcinoma invasion and metastasis via activating ERK signaling and stabilizing SNAIL1. J Exp Clin Cancer Res. 2015;34:101. doi: 10.1186/s13046-015-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park JW, Lee YS, Kim JS, Lee SK, Kim BH, et al. Downregulation of discoidin domain receptor 2 decreases tumor growth of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2015;141:1973–83. doi: 10.1007/s00432-015-1967-5. [DOI] [PubMed] [Google Scholar]
- 124.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–91. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife. 2014;3:e01308. doi: 10.7554/eLife.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fernandez-Sanchez ME, Barbier S, Whitehead J, Bealle G, Michel A, et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 130.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 131.Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 133.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–67. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. PNAS. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–83. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954–61. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 137.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–56. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 138.Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. 2002;97:425–31. doi: 10.1002/ijc.1635. [DOI] [PubMed] [Google Scholar]
- 139.Okazaki I. Novel cancer-targeting agents/application strategies developed from MMP science. Anticancer Agents Med Chem. 2012;12:687. [PubMed] [Google Scholar]
- 140.Jia YL, Shi L, Zhou JN, Fu CJ, Chen L, et al. Epimorphin promotes human hepatocellular carcinoma invasion and metastasis through activation of focal adhesion kinase/extracellular signal-regulated kinase/matrix metalloproteinase-9 axis. Hepatology. 2011;54:1808–18. doi: 10.1002/hep.24562. [DOI] [PubMed] [Google Scholar]
- 141.Zhao W, Su W, Kuang P, Zhang L, Liu J, et al. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol. 2012;41:457–64. doi: 10.3892/ijo.2012.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases? Hepatology. 2011;54:707–13. doi: 10.1002/hep.24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258–70. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 145.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141–48. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 146.Sanz-Cameno P, Martin-Vilchez S, Lara-Pezzi E, Borque MJ, Salmeron J, et al. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: role of HBV X protein. Am J Pathol. 2006;169:1215–22. doi: 10.2353/ajpath.2006.051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, et al. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40:799–807. doi: 10.1016/j.jhep.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 148.Geng ZM, Li QH, Li WZ, Zheng JB, Shah V. Activated human hepatic stellate cells promote growth of human hepatocellular carcinoma in a subcutaneous xenograft nude mouse model. Cell Biochem Biophys. 2014;70:337–47. doi: 10.1007/s12013-014-9918-3. [DOI] [PubMed] [Google Scholar]
- 149.Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, et al. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674–85. doi: 10.1053/jhep.2003.50068. [DOI] [PubMed] [Google Scholar]
- 150.Kang N, Yaqoob U, Geng Z, Bloch K, Liu C, et al. Focal adhesion assembly in myofibroblasts fosters a microenvironment that promotes tumor growth. Am J Pathol. 2010;177:1888–900. doi: 10.2353/ajpath.2010.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen JA, Shi M, Li JQ, Qian CN. Angiogenesis: multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol Int. 2010;4:537–47. doi: 10.1007/s12072-010-9192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang ZL, Liu ZS, Sun Q. Expression of angiopoietins, Tie2 and vascular endothelial growth factor in angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol. 2006;12:4241–45. doi: 10.3748/wjg.v12.i26.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wirz W, Antoine M, Tag CG, Gressner AM, Korff T, et al. Hepatic stellate cells display a functional vascular smooth muscle cell phenotype in a three-dimensional co-culture model with endothelial cells. Differentiation. 2008;76:784–94. doi: 10.1111/j.1432-0436.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 154.Shimizu S, Yamada N, Sawada T, Ikeda K, Kawada N, et al. In vivo and in vitro interactions between human colon carcinoma cells and hepatic stellate cells. Jpn J Cancer Res. 2000;91:1285–95. doi: 10.1111/j.1349-7006.2000.tb00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Song J, Ge Z, Yang X, Luo Q, Wang C, et al. Hepatic stellate cells activated by acidic tumor microenvironment promote the metastasis of hepatocellular carcinoma via osteopontin. Cancer Lett. 2015;356:713–20. doi: 10.1016/j.canlet.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 156.Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-β signaling in onset and progression of hepatocellular carcinoma. Dig Dis. 2012;30:514–23. doi: 10.1159/000341704. [DOI] [PubMed] [Google Scholar]
- 157.Stover DG, Bierie B, Moses HL. A delicate balance: TGF-β and the tumor microenvironment. J Cell Biochem. 2007;101:851–61. doi: 10.1002/jcb.21149. [DOI] [PubMed] [Google Scholar]
- 158.Toyoda H, Komurasaki T, Uchida D, Takayama Y, Isobe T, et al. Epiregulin: a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995;270:7495–500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- 159.Ljubimova JY, Petrovic LM, Wilson SE, Geller SA, Demetriou AA. Expression of HGF, its receptor c-met, c-myc, and albumin in cirrhotic and neoplastic human liver tissue. J Histochem Cytochem. 1997;45:79–87. doi: 10.1177/002215549704500111. [DOI] [PubMed] [Google Scholar]
- 160.Neaud V, Faouzi S, Guirouilh J, Le Bail B, Balabaud C, et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458–66. doi: 10.1053/jhep.1997.v26.pm0009397985. [DOI] [PubMed] [Google Scholar]
- 161.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–18. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao M, He HW, Sun HX, Ren KH, Shao RG. Dual knockdown of N-ras and epiregulin synergistically suppressed the growth of human hepatoma cells. Biochem Biophys Res Commun. 2009;387:239–44. doi: 10.1016/j.bbrc.2009.06.128. [DOI] [PubMed] [Google Scholar]
- 164.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 165.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–70. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 168.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 169.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–75. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 171.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 172.Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010;25:1011–16. doi: 10.3346/jkms.2010.25.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Modha K, Navaneethan U. Diagnosis and management of primary sclerosing cholangitis--perspectives from a therapeutic endoscopist. World J Hepatol. 2015;7:799–805. doi: 10.4254/wjh.v7.i5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guest RV, Boulter L, Kendall TJ, Minnis-Lyons SE, Walker R, et al. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014;74:1005–10. doi: 10.1158/0008-5472.CAN-13-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mu X, Pradere JP, Affo S, Dapito DH, Friedman R, et al. Epithelial transforming growth factor-β signaling does not contribute to liver fibrosis but protects mice from cholangiocarcinoma. Gastroenterology. 2016;150:720–33. doi: 10.1053/j.gastro.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–15. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–18. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–24. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–18. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chan-On W, Nairismagi ML, Ong CK, Lim WK, Dima S, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–78. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 184.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–73. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 186.Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–93. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 187.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59:2397–402. doi: 10.1002/hep.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim HJ, Kim JS, Suh SJ, Lee BJ, Park JJ, et al. Cholangiocarcinoma risk as long-term outcome after hepatic resection in the hepatolithiasis patients. World J Surg. 2015;39:1537–42. doi: 10.1007/s00268-015-2965-0. [DOI] [PubMed] [Google Scholar]
- 189.Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of ‘scirrhous-type’ and ‘nonscirrhous-type’ growth. Am J Surg Pathol. 1999;23:892–902. doi: 10.1097/00000478-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 190.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382:1587–99. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 195.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, et al. Liver fluke induces cholangiocarcinoma. PLOS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Qian MB, Utzinger J, Keiser J, Zhou XN. Clonorchiasis. Lancet. 2016;387:800–10. doi: 10.1016/S0140-6736(15)60313-0. [DOI] [PubMed] [Google Scholar]
- 197.Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:301–8. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- 198.Sripa B, Thinkhamrop B, Mairiang E, Laha T, Kaewkes S, et al. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLOS Negl Trop Dis. 2012;6:e1654. doi: 10.1371/journal.pntd.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Togo S, Polanska UM, Horimoto Y, Orimo A. Carcinoma-associated fibroblasts are a promising therapeutic target. Cancers. 2013;5:149–69. doi: 10.3390/cancers5010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. α-Smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957–69. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 201.Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–64. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 202.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, et al. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leyva-Illades D, McMillin M, Quinn M, Demorrow S. Cholangiocarcinoma pathogenesis: role of the tumor microenvironment. Transl Gastrointest Cancer. 2012;1:71–80. [PMC free article] [PubMed] [Google Scholar]
- 204.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Epithelial-to-mesenchymal transition and cancer invasiveness: What can we learn from cholangiocarcinoma? J Clin Med. 2015;4:2028–41. doi: 10.3390/jcm4121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042–53. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu Q, Tong S, Zhao X, Ding W, Gou Y, et al. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem. 2015;36:799–809. doi: 10.1159/000430139. [DOI] [PubMed] [Google Scholar]
- 209.Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28:181–86. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- 210.Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–75. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Campbell DJ, Dumur CI, Lamour NF, Dewitt JL, Sirica AE. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol Res. 2012;42:1119–30. doi: 10.1111/j.1872-034X.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dumur CI, Campbell DJ, DeWitt JL, Oyesanya RA, Sirica AE. Differential gene expression profiling of cultured neu-transformed versus spontaneously-transformed rat cholangiocytes and of corresponding cholangiocarcinomas. Exp Mol Pathol. 2010;89:227–35. doi: 10.1016/j.yexmp.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. 2009;3:287–310. doi: 10.1007/s12079-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr. 2015;9:96–104. doi: 10.1080/19336918.2015.1008332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aishima S, Taguchi K, Terashi T, Matsuura S, Shimada M, Tsuneyoshi M. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol. 2003;16:1019–27. doi: 10.1097/01.MP.0000086860.65672.73. [DOI] [PubMed] [Google Scholar]
- 216.Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, et al. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer. 2010;127:2576–87. doi: 10.1002/ijc.25266. [DOI] [PubMed] [Google Scholar]
- 217.Sirica AE, Dumur CI, Campbell DJ, Almenara JA, Ogunwobi OO, Dewitt JL. Intrahepatic cholangiocarcinoma progression: prognostic factors and basic mechanisms. Clin Gastroenterol Hepatol. 2009;7:S68–78. doi: 10.1016/j.cgh.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–44. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- 219.Ling H, Roux E, Hempel D, Tao J, Smith M, et al. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLOS ONE. 2013;8:e54499. doi: 10.1371/journal.pone.0054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–88. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2011;27:276–84. doi: 10.1097/MOG.0b013e32834405c3. [DOI] [PubMed] [Google Scholar]
- 223.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential expression of Sonic Hedgehog protein in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2015;21:901–8. doi: 10.1007/s12253-015-9918-7. [DOI] [PubMed] [Google Scholar]
- 224.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–10. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 226.Kim Y, Kim MO, Shin JS, Park SH, Kim SB, et al. Hedgehog signaling between cancer cells and hepatic stellate cells in promoting cholangiocarcinoma. Ann Surg Oncol. 2014;21:2684–98. doi: 10.1245/s10434-014-3531-y. [DOI] [PubMed] [Google Scholar]
- 227.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–85. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49:318–24. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–19. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Techasen A, Loilome W, Namwat N, Dokduang H, Jongthawin J, Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):115–18. [PubMed] [Google Scholar]
- 231.Techasen A, Namwat N, Loilome W, Bungkanjana P, Khuntikeo N, et al. Tumor necrosis factor-α (TNF-α) stimulates the epithelial-mesenchymal transition regulator Snail in cholangiocarcinoma. Med Oncol. 2012;29:3083–91. doi: 10.1007/s12032-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 232.Xu LB, Liu C, Gao GQ, Yu XH, Zhang R, Wang J. Nerve growth factor-β expression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma. World J Surg. 2010;34:1039–45. doi: 10.1007/s00268-010-0417-4. [DOI] [PubMed] [Google Scholar]
- 233.Ogasawara S, Yano H, Higaki K, Takayama A, Akiba J, et al. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res. 2001;20:97–113. doi: 10.1016/s1386-6346(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 234.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–25. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shirabe K, Shimada M, Tsujita E, Aishima S, Maehara S, et al. Prognostic factors in node-negative intrahepatic cholangiocarcinoma with special reference to angiogenesis. Am J Surg. 2004;187:538–42. doi: 10.1016/j.amjsurg.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 236.Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–32. doi: 10.1038/ajg.2009.674. [DOI] [PubMed] [Google Scholar]
- 237.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–29. [PubMed] [Google Scholar]
- 238.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 239.Claperon A, Mergey M, Aoudjehane L, Ho-Bouldoires TH, Wendum D, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013;58:2001–11. doi: 10.1002/hep.26585. [DOI] [PubMed] [Google Scholar]
- 240.Fingas CD, Mertens JC, Razumilava N, Bronk SF, Sirica AE, Gores GJ. Targeting PDGFR-β in cholangiocarcinoma. Liver Int. 2012;32:400–9. doi: 10.1111/j.1478-3231.2011.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wiedmann MW, Mossner J. Molecular targeted therapy of biliary tract cancer--results of the first clinical studies. Curr Drug Targets. 2010;11:834–50. doi: 10.2174/138945010791320818. [DOI] [PubMed] [Google Scholar]
- 242.Riener MO, Fritzsche FR, Soll C, Pestalozzi BC, Probst-Hensch N, et al. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology. 2010;56:600–6. doi: 10.1111/j.1365-2559.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 243.Sirica AE, Almenara JA, Li C. Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Exp Mol Pathol. 2014;97:515–24. doi: 10.1016/j.yexmp.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the β4 integrin and the PI3k pathway. Oncogene. 2007;26:2082–94. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 245.Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, et al. Periostin activates integrin α5β1 through a PI3K/AKTdependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–18. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- 246.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–30. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kawahara N, Ono M, Taguchi K, Okamoto M, Shimada M, et al. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512–17. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- 248.Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, et al. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525–32. [PubMed] [Google Scholar]
- 249.Distler JH, Jungel A, Pileckyte M, Zwerina J, Michel BA, et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56:4203–15. doi: 10.1002/art.23074. [DOI] [PubMed] [Google Scholar]
- 250.Sargiannidou I, Zhou J, Tuszynski GP. The role of thrombospondin-1 in tumor progression. Exp Biol Med. 2001;226:726–33. doi: 10.1177/153537020222600803. [DOI] [PubMed] [Google Scholar]
- 251.Lai GH, Radaeva S, Nakamura T, Sirica AE. Unique epithelial cell production of hepatocyte growth factor/scatter factor by putative precancerous intestinal metaplasias and associated “intestinal-type” biliary cancer chemically induced in rat liver. Hepatology. 2000;31:1257–65. doi: 10.1053/jhep.2000.8108. [DOI] [PubMed] [Google Scholar]
- 252.Miyamoto M, Ojima H, Iwasaki M, Shimizu H, Kokubu A, et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br J Cancer. 2011;105:131–38. doi: 10.1038/bjc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 254.Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, et al. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-α and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–68. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–68. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhao S, Wang J, Qin C. Blockade of CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma progression and metastasis via inactivation of canonical Wnt pathway. J Exp Clin Cancer Res. 2014;33:103. doi: 10.1186/s13046-014-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu H, Xue W, Ge G, Luo X, Li Y, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401:509–15. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 258.Penn MS. SDF-1:CXCR4 axis is fundamental for tissue preservation and repair. Am J Pathol. 2010;177:2166–68. doi: 10.2353/ajpath.2010.100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 260.Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer. 2012;131:2234–41. doi: 10.1002/ijc.27496. [DOI] [PubMed] [Google Scholar]
- 261.Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancer-associated fibroblasts in digestive tumors. World J Gastroenterol. 2014;20:17804–18. doi: 10.3748/wjg.v20.i47.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–15. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 263.Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–80. doi: 10.1002/ijc.11542. [DOI] [PubMed] [Google Scholar]
- 264.Korpos E, Wu C, Sorokin L. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15:1349–57. doi: 10.2174/138161209787846685. [DOI] [PubMed] [Google Scholar]
- 265.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–81. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]