Abstract
PURPOSE
ACRIN 6695 was a feasibility study investigating whether CT perfusion (CTP) biomarkers are associated with progression-free survival at 6 months (PFS-6) in patients with advanced ovarian cancer who were treated with carboplatin and either dose-dense (weekly) or conventional (3-weekly) paclitaxel, with optional bevacizumab in the prospective phase III GOG-0262 trial.
PATIENTS AND METHODS
ACRIN 6695 recruited participants with residual disease after primary cytoreductive surgery or planned interval cytoreduction following neoadjuvant therapy, to undergo CTP studies before (T0), 3-weeks (T1) and 4-weeks (T2) after chemotherapy initiation. Tumor blood flow (BF) and blood volume (BV) were derived with commercial software. Fisher’s exact tests assessed the associations of CTP biomarkers changes from T0 to T2 dichotomized at zero with PFS-6 and overall radiographic response rate, while Cox regression assessed the associations between CTP biomarkers changes and progression-free survival (PFS) and overall survival (OS). Bonferroni correction was used to account for multiple comparisons.
RESULTS
76 of 120 enrolled patients from 19 centers were evaluable with a median age of 61 years. BV increase was significantly associated with lower chance of PFS-6 (p=0.028), while BF achieves borderline significance (p=0.053). In addition, BF increase was associated with shorter PFS (HR 2.9, 95% CI 1.3–6.4, p=0.008) and remained significant after adjusting for age, change in tumor volume and surgery status (p=0.007). Neither BF nor BV changes were significantly associated with treatment response rate or OS.
CONCLUSION
Early CTP biomarkers measurement may provide early prognostic information for PFS in newly-diagnosed ovarian cancer.
Keywords: Ovarian cancer, Chemotherapy, Imaging biomarker, CT Perfusion, Progression free survival
Introduction
Of the estimated 21,980 women diagnosed each year with epithelial ovarian cancer (EOC) in the United States, more than 70% will present with advanced (FIGO stage III or IV) disease.1 Standard primary therapy consists of cytoreductive surgery in combination with platinum and taxane-based chemotherapy.2 In 60–85% of patients, relapse will occur after initial treatment,3 resulting in a poor 5-year survival rate of <50%4 and making EOC the 5th most common cause of cancer mortality in women.5 Recent strategies to improve progression-free survival (PFS) in both primary and recurrent settings have included combinations of biological agents, predominately those targeting angiogenesis, with standard of care chemotherapy.6–8 However, these agents have been associated with significant complications and expense, and despite demonstrating a modest prolongation of PFS, they have not improved overall survival (OS) in the setting of primary therapy. For example, bevacizumab is associated with higher rates of treatment-related toxicities vs chemotherapy alone, including grade >2 hypertension (20% vs.7%) and bowel perforation, fistula or abscess (7% vs. 0%) and increases treatment cost up to 7-fold.9 Thus, a biomarker to identify patients unlikely to benefit from a specific therapy would enable better patient selection to optimize the therapeutic ratio.10
CT perfusion (CTP) is a dynamic contrast enhanced computed tomography (CT) examination, in which a standard CT scanner is used to serially image the passage of a contrast bolus through a target lesion after intravenous injection. The acquired images are then processed with dedicated commercial software to calculate quantitative perfusion biomarkers that include tumor blood flow (BF), tumor blood volume (BV), and vessel permeability surface product (PS). When the treatment regimen involves inhibition of tumor associated angiogenesis,11,12 CT perfusion can measure treatment-induced microvascular changes. Furthermore, CTP studies can be readily incorporated into existing routine CT protocols used for morphologic assessments (RECIST). 13 We hypothesize that CTP derived BF, BV are PS are early imaging biomarkers of treatment response in advanced ovarian cancer. However, the feasibility of CT perfusion in women with newly-diagnosed advanced-stage ovarian cancer has not been determined, and the utility of CTP has not been validated as an early imaging biomarker of treatment response in a prospective multi-center trial setting.
ACRIN 6695 was a multi-center pilot imaging biomarker trial prospectively designed to use standardized CTP scanning and post-processing analysis protocols to test the hypotheses that: (1) CTP perfusion is feasible in a subpopulation of women with suboptimal residual disease or in those women electing to undergo neoadjuvant chemotherapy prior to interval cytoreductive surgery and (ii) CTP biomarkers are associated with progression-free survival at 6 months (PFS-6) in a cohort of GOG-0262 (clinicaltrials.gov: NCT01167712) patients.
Methods
(a) ACRIN 6695 Trial Design
Cohort, Study Schema and Outcomes
The patient cohort would be a subset of patients enrolled into the Phase III GOG-0262 trial, a prospective multi-center phase III clinical trial comparing progression-free survival in treatment naïve patients with advanced stage ovarian cancer who received carboplatin and either dose-dense (weekly) or conventional (3-weekly) paclitaxel, with optional concurrent bevacizumab followed by bevacizumab maintenance. Inclusion criteria for ACRIN 6695 included enrollment on GOG-0262 with histologic confirmation of epithelial ovarian peritoneal or fallopian cancer, and a suboptimal surgical cytoreduction (i.e. residual disease > 1 cm) or a planned interval cytoreduction following neoadjuvant chemotherapy (NACT). For brevity, the inclusion and exclusion of GOG-0262 are omitted here but can be assessed at https://www.clinicaltrials.gov/ct2/show/NCT01167712?term=NCT01167712&rank=1. The exclusion criteria for ACRIN 6695 were: contraindication to iodinated contrast for CTP imaging, metformin therapy, and prior treatment with radiotherapy, chemotherapy, and/or targeted therapy. Three CTP studies were incorporated into the ACRIN 6695 protocol as follows: baseline before chemotherapy (T0), in the last week of the first cycle of chemotherapy (T1), and in the second week of the second cycle of chemotherapy (T2) (Supplementary Figure 1). Primary outcome PFS-6 and secondary outcomes best overall radiographic response, progression free survival (PFS), and overall survival (OS) were analyzed with the CTP biomarker data. Disease progression was defined on the basis of radiographic imaging using the revised international Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)13, or increased CA-125 levels based on criteria from the Gynecologic Cancer Intergroup14.
Eligibility and Evaluability
Enrolled patients were eligible for completing the three CTP studies if they had at least one target lesion as determined and confirmed by the site and central body CT radiologist respectively before treatment on either the initial standard-of-care RECIST CT or the baseline CTP study. Target lesions were tumors greater than 1 cm in transaxial directions and had a CT attenuation greater than or equal to 10 Hounsfield units (HU) in at least half the lesion on unenhanced CT; and an enhancement greater than or equal to 5 HU in the CTP scan. The enhancement requirement ensured that cystic lesions or hematomas (which do not enhance) were excluded as targets and ensured good signal-noise ratio in the target for reliable derivation of CT Perfusion parameters. Enrolled subjects without eligible target lesions prior to treatment were excluded from further CTP studies and subsequent analysis. Since our primary objective centered on examining the association of primary and secondary outcomes with changes in tumor CT perfusion biomarker values from baseline (T0) to T2, we required successful completion and technically analyzable T0 and T2 CTP studies, of the same target lesion, for a patient to be considered evaluable for statistical analysis.
Power Analysis
Assuming a 30% difference in PFS-6 between patients with a positive and negative CTP biomarker change from T0 to T2 (65% vs. 95%), and equal number of patients in each group, it was estimated that 70 evaluable participants would provide 84% power with a two-sided hypothesis and type I error of 0.05 using Fisher’s exact test. Ethics approval for ACRIN 6695 was obtained centrally from the National Cancer Institute’s (NCI) central investigational review board (IRB) and the local IRBs.
Other details of the ACRIN 6695 protocol are available from https://www.clinicaltrials.gov/ct2/results?term=ACRIN6695&Search=Search.
(b) CT Perfusion Studies
The CTP studies were undertaken on routine standard-of-care CT scanners at each participating site, and consisted of sequential scans over the maximal cross-section of the target lesion in two CT scanning phases during the intravenous administration of CT contrast medium and free-breathing. The two CTP scan phases comprised 24 scans at 2.8 s scan interval, followed by 8 scans at 15 s scan interval for a total scan time of ~ 3 min. Contrast medium was 300–370 mgI·mL−1 concentration at a dose of 0.8 mL·kg−1 body weight to a maximum of 70 mL, injected at 2–4 mL·s−1, 4–5 s before scanning began. CT technical parameters were: 120 kVp, 50 mA and 1 s rotation speed, and 6 to 16 CT slices, thickness 4.5–5.0 mm (depending on the CT scanner type and model); axial “shuttle” (step and shoot) mode, which increases cranio-caudal coverage, was allowed if available.
In this study absolute perfusion values and absolute blood volume values were used. Because muscle perfusion and blood volume are within the ranges of 0–10 mL/min/100g and 0–5 mL/100g respectively, as an internal standard, the perfusion and blood volumes maps were accepted only when the pelvic muscle appeared ‘blue’ when these maps were displayed in the rainbow color scale as in Fig. 2. Other details of the imaging protocol can be found at: https://www.acrin.org/Portals/0/Protocols/6695/Imaging%20Materials/6695_IMPv2_03072013_FINAL.pdf
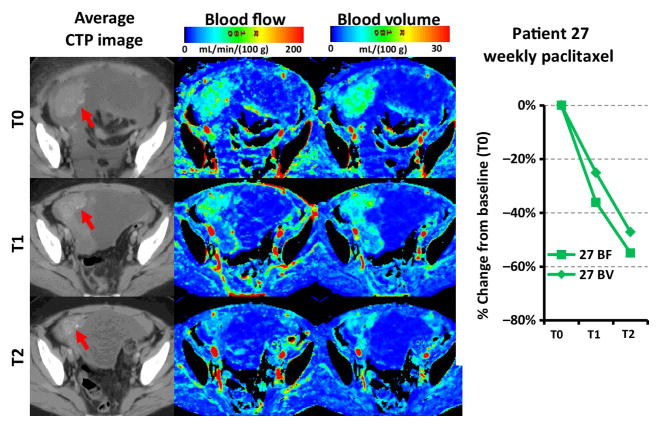
Figure 2.
Red arrows point to target lesion in T0, T1 and T2 CTP study of a neoadjuvant patient in the weekly paclitaxel treatment arm (Patient 27). The BF and BV maps are color coded from 0 (blue) to 200 (red) mL·min−1·(100g)−1 and 0 to 30 mL·(100g)−1 respectively. As shown there were a 36% and a 55% decrease in tumor BF and a 25% and a 47% decrease in tumor BV relative to T0 at T1 and T2 respectively.
(d) Analysis of CT Perfusion Scans
The CTP studies were analyzed centrally (Robarts Research Institute, London, Ontario and ACRIN). The target lesion criteria as prescribed in the study design described above were formally assessed on the baseline (T0) CTP study, and determined eligibility for patients to proceed to T1 and T2 CTP studies.
Target lesion eligibility at T0 was assessed and lesion outlines were provided by site radiologist and corroborated by the central radiologist (CNG & SIL) from the standard-of-care RECIST scan or the CTP scan according to pre-defined criteria detailed above. At the other time points (T1 and T2), the outlines of the same target lesion were provided by the central radiologist being careful to match the T0 outlines as much as possible and also taking into account the possible size change of the tumor from T0 to either T1 to T2 time point. This matching was facilitated by using the average images of the respective CTP studies. These images were generated by adding together all dynamic images of the same slice together to improve the lesion contrast from surrounding anatomical features. For each CT perfusion study, following delineation, the target lesion regions of interest (ROIs) in all slices were aligned using 3D rigid registration with ANALYZE software (Mayo Clinic, Rochester, MN) to correct for misregistrations from breathing motion during the scanning time of the study (~ 3 min). After registration, CT perfusion parametric maps were calculated from each study using CT perfusion software (body tumor protocol in CT Perfusion 4, GE Healthcare, Waukesha, WI), which is based on the modified Johnson-Wilson model,15 and the time-density curve from the largest visualized artery as the input function. Target lesion BF, BV and PS were calculated as the area-weighted average of the mean biomarker value in all target lesion ROIs.
(e) Statistical Analysis
Associations of primary outcome PFS-6 and secondary outcomes best overall radiographic response, progression free survival (PFS), and overall survival (OS) were CTP data were investigated. PFS-6 was the proportion of patients who had not progressed or died within 6 months of trial registration. PFS and OS were similarly calculated from the day of trial registration. Best overall radiographic response was assessed using RECIST criteria. The prognostic CTP biomarkers evaluated were changes of BF, BV, and PS relative to their baselines at T0, denoted by ΔBF, ΔBV and ΔPS, respectively. Angiogenesis is the hallmark of a proliferating tumor which leads to increase in blood flow, blood volume and PS; dichotomizing the perfusion parameter changes into either positive or negative i.e. “dichotomize at zero” would be appropriate as markers for patient response. The values of ΔBF, ΔBV and ΔPS between T0 and T1 or T2 are compared using Wilcoxon signed rank test.
Fisher’s exact tests were used to test the association between dichotomized CTP biomarkers and PFS-6 and best overall radiographic response. Univariate and multivariate Cox regression models were fitted for each biomarker, either with or without other covariates to test the association between dichotomized CTP biomarkers and PFS or OS. The unadjusted or adjusted hazard ratio of each biomarker, along with its 95% confidence interval and the p-value based on Wald’s statistic were reported. In addition, Kaplan-Meier survival curves were generated and the median survival time with its 95% confidence interval was provided. The log-rank test was used when appropriate. All tests were two-sided and p<0.05 was considered statistically significant for the primary aim of PFS-6. Bonferroni correction was used to account for multiple comparisons for the secondary analyses, where p< 0.017 was considered statistically significant.
Role of the funding source
This study was funded entirely by grants from US National Cancer Institute (NCI) to American College of Radiology Imaging Network (ACRIN) and Gynecological Oncology Group (GOG). NCI supplied the drug bevacizumab used in the study and provided centralized ethics review of the ACRIN 6695 study protocol. ACRIN also provided: image data storage and management and central statistical analysis. GOG collected and managed clinical and outcome data including overall response, progression-free survival and overall survival. The publication committees of both ACRIN and GOG reviewed and approved the manuscript before submission.
Results
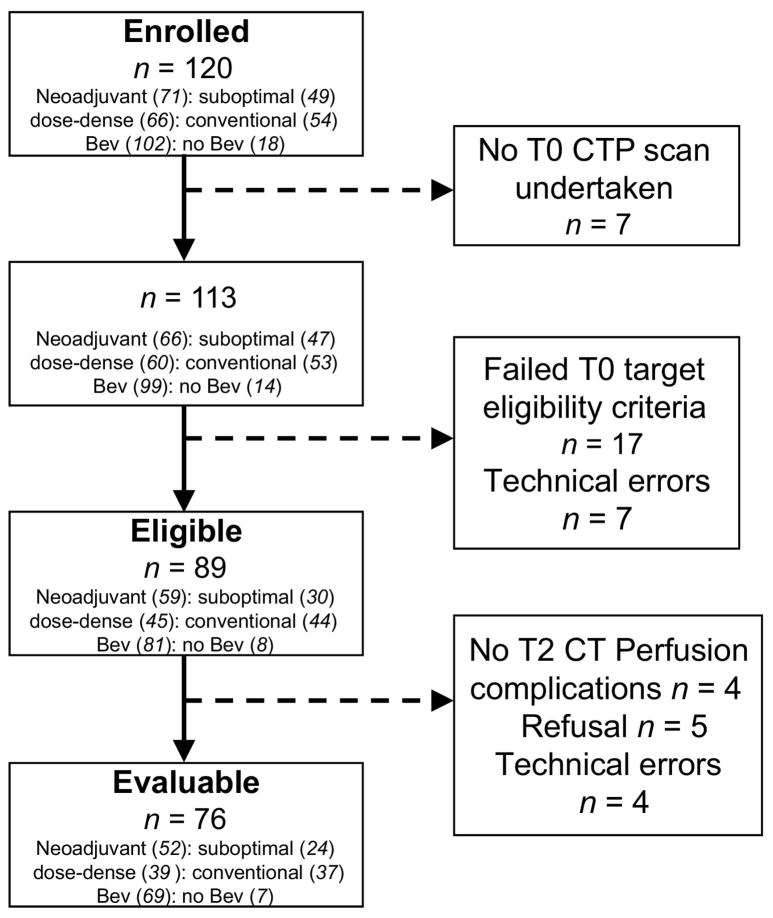
A total of 19 centers enrolled subjects into the ACRIN 6695 trial. Of the 120 patients enrolled from August 2011 to July 2013, 76 had analyzable T0 and T2 studies and were considered evaluable, Figure 1. Of these, 52/76 (68%) were neoadjuvant, and 24/76 (32%) were suboptimally debulked patients; 39/76 (51%) were randomized to dose-dense therapy and 37/76 (49%) to conventional therapy. 91% (69/76) evaluable patients received bevacizumab. The median age of patients was 61 years, range 25 to 87; and 70/76 (92%) were white. The histologic diagnoses were ovarian (n=63), primary peritoneal (7) and fallopian tube cancers (6), with the following histologic grades: grade 1 (n=2), grade 2 (7), grade 3 (55) and grade unknown (12). FIGO stage at diagnosis was stage II (n=4), stage IIIC (41) and stage IV (31). Demographic and clinical data of enrolled and evaluable patients are listed in Table 1.
Figure 1.
Flow diagram of patient recruitment in ACRIN 6695. Eligible: target lesion met target lesion criteria on central review; Evaluable: patient with technically analyzable CTP scans of the same target lesion from T0 and T2; Suboptimal: Suboptimally debulked tumor; Dose-dense: weekly paclitaxel; Conventional: 3-weekly paclitaxel; Bev =Bevacizumab
Table 1.
Demographic and clinical data of the enrolled and evaluable cohorts of the ACRIN 6695 study.
| Enrolled (n=120) | Evaluable (n=76) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 60.2 (10.5) | 60.1 (10.5) |
| Median (Range) | 61 (25 – 87) | 61 (25 – 87) |
| Race, N (%) | ||
| White | 109 (90.8) | 70 (92.1) |
| Black or African American | 6 (5.0) | 4 (5.3) |
| American Indian or Alaska Native | 2 (1.7) | 1 (1.3) |
| Asian | 2 (1.7) | 0 (0.0) |
| Native Hawaiian or Pacific Islander | 0 (0.0) | 0 (0.0) |
| Unknown | 2 (1.7) | 1 (1.3) |
| Bevacizumab Administration Status, N (%) | ||
| Bevacizumab | 102 (85.0) | 69 (90.8) |
| No Bevacizumab | 18 (15.0) | 7 (9.2) |
| Cohort, N (%) | ||
| Suboptimally Debulked | 49 (40.8) | 24 (31.6) |
| Neoadjuvant | 71 (59.2) | 52 (68.4) |
| Randomized Treatment Regimen, N (%) | ||
| Conventional: Crb+Tax Q3 Wks | 54 (45.0) | 37 (48.7) |
| Dose-Dense: Crb+Tax Weekly | 66 (55.0) | 39 (51.3) |
| Primary Site, N (%) | ||
| Ovary | 98 (81.7) | 63 (82.9) |
| Fallopian tube | 10 (8.3) | 6 (7.9) |
| Peritoneum | 12 (10.0) | 7 (9.2) |
| Stage at Diagnosis (FIGO), N (%) | ||
| 3 | 8 (6.7) | 4 (5.3) |
| 3A | 1 (0.8) | 0 (0.0) |
| 3B | 3 (2.5) | 0 (0.0) |
| 3C | 64 (53.3) | 41 (53.9) |
| 4 | 44 (36.7) | 31 (40.8) |
| Histologic Grade, N (%) | ||
| 1 | 4 (3.3) | 2 (2.6) |
| 2 | 11 (9.2) | 7 (9.2) |
| 3 | 88 (73.3) | 55 (72.4) |
| Unknown | 17 (14.2) | 12 (15.8) |
The overall radiographic response rate was 74% (56/76), consisting of 22 complete responders (CR) and 34 partial responders (PR). The PFS-6 rate was 96% (73/76), and the median PFS was 427 days (with 45/76 events), and median OS was 760 days (with 16/76 events).
For the cohort as a whole, BF (P<0.0001), BV (P<0.0001) and PS (P=0.0035) had significant decreases from T0 to T2. The decrease of BV from T0 to T1 (P=0.0069) was also significant (Table 2). On an individual patient basis, the T0-T2 period was associated with an increase in BF (i.e. ΔBF was positive) in 11 (14%) patients, and a decrease (i.e. ΔBF was negative) in 65 (86%) patients (Table 3). Similarly, ΔBV was positive in 8 (11%) patients and negative in 68 (89%) patients, and ΔPS was positive in 20 (26%) patients, and negative in 56 (74%) patients. Table 3 summarizes the association of change in CTP biomarkers in the periods of T0-T1 and T0-T2 with PFS-6, best overall response, PFS and OS. Figure 2 shows the results from a neoadjuvant patient in the weekly paclitaxel treatment arm whose blood flow and volume at the two follow-up CTP studies (T1 and T2) decreased relative to baseline (T0).
Table 2.
Summary of percentage changes of CT perfusion biomarkers changes from T0 to T2 and from T0 to T1 for the entire cohort of ACRIN 6695 evaluable patients.
| ΔBF (%) | ΔBV (%) | ΔPS (%) | |
|---|---|---|---|
| T0-T2 * | |||
| Mean | −22.2 | −29.9 | −9.5 |
| SD | 39.3 | 38.2 | 52.5 |
| Median | −25.1 | −33.6 | −21.5 |
| Minimum | −72.8 | −84.1 | −87.0 |
| Maximum | 172.0 | 159.2 | 175.5 |
| p-value§ | <0.0001 | <0.0001 | 0.0035 |
| T0-T1 # | |||
| Mean | −2.2 | −9.5 | −3.7 |
| SD | 41.1 | 30.1 | 39.5 |
| Median | −9.0 | −12.3 | −6.6 |
| Minimum | −66.4 | −63.1 | −74.3 |
| Maximum | 183.7 | 59.3 | 164.4 |
| p-value§ | 0.092 | 0.0069 | 0.14 |
ΔBF(%), ΔBV(%) or ΔPS(%) = T2 value minus T0 value expressed as percentage of T0 value
ΔBF(%), ΔBV(%) or ΔPS(%) = T1 value minus T0 value expressed as percentage of T0 value
Wilcoxon Signed Rank Test
Table 3.
Association of changes of CTP biomarkers (BF, BV and PS) with clinical outcomes
| ΔBF (+ vs. −) | ΔBV (+ vs. −) | ΔPS (+ vs. −) | |
|---|---|---|---|
| T0-T2 * | |||
| PFS-6 | 82% vs. 98%, P=0.053 | 75% vs. 99%, P=0.028¶ | 90% vs. 98%, P=0.17 |
| Best response | 45% vs. 78%, P=0.032 | 38% vs. 78%, P=0.026 | 75% vs. 73%, P=1.0 |
| PFS | HR= 2.86 (1.28, 6.42), P=0.008¶ | HR = 1.61 (0.68, 3.82), P=0.28 | HR=0.72 (0.35, 1.51), P=0.39 |
| OS | HR= 1.88 (0.59, 5.93), P=0.28 | HR=1.71 (0.46, 6.29), P=0.42 | HR=0.57 (0.15, 2.13), P=0.40 |
| T0-T1 # | |||
| PFS-6 | 92% vs. 98%, P=0.27 | 96% vs. 96%, P=1.0 | 97% vs. 96%, P=1.0 |
| Best response | 65% vs. 78%, P=0.28 | 60% vs. 80%, P=0.09 | 66% vs. 79%, P=0.28 |
| PFS | HR= 0.88 (0.47, 1.66), P=0.70 | HR = 0.86 (0.46, 1.60), P=0.63 | HR=0.85 (0.46, 1.56), P=0.59 |
| OS | HR= 1.12 (0.40, 3.16), P=0.83 | HR=0.88 (0.30, 2.58), P=0.81 | HR=1.09 (0.37, 3.18), P=0.88 |
ΔBF, ΔBV or ΔPS calculated as T2 value minus T0 value
ΔBF, ΔBV or ΔPS calculated as T2 value minus T0 value
Statistically significant (P<0.05 for primary analysis; P<0.017 for secondary analyses).
Association of Change of CTP Biomarkers from T0 to T2 with PFS-6
There were 3 patients who progressed or died within 6 months of their trial registration. Patients whose BV increased had a lower chance of being progression-free at 6 months (PFS-6) (6/8, 75%), compared with patients whose BV decreased (67/68, 98.5%, p=0.028). Similarly, patients whose BF increased had a lower chance of PFS-6 (9/11, 81.8%), compared with patients whose BF decreased with borderline significance (64/65, 98.5%, p=0.053). Of the three patients who progressed or died within 6 months of their trial registration, two had increased while one had decreased BF and BV. No significant association was found between ΔPS and PFS-6 (p=0.17). All these results are summarized in Table 3.
Association of Change of CTP Biomarkers from T0 to T2 with best overall radiographic response
56 (74%) patients had either a complete or partial radiographic response. Patients whose BF increased (ΔBF positive) had a lower chance of being responders (5/11, 45.5%), compared with patients whose BF decreased (ΔBF negative) (51/65, 78.5%), but this did not attain significance after applying Bonferroni’s correction (p=0.032). Similarly, patients whose BV increased had a lower chance of being responders (3/8, 37.5%), compared with patients whose BV decreased (53/68, 77.9%), again not significantly after correction for multiple comparisons in these secondary analyses (p=0.026). No significant association was found between ΔPS and best overall response (p=1.0). Results are summarized in Table 3.
Association of Change of CTP Biomarkers from T0 to T2 with PFS and OS
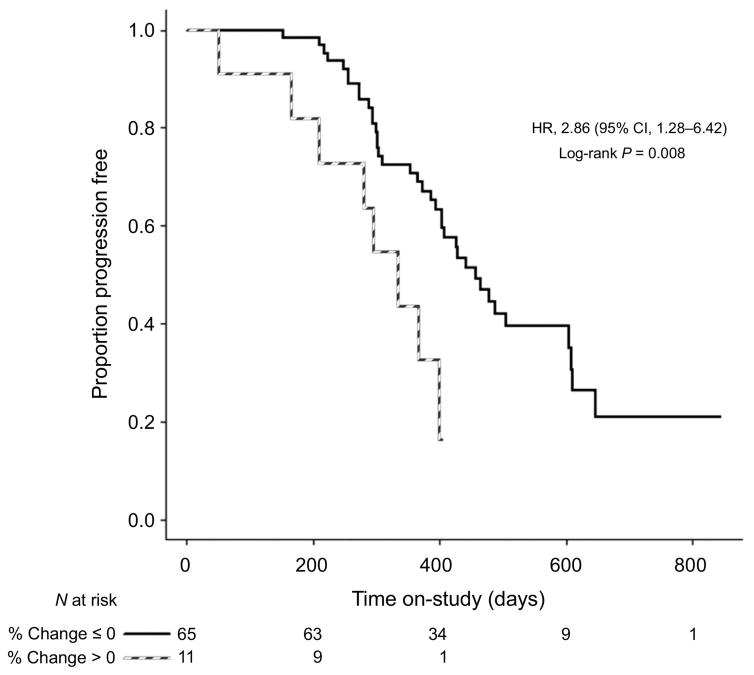
An increase in BF between T0 and T2 (ΔBF positive) was associated with significantly shorter PFS, yielding a hazards ratio of 2.86 (95% CI, 1.28 to 6.42, p=0.008) (Figure 3). The median PFS for patients whose BF increased was 335 days (95% CI, 166 to 399), and for the patients whose BF decreased was 457 days (95% CI, 394 to 605). Similar results was obtained for patients who received bevacizumab (HR 2.87, 95% CI: 1.20 to 6.89, p=0.013) (Supplementary Figure 2). The number of patients who did not receive bevacizumab is too small (n = 7) to support a separate analysis. The significant association between change in BF and PFS remained after adjusting for age, change in tumor volume from T0 to T2 (increase vs. decrease) and surgery status (adjuvant vs. neo-adjuvant) (p=0.007 for the whole cohort, p=0.013 for the bevacizumab group and p=0.0017 for the conventional group). No significant associations were found between the other two CTP biomarkers and PFS. No significant association was found between OS and BF (p=0.28) or BV (p=0.42) or PS (p=0.40), but the study was not adequately powered to detect small differences in OS.
Figure 3.
Kaplan-Meier PFS curve for patients who had increase vs. decrease in BF from T0 to T2.
Change of CTP Biomarkers from T0 to T1
No associations in changes of CTP biomarkers from T0 to T1 were observed with any of the four outcomes.
DISCUSSION
In this prospectively designed pilot imaging biomarker study, we demonstrated that CTP is feasible in patients with residual lesions measuring at least 1 cm following primary cytoreductive surgery or prior to interval cytoreductive surgery. Temporal changes as early as within four weeks of initiating therapy in one or more CTP biomarkers of perfusion were associated with long-term outcomes of tumor treatment success, including PFS-6 and PFS; the latter association present even after adjusting for age, change in tumor volume between T0 and T2 CTP scans, and surgical status (neoadjuvant vs suboptimally debulked).
The development of “early-in-treatment” biomarkers for treatment outcomes are becoming increasingly important as the portfolio of novel agents expands. This development has proceeded along several different paths including, serial tissue biopsies, repeated evaluation of circulating factors and non-invasive imaging. The latter two options provide the most acceptable form of evaluation but validation has been difficult due to the large dynamic variability of biomarkers in body fluids in the former and the need for standardization of image acquisition and analysis in the latter. CTP has the advantage that it is quantitative and more amenable to standardized acquisition. However, CT perfusion has not been validated under centralized review and standardized analysis as an early imaging biomarker of treatment response in a prospective multi-center trial setting.
ACRIN 6695 is a pilot trial designed to demonstrate the feasibility of CTP as an early imaging biomarker before a formal validation trial is undertaken. It required a multi-slice CT (64 or more slice) scanner, capable of imaging a 2.8cm or wider section of the abdomen/pelvis repeatedly every 1–3 seconds for a period up to 3 minutes, which in terms of present day CT technology is relatively standard and is widely available as a second-tier rather than premium model scanner from all commercial vendors. As such, the required CT scanner is likely to be available in most cancer treatment centers. A pilot trial is limited by nature in the number of patients and follow-up time available as well as in prior data to guide the statistical analysis of the acquired imaging biomarker data. These limitations were reflected in the design of the ACRIN 6695 trial in three important aspects: (i) the different treatment arms of GOG-0262 were treated as a single treatment and CTP was tested as an imaging biomarker of treatment response; (ii) PFS-6 was used as the primary outcome to limit the follow-up time required; and, (iii) CTP-derived biomarkers were dichotomized as positive vs. negative changes to access treatment response in the statistical analysis plan due to the lack of known thresholds in these imaging biomarkers. Nevertheless, results from ACRIN 6695 show that CTP did not require specialized equipment except standard clinical CT scanners, could be carried out in the multi-institutional setting with standardized acquisition and analysis protocol with good patient compliance, and demonstrated biological precision to the anticipated effects of successful (or unsuccessful) treatment under centralized review.
ACRIN 6695 focused on CTP-derived parameters-BF, BV and PS- because one rationale for anti-VEGF based therapy was normalization of the microvasculature in tumors leading to reduction in edema (PS) and interstitial fluid pressure which could enhance chemotherapy delivery thereby enhancing its effect.16 This would also be represented by decreases in tumor blood volume, perfusion and edema. Previous studies of perfusion imaging have suggested that effective neoangiogenesis targeting can impact these biomarkers.17 However, inter-observer variability and requirements for specialized imaging equipment and protocols have limited the generalization of these early successes.18
GOG-0262 was a phase III trial assessing the impact of dose-dense paclitaxel and carboplatin relative to standard, every 3-week paclitaxel and carboplatin on progression-free survival. Bevacizumab use was left to physician’s discretion and was overwhelmingly preferred based on two Phase III clinical trials in patients with predominately advanced ovarian cancer. Both of these trials demonstrated a significant benefit in PFS with the use of bevacizumab combined with standard (3-weekly paclitaxel/carboplatin) chemotherapy and in maintenance.19,20 Neither trial demonstrated a benefit in OS, raising a risk:benefit discussion for unselected patients, particularly since significant adverse effects from therapy were observed in both trials. Indeed, a recent genomic analysis of one of these trials (ICON7) suggested that patients with an immune gene upregulation actually did worse if treated with bevacizumab.21 While reproducibility of genomic characterization to predict patient outcomes a priori is still challenging, characterization of lack of treatment effects with non-invasive imaging would be of great value in avoiding unnecessary treatment exposure of agents unlikely to benefit an individual patient, and to potentially facilitate early transition to alternative treatments.
One limitation of the ACRIN 6695 study is that bevacizumab use was not randomized per GOG-0262 trial protocol. There was significant physician choice for its incorporation; this therapy bias was represented in the ACRIN 6695 results. In the parent GOG-0262 trial, among the patient cohort who did not receive bevacizumab, weekly (dose-dense) paclitaxel was associated with a 3.9 month improvement on the median of PFS over every-3-week paclitaxel (14.2 vs. 10.3 months; HR=0.62; 95%CI: 0.4–0.95; p=0.03).22 Dose-dense chemotherapy has been hypothesized to impact angiogenesis by upregulating the production of endogenous anti-vascular glycoproteins, such as thrombospondin I, and by directly targeting the tumor endothelium. Additional purported mechanisms by which dose-dense chemotherapy may impact the tumor microenvironment are suppression of HIF-1α, reduction of T regulatory cells, maturation of dendritic cells and inhibition of tumor cell extravasation.23 However, in the ACRIN 6695 cohort, approximately 91% of patients received bevacizumab starting at cycle 2, so it was not possible to evaluate whether dose-dense or every-3-week paclitaxel with bevacizumab vs. without the agent had effects on PFS or OS as in the parent trial. In addition, changes of CTP biomarkers from T0 to T1, unlike those from T0 to T2, were not associated with overall response, PFS-6, PFS or OS. This can be explained by: first, in the former time interval, only 51% of patients were treated with anti-angiogenesis dose-dense chemotherapy, thus the treatment effect on BF and BV could be diluted by the remaining patients that were on standard chemotherapy; second, in the latter time interval all patients would have had anti-angiogenesis treatment with either bevacizumab alone or dose-dense chemotherapy plus bevacizumab. Another limitation is the high rate (26/113) of patients that failed the target criteria at T0. These criteria were instituted in the design of ACRIN 6695 as CTP parameters may not be reliably determined if the lesion is smaller than 1 cm. As shown in Figure 1, 17 out of 47 suboptimally debulked patients while only 7 out of 66 neoadjuvant patients failed the target criteria at T0. Since the lesion was smaller in suboptimally debulked than neoadjuvant patients, this result was not unexpected. In terms of patient compliance with the CTP protocol, 13/89 (14.9%) patients did not complete the T2 scan. However, this estimate of non-compliance is an overestimate because four cases were due to treatment complications, another four due to technical problems with the T2 scan, only five subjects were unwilling to continue with the CTP protocol.
The next step in developing CTP imaging biomarkers would be their use to identify the subgroup most likely to gain a PFS or OS benefit in future adaptive therapy trials, especially in view of emerging molecular analysis that suggests a subgroup of patients in whom survival outcomes may be adversely affected by bevacizumab treatment.20 Clinically, these imaging biomarkers will guide the use of newer treatment regimens in the targeted subgroup of patients most likely to benefit from the treatment and minimize the overall morbidity and cost of the treatment, by sparing patients who are unlikely to benefit from the regimen It may also help guide more aggressive surveillance for the group of patients who are likely to recur early, so that novel therapeutics can be considered for this poor risk group, either as a maintenance strategy, or in the setting of recurrence.
Supplementary Material
Statement of Translational Relevance.
Our study (ACRIN 6695) demonstrated that abdominopelvic CT perfusion (CTP) biomarkers can be measured successfully with sufficient uniformity across multiple sites and scanner platforms to yield positive results in a multicenter biomarker trial. CTP biomarkers measured within 4 weeks of initiating therapy may provide early prognostic information for treatment response and time to progression, and could be used to refine early treatment interventions for advanced ovarian cancer in future clinical trials, particularly in removing ineffective therapeutic modalities for patients. Furthermore, since CT Perfusion can be incorporated easily into routine CT imaging for the follow-up of cancer treatment, our results suggest that CTP biomarkers not only can be used in clinical trials but can also be considered for routine monitoring of cancer treatment.
Acknowledgments
Financial Support: Grants from US National Cancer Institute (NCI) to American College of Radiology Imaging Network (ACRIN) and Gynecological Oncology Group (GOG).
This study was funded by grants from US National Cancer Institute to American College of Radiology Imaging Network (ACRIN, U01 CA079778 and U01 CA080098) and Gynecological Oncology Group (GOG). Participating Centers of the ACRIN 6695 study include: Brown University, Rhode Island; Duke University Medical Center, Durham, North Carolina; Fox Chase Cancer Center, Philadelphia, Pennsylvania; Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania; Indiana University Medical Center, Indianapolis, Indiana; Massachusetts General Hospital, Boston, Massachusetts; Ohio State University Medical Center, Columbus, Ohio; St. Joseph’s Hospital and Medical Center, Phoenix, Arizona; University of Alabama Medical Center, Tuscaloosa, Alabama; University of Arizona Health Sciences Center, Tucson, Arizona; University of California San Francisco, California; University of Colorado Hospital, Aurora, Colorado; University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; MD Anderson Cancer Center, Houston, Texas; University of Texas Southwestern Medical Center, Dallas, Texas; University of Virginia Medical Center, Charlottesville, Virginia; University of Wisconsin Hospital, Madison, Wisconsin; Wake Forest University Medical Center, Winston-Salem, North Carolina; Washington University Medical School, St. Louis, Missouri.
Footnotes
Contributions
TYL, SIL, CSN, RLC and ZZ contributed to the design of the study, including site selection. TYL designed the CT Perfusion scanning protocol and the analysis protocol of the acquired studies. CSN and SIL evaluated and approved the initial target selection at T0 by participating sites. CSN and SIL also outlined target lesions for subsequent T1 and T2 CT Perfusion studies. FS, KB and JB performed analysis of CT Perfusion studies under the supervision of TYL to derive the perfusion biomarker values of the target lesion. TYL reviewed the perfusion biomarker results before submission to ACRIN for central statistical analysis. ZZ and HSM did the statistical analysis. CSN, ZZ, RLC and TYL performed literature search and wrote the manuscript with input from SIL and MAB. All other co-authors enrolled patients, supervised the acquisition of CT Perfusion studies on patients at their sites and defined the target lesions in those studies, and reviewed and edited the manuscript.
Potential Conflict of Interest: TYL licenses the CT Perfusion software to GE Healthcare. RLC is supported in part by CPRIT RP120214, Ovarian Cancer Research Fund Program Project Development Grant, the Judy Reis Ovarian Cancer Fund, and the Ann Rife Cox Chair in gynecology. All other co-authors declare no competing interests.
References
- 1.Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–979. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 2.Wimberger P, Lehmann N, Kimmig R, et al. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecol Oncol. 2007;106:69–67. doi: 10.1016/j.ygyno.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park) 2013;27:288–294. [PubMed] [Google Scholar]
- 4.Cancer Research UK. [Accessed June 20, 2015];Ovarian Cancer Survival Statistics. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/ovary/survival/ovarian-cancer-survival-statistics.
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 6.Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010:497429. doi: 10.1155/2010/497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 9.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29:1247–1251. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]
- 10.Hensley ML. Big costs for little gain in ovarian cancer. J Clin Oncol. 2011;29:1230–1232. doi: 10.1200/JCO.2010.34.0489. [DOI] [PubMed] [Google Scholar]
- 11.Laquente B, Viñals F, Germà JR. Metronomic chemotherapy: an antiangiogenic scheduling. Clin Transl Oncol. 2007;9:93–98. doi: 10.1007/s12094-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 12.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 15.St Lawrence K, Lee T-Y. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain I. Theoretical derivation. J Cereb Blood Flow Metab. 1998;18:1365–1377. doi: 10.1097/00004647-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increasestumor cell invasion in glioblastoma. Proc Natl Acad Sci. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paldino MJ, Barboriak DP. Fundamentals of quantitative dynamic contrast-enhanced MR imaging. Magn Reson Imaging Clin N Am. 2009;17:277–289. doi: 10.1016/j.mric.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 20.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 21.Gourley C, McCavigan A, Perren T, Paul J, Michie CO, Churchman M, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol. 2014;32:5s. (abstr 5502) [Google Scholar]
- 22.Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, DiSilvestro PA, Rubin SC, Martin LP, Davidson SA, Huh WK, O’Malley DM, Boente MP, Michael H, Monk BJ. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N Engl J Med. 2016;374:738–48. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnoni A, Silvestris N, Licchetta A, Santini D, Scartozzi M, Ria R, et al. Metronomic chemotherapy from rationale to clinical studies: A dream or reality? Crit Rev Oncol Hematol. 2015;95:46–61. doi: 10.1016/j.critrevonc.2015.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.