Abstract
Magnetic nanoparticles can be made to dissipate heat to their immediate surroundings in response to an applied alternating magnetic field. This property, combined with the biocompatibility of iron oxide nanoparticles and the ability of magnetic fields to penetrate deep in the body, makes magnetic nanoparticles attractive in a range of biomedical applications where thermal energy is used either directly to achieve a therapeutic effect or indirectly to actuate the release of a therapeutic agent. Although the concept of bulk heating of fluids and tissues using energy dissipated by magnetic nanoparticles has been well accepted and applied for several decades, many new and exciting biomedical applications of magnetic nanoparticles take advantage of heat effects that are confined to the immediate nanoscale vicinity of the nanoparticles. Until recently the existence of these nanoscale thermal phenomena had remained controversial. In this short review we summarize some of the recent developments in this field and emerging applications for nanoscale thermal phenomena in the vicinity of magnetic nanoparticles in alternating magnetic fields.
Keywords: iron oxide, magnetic nanoparticle, hyperthermia, magnetically triggered drug release, local heating
TOC Image
Magnetic nanoparticles (MNPs) respond to applied alternating magnetic fields (AMFs) by dissipating heat. Recent experimental evidence demonstrates that nanoscale thermal phenomena in the vicinity of MNPs can be harnessed to kill cancer cells and to trigger release of a drug cargo.
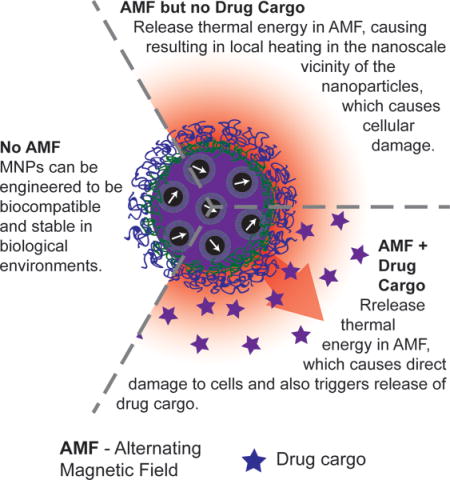
1. Introduction
There has been a growing interest in the use of magnetic nanoparticles (MNPs) due to the capability for the particles to dissipate heat in response to applied alternating magnetic fields (AMFs) of appropriate amplitude and frequency. Early theory suggests that heating results through magnetic relaxation losses.[1] MNPs have two mechanisms of magnetic relaxation: a Brownian mechanism in which the magnetic moment is locked to the crystal axis and the particle rotates to align with the instantaneous field, and a Néel mechanism in which the magnetic moment rotates within the crystal.[1] When time-varying magnetic fields are applied to single-domain MNPs, whether they relax by the Brownian and/or the Neel mechanism, magnetic work is converted to internal energy that is dissipated to the surroundings.[1] The energy dissipated by these single-domain MNPs corresponds to the area of the hysteresis loop.[1–2] The hysteresis loop can be described using the Linear Response theory, which uses the Neel-Brownian relaxation time, or Stoner-Wohlfarth model based theory (SWMBTs),[2] According to these theories and extensive experimentation, the heat dissipated by MNPs is highly dependent on their size and composition, as well as the temperature and frequency of the applied magnetic field. The ability of MNPs to dissipate heat in response to an applied magnetic field has become very useful for various applications, including cancer treatment[3], magnetically triggered drug delivery[4], and other applications outside of biomedicine, such as in driving chemical reactions[5] and repair of nanocomposites.[5b, 6]
2. Hyperthermia – The Current Paradigm in Thermal Cancer Therapy Using Magnetic Nanoparticles
The use of magnetic implants and seeds to deliver heat to cancer tumors has been an active area of research for several decades.[7] Since the 1990’s, attention has shifted to the use of magnetic nanoparticles, because of their potential to accumulate preferentially in tumors after systemic delivery.[8] The paradigm behind these approaches has been the idea that cancer cells are more susceptible to elevated tissue temperatures than normal cells. This is the cornerstone of the application of hyperthermia in oncology, where the goal is to raise the temperature of cancer tissues to the range of 43–47°C, while sparing surrounding healthy tissue (i.e., locoregional hyperthermia).[9]
There are several potential approaches to achieve controlled locoregional hyperthermia, including high intensity focused ultrasound[10], photothermal therapy using gold nanoparticles and shells[11], radiofrequency ablation using carbon nanotubes[12], and, as mentioned above, energy dissipated by magnetic nanoparticles in what is commonly called magnetic fluid hyperthermia (MFH)[8a] (see Figure 1). These approaches have their relative advantages and disadvantages. Here we focus on the use of magnetic nanoparticles because of their intrinsic biocompatibility and the ease with which magnetic fields can penetrate deep into tissue to actuate energy release even in deep-seated tumors.
Figure 1.
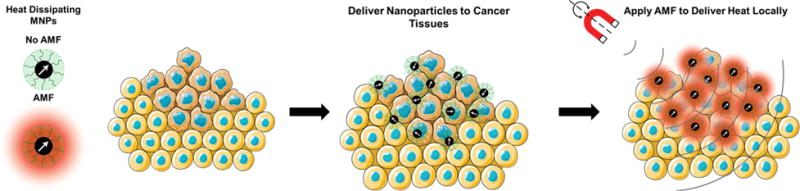
In magnetic fluid hyperthermia (MFH), magnetic nanoparticles dissipate heat in the presence of an alternating magnetic field (AMF). The particles are delivered to cancer tissues and cells, and can be used to achieve locoregional hyperthermia upon application of an AMF to the targeted region.
MFH has shown success in vitro, in vivo, in pre-clinical studies, and in clinical trials. The first study in vitro was that reported by Jordan et al. (1996), who demonstrated that MFH was able to inactivate malignant cells to at least the same extent as water bath hyperthermia.[8a] Contemporaneously, Shinkai et al. (1996) demonstrated cell death after a high frequency AMF was applied to magnetic cationic liposomes.[13] It was later demonstrated by Rinaldi, Torres-Lugo and their collaborators that MFH is superior to water bath hyperthermia both alone and in combination with drug.[14] One possible mechanism for this enhanced effect is the observation that MFH causes additional stress on the cellular membrane,[15] which can cause direct damage and simultaneously enhance cytotoxicity of drugs even in resistant cells.
One of the first successes of MFH in vivo was reported by Jordan et al. (1997), who showed that after MFH some tumors had no regrowth or a smaller tumor volume 50 days after treatment.[8b] Later, Yanase et al. (1998) showed similar results using magnetic cationic liposomes, observing regression of most tumors.[16] In another contribution, Yanase et al. (1998) observed evidence of an antitumor immune response when tumors were treated with hyperthermia.[17] Subcutaneous tumors grown on both femurs of a rat disappeared completely after only a single side was treated.[17] Furthermore, treated rats that were rechallenged with cancer cells 3 months later had slow tumor growth, and eventually the tumors disappeared without any further treatment.[17] A similar recent study by Toraya-Brown et al. (2014) considered different tumor models but observed similar results, concluding that MFH induces an immune effect to contralateral tumors.[18]
MFH is currently approved for use in Europe to treat glioblastoma multiforme by injecting non-targeted MNPs directly into brain tumors followed by the application of AMFs combined with radiotherapy.[19] Clinical studies demonstrated an increase in survival time from 15 months to 23 months,[20] which was attributed to achievement of hyperthermia temperature in the tumors due to the heat dissipated by the MNPs. MagForce, a company established after successes were shown in vivo for the treatment of Glioblastoma multiforme, is continuing clinical trials in treatment of prostate cancer[21], esophageal cancer, pancreatic cancer, and others[22].
Currently, MNPs are not only being used for MFH for direct treatment of cancer, but are also of interest for magnetically-triggered drug delivery. Ruiz-Hernandez et al. (2011) created mesoporous silica nanoparticles wherein the silica network was loaded with iron oxide and a single-stranded DNA was attached onto the surface.[23] The particles were loaded with fluorescein before capping and the complementary DNA strand was attached separately to other iron oxide particles and used to cap the pores of the silica nanoparticles.[23] Placing the particles in an incubator shaker or applying a magnetic field to hyperthermia level showed the same level of fluorescein release.[23] The authors showed on-off release of fluorescein due to the reversibility of the DNA linkage.[23] Baeza et al. (2012) prepared mesoporous silica nanoparticles with iron oxide decorated with a thermoresponsive copolymer.[24] The copolymer acted as a gatekeeper keeping drugs trapped in the silica matrix and retaining other compounds through electrostatic interactions or hydrogen bonds.[24] They used fluorescein as a model drug, and soybean trypsin inhibitor type II-S as the compound attached to the copolymer.[24] The authors demonstrated that at a temperature above 35°C there was similar release by the use of AMF or regular hot plate of the protein. However, they did see a slight increase of fluorescein release, which they attributed to the increase in pore size due to rapid rotation of the nanoparticles.[24] Guo et al. (2014) made particles consisting of iron oxide cores and a mesoporous silica shell, loaded with Doxorubicin hydrochloride (DOX).[25] They showed controlled release of DOX only by reaching hyperthermia temperature under AMF, and compared free DOX to their nanocarriers and saw enhanced cytotoxicity.[25]
Despite these promising successes of MFH in vitro, in vivo, and in the clinic, there are still several challenges to be overcome in order to achieve the full potential of thermal cancer therapy using magnetic nanoparticles. The main reason for success thus far is the achievement of hyperthermia. The question becomes: Is thermal therapy using magnetic nanoparticles limited to the hyperthermia paradigm? There has been theoretical controversy and experimental support for nanoscale thermal phenomena on the surface of magnetic nanoparticles, both of which are summarized below.
3. Theoretical Arguments Against Nanoscale Thermal Phenomena
Rabin (2002) used theoretical arguments based on the macroscopic theory of heat transfer to test whether intracellular hyperthermia via magnetic heating in a single cell is possible.[26] There are various assumptions associated with this theoretical analysis, the main assumptions being that the thermophysical properties of water were taken as representative values for biological solutions, nanoparticles were treated as perfect conductors, and the classical Fourier law for heat transfer was used.[26] Rabin (2002) considered nano, micro, and macro-scale heat transfer assuming nanoparticles are perfect conductors for the nano-scale heat transfer analysis.[26] The most relevant result to the present discussion is Rabin’s predictions that for a particle diameter of 100 nm, the temperature difference between the bulk fluid and nanoparticle surface at steady state will not be greater than 10−5 °C, implying nanoscale thermal phenomena should be negligible.[26] Note that because the theoretical temperature difference is proportional to the size of the particles, it would be even smaller for smaller diameter nanoparticles. Rabin further concludes that a cell that is densely packed with nanoparticles will have a surface temperature that is only approximately 0.1°C higher than their surroundings.[26] Thus, Rabin concludes that hyperthermia levels cannot be reached by intracellular heating alone.[26] Finally, Rabin considered the smallest tumor diameter that could be effectively treated by MFH at a tumor surface temperature of 43°C.[26] Assuming a spherical tumor and MNPs to be uniformly distributed, a minimal diameter of 0.9 mm occupied by the nanoparticles is required, and in order to ensure a temperature rise of 6°C at the edge of the region occupied by the MNPs, the minimal diameter of the tumor needs to be 1.1 mm assuming no convective cooling due to blood perfusion.[26]
Later, Keblinski (2006) concluded that the immediate, nanoscale environment surrounding nanoparticles will not experience a temperature rise to hyperthermia levels.[27] According to Keblinski, steady state is achieved within 100 ns with a 100 nm particle and the temperature rise at the surface due to relaxation losses will only be 0.1 mK, whereas the temperature rise due to eddy current heating will be even less.[27] As with the theory due to Rabin, the temperature difference would be even smaller for smaller diameter nanoparticles. Thus, in dilute solutions it should be nearly impossible for the nanoparticle surface temperature to be higher than the bulk temperature of the surrounding medium.[27]
More recently, Kozissnik et al. (2013) reported predictions from a simplified steady state heat conduction analysis, similar to that Rabin and Keblinski but including the effects of tissue blood perfusion by solving Penne’s bioheat equation for a solid spherical tumor, and considering the currently-reported ranges for MNP energy dissipation, parameterized through the specific absorption rate (SAR).[3c] The calculations corresponding to MFH in Figure 2 were generated using the analysis reported in Kozissnik et al. (2013) and illustrates the estimated iron oxide concentration required to achieve a hyperthermia temperature of 45 °C at the interface between tumor and healthy tissue, as a function of tumor volume, for spherical tumors. In Figure 2 we consider typical ranges for tumor volumes used in rodent models (100–800 mm3). Furthermore, we identify a plausible feasible range of MNP accumulation of MNPs in tumors after systemic delivery, based on recent reports of MNP accumulation with and without magnetic targeting that observed MNP concentrations in tumor tissue of up to ~100–150 μgFe/g in the absence of magnetic targeting (green shaded region in Figure 2) and up to ~400–450 μgFe/g with magnetic targeting (blue shaded region in Figure 2).[28] This simple analysis seems to indicate that achieving hyperthermia temperatures through MFH using systemically-delivered MNPs may be impossible with the currently reported ranges of SAR and for the tumor volumes typically used in rodent models, even with these arguably optimistic assumptions. Perhaps this is one reason why most in vivo demonstrations of MFH are done using intratumorally-injected MNPs, as this mode of delivery can achieve the required nanoparticle concentrations at the tumor site. However, intratumoral delivery is not practical for many important classes of cancer and would not be feasible for metastatic disease. Figure 2 also includes calculated required concentrations for treatment using intracellular energy delivery, which will be discussed below.
Figure 2.
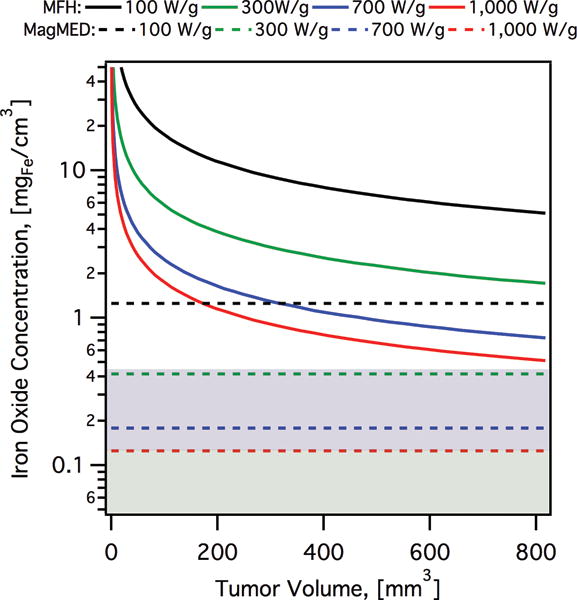
Estimated tissue iron oxide concentrations required to eradicate a spherical tumor as a function of tumor volume by hyperthermia (MFH, solid lines) or by intracellular heating (MagMED, dashed lines). MFH calculations are based on the solution of Penne’s bioheat equation, achieving a surface tumor temperature of 45°C, and reported values of the specific absorption rate (SAR) of magnetic nanoparticles. Intracellular heating calculations assume a thermal dose of 18 μJ/cell required, over 2 hr of AMF application, and a cancer cell density of 5×107 cells/cm3. The green shaded region corresponds to the range of MNP accumulation that seems potentially feasible without magnetic targeting, and the blue shaded region corresponds to the range of MNP accumulation that seems potentially feasible with magnetic targeting, based on recent reports.[28]
In summary, the theories and analyses discussed above cast doubt on the feasibility of MFH for small tumors and suggest that it may be impossible to raise the temperature of single nanoparticles, cells, or small tumors significantly above the bulk temperature of their surroundings. However, as we will summarize below, recent experimental studies performed by different groups have provided direct and indirect evidence of nanoscale thermal phenomena that directly contradict these theoretical predictions.
4. Direct Experimental Evidence of Nanoscale Thermal Phenomena
The first experimental study to purposefully attempt to address this question, that we are aware of, is that of Gupta et al. (2010), who used fluorescent Quantum Dots as so-called nanoscale thermometers to sense the temperature in the immediate environment of gold and magnetic nanoparticles.[29] The thermosensitive QDs were either mixed with or covalently tethered to the Au/MNPs.[29] They concluded that the difference between the temperature in the nanoscale vicinity of the nanoparticles and the bulk temperature was negligible.[29] However, this study lacked thorough characterization of the nanoparticles used, for example, of the intrinsic rate of energy dissipation of the nanoparticles.
Although Gupta et al. (2010)[29] did not observe evidence of nano-scale energy delivery, there is mounting experimental evidence, acquired by several independent labs around the world using a wide range of complementary techniques, that supports the notion that heat released by magnetic nanoparticles in an alternating magnetic field causes nanoscale thermal phenomena in the immediate vicinity of the nanoparticles and without the need of a macroscopic, or bulk, temperature rise.
Huang et al. (2010) performed a similar study to Gupta et al., but conjugating a fluorescent dye, DyLight549, to the particles while also placing free yellow fluorescent protein, YFP, in solution.[30] They showed that the intensity of Dylight549 decreased while the fluorescence intensity of the free dye did not change as AMFs were applied, clearly suggesting substantial heating limited to the nanoscale vicinity of the particles.[30] Using the correlation between dye fluorescence and temperature, Huang et al. (2010) estimated the MNP surface temperature. Figure 3, from Huang et al. (2010), suggests a temperature rise of approximately 5 °C within only 45 s of application of an AMF. They also targeted the nanoparticles to the temperature-sensitive cell surface protein TRPV1, responsible for influx of calcium ions, and saw an influx of calcium ions upon application of AMFs, which they reasoned meant that MNPs were able to increase the local temperature to 42°C, activating TPRV1.[30]
Figure 3.
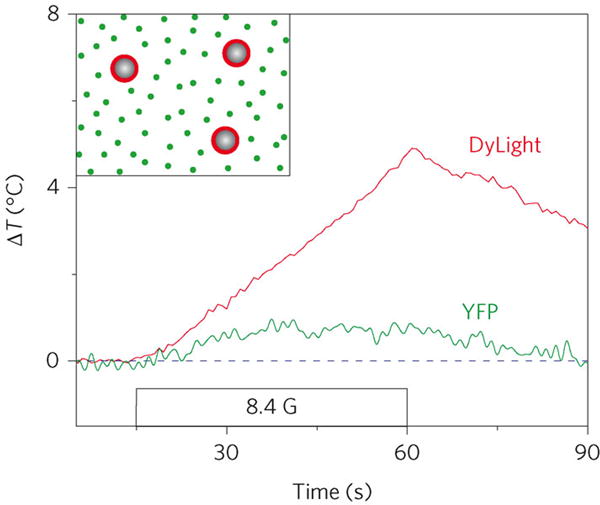
Observations of local heating in the vicinity of magnetic nanoparticles when an AMF (8.4 G, 40 MHz) is applied. The surface of the magnetic nanoparticles was functionalized with DyLight fluorophore, whereas yellow fluorescent protein (YFP) was present in the surrounding fluid. Reproduced with permission.[30] Copyright 2013, Nature Publishing Group.
In another study, Polo-Corrales and Rinaldi (2012) used a thermoresponsive polymer conjugated to a fluorescent dye as a means to monitor the surface environment of the particles.[31] With the combination of poly(N-isopropylacrylamide) (pNIPAM) and benzofurazan, the fluorescence intensity of the nanoparticles increased with decreasing polarity of the pNIPAM as it was heated.[31] Thus, fluorescence intensity changes of the polymer could be used to track the temperature in the nanoscale vicinity of the MNPs.[31] With external heating, the medium had to reach a temperature of 35 °C before an increase in fluorescence intensity was observed, while an increase in fluorescence intensity was immediately observed upon applying an AMF, even though the bulk temperature was 20 °C, approximately 15 °C below the transition temperature (Figure 4).[31] These observations suggested that the temperature in the vicinity of the MNPs under AMFs can be much higher than that of the bulk.[31]
Figure 4.
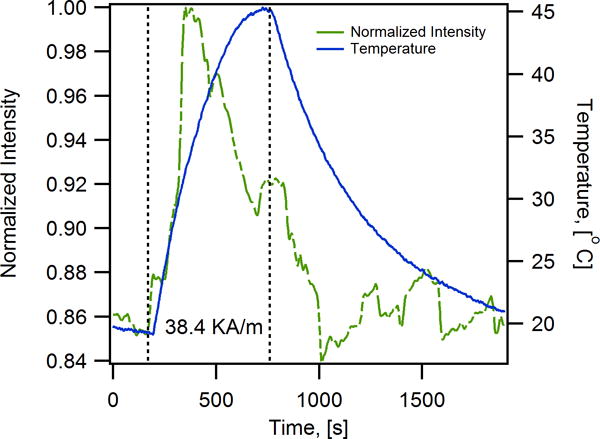
Iron oxide magnetic nanoparticles coated with a thermoresponsive-fluorescent polymer consisting of a copolymer of benzofuranzan and N-isopropylacrylamide experience nanoscale heating upon application of an AMF (38.4 kA/m, 233 kHz). The fluorescence of the benzofuranzan dye increases with change in environment polarity, which changes with temperature due to the thermoresponsive properties of pNIPAM. With external heating the fluorescence is observed to increase at a temperature of ~40 °C, whereas upon application of an AMF the fluorescence is observed to increase even though the bulk temperature is 20 °C. Reproduced with permission.[31] Copyright 2013, Nature Publishing Group.
Riedinger et al. (2013) used thermally-labile linkers as probes of local temperature changes, with spatial resolution at subnanometer scale.[32] For this purpose, they used the thermally-labile azo bond (2,2′-azobis[N-(2-carboxyethyl)-2-methylpropionamidine]) as a crosslinker between fluoresceineamine dye and MNPs, with distance between the azo bond and nanoparticle surface controlled using PEG spacers of different molecular weights.[32] Release of the fluorophore due to the breakage of the thermally-labile azo linker was monitored and enhanced release under an AMF as compared to release under external heating was interpreted as evidence of a local temperature rise.[32] This local temperature rise was found to scale linearly with increasing magnetic field amplitude and decreases exponentially with increasing molecular weight of the PEG spacer.[32] This study is relevant because it shows evidence of local heating phenomenon as a function of distance from the MNP surface (Figure 5).
Figure 5.
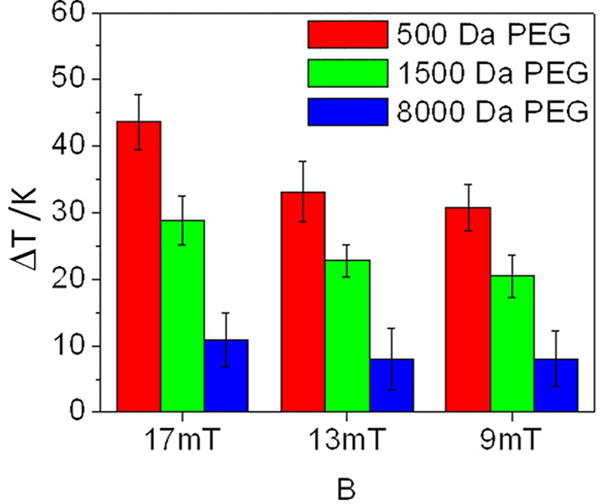
Change in temperature as a function of different applied magnetic field amplitudes and PEG spacer molecular weight, as determined from the rate of release of a dye conjugated to magnetic nanoparticles through a thermally labile crosslinker.
Reproduced with permission.[32] Copyright 2013, American Chemical Society.
Later, Dong et al. (2014) encapsulated MNPs with a crystalline optical nanothermometer consisting of NaYF4:Yb3+, Er3+ in silica nanoparticles in order to monitor the temperature inside MNP-loaded silica nanoparticles.[33] The temperature inside the silica nanoparticles was determined based on the temperature dependent intensity ratio of the two luminescence bands in the upconversion emission spectrum of the nanothermometer.[33] They observed a rise in internal temperature of the silica nanoparticles above that of the bulk solution over time scales of minutes, reaching saturation 2–3 minutes after turning on the AMF.[33]
More recently, Piñol et al. (2015) co-encapsulated MNPs and a Eu/Tb complex inside polymer shells to monitor the temperature inside the nanoparticles.[34] The absolute local temperature close to the MNPs was inferred through the changes in fluorescence from the ratiometric thermometric dyes.[34] Transient changes in temperature were correlated with application of an AMF, demonstrating a direct correlation between application of the AMF and substantial heating inside the nanoparticles, above the bulk temperature of their environment.[34]
The aforementioned papers are only a few of the studies that suggest nanoscale thermal phenomena in the vicinity of MNPs, in stark contradiction of the theoretical models of Rabin[26] and Keblinski[27]. Although initial experiments failed to observe nanoscale thermal phenomena suggested by theory, a suite of complementary techniques developed since all agree that there is a significant nanoscale thermal phenomena on the surface of the MNPs in AMFs; in other words, it certainly appears that nanoscale heating in the vicinity of MNPs in AMFs is real.
5. Can nanoscale thermal phenomena in the vicinity of magnetic nanoparticles be harnessed for biomedical application?
Having established that there is real experimental evidence of nano-scale thermal phenomena, it is natural to wonder if such effects lead to biologically relevant responses. If so, does it enhance traditional hyperthermia and the ability to kill cells without a macroscopic temperature rise? Such observations could also be construed as indirect evidence of nano-scale thermal phenomena.
There have been many works that show that heating using MNPs is superior over regular hyperthermia in terms of killing cancer cells. Rodriguez-Luccioni et al. (2011) showed in vitro that heating with MNPs resulted in greater decrease in cell viability over hot water hyperthermia under similar time-temperature conditions.[35] Lee et al. (2011) showed that much lower doses of cisplatin are effective in combination with MFH, enhancing the anticancer activity of cisplatin more than hot water bath hyperthermia.[14a] Alvarez-Berrios et al. (2013) demonstrated that fluidization of the cell membrane occurs due to heating with MNPs, which enhances drug uptake[15] and that MFH has synergy with cisplatin even in resistant cell lines.[14b]
Besides showing that there is an enhanced effect due to hyperthermia using MNPs, other work has shown that cell death can occur without a temperature rise. Villanueva at al. (2010) reported that viability of HeLa cells was reduced by manganese oxide perovskite nanoparticles, suggesting that cell death was triggered even though the temperature rise of the cell medium was less than 0.5°C.[36] According to Villanueva et al., cell death was due to an apoptotic mechanism because of extensive cellular damage done by the MNPs.[36] Although promising, assessment of effect of heating on cells was limited to observations of morphological changes in a single cancer cell line, with no comparisons to non-cancer cell lines. Also, the colloidal stability of the particles in media was not demonstrated, the extent of particle internalization in the cells was not quantified, and the actual degree of cell death achieved was not determined. Thus, it was not clear from this study if intracellular heating by MNPs can be made to be selective against cancer cells and can achieve clinically-relevant levels of cancer cell death.
Creixell et al. (2011), were the first to demonstrate that receptor targeted MNPs could significantly reduce viability of cancer cells, with selectivity toward cells that overexpress targeted receptors. For this purpose, Creixell et al. used colloidally stable, carboxymethyl-dextran coated iron oxide MNPs conjugated to the peptide epidermal growth factor (EGF) to target the epidermal growth receptor (EGFR), which is overexpressed in many cancer cells. They used two different cell lines to compare results for two levels of EGFR expression,[37] and observed that targeted nanoparticles were localized on the cell membrane, the cytoplasm, and lysosomes after 1 hour incubation while they did not observe any specific localization of non-targeted nanoparticles.[37] Non-targeted MNPs internalized up to 2–5 pg/cell whereas EGFR targeted MNPs internalized at different amounts depending on cell expression of EGFR and incubation time. After removing unbound MNPs, AMFs were applied for at different field strengths and cell death was assessed by clonogenic assay.[37] They reported a 99.9% reduction in cell clonogenic survival after application of an AMF, even though there was no macroscopic temperature rise.[37] Also, they showed that reduction in clonogenic survival correlated with applied field strength/SAR of the MNPs (Figure 6) and cell type. Later, Domenech et al. (2013) studied the mechanism underlying the observations of Creixell et al. (2011) further demonstrating that EGFR targeted iron oxide MNPs selectively kill EGFR-overexpressing cancer cells in AMFs without a perceptible temperature rise.[37–38] Domenech et al. (2013) compared effects on a non-cancerous breast cell line to a breast cancer cell line, demonstrating minimal cell death in the normal tissue line compared to a 5 fold change in viability in the cancer cells.[38] Further, they demonstrated that cell death occurred due to lysosomal membrane permeabilization, which correlated with an increase in Cathepsin B levels in the cytoplasma and the release of Reactive Oxygen Species (ROS) only in cancer cells treated with targeted particles and AMF[38]. Of note, in their study, Domenech et al. did not observe evidence of increased ROS in cells exposed to MNPs alone, or cells exposed to non-targeted MNPs and AMFs, thus, the observed increase in ROS for cancer cells treated with targeted nanoparticles can be construed to result from lysosomal damage. This in turn suggests that one of the underlying mechanisms is magnetically-mediated activation of lysosomal death pathways,[39] illustrated in Figure 7. This is a noteworthy result, as lysosomal death pathways are currently believed to be upregulated in many cancer cells and activating them may prove to be a viable approach to cancer treatment, especially in cancers resistant to other therapies.[40] Figure 2 illustrates the expected nanoparticle concentrations needed to treat tumors using magnetically-mediated activation of lysosomal pathways as a function of the volume of the tumor and for typically reported values of the SAR (dashed lines, MagMED). For hyperthermia, the parameter used to compare cell damage is cumulative equivalent minutes (CEM)[41], but for nanoscale hyperthermia effect, where temperature is not measurable, total heat dose (THD) is used as reported by DeNardo et al. (2007)[42] and used by Creixell et al. (2011)[37]. Total heat dose is in joules per cell is calculated using the SAR of the internalized nanoparticles, times the mass of nanoparticles per cell (mcells) times the duration of treatment (t): THD = (mcells)(SAR)(t). Creixell et al. (2011) determined that THD = 18 μJ/cell was required for 99% cell death[37]. Figure 2 is based on the value of 18 μJ/cell and a cell density of 5×107 cells/cm3 of cancer tissue.[43] Figure 2 is suggestive that cancer treatment by magnetically-mediated activation of lysosomal death pathways may be feasible in vivo for typical tumor volumes used in rodent models.
Figure 6.
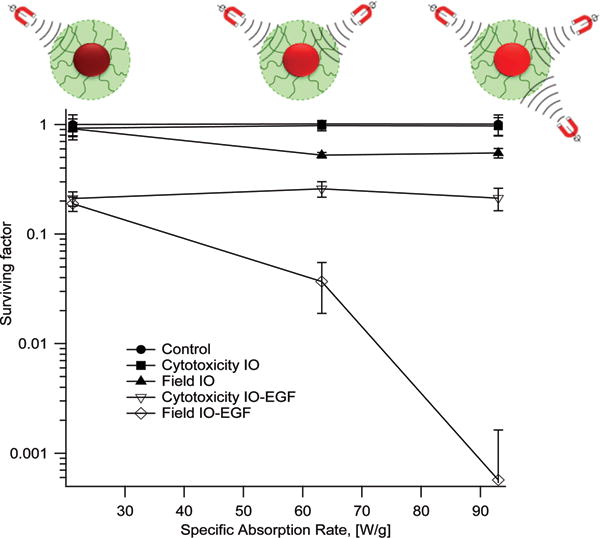
Clonogenic surviving fraction as a function of specific absorption rate for cancer cells (MDA-MB-468) exposed to iron oxide (IO) magnetic nanoparticles without/with the peptide epidermal growth factor (EGF). Unbound/uninternalized nanoparticles were removed prior to field application and the temperature remained constant at 37°C during field application. Increasing SAR has a slight effect on clonogenic survival of cells treated with the non-targeted particles (Field IO), but has a significant effect on clonogenic survival of cells treated with targeted nanoparticles (Field IO-EGF).
Reproduced with permission.[37] Copyright 2011, American Chemical Society.
Figure 7.
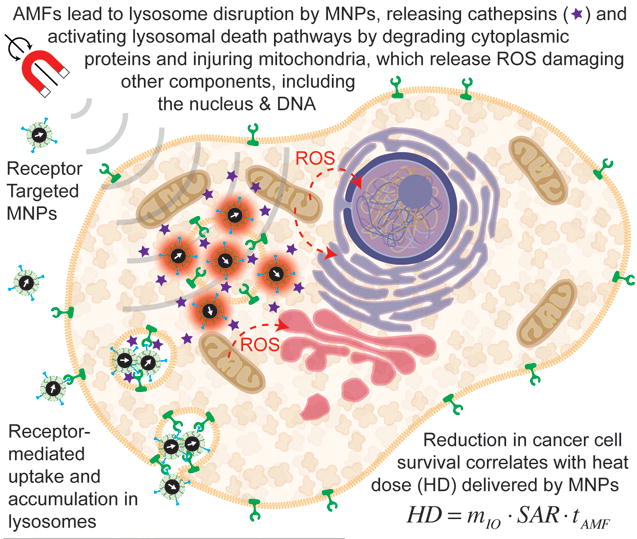
Magnetically-mediated activation of lysosomal death pathways through intracellular energy delivery by magnetic nanoparticles.
Other groups have recently reported additional studies that support the broad potential of targeted nanoscale energy delivery for the treatment of cancer. Sanchez et al. (2014)[44] reproduced the work of Creixell et al. (2011) and Domenech et al. (2013) using different cell lines and different cell surface targets and also found evidence of substantial reduction in viability of cells treated with targeted nanoparticles and of involvement of lysosomal death pathways. Connord et al. (2015) reported real-time observations of the behavior of cells after particle internalization and during application of AMFs using a novel AMF actuator compatible with optical microscopy.[45] They saw evidence of lysosome permeabilization and increased ROS production in cells with internalized nanoparticles in the AMF in a span of minutes, even though there was no change in the bulk temperature. Their observations led them to conclude that cell death occurred at the single-cell level even without a global temperature rise.
Stanley et al. (2012) and Chen et al. (2015) followed up on the work of Huang et al. (2010), by targeting the cellular TRPV1 receptor. Stanley et al. (2012), used iron oxide NPs targeted to the temperature sensitive channel, TRPV1, to show actuation of TRPV1 gates allowing calcium to enter the cytoplasm, stimulating synthesis and release of insulin.[46] Chen et al. (2015) targeted the TRPV1 receptor to see if they could activate a subpopulation of neurons in the brain.[47] They targeted the ventral tegmental area (VTA) first which had low endogenous expression of TRPV1 and saw no activity in the region when injected with MNPs and exposed to AMFs. On the other hand, when the VTA was sensitized by lentiviral delivery of TRPV1, they saw an increase in activity when the region was injected with MNPs and exposed to AMFs.
Beyond directly triggering biological responses, nanoscale heating by MNPs in AMFs has been applied for magnetically triggered drug release, providing further indirect evidence of nanoscale thermal phenomena. Thomas et al. (2010) prepared silica nanoparticles containing zinc iron oxide nanocrystals within the silica network.[48] Rhodamine B was loaded into the nanoparticles and capped with a nanovalve that remains closed at room temperature and opens only due to heating.[48] During release experiments, they kept the sample at 0 °C while applying an AMF and saw release of the dye, suggesting there is local internal heating of the particles, sufficient for valve actuation without macroscopic heating.[48] Amstad et al. (2011) demonstrated that liposomes with NitroDOPA-palmityl stabilized iron oxide nanoparticles embedded in the lipid bilayer release their cargo in response to AMFs.[49] Amstad et al. encapsulated calcein and saw fluorescence increase when an AMF was applied and ceased fluorescence when the AMF was removed.[49] It is important to note that release of calcein occurred even though the bulk temperature was below the liposome melting temperature. N’Guyen et al. (2013), made use of the thermoreversible Diels-Alder (rDA) reaction to achieve magnetically triggered drug release from iron oxide NPs.[50] Of note, the rDA is too slow below 90–110°C, but conjugated to the MNPs they saw release of their fluorescent probe with minimal increase in temperature in the medium, suggesting a greater local thermal effect.[50] Saint-Cricq et al. (2015) used mesoporous silica nanoparticles with an iron oxide core and coated with a thermodegradable polymer that breaks with an elevation in temperature.[51] The polymer was conjugated with rhodamine 6G and they saw an increase in fluorescence signal.[51] The bulk temperature when AMF was applied never exceeded 30 °C when previous experiments showed that they needed a bulk solution temperature of 55 °C before they saw fluorescence signal increase.[51] Ruhle et al. (2015) prepared mesoporous silica nanoparticles doped with manganese zinc iron oxide and used a thermally reversible cycloaddition reaction with a small molecular snaptop to block the pores of the silica nanoparticles.[52] They loaded the particles with fluorescein and saw fluorescein release when the bulk temperature went from 37 °C to 65 °C.[52] When AMFs were applied to the particles, to conclude that the increase in fluorescein release was not due to bulk heating the experiments were carried out in an ice bath at 0 °C at all times and the fluorescence intensity still increased, suggesting localized heating.[52]
6. Outlook for the field – the future of nanoscale thermal energy delivery using magnetic nanoparticles in biomedicine
As shown above, there is clear direct and indirect evidence that nano-scale thermal phenomena in the vicinity of MNPs in AMFs is real. One obvious gap in the field is the lack of theoretical models that explain experimental observations. Clearly, the underlying assumptions of past models[26–27] must be re-evaluated. Furthermore, the reasons for discrepancies between groups that do and do not experimentally observe nanoscale thermal phenomena remains unknown, but could be due to differences in rate and mechanism of energy dissipation of the MNPs used in the studies. Unfortunately, MNPs used in most studies of nanoscale thermal phenomena are poorly characterized physically and magnetically.
While a theoretical understanding of how MNPs behave in AMFs is incomplete, many researchers continue to pursue applications of these particles, especially in cancer therapy. Major challenges in thermal cancer therapy using MNPs involves engineering MNPs with predictable and reproducible properties, including high energy dissipation rates, achieving significant tumor accumulation after systemic delivery, controlling temperature rise and achieving reproducible therapeutic doses, and, as discussed above, overcoming heat transfer limitations in small tumors. Recent understanding of the potential role of nanoscale heating by magnetic nanoparticle seems to indicate that it has the potential of overcoming some of these challenges, as it may be a viable route to treating small tumors where traditional hyperthermia would require unachievable doses.
While the studies discussed above are exciting in terms of demonstrating therapeutic potential of intracellular energy delivery and magnetically-triggered drug release, there is much work to be done still. For example, to date there have been no demonstrations that intracellular heating can affect tumor growth in vivo, without the need for a tissue-level temperature rise. Similarly, there are few studies testing the efficacy of magnetic-triggered drug delivery using preclinical animal models, and studies addressing the passive and active rates of drug release from such particles remain limited. Finally, to date the focus on magnetic thermal therapy and magnetically-triggered drug delivery has been on killing cells, such as in the context of cancer. It remains to be determined if these nanoscale thermal phenomena can be harnessed to treat diseases where the goal is to repair tissue or redirect cellular pathways. For example, it remains to be determined if magnetically triggered drug delivery can be applied to the delivery of disease-modifying drugs (i.e., those that alter cells or tissues without killing them) without affecting cell viability due to the heat released by the nanoparticles, or if thermal energy can be used to promote a positive change in the behavior of cells. Such demonstrations would open the door to many new and exciting biomedical applications for MNPs.
Biographies

Andreina Chiu-Lam received her B.S. in Chemical and Biomolecular Engineering (2010) and M.S. in Technology Management (2011) from the University of Illinois at Urbana-Champaign, USA. Currently, she is pursuing her Ph.D. degree in Chemical Engineering at the University of Florida, USA under the supervision of Professor Carlos Rinaldi. Her research focuses on the use of superparamagnetic iron oxide nanoparticles, for the targeted selective destruction of triple negative breast cancer through local heating effects in vitro and in vivo.

Carlos Rinaldi received his B.S. in Chemical Engineering (1998) from the University of Puerto Rico, Mayagüez, M.S. degree in Chemical Engineering and Chemical Engineering Practice (2001) and Ph.D. in Chemical Engineering (2002) from the Massachusetts Institute of Technology. From 2002 to 2012 he was a Professor in the Department of Chemical Engineering at the University of Puerto Rico, Mayagüez. In 2006 he received the Presidential Early Career Award for Scientists and Engineers (PECASE). Since 2012 he has been a Professor in the J. Crayton Pruitt Family Department of Biomedical Engineering and in the Department of Chemical Engineering at the University of Florida. His research interests are in the biomedical applications of magnetic nanoparticles and in understanding the interplay of magnetic, hydrodynamic, and thermal forces and torques in determining the response of magnetic nanoparticles to time-varying magnetic fields.
Contributor Information
Andreina Chiu-Lam, Department of Chemical Engineering, University of Florida, Gainesville, Florida, 32611-6005, USA.
Carlos Rinaldi, Department of Chemical Engineering, University of Florida, Gainesville, Florida, 32611-6005, USA; J. Crayton Pruitt Family, Department of Biomedical Engineering, University of Florida, Gainesville, Florida, 32611-6131, USA.
References
- 1.Rosensweig RE. J Magn Magn Mater. 2002;252:370. [Google Scholar]
- 2.Carrey J, Mehdaoui B, Respaud M. J Appl Phys. 2011;109:083921. [Google Scholar]
- 3.a) Frazier N, Ghandehari H. Biotechnol Bioeng. 2015;112:1967. doi: 10.1002/bit.25653. [DOI] [PubMed] [Google Scholar]; b) Lima-Tenorio MK, Pineda EA, Ahmad NM, Fessi H, Elaissari A. Int J Pharm. 2015;493:313. doi: 10.1016/j.ijpharm.2015.07.059. [DOI] [PubMed] [Google Scholar]; c) Kozissnik B, Bohorquez AC, Dobson J, Rinaldi C. Int J Hyperthermia. 2013;29:706. doi: 10.3109/02656736.2013.837200. [DOI] [PubMed] [Google Scholar]; d) Kobayashi T. Biotechnol J. 2011;6:1342. doi: 10.1002/biot.201100045. [DOI] [PubMed] [Google Scholar]
- 4.a) Reimhult E. N Biotechnol. 2015;32:665. doi: 10.1016/j.nbt.2014.12.002. [DOI] [PubMed] [Google Scholar]; b) Ridi F, Bonini M, Baglioni P. Adv Colloid Interface Sci. 2014;207:3. doi: 10.1016/j.cis.2013.09.006. [DOI] [PubMed] [Google Scholar]; c) Bonini M, Berti D, Baglioni P. Curr Opin Colloid Interface Sci. 2013;18:459. [Google Scholar]; d) Amstad E, Reimhult E. Nanomedicine (Lond) 2012;7:145. doi: 10.2217/nnm.11.167. [DOI] [PubMed] [Google Scholar]
- 5.a) Leng JS, Lan X, Liu YJ, Du SY. Prog Mater Sci. 2011;56:1077. [Google Scholar]; b) Medford JA, Hubbard JW, Orange F, Guinel MJF, Calcagno BO, Rinaldi C. Colloid Polym Sci. 2014;292:1429. [Google Scholar]; c) Puig J, Hoppe CE, Fasce LA, Perez CJ, Pineiro-Redondo Y, Banobre-Lopez M, Lopez-Quintela MA, Rivas J, Williams RJJ. J Phys Chem C. 2012;116:13421. [Google Scholar]; d) Yu X, Zhou S, Zheng X, Guo T, Xiao Y, Song B. Nanotechnology. 2009;20:235702. doi: 10.1088/0957-4484/20/23/235702. [DOI] [PubMed] [Google Scholar]; e) Corten CC, Urban MW. Adv Mater. 2009;21:5011. doi: 10.1002/adma.200901940. [DOI] [PubMed] [Google Scholar]; f) Adzima BJ, Kloxin CJ, Bowman CN. Adv Mater. 2010;22:2784. doi: 10.1002/adma.200904138. [DOI] [PubMed] [Google Scholar]
- 6.a) Razzaq MY, Behl M, Lendlein A. Nanoscale. 2012;4:6181. doi: 10.1039/c2nr31332d. [DOI] [PubMed] [Google Scholar]; b) Cai Y, Jiang JS, Zheng B, Xie MR. J Appl Polym Sci. 2013;127:49. [Google Scholar]
- 7.a) Lay EH, Chvapil M, Cetas C. Clin Res. 1985;33:A73. [Google Scholar]; b) Cetas TC, Dewhirst MW, Deyoung DW, Fletcher AM, Oleson JR, Stauffer PR. Radiat Res. 1982;91:420. [Google Scholar]; c) Matloubieh AY, Roemer RB, Cetas TC. Radiat Res. 1982;91:416. [Google Scholar]
- 8.a) Jordan A, Wust P, Scholz R, Tesche B, Fahling H, Mitrovics T, Vogl T, CervosNavarro J, Felix R. Int J Hyperthermia. 1996;12:705. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]; b) Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Int J Hyperthermia. 1997;13:587. doi: 10.3109/02656739709023559. [DOI] [PubMed] [Google Scholar]
- 9.Goldman L, Dreffer R. 1976;257:227. doi: 10.1007/BF00558096. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy JE. Nat Rev Cancer. 2005;5:321. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 11.a) Sasidharan A, Monteiro-Riviere NA. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2015;7:779. doi: 10.1002/wnan.1341. [DOI] [PubMed] [Google Scholar]; b) Dykman L, Khlebtsov N. Chem Soc Rev. 2012;41:2256. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]; c) Lal S, Clare SE, Halas NJ. Acc Chem Res. 2008;41:1842. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 12.a) Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Cancer. 2007;110:2654. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]; b) Ghosh S, Dutta S, Gomes E, Carroll D, D’Agostino R, Jr, Olson J, Guthold M, Gmeiner WH. ACS Nano. 2009;3:2667. doi: 10.1021/nn900368b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Jpn J Cancer Res. 1996;87:1179. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Lee JS, Rodriguez-Luccioni HL, Mendez J, Sood AK, Lpez-Berestein G, Rinaldi C, Torres-Lugo M. J Nanosci Nanotechnol. 2011;11:4153. doi: 10.1166/jnn.2011.3821. [DOI] [PubMed] [Google Scholar]; b) Alvarez-Berrios MP, Castillo A, Rinaldi C, Torres-Lugo M. Int J Nanomedicine. 2014;9:145. doi: 10.2147/IJN.S51435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Berrios MP, Castillo A, Mendez J, Soto O, Rinaldi C, Torres-Lugo M. Int J Nanomedicine. 2013;8:1003. doi: 10.2147/IJN.S38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. 1998;89:463. doi: 10.1111/j.1349-7006.1998.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Jpn J Cancer Res. 1998;89:775. doi: 10.1111/j.1349-7006.1998.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S. Nanomedicine. 2014;10:1273. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A. J Neurooncol. 2007;81:53. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 20.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. J Neurooncol. 2011;103:317. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. 2005;21:637. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- 22.Wust P, Gneveckow U, Johannsen M, Boehmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. 2006;22:673. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Hernandez E, Baeza A, Vallet-Regi M. ACS Nano. 2011;5:1259. doi: 10.1021/nn1029229. [DOI] [PubMed] [Google Scholar]
- 24.Baeza A, Guisasola E, Ruiz-Hernandez E, Vallet-Regi M. Chem Mater. 2012;24:517. [Google Scholar]
- 25.Guo W, Yang C, Lin H, Qu F. Dalton Trans. 2014;43:18056. doi: 10.1039/c4dt02441a. [DOI] [PubMed] [Google Scholar]
- 26.Rabin Y. Int J Hyperthermia. 2002;18:194. doi: 10.1080/02656730110116713. [DOI] [PubMed] [Google Scholar]
- 27.Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. J Appl Phys. 2006;100 [Google Scholar]
- 28.a) Hashemi-Moghaddam H, Kazemi-Bagsangani S, Jamili M, Zavareh S. 2016;497:228. doi: 10.1016/j.ijpharm.2015.11.040. [DOI] [PubMed] [Google Scholar]; b) Li WM, Chiang CS, Huang WC, Su CW, Chiang MY, Chen JY, Chen SY. 2015;220:107. doi: 10.1016/j.jconrel.2015.10.020. Part A. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Kane RS, Borca-Tasciuc DA. J Appl Phys. 2010;108 [Google Scholar]
- 30.Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Nat Nanotechnol. 2010;5:602. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- 31.Polo-Corrales L, Rinaldi C. J Appl Phys. 2012;111 [Google Scholar]
- 32.Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, Pellegrino T. Nano Lett. 2013;13:2399. doi: 10.1021/nl400188q. [DOI] [PubMed] [Google Scholar]
- 33.Dong J, Zink JI. ACS Nano. 2014;8:5199. doi: 10.1021/nn501250e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinol R, Brites CD, Bustamante R, Martinez A, Silva NJ, Murillo JL, Cases R, Carrey J, Estepa C, Sosa C, Palacio F, Carlos LD, Millan A. ACS Nano. 2015;9:3134. doi: 10.1021/acsnano.5b00059. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, Torres-Lugo M. Int J Nanomedicine. 2011;6:373. doi: 10.2147/IJN.S14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villanueva A, de la Presa P, Alonso JM, Rueda T, Martinez A, Crespo P, Morales MP, Gonzalez-Fernandez MA, Valdes J, Rivero G. J Phys Chem C. 2010;114:1976. [Google Scholar]
- 37.Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. ACS Nano. 2011;5:7124. doi: 10.1021/nn201822b. [DOI] [PubMed] [Google Scholar]
- 38.Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. ACS Nano. 2013;7:5091. doi: 10.1021/nn4007048. [DOI] [PubMed] [Google Scholar]
- 39.Iovino N, Bohorquez AC, Rinaldi C. 2014;9:937. doi: 10.2217/nnm.14.52. [DOI] [PubMed] [Google Scholar]
- 40.a) Appelqvist H, Waster P, Kagedal K, Ollinger K. 2013;5:214. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]; b) Groth-Pedersen L, Jaattela M. 2013;332:265. doi: 10.1016/j.canlet.2010.05.021. [DOI] [PubMed] [Google Scholar]; c) Gyparaki MT, Papavassiliou AG. 2014;20:239. doi: 10.1016/j.molmed.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Sapareto SA, Dewey WC. Int J Radiat Oncol, Biol, Phys. 1984;10:787. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 42.DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R. J Nucl Med. 2007;48:437. [PubMed] [Google Scholar]
- 43.Del Monte U. 2014;8:505. [Google Scholar]
- 44.Sanchez C, El Hajj Diab D, Connord V, Clerc P, Meunier E, Pipy B, Payre B, Tan RP, Gougeon M, Carrey J, Gigoux V, Fourmy D. ACS Nano. 2014;8:1350. doi: 10.1021/nn404954s. [DOI] [PubMed] [Google Scholar]
- 45.Connord V, Clerc P, Hallali N, El Hajj Diab D, Fourmy D, Gigoux V, Carrey J. Small. 2015;11:2437. doi: 10.1002/smll.201402669. [DOI] [PubMed] [Google Scholar]
- 46.Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Science. 2012;336:604. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Science. 2015;347:1477. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 48.Thomas CR, Ferris DP, Lee JH, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. J Am Chem Soc. 2010;132:10623. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 49.Amstad E, Kohlbrecher J, Muller E, Schweizer T, Textor M, Reimhult E. Nano Lett. 2011;11:1664. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 50.N’Guyen TT, Duong HT, Basuki J, Montembault V, Pascual S, Guibert C, Fresnais J, Boyer C, Whittaker MR, Davis TP, Fontaine L. Angew Chem Int Ed Engl. 2013;52:14152. doi: 10.1002/anie.201306724. [DOI] [PubMed] [Google Scholar]
- 51.Saint-Cricq P, Deshayes S, Zink JI, Kasko AM. Nanoscale. 2015;7:13168. doi: 10.1039/c5nr03777h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruhle B, Datz S, Argyo C, Bein T, Zink JI. Chem Commun (Camb) 2016;52:1843. doi: 10.1039/c5cc08636a. [DOI] [PubMed] [Google Scholar]


