Abstract
Normative aging is associated with deficits in visual acuity and cognitive control that impact the allocation of visual attention, but little is known about how those changes affect information extraction and integration during visual language comprehension in older adulthood. In the current study, we used a visual hemi-field flanker RSVP paradigm with event-related brain potentials to study how older readers process fine-grained aspects of semantic expectancy in parafoveal and foveal vision. Stimuli consisted of high constraint sentences with expected, unexpected but plausible, or anomalous parafoveal target words, as well as low constraint sentences with neutral but expected target words. Older adults showed graded parafoveal N400 effects that were strikingly similar to younger readers, indicating intact parafoveal semantic processing. However, whereas young adults were able to use this parafoveal pre-processing to facilitate subsequent foveal viewing, resulting in a reduced foveal N400 effect, older adults were not able to. Instead, older adults re-processed the semantics of words in foveal vision, resulting in a larger foveal N400 effect relative to the young. Collectively, our findings suggest that although parafoveal semantic processing per se is preserved in aging, there exists an age-related deficit in the ability to rapidly integrate parafoveal and foveal visual semantic representations.
Keywords: aging, sentence processing, ERP, parafoveal processing
Although skilled readers may have the subjective experience that they can clearly perceive words across a large portion of the visual field in a single fixation (e.g., a line or even an entire page of text), in reality, readers are unable to fully process information outside of foveal vision. Acuity drops off rapidly in the parafoveal region, corresponding to approximately 2° to 5° on either side of the vertical meridian, and further so in peripheral vision. The perceptual span, which is the region in which useful visual features can be processed in a given fixation in reading, is asymmetric—skewing in the direction of reading. In English, it ranges from about 3–4 character spaces to the left of a fixation to upwards of 15 characters to the right of fixation (see Rayner, 2009 for a review). Current questions in reading research concern delineating the nature and quality of information that can be extracted from parafoveal vision and understanding how that information is used in the course of subsequent reading (e.g., Schotter et al., 2012 for a review).
One factor that may importantly affect parafoveal processing during reading is aging. Age-related changes in sensory and cognitive systems have a substantial impact on multiple aspects of language comprehension (see Shafto & Tyler, 2014; Stine-Morrow & Payne, 2016; Wlotko et al., 2012). These changes are likely to influence older adults’ allocation of visual attention during reading and their ability to process information in the parafovea. However, few studies have examined age-related changes in parafoveal processing or the ability to use parafoveal preview information during reading. In the current study, we examined age-related changes in neural indices of parafoveal processing during reading by measuring event-related brain potentials (ERPs) in a modified flanker-RSVP paradigm that affords the simultaneous investigation of neural indices of parafoveal and foveal processing in real time during reading. Specifically, we examined how normative aging impacts (1) the processing of words in parafoveal vision that vary in semantic expectancy and congruity with respect to their preceding context, and (2) how parafoveal semantic processing influences subsequent foveal viewing (i.e., parafoveal-to-foveal integration).
Parafoveal Vision and Reading
Eye-movement research forms the primary evidence base for the current understanding of parafoveal processing during reading and has provided valuable insight into how information across foveal and parafoveal vision is processed and integrated during reading (Clifton et al., 2016; Rayner et al., 2009; Schotter et al., 2012). A number of clever eye-tracking paradigms have been developed to study the dynamics of the perceptual span and parafoveal processing in natural reading (see Schotter et al., 2012, for a review). For example, in the gaze-contingent boundary change paradigm (Rayner, 1975), an invisible boundary is placed between the fixated word (n) and the word to the right of fixation (n + 1). When the eyes cross the invisible boundary, a change in the display is triggered, which is used to manipulate the availability of useful parafoveal information. On selected trials, n + 1 is initially replaced by a masking stimulus (e.g., a different word or a string of characters or X’s) and, as the reader saccades from n to n + 1, the mask is replaced with the target word. Thus, the logic of this paradigm is that by comparing fixation durations and eye-movements on word n+1 between conditions where different information was presented parafoveally, one can infer what kind of information was extracted from parafoveal vision. Indeed, this paradigm has revealed a characteristic parafoveal preview benefit: When a reader receives a valid preview of word n + 1, fixation durations on that word are 30 to 50 ms shorter relative to trials in which word n was initially replaced with an invalid mask, suggesting not that some level of preprocessing of n + 1 occurred when the eyes were fixating word n and that features of parafoveal and foveal words are rapidly integrated across successive saccades (Hyönä, Bertram, & Pollatsek, 2004; Schotter et al., 2012), though at least a portion of this reflects a preview cost due to processing the invalid parafoveal masking stimulus (e.g., Kliegl et al., 2013).
Research using the boundary change paradigm while manipulating linguistic features of the parafoveal mask (orthographic, phonological, semantic) has largely formed the basis of our knowledge of the kind of information that can be extracted from parafoveal vision and integrated with subsequent foveal processing. This work has converged on the finding that readers can extract and integrate parafoveal orthographic information as well as abstract phonological codes (see Vasilev & Angele, 2017 for a recent meta-analysis). However, early on, semantic information was thought to not be extracted from parafoveal vision. For example, a number of studies found no evidence for lexical priming from the parafoveal word (Rayner et al., 1986; Dimigen, Kliegl, & Sommer, 2012). Moreover, there was a lack of evidence for lexical or morphological parafoveal preview benefits in reading (Bertram & Hyönä, 2007; Inhoff & Rayner, 1989; Altarriba, Kambe, Pollatsek, & Rayner, 2001; Rayner, Balota, & Pollatsek, 1986; Rayner et al., 2014).
More recent work, however, has found evidence for the extraction of semantic features from parafoveal vision. For example, morphologically rich languages such as Mandarin (Yan, Richter, Shu, & Kliegl, 2009) and German (Hohenstein, Laubrock, & Kliegl, 2010) have provided evidence for semantic parafoveal preview. In English as well, a growing literature suggests that attention can be allocated to semantic processing in parafoveal vision. For example, semantic parafoveal effects are obtained when previews are available for short periods of time (Hohenstein et al., 2010), when parafoveal previews are synonyms (Schotter, 2013), or when previews are embedded within highly constraining sentence contexts (Schotter et al., 2015; Barber, 2010, 2013; Veldre & Andrews, 2015), with congruency-based parafoveal preview benefits observed in eye-movement behavior and electrophysiology (discussed in more detail below). Nevertheless, there is still ongoing debate regarding the nature and scope of semantic extraction from parafoveal vision (Rayner et al., 2014; Clifton et al., 2016; Dimigen et al., 2012; Johnson & Dunne, 2012; Snell, Vitu, & Grainger, 2016).
Aging, Parafoveal Processing, and “Risky” Reading
Normative aging is associated with widespread changes in central and peripheral visual acuity (e.g., contrast sensitivity, retinal illumination, visual processing speed, and useful field of view), as well as an increased risk for the development of visual pathology (e.g., macular degeneration, glaucoma, and cataracts; Owsley, 2011 for a review), all of which can lead to difficulties with reading fluency and comprehension with advancing age (e.g., Sass, Legge, & Lee, 2006; Legge, Ross, Isenberg, & Lamay, 1992; Payne & Stine-Morrow, 2014). At the same time, normative age-related neuroanatomical and functional changes, particularly in frontal and pre-frontal cortex, result in a range of declines in cognitive functioning, particularly for tasks that place strong demands on attention, executive control, and working memory (e.g., Fabiani, 2012; Grady, 2008, Raz, 2009; Fjell & Walhovd, 2010).
One way that normative age-related changes in sensory and cognitive function may affect visual language comprehension is by influencing how attention is allocated to foveal and parafoveal information (Rayner et al., 2006; Payne & Stine-Morrow, 2012). Older adults not only read more slowly (Stine-Morrow et al., Rayner et al., 2006), but also more variably (Payne & Stine-Morrow, 2014), and sometimes show greater rates of regressive eye-movements (Rayner et al., 2006, 2009, 2013). In non-reading domains, aging is associated with less efficient uptake of extra-foveal information (Ball et al., 1988; Sekuler, Bennett, & Mamelak, 2000). At the same time, only a small number of studies have examined the effects of aging on parafoveal processing in reading. These studies have revealed that older adults have a reduced and less asymmetric perceptual span (Rayner et al., 2009), extract less information from the word immediately to the right of fixation in parafoveal vision (Rayner et al., 2010; Payne & Stine-Morrow, 2012), and are less capable of using parafoveal vision to guide reading when foveal processing is impaired (via an artificial foveal scotoma; Rayner et al., 2014). At the same time, other research has found evidence for preserved parafoveal processing or even differential sensitivity to extra-foveal information in reading. For example, Risse and Kliegl (2014) found that older adults were sensitive to lexical status two words to the right of fixation (n + 2), and Paterson et al. (2013) found that older adults showed sensitivity to manipulations of spatial frequency in reading that were more diffuse across the visual field than younger adults. Importantly, the amount of information derived from parafoveal vision in aging may be largely dependent upon the concurrent cognitive workload of foveal processing. For example, Payne and Stine-Morrow (2012) found that older adults showed comparable parafoveal preview benefits to young adults when the difficulty of foveal processing was low. However, when a substantial foveal load (i.e, Henderson & Ferrieira, 1990; Payne et al., 2016) was induced, older adults’ parafoveal preview benefits were differentially reduced relative to the young (Payne & Stine-Morrow, 2012), suggesting that either the extraction or integration of parafoveal information was disrupted in aging.
Rayner and colleagues (2006, 2009, 2013) have argued that older adults adopt a series of strategies to compensate for age-related slowing of lexical processing, resulting in what has been termed risky reading. According to the risky reader theory, to maintain a fluent reading rate older adults rely differentially on noisy and incomplete parafoveal visual information to “guess” the identity of upcoming words, leading to increased word skipping rates. However, because of age-related declines in parafoveal visual acuity, parafoveal words are often incorrectly identified, resulting in a greater rate of disruption (e.g., regressions) to normal reading (though see Choi et al., 2017). In particular, Rayner and colleagues (2006) have argued that older readers will differentially rely on prior contextual constraints to make inferences about lexical items in parafoveal vision, despite reductions in parafoveal perception. However, a substantial electrophysiological literature has amassed indicating that older adults do not use context as effectively as do young adults, and, in particular, are less likely to use contextual constraints to engage in anticipatory or predictive processing (Federmeier et al., 2002; Federmeier, Kutas, & Schul, 2010; Payne & Federmeier, 2017; Wlotko et al., 2012; see Wlotko et al., 2010 for a review). One limitation in comparing these bodies of work is that nearly all electrophysiological and neuroimaging research on age differences in visual language processing has used single word presentation, which precludes the utilization of parafoveal information during reading. At the same time, eye-tracking and ERP studies infer real-time context use from very different outcome measures in very different paradigms, and only a small number of studies have attempted to find converging evidence between methods (e.g., Payne et al., 2016). Moreover, almost no research has directly investigated whether older adults show a qualitatively different reliance on or recruitment of parafoveal visual attention during reading, as predicted by the risky reader theory. Thus, it is unclear whether older adults are capable of using contextual constraints in real-time to identify parafoveal targets or to facilitate subsequent foveal processing. In the current study, we report the first event-related brain potential (ERPs) study to examine age-related differences in parafoveal processing during reading.
Event-Related Brain Potential Indices of Parafoveal Processing
One limitation of eye-tracking studies of parafoveal processing is that they almost all rely on paradigms that study the aftermath of that parafoveal processing. For example, the parafoveal preview benefit is used to draw inferences about the nature of information that was extracted from parafoveal vision based on measures derived from the subsequent foveal processing of words that were previously masked in parafoveal vision. In this way, it is difficult to dissociate stages of parafoveal perception per se from the integration of parafoveal and foveal representations using the parafoveal preview benefit alone (see also Payne et al., 2016). Indeed, effects of parafoveal manipulations during parafoveal processing (i.e., parafoveal-on-foveal effects) are rare in eye-movement studies. Such effects may only be rarely observed because of a lack of sensitivity of fixation durations to parafoveal processing, as parafoveal-on-foveal effects are sometimes observed for strong manipulations such as orthographic legality (e.g., Drieghe, 2011; Drieghe, Brysbaert, & Desmet, 2005; Inhoff, Starr, & Shindler, 2000; Payne & Stine-Morrow, 2012; Pynte, Kennedy, & Ducrot, 2004). Real-time parafoveal effects may also be obscured because of the timing properties of oculomotor programming, which can “smear” parafoveal effects across multiple words (e.g., Risse & Kliegl, 2014; Stites et al., 2014; Payne, Stites, & Federmeier, 2016). In contrast, ERPs can provide a continuous ongoing measure of neural activity during both parafoveal and foveal visual processing, allowing for the separation of parafoveal perception from the integration of parafoveal and foveal representations (Payne et al., 2016). A major theoretical implication of the parafoveal preview benefit is that the effect arises through trans-saccadic integration (Rayner, 1975; Cutter, Drieghe, & Liversedge, 2015), whereby information that is derived from parafoveal vision is used to facilitate subsequent foveal viewing through the integration of parafoveal and foveal visual representations across successive saccades. However, there is a lack of direct evidence that information derived during parafoveal processing directly influences subsequent foveal processing. That is, the parafoveal preview benefit in eye-tracking is used to infer what information was obtained during parafoveal processing based on measures obtained from foveal processing of that word, thus necessarily conflating parafoveal extraction and the integration of parafoveal and foveal information (see e.g., Risse & Kliegl, 2014), ERPs may provide a useful tool for decomposing these separate stages of processing.
A number of recent studies have examined parafoveal processing in reading by using ERPs (e.g., Barber, Benzvi, Bentin, & Kutas, 2011; Barber, van der Meij, & Kutas, 2013; Dimigen, Kliegl, & Sommer, 2012; Dimigen, Sommer, Hohlfeld, Jacobs, & Kliegl, 2011, Kretzschmar, Bornkessel-Schlesewsky, & Schlesewsky, 2009; Li, Niefind, Wang, Sommer, & Dimigen, 2015). One approach to examining ERPs in reading has been to co-register electroencephalogram (EEG) and eye-tracking data within the same experiment to examine fixation-related potentials (Dimigen et al., 2012; Kornrumpf et al., 2016; Lopez-Perez et al., 2016). An alternative approach, first developed by Barber, Kutas, and colleagues (Barber et al., 2011, 2013; Barber, Doñamayor, Kutas, & Münte, 2010), has been to modify the traditional ERP–RSVP paradigm by adding hemifield flankers in the left and right visual field, corresponding to the prior and subsequent word in the sentence, respectively. In this paradigm, participants fixate on a centrally presented word that is flanked in the right and left visual field by the preceding and following word, separated by 2° of visual angle (see “Procedure” below and Barber et al., 2011, 2013). On the immediately following trial, each word is shifted to the left, such that the sentence appears to progress successively across the screen from right to left. Although not naturalistic, this method has proven to be a useful tool for examining parafoveal processing during reading, providing a bridge between eye movement and ERP studies of language processing, while maintaining much of the experimental and EEG artifact control that is a strength of traditional ERP studies (i.e., homogeneity in visual input across trials, control and assessment of component overlap, reduction of eye-movement artifacts).
Moreover, ERPs provide a clear component to study manipulations of semantic features in parafoveal vision: the N400. The N400 is a component linked to meaning processing and initial access to semantic memory. It is part of the default response to any meaningful or potentially meaningful stimulus and is broadly sensitive to a whole host of factors that impact the ease of semantic memory access in non-linguistic domains, visual word recognition, and higher-order sentence processing (see reviews in Kutas & Federmeier, 2000, 2011). Indeed, one of the strongest determinants of N400 amplitude in sentence processing is a word’s expectancy (as indexed by cloze probability; the proportion of people who give a particular word as the most likely completion of a sentence fragment; Taylor, 1953). The N400 shows strong correlations with cloze-probability, with reduced (more positive) N400s to words with increasingly higher cloze probability, reflecting facilitation of semantic memory access based on the prior context (Kutas & Hillyard, 1984).
It is not surprising then, that a number of FRP/ERP studies have focused on the N400 component to study parafoveal semantic processing. Indeed, studies using the flanker-word ERP paradigm have reliably found evidence for semantic modulations of the N400 to words presented in parafoveal vision in young adults (Barber et al., 2010, 2013; Kornrumpf & Sommer, 2015; Li, Niefind, Wang, Sommer, & Dimigen, 2015; Stites, Payne, & Federmeier, 2017; Zhang, Li, Wang, & Wang, 2015). For example, semantically incongruent or unexpected parafoveal words embedded in moderately and strongly constraining sentence contexts elicit larger N400 amplitudes than do congruent words, an effect that mimics the semantic processing of words in foveal vision. In contrast, findings from FRP studies are more variable, with some studies finding evidence consistent with parafoveal semantic processing (Baccino & Manunta, 2005; Lopez-Perez et al., 2016; Kretzschmar, Bornkessel-Schlesewsky, & Schlesewsky, 2009), but others finding no clear effects of semantic manipulations in parafoveal vision (i.e., Dimigen et al., 2012; Ni et al., 2015).
Recently, Stites, Payne, and Federmeier (2017) used the flanker ERP paradigm to examine whether the parafoveal N400 responds in a manner that is graded with respect to semantic expectancy (as is observed for the foveal N400) and to directly examine the effects of parafoveal processing on subsequent foveal viewing. Young participants read highly constraining sentence contexts wherein a target word was expected, unexpected but plausible, or anomalous, as well as neutral (low constraint) sentences, in which the target word was moderately expected. Findings revealed an N400 effect to the target word when it appeared in the parafovea that was clearly graded with respect to the target’s cloze probability within the sentence context, showing that parafoveal N400 effects were impacted by target word expectancy in a manner that was highly consistent with prior research using only foveal presentation (see Kutas & Federmeier, 2011 for a review). This finding provided strong evidence for graded semantic processing in parafoveal vision in the young. Importantly then, when parafoveal targets subsequently appeared at central fixation, N400 amplitudes were reduced, and the graded cloze effect was substantially mitigated, suggesting that parafoveal semantic processing facilitated subsequent foveal semantic processing.
These findings are consistent with the claim that parafoveal and foveal visual representations are rapidly integrated (cf. Cutter et al., 2015). Moreover, the ERP findings suggest a mechanism through which this rapid integration of parafoveal and foveal representations might occur: parafoveal words are activated in semantic memory as indexed by the N400, and this spreading activation primes the foveal presentation of the word, not unlike the effects of foveal repetition priming on the N400 (Rugg, 1985). Thus, once the parafoveal semantic representation is activated, it does not have to be activated again to the same degree in foveal vision (see expanded discussion in Stites, Payne, & Federmeier, 2017).
Current Study
To examine how neural indices of parafoveal and foveal word processing change with age during sentence reading, in the current study we used the materials and procedure from Stites, Payne, and Federmeier (2017) with healthy and neurologically intact older adult participants. As described above, studies examining parafoveal semantic processing using ERPs have replicated sentential congruency effects on the N400 in parafoveal vision that are well-established in central vision. These well-characterized parafoveal N400 effects found in younger adults thus provide a reliable effect against which to compare older adults’ neural responses to parafoveal semantic processing. Specifically, we aimed to examine (1) whether older adults are sensitive to semantic constraints in parafoveal vision, as revealed by the parafoveal N400 and (2) whether parafoveal semantic representations impact ERPs to subsequent foveal viewing in aging. If older adults are less likely to extract useful lexical information from parafoveal words, as would be suggested by the existing eye-movement literature (e.g., Rayner et al., 2006), then we would expect reduced parafoveal N400 effects in older age. If older adults do, however, extract semantics from parafoveal vision, it remains an open question whether they can use that parafoveal information to actively facilitate subsequent foveal processing, as we have previously demonstrated in young adults (Stites et al., 2017).
Method
Participants
Twenty-four community dwelling older adults (M = 68.4; Range: 65–79, 16 female) participated in the experiment and were compensated with a cash payment. All participants were right-handed as assessed by the Edinburgh inventory (Oldfield, 1971) and had an average of 15.7 years of education (Range: 12 – 18 years). Participants scored within normal corrected or uncorrected near visual acuity range, as assessed by the Rosenbaum vision test (M = 3.13 points, Range = 3–5) and no participants reported visual impairments. All readers were monolingual speakers of English, with no consistent exposure to other languages before the age of five. Participants had no history of neurological or psychiatric disorders or brain damage and scored in the normal range on two common tests for clinical cognitive impairment. The Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), a brief 30-item cognitive impairment assessment often used in clinical settings to assess general cognitive function and risk for Alzheimer’s dementia, was administered to the older adult participants. A score below 24 often indicates some risk for clinically relevant cognitive impairment. All participants in the current study scored in the normal range (M = 29.3, range 26–30), indicating that the sample was cognitively intact. Participants also completed the Montreal Cognitive Assessment (MoCA, Nassredine et al., 2005), which has been used as a clinical instrument to assess the presence of mild cognitive impairment. Moreover, the MoCA and has been used to predict amnestic MCI-related language comprehension difficulties in prior work (Payne & Stine-Morrow, 2014). Although the appropriate cutoff score for cognitive impairment in the MoCA is more variable across samples (e.g., Waldron-Perrine & Axelrod, 2012), individuals scoring below 20 are generally considered to be at increased risk for MCI. The participants in the current experiment again scored in the normal range on the MoCA (M = 27.2, range 24–30). Finally, participants completed two tests of verbal fluency (phonemic and category fluency; Benton & Hamsher, 1978). Participants generated 50.46 words in the phonemic fluency task (range: 27–69) and 66.13 words (range: 37–94) in the category fluency task. These scores are comparable to the performance of age and education matched samples (Tombaugh, Kozak, & Rees, 1999).
To directly compare ERP and performance data of the older adults in the current study to a healthy young sample, we present an additional supplementary age-comparative analysis by pooling data from the prior experiment in our lab adopting the exact same stimuli, protocol, and analysis procedures as this experiment with a sample of healthy young adults (Stites et al., 2017). In that study, twenty-four participants (19 male, mean age = 20.7 years, range: 18–25 years) from the University of Illinois participated for course credit. All participants were native speakers of English, were right-handed as assessed by Edinburgh handedness inventory (Oldfield, 1971), and had no history of neurological disease or psychiatric disorders. Young adults had an average of 13.37 years of education (Range: 12 – 18 years) which was significantly lower than that of the old, t(46) = 3.96, p < .001.
Materials
Experimental sentences included 180 highly constraining sentences and 60 low constraint control sentences. These stimuli were identical to those used in Stites, Payne, and Federmeier (2017) and Payne, Stites, & Federmeier (2016) and were adapted from Federmeier et al. (2007). For the highly constraining sentence contexts, sentences were identical up to the target word across all conditions. Highly constraining sentence contexts were continued with either the most expected word (mean cloze = .87), an unexpected but plausible word of the same grammatical class (mean cloze = .06), or a semantically anomalous word. Semantically anomalous words were drawn from the same grammatical class as the unexpected target words and were matched on perceived concreteness and imageability, based on norms collected from the MRC Psycholinguistics Database (Wilson, 1988), as well as on frequency measures derived from multiple sources: Francis & Kucera norms (1982), the English Lexicon Project Hyperspace Analogue to Language norms (Balota et al., 2007), and the Corpus of Contemporary American English (CoCa, Davies, 2008). The expected words, however, were slightly shorter than the incongruent words, which, in turn, were slightly shorter than the unexpected words. Note, however, that because the parafoveal manipulation is defined based on degrees of visual angle (see “Procedure” below), there is no confound between target word length and degree of parafoveal eccentricity.
Additionally, there were 60 neutral low constraint experimental sentence frames that always contained their most expected completion (mean cloze = .23). Including these items allows us to examine the parafoveal N400 to low-to-moderately expected continuations and also ensures that half of the sentences presented to subjects did not violate readers’ predictions about upcoming targets, and contained no unexpected or anomalous words. Expected words in the low constraint sentences also did not differ from the high constraint expected words in terms of frequency, concreteness, or imageability, although they were longer than the targets in the high constraint sentences, t(59) = −2.76, p < .01 (which again, is not confounded with visual eccentricity). In each of the three high constraint conditions, the parafoveal target word was preceded by an identical pre-target word (mean length: 3.5 characters), which, in most sentences, was a short function word or pronoun (i.e., a, the, to). The targets in the low constraint sentences were preceded by similar short pre-target function words or pronouns (mean length: 3.0 characters).
Sentences ranged in length from 7 to 27 words (M = 14.58). To assess ERP effects following the target word’s appearance in the parafovea, continuations were written for every sentence (high constraint contexts mean continuation length = 5 words, range: 2–11; low constraint contexts mean = 4.4 words, range: 3–7). For the high constraint sentences, the same continuations were used after expected, unexpected but plausible, and anomalous words; these were judged by the three experimenters to be plausible following both the expected and unexpected endings. Similar continuations were created for the low constraint contexts. The majority (85%) of the immediately post-target words were high frequency function/closed class words (e.g., after, because, with), and the rest of the post-target words were distributed across nouns (2%), verbs (2%), adjectives (4%), and adverbs (7%). Further detailed stimulus characteristics and the results of a full norming study on these stimuli in younger adults are described in detail in Stites et al. (2017) and are thus not repeated here.
Procedure
The procedure adopted here was identical to that in Payne et al. (2016) and Stites et al. (2017). Participants were seated 85 cm from a 21″ CRT computer monitor in a dim, quiet testing room. As in prior flanker ERP studies (Barber et al., 2011, 2013; Stites, Payne, & Federmeier, 2017; Payne, Stites, & Federmeier, 2016) sentences were presented serially in triads, with the target word appearing at central fixation, flanked bilaterally by the upcoming word in the sentence to the right, and the preceding word to the left. Participants were informed that multiple words and symbols would appear to the left and right of the central word but to keep their fixation on the word presented at the center of the screen and read each sentence for comprehension, because there would be a recognition memory test at the end.
At the viewing distance, 3.5 letters subtended one degree of visual angle. The beginning letter of the right parafoveal word and the final letter of the left parafoveal word were anchored so as to appear at 2° of visual angle from the center of the screen. Each trial began with a series of fixation crosses (“+++++”) that remained on the screen for a duration that was jittered from 500–1500 ms. Each triad was visible on the screen for 100ms, with an inter-stimulus interval of 350 ms (cf. Barber et al., 2013). This short stimulus duration also minimized the possibility that participants could make a saccade to the parafoveal word within the amount of time that it was visible on the screen. The experiment began with a practice block of nine sentences to allow participants to become accustomed to the experimental procedure. The practice block could be repeated if participants made excessive numbers of eye movements, and they were continually given feedback about reducing eye movements throughout the experiment. The 240 sentences were divided into 6 blocks of 40 sentences each. Each recording block took approximately 7 minutes to complete, and short breaks were offered between each block. At the end of the experiment, participants completed a sentence recognition memory test. This test consisted of 160 sentences frames with a sentence-medial word left out, 80 of which appeared in the study (20 each from the high constraint expected, unexpected, and anomalous, and the low constraint sentences) and 80 that did not. For each sentence, subjects had to first indicate whether or not they saw the sentence frame in the study. For items they marked as having seen, they then had to fill in the blank with the word that completed the sentence in the study.
EEG Recording and Processing
EEG was recorded from twenty-six evenly-spaced silver-silver chloride electrodes embedded in an Electro-Cap (following the same montage as in Federmeier et al., 2007). Electrodes were referenced on-line to the left mastoid and re-referenced off-line to the average of the right and left mastoids. In addition, one electrode (referenced to the left mastoid) was placed on the left infraorbital ridge to monitor for vertical eye movements and blinks, and another two electrodes (referenced to one another) were placed on the outer canthus of each eye to monitor for horizontal eye movements. Electrode impedances were kept below 5 kΩ. The continuous EEG was amplified through a bandpass filter of .02–100 Hz and recorded to hard disk at a sampling rate of 250 Hz.
Epochs of EEG data were taken from 100 ms before stimulus onset to 1600 ms post-stimulus onset, such that the epoch contained target words in parafoveal and foveal vision. EEG epochs were examined for artifacts (amplifier blocking, signal drift, eye movements, eye blinks, or muscle activity), which were rejected off-line before averaging, using individually selected thresholds through visual inspection of the data. Special care was taken to set very sensitive thresholds for detecting and rejecting trials that contained lateral eye movements within the time-window that the parafoveal stimuli were presented on the screen, to ensure that flanker words were not directly foveated. We took steps to ensure that data contained as few eye movements as possible, to ensure that our findings represent circumstances in which readers were actually fixating the central word (and did not, for example, only fixate the right-most word). First, triads were only visible on the screen for 100 ms with a 350 ms ISI. Because it takes approximately 200ms to program and execute an eye movement, this short stimulus duration would have provided insufficient time for participants to make a saccade from the centrally presented word to the parafoveal word. Second, stringent artifact rejection procedures were undertaken to ensure the removal of trials that contained eye movements while the target word was present on the screen. Individualized thresholds were separately determined for each participant through visual inspection of the ERPs to identify trials with obvious eye movements in the bipolar HEOG; these thresholds were then applied to every trial for that participant, such that all trials with eye movements were removed prior to performing data analysis. Average residual eye movements in right and left EOG within the stimulus presentation window (100ms following stimulus onset) were then calculated. Residual EOG was less than 3μV for the congruity violation effect for all subjects, corresponding to an eye rotation of less than ± 0.1° (see Woodman & Luck, 2003; Lins, Picton, Berg, & Scherg, 1993).
For participants who had an excessive number of trials containing eye blinks (n = 4), those epochs were blink corrected using a PCA-based Wiener linear spatial filter (Dale, 1994) and added back into the artifact-free epochs for averaging. One subject was dropped following artifact detection for an excessive number of artifacts (> 50%), leaving N = 23 older adults for analysis. Artifact-free ERPs were averaged separately for each subject by stimulus type after subtraction of the 100 ms pre-stimulus baseline. On average, a total of 19% (SD = 11%; range across subjects 2%–44%) of words were marked as artifacts and not included in subsequent analyses. Prior to statistical analyses, ERPs were digitally filtered with a low-pass filter of 30Hz.
Results
Recognition Memory
The older adult participants correctly recognized an average of 43% of the experimental sentences and false alarmed to an average of 8% of lure sentences. Signal detection sensitivity was calculated using the A-index (see Zhang & Mueller, 2005), a non-parametric alternative to the common d’ index. Mean A was .79, 95% CI [.76, .81], indicating that the older adult participants were successfully discriminating between old and new sentences. Thus, participants appeared to be attending to the experimental materials. To compare sentence recognition memory of the current older adult sample to the performance of young adults, we compared signal detection sensitivity to the younger adults from Stites, Payne, & Federmeier (2017). Young adults from that sample showed a 53% hit rate for correctly recognizing experimental sentences, but showed a slightly higher false alarm rate than the old, at 13%, yielding a numerically smaller but not statistically significantly different A-index of .77, 95% CI [.69, .85]. Therefore, younger and older adults showed quite comparable overall behavioral performance.
Event-Related Brain Potentials
Figure 1a shows grand-average ERPs at representative electrode sites (left medial and right medial: prefrontal, central, parietal, and occipital) time-locked to the onset of the critical word triad for all four target word conditions presented initially in parafoveal vision. Typical neural responses were observed for visual stimulation in older adults: early sensory components included the posterior P1, N1, and P2, and the anterior N1 and P2. Following the sensory components, a late negative-going wave, the N400, was observed, onsetting around 350ms with a peak around 500ms, displaying a somewhat delayed latency relative to younger adults, a pattern commonly observed in aging studies (Federmeier et al., 2003; Wlotko et al., 2012). Replicating prior work on the parafoveal N400 with younger adults (Stites, Payne, & Federmeier, 2017), the N400 was graded in amplitude based on the expectancy of the parafoveal target.
Figure 1.
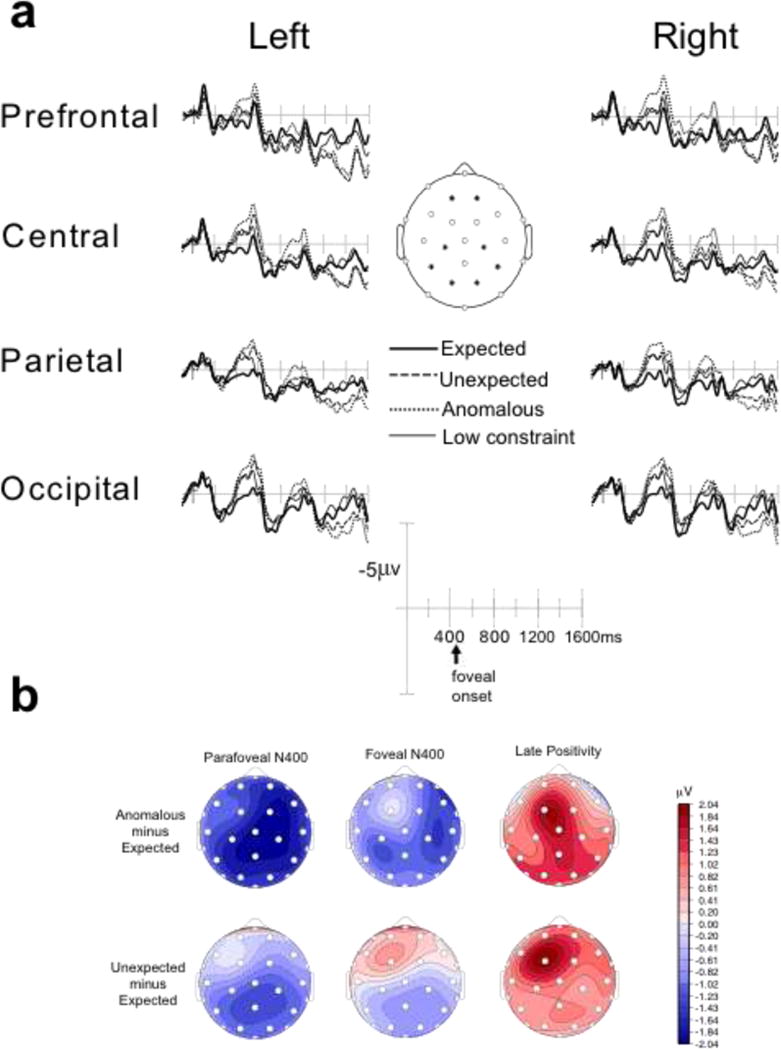
(a) Grand average event-related brain potentials at representative electrode sites. The time window encompasses both parafoveal and foveal viewing for high constraint sentences with expected, unexpected, and anomalous target words and target words in low constraint contexts. Time 0 indicates when the critical target appeared in parafoveal vision. This is followed at 450 ms by the subsequent triad, in which the target appeared in foveal vision, marked in the diagram as “foveal onset”. (B) Scalp distribution of the effect of expectancy (unexpected – expected) and congruity (anomalous – expected) violations on parafoveal and foveal N400 responses and on the late positivity following foveal viewing.
The following triad, in which the parafoveal target moved into foveal vision, was presented at 450ms following onset of the target triad. Sensory potentials to the foveal target (most notably the posterior-occipital P2) were observed following the offset of the parafoveal N400 effect. The N400 to the foveal target onset at approximately 800ms post parafoveal target-onset, which corresponded to 350ms post foveal-target onset. Interestingly, the pattern in foveal vision appeared quite similar to the pattern observed in parafoveal vision, with older adults again showing a robust and graded, although slightly reduced, N400 effects of expectancy in foveal vision. Finally, a broadly distributed positivity followed the foveal N400, with unexpected and anomalous conditions more positive than the expected and low constraint conditions. This effect is generally consistent with other reports finding late positive potentials indexing costs of semantic violations (DeLong et al., 2014; Federmeier et al., 2007, 2010; Kuperberg et al., 2013; Payne & Federmeier, 2017; Van Petten & Luka, 2012), but may also (or additionally) reflect a sustained negativity to the expected items, which has been observed in both younger (Wlotko & Federmeier, 2012) and older adults (Wlotko & Federmeier, 2012) during the processing of strong contexts. Figure 1b presents the scalp distribution of the canonical expectancy violation (unexpected – expected) and congruity violation (anomalous – expected) effects for the parafoveal N400, the foveal N400, and the late positivity following the foveal N400.
Parafoveal and Foveal N400 Condition Differences
Time-window ROI selection of the N400 was conducted based on inspection of the aggregate grand average across all trials (see Brooks et al., 2017), a waveform computed by aggregating all trials across all subjects and conditions, which allows for visualization of overall ERP component morphology independently from condition differences in ERP components, thus reducing the risk for increased Type-I error. An a priori ROI window length (200ms) was used based on prior work defining N400 component activity as maximal between 300–500ms (cf. Kutas & Federmeier, 2011). The aggregate waveform revealed that the N400 component peak occurred at approximately 500ms, later than what is typically observed in younger adults. This is consistent with a substantial number of studies showing a later peak amplitude of the N400 in aging (see Wlotko et al., 2010 for a review). As such, the parafoveal N400 window was chosen from 400–600ms post parafoveal onset. The foveal N400 window was then computed by aligning the N400 window to foveal onset (400–600ms + 450ms SOA = 850–1050ms). The same 15 central and posterior electrode sites as in Stites et al., (2017), were selected for analyses of the N400.
We adopted a two-stage approach to analyzing condition differences on parafoveal and foveal N400 effects (see also Stites et al., 2017). First, effects of expectancy are reported in a traditional sentence-condition level analysis on the subject grand-averaged ERPs. Subject mean amplitudes for the parafoveal and foveal N400 effect were submitted to separate linear mixed-effects models using the lme4 package (Bates et al., 2010) in the R language for statistical computing. The (sum-contrast coded) fixed factor of expectancy was the critical factor of interest (with 4 levels: high constraint expected, low constraint expected, unexpected but plausible, anomalous). Random intercepts were defined for subjects and electrodes, and a random slope for the Expectancy factor was included in the models, excluding covariance terms between random intercepts and slopes (cf. Barr, 2013; Barr et al., 2013; Bates et al., 2016; Matuschek et al., 2017). Note that, because analyses are conducted on subject-mean amplitudes, as in traditional ERP experiments, these models are analogous to repeated-measures ANOVAs, but with an additional random effect for channel site (see Payne, Lee, & Federmeier, 2015), and do not require a sphericity correction because the homogeneity of effect variances is modeled specifically in the random-effects structure (see Barr et al., 2013 for more discussion).1
We then followed up on effects of the expectancy factor by specifically testing the prediction that N400 amplitudes are graded as a function of expectancy. Single-trial ERP amplitudes from the same fifteen posterior channels were submitted to an item-level linear mixed- effects model (cf. Payne et al., 2015) with single-word target cloze probability treated as an item-varying continuous predictor, using the same procedure as in Stites et al. (2017). Models were fit with random intercepts for subjects, items, and channels, and slope adjustments for the cloze predictor across subjects and channels (excluding correlations between random intercepts and slopes to reduce model complexity; Barr, 2013). There were not random slopes for cloze at the item level because each item had only one cloze value. Additionally, the covariates of word length, frequency, concreteness and imageability were also included in the model to adjust for the influence of these item-level factors on the N400. All covariates were standardized. All trials with artifacts that were excluded from the subject-mean analyses were also excluded from the linear mixed model, as well as single trials with extreme amplitudes (> +/−3 SD).
Results on the parafoveal N400 revealed a robust omnibus effect of the expectancy factor, χ2(3) = 47.85, p < .001, indicating differences in the N400 amplitude elicited across these four conditions. Table 1 shows mean amplitudes across all four conditions, which were clearly graded by contextual fit in parafoveal vision, with the largest (most negative) N400 amplitudes observed to anomalous items, followed by unexpected but plausible, low constraint, and then expected items. Supporting this, results of the single-trial analysis showed a significant effect of target word cloze probability on the parafoveal N400 (b = .36, 95% CI [.20, .52], t = 4.37; χ2(1) = 14.38, p < .001) such that more expected words showed smaller single-trial N400 amplitudes (i.e., more positive, see Figure 2). In general then, the N400 amplitude elicited by parafoveal target words showed the same graded sensitivity to cloze probability as has been documented in younger adults.
Table 1.
Mean N400 amplitudes in older adults as a function of Expectancy and Foveality
| Constraint | Parafoveal
|
Foveal
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Expected | .37 | 1.30 | 1.31 | 1.52 |
| Low Constraint | −.58 | 1.34 | .46 | 1.42 |
| Unexpected | −.80 | 1.39 | .22 | 1.88 |
| Anomalous | −1.26 | 1.34 | −.26 | 1.70 |
Figure 2.
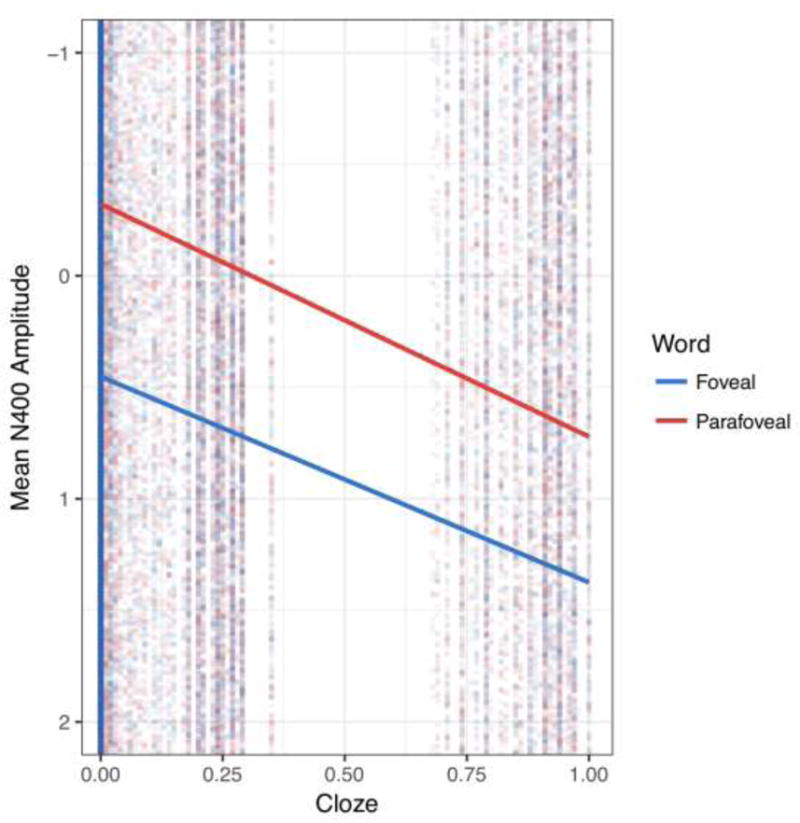
Results of individual item-level N400 amplitude by cloze probability and foveality, overlaid with the slope of the cloze effect for the parafoveal N400 (red) and foveal N400 effect (blue). Note that negative is plotted up, for ease of comparison with the ERP plots. N400 amplitudes were continuously graded with respect to cloze probability to a similar degree in both foveal and parafoveal vision.
When the target word was subsequently foveated, results again revealed a robust effect of expectancy on the subject-average ERPs, χ2(3) = 28.26, p < .001. Table 1 shows that mean amplitudes of the conditions were again clearly graded by contextual fit of the foveal word, with the largest (most negative) N400 amplitudes to anomalous items, followed by unexpected but plausible, low constraint, and then expected items. Note that there was an overall mean-shift of N400 activity, with all N400s appearing more positive in foveal vision. This reflects the overall low-frequency positive shift on the entire ERP (observed in Figure 1) that impacted all conditions similarly, and is not uncommon in examining long-average multi-word ERP epochs (e.g., Van Petten & Kutas, 1990). The N400 effects riding on top of the slow positive deflection show reliable condition differences at the foveal word. Indeed, results from the single trial analysis again showed a reliable, graded foveal cloze probability effect (b = .25, 95% CI [.04, .46], t = 2.30; χ2 (1) = 4.95, p < .01), indicating decreasing N400 amplitudes with increasing expectancy. This robust foveal N400 effect is in striking contrast to the pattern observed for young adults (Stites et al., 2017), wherein the N400 showed no reliable effect of cloze probability at foveal vision following a reliable parafoveal preview (see also Barber et al., 2010, Dimigen et al., 2012, Li et al., 2015, Kornrumpf et al. 2016 for evidence of a global reduction of the foveal N400 with valid parafoveal previews in the young). Figure 2 graphically represents the reliable effects of cloze probability on single-trial N400 amplitudes in both parafoveal and foveal vision in older adults, illustrating best fit linear functions of cloze probability on single-trial N400 amplitudes.
To more directly compare the young and old N400 effects across parafoveal and foveal vision, in the next section, we describe a supplemental analysis pooling data from the current experiment and from Stites et al., (2017).
Direct Age-Group Comparison of Foveal-Parafoveal N400
Figure 3a plots the ERPs from a representative midline parietal channel in the older sample and in the young sample from Stites et al. (2017). This figure clearly shows that the graded parafoveal N400 effect is similar in both young adults and older adults. In foveal vision, younger adults show an extinction of the graded N400 effect in foveal vision, suggesting that parafoveal viewing facilitated subsequent foveal processing. However, older adults do not show such facilitation, instead showing a similar graded N400 response in foveal vision, as described previously.
Figure 3.
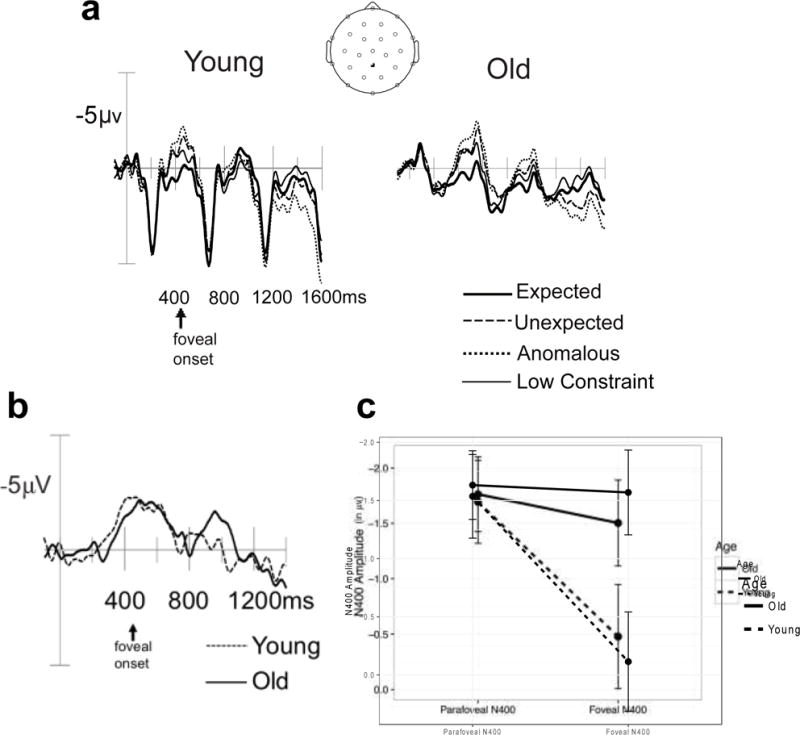
Age-group comparison of foveal and parafoveal N400 effects. a) Long grand -average ERPs at a representative midline parietal electrode as a function of target word expectancy and Age. b) Difference wave of the canonical N400 congruity violation (Anomalous vs Expected) overlaid separately for young and old. c) Age-group comparison of the mean N400 congruity violation effect in foveal and parafoveal vision. Young group data original reported in Stites et al. (2017).
To directly compare age-related changes in the parafoveal and foveal N400, we report an analysis focused on quantifying age-group differences in the magnitude of the prototypical N400 incongruity effect—that is the difference wave between expected and anomalous items— in parafoveal and foveal vision. By focusing the age difference on the N400 incongruity effect difference wave, we can more directly compare between-group differences in semantic processing while reducing concerns about overall age-group differences in biophysical properties (e.g., developmental changes in brain morphology, skull density, neural variability) that would impact the overall event-related potential. Focusing on the difference wave isolates the component of interest and reduces the complexity of the resultant statistical tests (from a 3-way to a 2-way interaction), all of which increase the power to detect reliable between-group differences (Luck, 2005). Figure 3b plots the anomalous – expected difference wave and Figure 3c graphically presents the results from the age-group comparative analysis of the N400 incongruity effect across parafoveal and foveal vision.
The mean amplitude of the N400 difference wave was measured both parafoveally and foveally in young and old adults. These mean amplitudes were submitted to an Age Group (Young, Old) × Foveality (Parafovea, Fovea) linear mixed-effects model with random slopes for the within-subjects foveality factor. Results of this analysis are presented in Figure 3c. There was a reliable Age × Foveality interaction, χ2(1) = 4.07, p < .05, such that young adults showed a reliable reduction in the magnitude of the N400 effect from parafoveal to foveal vision, t (23) = −3.02, p < .01, whereas the older adults did not show a reliable difference in the N400 effect between parafoveal and foveal vision, t (22) = −.13.
Finally, mean latency of the N400 effect was measured in both the old adults and young adults to compare age-related change in the latency of the parafoveal N400 effect. Because latency measures are nonlinear and exhibit a high degree of measurement error at the single-subject level, we adopted a jackknife approach to assessing midpoint latency of the N400 (Kiesel, Miller, Jolicoeur, & Brisson, 2008; Ulrich & Miller, 2001). The 50% fractional area latency (i.e., the time point before which 50% of the total area was observed) was measured separately for each jackknife sub-sample, within a temporal ROI from 300–600ms (the beginning of the young latency band to the end of the old latency band) and jackknife corrected t-test was conducted to compare the group differences (see Ulrich & Miller, 2001, Kiesel et al. 2008). The mean latency of the N400 incongruity effect was 413ms in the young and 493ms in the old (tcorrected = 4.29, p < .001), consistent with the common finding of age-related slowing of the N400 (cf. Wlotko et al., 2010).
Exploratory Analysis of Late Positivity
Finally, we conducted an exploratory analysis of the expectancy violation and congruity violation effects on the late positive potentials observed following the foveal N400 effect in the older adults, utilizing a mass univariate analysis (Groppe et al., 2011a, 2011b). The mass univariate approach allows for a broader statistical exploration of ERP dynamics compared to traditional approaches, but is limited by reduced power compared with a priori selection of analysis parameters (see Groppe et al., 2011a,b for an in-depth discussion and tutorial in the context of ERP data). This is particularly useful for a conservative exploratory analysis of the sustained potentials (anterior positivities/negativities) that are sometimes found to follow the N400 (Federmeier et al., 2007; Wlotko et al., 2012; Van Petten & Luka, 2012; Payne & Federmeier, 2017), as both the scalp distribution and timing of these late potentials are not well understood in aging. Mean amplitudes were measured across a 20-ms moving window separately in each context condition from 1000 ms (approximately following the offset of the foveal N400) to 1,600 ms post parafoveal onset, across all electrodes. Congruity and expectancy violation effects were then computed by conducting paired-sample t-tests between the anomalous and expected conditions and between the unexpected and expected conditions across all time bins and electrodes. We adopted directional one-tailed t-tests to specifically characterize the increased positivity following the N400 (cf. Cho & Abe, 2014). To protect against a large proportion of false-positives due to the massive number of multiple comparisons, we adopted a false discovery rate (FDR) control (see Benjamini & Hochberg, 1995). The local FDR was estimated as described in Strimmer (2008a), and positive t values that did not exceed the FDR-corrected critical threshold were considered nonsignificant. FDR control was conducted using the fdrtool package in R (Strimmer, 2008).
Figure 4 graphically represents the results from the mass univariate analysis of the time-window following the foveal N400. The figure is a raster-style heat map, plotting thresholded t-statistics for the congruity violation effect (Figure 4a) and the expectancy violation effect (Figure 4b) for each electrode and time-bin point. Values exceeding the 95% local FDR threshold are considered statistically significant. Values falling underneath the critical threshold are set equal to t = 0. As can be seen, a late positivity was observed to congruity violations, primarily over posterior electrodes, appearing between 1300–1500ms post parafoveal word onset (approximately 850–1050 post-foveal onset). A somewhat similar but much smaller effect pattern was observed for the expectancy violations. There was an interesting trend for the expectancy violation contrast to show a more anterior distribution relative to the congruity violation contrast (see Figure 1a,b as well), particularly between 1400–1500 ms (950–1150ms post foveal onset). This effect, however, may be partially driven by the expected condition, which often shows a sustained anterior negativity, in younger as well as older adults (Wlotko et al., 2012).
Figure 4.
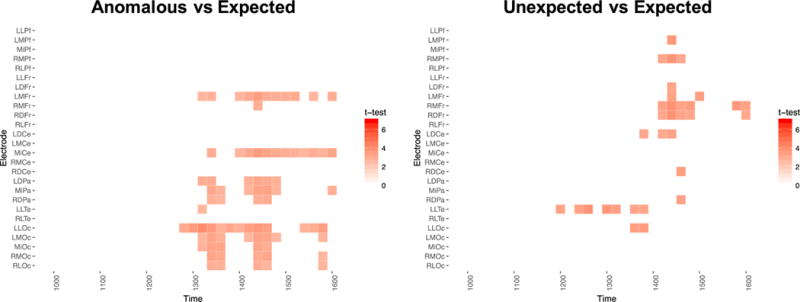
Results of the mass-univariate test of the congruity and expectancy violations in parafoveal and foveal vision in older adults. Raster-style heat-map plots the local FDR controlled one-tailed t-tests of the expectancy and congruity violation effects. Results are presented in 20-ms bins beginning around the offset of the foveal N400 and extending until the end of the epoch. Anterior electrodes are depicted in the upper portion of the figure, medial electrodes are presented in the center, and posterior electrodes are presented in the lower portions of each panel. Significant positive t-tests are plotted in red.
Discussion
The aim of the current study was to investigate the impact of contextual constraints on electrophysiological indices of parafoveal and foveal semantic processing in aging. Specifically, we tested whether older adults showed evidence for a graded sensitivity to semantic constraints in parafoveal vision, as in the young, and whether older adults could use parafoveal preview information to facilitate subsequent foveal semantic processing, as has been observed in young adults (Stites et al., 2017). First, we observed robust and graded context-related N400 modulations in parafoveal vision in older adults, suggesting that semantic access to parafoveally presented words is preserved in aging. The N400 to parafoveally presented targets was largest for words that violated the semantic congruity of the prior context, but was also large to words that were plausible but violated strong semantic expectations. Moreover, a single-trial analysis revealed a reliable graded impact of cloze probability on the N400 to parafoveally presented words, consistent with prior findings in the young. Importantly, the pattern and magnitude of this effect in the older adults was remarkably similar to what is found in young adults reading the same exact sentences (Figure 3 and Stites et al., 2017). Thus, despite age-related changes in visual acuity and parafoveal word processing (Rayner et al., 2006; Payne & Stine-Morrow, 2012), these findings suggest that, at least under some circumstances, older readers can extract high-level aspects of semantics from parafoveal vision to a similar degree as their younger counterparts.
Although parafoveal semantic processing per se was preserved in aging, age differences were found in the subsequent foveal viewing of the critical word. When the targets appeared subsequently in foveal vision (on the following triad), there was again a robust N400 effect, similar in magnitude to what had been observed when those words had first appeared in parafoveal vision. This pattern is strikingly different from what is observed in younger adults, who show facilitation in the foveal N400 effect when the word is preceded by a valid parafoveal preview (Barber et al., 2011; Stites, Payne, & Federmeier, 2017; Dimigen et al., 2012; Li et al., 2015; Kornrumpf et al. 2016). Importantly, in addition to the global reduction in the magnitude of the N400 previously reported in the literature on young adults, Stites, Payne, and Federmeier (2017) additionally showed that the graded semantic expectancy effects seen for items when they were parafoveally previewed were nearly abolished when young adults then viewed these items foveally. These findings have been taken as evidence that parafoveal semantic representations are rapidly integrated with foveal processing to facilitate subsequent semantic access. In contrast, the robust foveal N400 effect observed in older adults in the current study suggests that the semantic features of the critical word are being re-accessed and reactivated in foveal vision, despite a robust parafoveal semantic preview.
Thus, the locus of parafoveal processing difficulties in aging do not appear to occur during parafoveal perception per se, as had been previously suggested (Rayner et al., 2006, 2009, 2010; Payne & Stine-Morrow, 2012), but rather in the rapid use of parafoveal information to facilitate foveal semantic processing. Because the parafoveal preview benefit in eye-tracking at least partially conflates these stages of processing, ERPs provide a useful tool for decomposing these separate stages. One interesting implication of these findings is that the previously observed attenuated preview benefit in aging (Rayner et al., 2010; Payne & Stine-Morrow, 2012) may not be due to a reduced perceptual span but rather to a reduced efficiency in rapidly using the information derived from parafoveal vision to directly modulate foveal processing. An open question is whether the facilitation of foveal N400 effects found in the presence of a valid semantic parafoveal preview reflects a kind of repetition priming (e.g., Holcomb & Grainger, 2007) across parafoveal and foveal vision, and whether this effect reflects age-related changes in the magnitude of repetition priming effects across the visual field.
Within current models of attentional control and reading, such as the EZ-Reader model (Reichle et al., 2003), age-related changes in foveal and parafoveal processing have been modeled by assuming older adults adopt a riskier reading strategy (Rayner et al., 2006). According to this argument, older adults will adopt a strategy of relying more on parafoveal information coupled with higher-order contextual constraints to “guess” the identity of upcoming words. However, because of age-related declines in parafoveal vision, parafoveal information is incomplete and noisy, and thus less effective in aiding older adults’ ongoing processing, resulting in a higher degree of disruption to normal reading. If older adults did have more difficulty with parafoveal perception per se, we would have expected to observed a reduced influence of context on the parafoveal N400 in aging. Instead, we saw robust parafoveal N400 effects in aging, suggesting that preserved semantic access of parafoveal words in aging. Moreover, if older adults were simply using the constraining contexts to “guess” the identity of the parafoveally presented word and then using partial orthographic information from parafoveal vision to confirm or deny that guess, then we should have observed that all non-predictably target words elicited similarly strong N400 activity and not a clearly contextually graded N400 (see also Barber et al., 2011; Stites et al., 2017. Instead, we found that parafoveal semantic processing was well preserved in older adulthood, with both young and old adults showing qualitatively similar, graded sensitivity to parafoveal targets. We take these findings as arguing against this idea that older adults can’t access information effectively in the parafovea and thus must simply guess. Indeed, our finding that parafoveal processing is preserved in aging is also consistent with recent work indicating that older adults can show sensitivity to words in extreme parafoveal vision, as far out as two words to the right of the currently foveated word (Risse and Kliegl, 2011), that older adults do not always elicit such “risky” reading behavior (Choi et al., 2017), and that they show robust parafoveal preview benefits when foveal difficulty is reduced (Payne & Stine-Morrow, 2012), as would be the case in the highly constraining sentence contexts and short simple foveal pre-target words of the current study.
Drawing on theories of parafoveal-foveal processing from eye-movement studies, which posit a stage of trans-saccadic parafoveal-to-foveal integration (Rayner et al., 2009), we hypothesize that the reduced N400 in foveal vision following parafoveal processing of the same stimulus at least partially reflects this integration process, wherein a parafoveal semantic representation is activated and thus does not have to be activated again to the same degree in foveal vision (Stites, Payne, & Federmeier, 2017). Crucially, older adults do not show this priming in foveal vision, suggesting that aging impairs this process of parafoveal-foveal integration—that is, using semantic features activated to parafoveal words to facilitate semantic processing of that same word when it appears in foveal vision.
Risse and Kliegl (2011) have argued that, rather than an overall reduction in parafoveal processing, older adults instead show reductions in rapidly modulating distributed attention in response to variations in lexical properties of words in parafoveal and foveal vision. This argument is consistent with other work suggesting that age-related changes in foveal and parafoveal inhibition result in reduced flexibility to respond to changes in foveal and parafoveal vision (Laubrock et al., 2006). This idea that older adults are less flexible in modulating current foveal processing in response to parafoveal demands is somewhat consistent with this work, but the present findings isolate the stage of difficulty not to difficulties in the allocation of visual attention per se but to a difficulty in integrating parafoveal information into subsequent foveal processing, a feature which Risse and Kliegl (2011) have suggested may be preserved in aging. Thus, even if older adults are capable of dynamically modulating visual attention in a compensatory manner, such as relying more strongly on parafoveal visual representations (Rayner et al., 2013; Risse and Kliegl, 2011), this does not appear to result in a facilitation of subsequent foveal processing of these targets.
We speculate that this reduction in parafoveal-to-foveal integration may be driven in part by changes in properties of visual short-term sensory memory in normative aging. Under this account, although parafoveal information is processed without major decrements in older adults, the parafoveal visual representation is not sustained to be carried over to foveal vision in such a way as to aid in subsequent foveal processing. Aging has been associated with declines in multiple aspects of visual STM, including capacity (Brockmole & Logie, 2013), maintenance in the face of decay (Cowan et al., 2006), and feature binding (i.e., maintaining the associations between object features; Peich, Husain, & Bays, 2013), as well as in low-level aspects of maintaining temporal order in visual and auditory iconic sensory memory (Fogerty et al., 2010, 2016; Walsh & Thompson, 1978). One possibility is that age-related deficits in medial-temporal-lobe mediated associative memory functioning (Naveh-Benjamin, 2001; Mayes, Maltadi, & Migo, 2007) result in the loss of order information that binds parafoveal visual representations to subsequent foveal representations. On this view, in younger adults, parafoveal and foveal visual representations are linked across successive saccades, resulting in fluent reading. However with aging, foveal lexical processing is carried out automatically, activating a novel event representation that is not dependent upon the prior parafoveal information.
Related to the finding of reduced neural facilitation from sensory STM representations, Fabiani and colleagues (Fabiani et al., 2006; Kazmerski, Lee, Gratton, & Fabiani, 2005; Fabiani, 2012) have shown that older adults show reduced flexibility in adapting to rapidly presented trains of sensory stimuli, which they argue underpins poor attentional control in older adults. In these studies, young and older adults are instructed to ignore repeated trains of auditory stimuli while performing a secondary task. Whereas young adults are able to suppress sensory processing of these stimuli, as indicated by reduced sensory potentials, older adults do not habituate sensory processing even after trains of thousands of stimuli, processing each incoming auditory stimulus as a novel event. This lack of sensory-memory-based habituation may additionally contribute to the foveal semantic effects observed in the current study: despite robust parafoveal semantic access of the targets immediately prior to foveal viewing, the N400 does not habituate in aging, and instead is reprocessed as a novel event. Whether or not these age-related deficits in parafoveal-foveal integration during reading reflect generalized age-related deficits of top-down attentional control or visual-sensory STM remain to be seen. However, given the striking similarity of these findings and the robust effects of aging on cortical networks of top-down attentional control (see Madden, 2007 for a review), such broader attentional mechanisms should be considered in the research on aging and visual language processing. One reason why there may be a dearth of consideration of such sensory STM mechanisms in the current literature is that these iconic visual STM-dependent processes are currently not represented in most models of attention control in reading, which instead focus largely on the allocation of visual attention (see Payne et al., 2016 for a related discussion).
In our exploratory analysis of potentials following the foveal N400 effects, we also observed a sustained late positivity that varied based on the relationship of the target word to the sentential context. Prior work has implicated these late positive potentials in aspects of higher-level semantic processing, establishing the existence of additional neural activity engaged as a result of encountering semantic expectancy and congruity violations. At the same time, the mechanisms underlying these effects are not well understood. For instance, late positivites with an anterior distribution have been linked to (plausible) prediction violations (e.g., Federmeier et al., 2007; Payne & Federmeier, 2017), whereas semantic anomalies tend to elicit a posterior P600-like potential following the N400 (see Van Petten & Luka, 2012 for a review). However, these late positivites are not always observed (Van Petten & Luka, 2012), and their role in language comprehension in aging is not well understood. The fact that older adults showed a posterior late positive potential following foveal (but not parafoveal) presentation of semantically anomalous targets suggests that foveal viewing appears to be necessary to elicit such late P600-like activity and is consistent with the idea that these potentials may reflect integration processes that require central attention (cf. Batternick & Neville, 2013), whereas the N400 may reflect more automatic access to semantics (Kutas & Federmeier, 2011). Whether the late anteriorly distributed positivity to foveal prediction violations observed in older adults in the current study reflects a sensitivity to prediction violations or instead a negativity that has been observed to expected words completing strongly constraining contexts (Wlotko & Federmeier, 2012) remains an open question for future research.
One important criticism of the flanker ERP paradigm – and, indeed, all RSVP ERP studies of language – is that they are not naturalistic, and, as such, that task-related demands may result in effects that are not associated with normal processing during reading. For instance, fixed presentation rates mean that the distribution of processing time across words is different from that in natural reading and that people cannot pace the input to meet with processing needs. Here, stimulus durations were comparable with fixation times during natural reading (Rayner, 1998; 2009), but word-to-word presentation rates were slower than typical, albeit similar to the pacing that participants tend to self-select for RSVP reading (Payne & Federmeier, 2017). Indeed, this difference between the slower SOA of ERP studies and fixation durations may in part account for differences between eye-tracking and ERP studies. However, a growing eye-tracking literature using constraining sentence contexts (as in the current study) and plausibility violations have reliably found evidence for parafoveal semantic processing (Schotter et al., 2015; Veldre & Andrews, 2015), consistent with the ERP literature (Barber, 2010, 2013; Stites et al., 2017). Additionally, the lack of motor programming in the flanker ERP paradigm may impact the allocation of attention during reading. For instance, Kornrumpf and colleagues (2016) compared results in a word recognition task using both the flanker ERP paradigm and through co-registering ERPs and eye-movements. They found stronger parafoveal modulation during eye-movement control than when the eyes were fixed, and thus argued that motor programming modulates the magnitude of attentional allocation to parafoveal vision. In this sense, our findings may be overly conservative with respect to the allocation of attention to parafoveal processing, Nevertheless, other studies have found considerable overlap between eye-movement-based and flanker ERP paradigms (Payne et al., 2016; López-Peréz et al., 2016), and have shown that the flanker ERP paradigm elicits a right visual-field bias (in English), consistent with the perceptual span in reading (Simola et al., 2009; Barber et al., 2010).
Finally, one important benefit to the flanker ERP paradigm is that it provides a method for continuously tracking brain activity during (and between) parafoveal and foveal processing across long time-scales. Although there is a growing literature using electrophysiological methods to examine parafoveal processing in reading, very few of these studies have been able to measure longer-latency and/or sustained responses that may play a critical role in attentional control during reading. When FRPs are used, eye-movement activity obscures the examination of long-latency responses (see e.g., Dimigen et al., 2012). In free reading, progressive and regressive eye-movements quickly follow the onset of fixations, such that the observation of activity after ~350–450 ms post-fixation is contaminated by cortical component overlap due to subsequent sensory processing of the following saccade target, which will vary across subjects and items in a largely unpredictable manner. FRP studies have thus focused on very early activity. Because the flanker ERP paradigm utilizes a linear fixed presentation, subsequent stimulus-aligned processes are additive, and thus flanker ERPs can be examined across multiple words. Therefore, long-latency components can be examined without considerable unpredictable component overlap. The ability to examine such late slow potentials across parafoveal and foveal viewing is particularly important given that these late post-N400 potentials have played a growing role in understanding the neural basis of higher-order language processing (Van Petten & Luka, 2012).
In conclusion, information extraction and integration across parafoveal and foveal vision during reading is a complex, interactive, multi-staged, and temporally extended process, and as such, there are likely to be multiple sources of age-related changes in these processes that impact comprehension. The current study suggests that although parafoveal semantic processing per se appears to be preserved with advancing age, the rapid integration of parafoveal and foveal semantic representations is compromised with aging, resulting in older adults re-processing the semantics of foveally presented words despite intact parafoveal semantic access. Although there is a substantial behavioral literature describing basic aspects of visual processing and attention allocation in reading, one issue that has been elusive is how higher-level language processes interact with visual processing and attention mechanisms, particularly in aging. Event-related brain potentials offer a window into such parafoveal-foveal neural dynamics that are not readily observed with other behavioral or neuroimaging measures of language comprehension.
Highlights.
Age-related changes in parafoveal and foveal semantic processing were examined via event-related brain potentials in an RSVP-Flanker paradigm.
Old adults showed robust and contextually-graded parafoveal N400 effects of a similar magnitude to the young, indicating preserved parafoveal lexical processing in aging.
In the young, valid parafoveal previews facilitate subsequent foveal N400 effects, suggesting that parafoveal and foveal visual representations are rapidly integrated.
Older adults, despite showing intact parafoveal processing, do not show facilitated foveal N400 effects, indicating an age-related deficit in rapidly integrating parafoveal and foveal visual representations.
Acknowledgments
This work was supported by a James S. McDonnell Foundation Scholar Award and NIH grant AG026308 to Kara D. Federmeier. Portions of this research were presented at the Annual Meeting of the Society for the Neurobiology of Language.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In Stites et al., (2017), analyses of condition differences in the subject-average ERPs were conducted via RM-ANOVA. In the current study, linear mixed-effects models were used to analyze not only the single-trial mean amplitudes, but also the subject-average ERPs, given the benefits of the increased flexibility to accommodate a more complex random-effects variance-covariance structure (see text for details). Note however, that applying a traditional RM-ANOVA model to the current data yields nearly identical results to the linear mixed-effects models. The main effect of Expectancy was significant in both parafoveal vision (F(3, 66) = 10.82, p < .001) and foveal vision (F(3, 66) = 6.22, p < .001), consistent with the mixed-model analyses.
References
- Altarriba J, Kambe G, Pollatsek A, Rayner K. Semantic codes are not used in integrating information across eye fixations in reading: Evidence from fluent Spanish-English bilinguals. Perception & Psychophysics. 2001;63:875–890. doi: 10.3758/bf03194444. [DOI] [PubMed] [Google Scholar]
- Baccino T, Manunta Y. Eye-fixation-related potentials: Insight into parafoveal processing. Journal of Psychophysiology. 2005;19:204–215. [Google Scholar]
- Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: Expanding the useful field of view. JOSA A. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, Treiman R. The English lexicon project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Barber HA, Ben-Zvi S, Bentin S, Kutas M. Parafoveal perception during sentence reading? An ERP paradigm using rapid serial visual presentation (RSVP) with flankers. Psychophysiology. 2011;48:523–531. doi: 10.1111/j.1469-8986.2010.01082.x. http://dx.doi.org/10.1111/j.1469–8986.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber HA, Doñamayor N, Kutas M, Münte T. Parafoveal N400 effect during sentence reading. Neuroscience Letters. 2010;479:152–156. doi: 10.1016/j.neulet.2010.05.053. http://dx.doi.org/10.1016/j.neulet.2010.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber HA, van der Meij M, Kutas M. An electrophysio- logical analysis of contextual and temporal constraints on parafoveal word processing. Psychophysiology. 2013;50:48–59. doi: 10.1111/j.1469-8986.2012.01489.x. http://dx.doi.org/10.1111/j.1469–8986.2012.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DJ. Random effects structure for testing interactions in linear mixed-effects models. Frontiers in psychology. 2013;4 doi: 10.3389/fpsyg.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language. 2013;68:255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DM. lme4: Mixed-effects modeling with R 2010 [Google Scholar]
- Batterink L, Neville HJ. The human brain processes syntax in the absence of conscious awareness. Journal of Neuroscience. 2013;33:8528–8533. doi: 10.1523/JNEUROSCI.0618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B: Methodological. 1995;57:289–300. [Google Scholar]
- Brockmole JR, Logie RH. Age-related change in visual working memory: a study of participants aged 8–75. Frontiers in Psychology. 2013;4:12. doi: 10.3389/fpsyg.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JL, Zoumpoulaki A, Bowman H. Data-driven region-of-interest selection without inflating Type I error rate. Psychophysiology. 2017;54:100–113. doi: 10.1111/psyp.12682. [DOI] [PubMed] [Google Scholar]
- Choi W, Lowder MW, Ferreira F, Swaab TY, Henderson JM. Effects of word predictability and preview lexicality on eye movements during reading: A comparison between young and older adults. Psychology and Aging. 2017;32:232–242. doi: 10.1037/pag0000160. [DOI] [PubMed] [Google Scholar]
- Clifton C, Ferreira F, Henderson JM, Inhoff AW, Liversedge SP, Reichle ED, Schotter ER. Eye movements in reading and information processing: Keith Rayner’s 40year legacy. Journal of Memory and Language. 2016;86:1–19. [Google Scholar]
- Cowan N, Naveh-Benjamin M, Kilb A, Saults JS. Life-span development of visual working memory: When is feature binding difficult? Developmental Psychology. 2006;42:1089. doi: 10.1037/0012-1649.42.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter MG, Drieghe D, Liversedge SP. The Oxford Handbook of Reading. Oxford University Press; USA: 2015. How is information integrated across fixations in reading? p. 245. [Google Scholar]
- Davies M. The corpus of contemporary American English: 450 million words, 1990–2012. 2008 2011-11-12)[2012-03-03]. http://corpus.byu.edu.
- DeLong KA, Quante L, Kutas M. Predictability, plausibility, and two late ERP positivities during written sentence comprehension. Neuropsychologia. 2014;61:150–162. doi: 10.1016/j.neuropsychologia.2014.06.016. http://dx.doi.org/10.1016/j.neuropsychologia.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimigen O, Kliegl R, Sommer W. Trans-saccadic parafoveal preview benefits in fluent reading: A study with fixation-related brain potentials. NeuroImage. 2012;62:381–393. doi: 10.1016/j.neuroimage.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Dimigen O, Sommer W, Hohlfeld A, Jacobs AM, Kliegl R. Coregistration of eye movements and EEG in natural reading: analyses and review. Journal of Experimental Psychology: General. 2011;140:552. doi: 10.1037/a0023885. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. Journal of Cognitive Neuroscience. 2006;18:637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M, Schul R. Age-related and individual differences in the use of prediction during language comprehension. Brain and Language. 2010;115:149–161. doi: 10.1016/j.bandl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, McLennan DB, Ochoa E, Kutas M. The impact of semantic memory organization and sentence context information on spoken language processing by younger and older adults: An ERP study. Psychophysiology. 2002;39:133–146. doi: 10.1017/S0048577202001373. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–222. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fogerty D, Humes LE, Busey TA. Age-related declines in early sensory memory: Identification of rapid auditory and visual stimulus sequences. Frontiers in Aging Neuroscience. 2016;8 doi: 10.3389/fnagi.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology. 2011a;48:1711–1725. doi: 10.1111/j.1469-8986.2011.01273.x. http://dx.doi.org/10.1111/j.1469–8986.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Ferreira F. Effects of foveal processing difficulty on the perceptual span in reading: implications for attention and eye movement control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:417. doi: 10.1037//0278-7393.16.3.417. [DOI] [PubMed] [Google Scholar]
- Hohenstein S, Laubrock J, Kliegl R. Semantic preview benefit in eye movements during reading: A parafoveal fast-priming study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1150. doi: 10.1037/a0020233. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Grainger J. Exploring the temporal dynamics of visual word recognition in the masked repetition priming paradigm using event-related potentials. Brain Research. 2007;1180:39–58. doi: 10.1016/j.brainres.2007.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyönä J, Bertram R, Pollatsek A. Are long compound words identified serially via their constituents? Evidence from an eye movement-contingent display change study. Memory & Cognition. 2004;32:523–532. doi: 10.3758/bf03195844. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Rayner K. Parafoveal word processing during eye fixations in reading: Effects of word frequency. Perception & Psychophysics. 1986;40:431–439. doi: 10.3758/bf03208203. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Starr M, Shindler KL. Is the processing of words during eye fixations in reading strictly serial? Attention, Perception, & Psychophysics. 2000;62:1474–1484. doi: 10.3758/bf03212147. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Dunne MD. Parafoveal processing of transposed-letter words and nonwords: Evidence against parafoveal lexical activation. Journal of Experimental Psychology: Human Perception and Performance. 2012;38:191. doi: 10.1037/a0025983. [DOI] [PubMed] [Google Scholar]
- Kazmerski V, Lee Y, Gratton G, Fabiani M. Evidence for inefficient sensory filtering mechanisms in aging. Journal of Cognitive Neuroscience. 2005:89–90. [Google Scholar]
- Kliegl R, Hohenstein S, Yan M, McDonald SA. How preview space/time translates into preview cost/benefit for fixation durations during reading. The Quarterly Journal of Experimental Psychology. 2013;66:581–600. doi: 10.1080/17470218.2012.658073. [DOI] [PubMed] [Google Scholar]
- Kornrumpf B, Niefind F, Sommer W, Dimigen O. Neural correlates of word recognition: A systematic comparison of natural reading and rapid serial visual presentation. Journal of Cognitive Neuroscience. 2016;28:1374–1391. doi: 10.1162/jocn_a_00977. [DOI] [PubMed] [Google Scholar]
- Kretzschmar F, Bornkessel-Schlesewsky I, Schlesewsky M. Parafoveal versus foveal N400s dissociate spreading activation from contextual fit. NeuroReport. 2009;20:1613–1618. doi: 10.1097/WNR.0b013e328332c4f4. http://dx.doi.org/10.1097/WNR.0b013e328332c4f4. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: Finding meaning in the N400 component of the event related brain potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. http://dx.doi.org/10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Kliegl R, Engbert R. SWIFT explorations of age differences in eye movements during reading. Neuroscience & Biobehavioral Reviews. 2006;30:872–884. doi: 10.1016/j.neubiorev.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Legge GE, Ross JA, Isenberg LM, Lamay JM. Psychophysics of reading. Clinical predictors of low-vision reading speed. Investigative Ophthalmology & Visual Science. 1992;33:677–687. [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in EEG and event-related potentials I: Scalp topography. Brain Topography. 1993;6:51–63. doi: 10.1007/BF01234127. [DOI] [PubMed] [Google Scholar]
- López-Peréz PJ, Dampuré J, Hernández-Cabrera JA, Barber HA. Semantic parafoveal-on-foveal effects and preview benefits in reading: Evidence from Fixation Related Potentials. Brain and Language. 2016;162:29–34. doi: 10.1016/j.bandl.2016.07.009. [DOI] [PubMed] [Google Scholar]
- López-Peréz PJ, Dampuré J, Hernández-Cabrera JA, Barber HA. Semantic parafoveal-on-foveal effects and preview benefits in reading: Evidence from Fixation Related Potentials. Brain and Language. 2016;162:29–34. doi: 10.1016/j.bandl.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and visual attention. Current Directions in Psychological Science. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek H, Kliegl R, Vasishth S, Baayen H, Bates D. Balancing Type I error and power in linear mixed models. Journal of Memory and Language. 2017;94:305–315. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision Research. 2011;51:1610–1622. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Federmeier KD. Pace yourself: Intraindividual variability in context use revealed by self-paced event-related brain potentials. Journal of Cognitive Neuroscience. 2017;29:837–854. doi: 10.1162/jocn_a_01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stine-Morrow EA. Risk for mild cognitive impairment is associated with semantic integration deficits in sentence processing and memory. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2016;71:243–53. doi: 10.1093/geronb/gbu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stites MC, Federmeier KD. Out of the corner of my eye: Foveal semantic load modulates parafoveal processing in reading. Journal of Experimental Psychology: Human Perception and Performance. 2016;42:1839–1857. doi: 10.1037/xhp0000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stine-Morrow EA. Aging, parafoveal preview, and semantic integration in sentence processing: testing the cognitive workload of wrap-up. Psychology and Aging. 2012;27:638–649. doi: 10.1037/a0026540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Lee CL, Federmeier KD. Revisiting the incremental effects of context on word processing: Evidence from single-word event-related brain potentials. Psychophysiology. 2015;52:1456–1469. doi: 10.1111/psyp.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peich MC, Husain M, Bays PM. Age-related decline of precision and binding in visual working memory. Psychology and Aging. 2013;28:729–743. doi: 10.1037/a0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynte J, Kennedy A, Ducrot S. The influence of parafoveal typographical errors on eye movements in reading. European Journal of Cognitive Psychology. 2004;16:178–202. [Google Scholar]
- Rayner K. Eye movements and attention in reading, scene perception, and visual search. The Quarterly Journal of Experimental Psychology. 2009;62:1457–1506. doi: 10.1080/17470210902816461. [DOI] [PubMed] [Google Scholar]
- Rayner K, Schotter ER, Drieghe D. Lack of semantic parafoveal preview benefit in reading revisited. Psychonomic Bulletin & Review. 2014;21:1067–1072. doi: 10.3758/s13423-014-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risse S, Kliegl R. Adult age differences in the perceptual span during reading. Psychology and Aging. 2011;26:451. doi: 10.1037/a0021616. [DOI] [PubMed] [Google Scholar]
- Risse S, Kliegl R. Dissociating preview validity and preview difficulty in parafoveal processing of word n+ 1 during reading. Journal of Experimental Psychology: Human Perception and Performance. 2014;40:653. doi: 10.1037/a0034997. [DOI] [PubMed] [Google Scholar]
- Rugg MD. The effects of semantic priming and word repetition on event-related potentials. Psychophysiology. 1985;22:642–647. doi: 10.1111/j.1469-8986.1985.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Sass SM, Legge GE, Lee HW. Low-vision reading speed: Influences of linguistic inference and aging. Optometry & Vision Science. 2006;83:166–177. doi: 10.1097/01.opx.0000204752.43520.17. [DOI] [PubMed] [Google Scholar]
- Schotter ER, Angele B, Rayner K. Parafoveal processing in reading. Attention, Perception, & Psychophysics. 2012;74:5–35. doi: 10.3758/s13414-011-0219-2. [DOI] [PubMed] [Google Scholar]
- Sekuler B, Bennett PJ, Mamelak MA. Effects of aging on the useful field of view. Experimental aging research. 2000;26:103–120. doi: 10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK. Language in the aging brain: the network dynamics of cognitive decline and preservation. Science. 2014;346:583–587. doi: 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- Simola J, Holmqvist K, Lindgren M. Right visual field advantage in parafoveal processing: Evidence from eye-fixation-related potentials. Brain and Language. 2009;111:101–113. doi: 10.1016/j.bandl.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Snell J, Vitu F, Grainger J. Integration of parafoveal orthographic information during foveal word reading: beyond the sub-lexical level? The Quarterly Journal of Experimental Psychology. 2017;70:1984–1996. doi: 10.1080/17470218.2016.1217247. [DOI] [PubMed] [Google Scholar]
- Stine-Morrow EAL, Payne BR. Age differences in language segmentation. Experimental Aging Research. 2016;42:107–125. doi: 10.1080/0361073X.2016.1108751. [DOI] [PubMed] [Google Scholar]
- Stites MC, Payne BR, Federmeier KD. Getting ahead of yourself: Parafoveal word expectancy modulates the N400 during sentence reading. Cognitive, Affective, & Behavioral Neuroscience. 2017;17:475–490. doi: 10.3758/s13415-016-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology. 1999;14:167–177. [PubMed] [Google Scholar]
- Van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Memory & Cognition. 1990;18:380–393. doi: 10.3758/bf03197127. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ. Prediction during language comprehension: Benefits, costs, and ERP components. International Journal of Psychophysiology. 2012;83:176–190. doi: 10.1016/j.ijpsycho.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Vasilev MR, Angele B. Parafoveal preview effects from word N+ 1 and word N+ 2 during reading: A critical review and Bayesian meta-analysis. Psychonomic Bulletin & Review. 2016:1–24. doi: 10.3758/s13423-016-1147-x. [DOI] [PubMed] [Google Scholar]
- Vasilev MR, Angele B. Parafoveal preview effects from word N+ 1 and word N+ 2 during reading: A critical review and Bayesian meta-analysis. Psychonomic Bulletin & Review. 2017;24:666–689. doi: 10.3758/s13423-016-1147-x. [DOI] [PubMed] [Google Scholar]
- Veldre A, Andrews S. Parafoveal lexical activation depends on skilled reading proficiency. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41(2):586. doi: 10.1037/xlm0000039. [DOI] [PubMed] [Google Scholar]
- Veldre A, Andrews S. Parafoveal lexical activation depends on skilled reading proficiency. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41:586–595. doi: 10.1037/xlm0000039. http://dx.doi.org/10.1037/xlm0000039. [DOI] [PubMed] [Google Scholar]
- Waldron-Perrine B, Axelrod BN. Determining an appropriate cutting score for indication of impairment on the Montreal Cognitive Assessment. International journal of geriatric psychiatry. 2012;27(11):1189–1194. doi: 10.1002/gps.3768. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Thompson LW. Age differences in visual sensory memory. Journal of Gerontology. 1978;33:383–387. doi: 10.1093/geronj/33.3.383. [DOI] [PubMed] [Google Scholar]
- Wilson M. MRC psycholinguistic database: Machine-usable dictionary, version 2.00. Behavior Research Methods. 1988;20:6–10. [Google Scholar]
- Wlotko EW, Federmeier KD. So that’s what you meant! Event-related potentials reveal multiple aspects of context use during construction of message-level meaning. NeuroImage. 2012;62:356–366. doi: 10.1016/j.neuroimage.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotko EW, Lee CL, Federmeier KD. Language of the aging brain: Event-related potential studies of comprehension in older adults. Language and Linguistics Compass. 2010;4:623–638. doi: 10.1111/j.1749-818X.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:121. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Yan M, Richter EM, Shu H, Kliegl R. Readers of Chinese extract semantic information from parafoveal words. Psychonomic Bulletin & Review. 2009;16:561–566. doi: 10.3758/PBR.16.3.561. [DOI] [PubMed] [Google Scholar]
- Zhang W, Li N, Wang X, Wang S. Integration of sentence-level semantic information in parafovea: Evidence from the RSVP-flanker paradigm. PloS One. 2015;10:e0139016. doi: 10.1371/journal.pone.0139016. [DOI] [PMC free article] [PubMed] [Google Scholar]


