Abstract
Background
Major depressive disorder is a leading cause of disability worldwide; however, little is known about pathological mechanisms involved in its development. Research in adolescent depression has focused on reward sensitivity and striatal mechanisms implementing it. The contribution of loss sensitivity to future depression, as well as the orbitofrontal cortex (OFC) mechanisms critical for processing losses and rewards, remain unexplored. Furthermore, it is unclear whether OFC functioning interacts with familial history in predicting future depression.
Methods
In this longitudinal study we recorded functional magnetic resonance imaging (fMRI) data while 229 adolescent females with or without parental history of depression completed a monetary gambling task. We examined if OFC blood-oxygen-level-dependent (BOLD) response and functional connectivity during loss and win feedback was associated with depression symptoms concurrently and prospectively (9 months later), and whether this relationship was moderated by parental history of depression.
Results
Reduced OFC response during loss was associated with higher depression symptoms concurrently and prospectively, even after controlling for concurrent depression, specifically in adolescents with parental history of depression. Similarly, increased OFC-posterior insula connectivity during loss was associated with future depression symptoms but this relationship was not moderated by parental history of depression.
Conclusions
This study provides the first evidence for loss-related alterations in OFC functioning and its interaction with familial history of depression as possible mechanisms in the development of depression. While the current fMRI literature has mainly focused on reward, the present findings underscore the need to include prefrontal loss processing in existing developmental models of depression.
Keywords: depression, orbitofrontal, fMRI, loss, adolescence, parental history
Introduction
Major depressive disorder (MDD) is among the leading causes of disability worldwide. However, knowledge of pathological neural mechanisms involved in the development of MDD is limited, frustrating efforts aimed at early detection and intervention. A promising pathophysiological mechanism in depression is that of sensitivity to losses and rewards, with some studies showing either reduced reactivity to rewards (1), or increased reactivity to losses (2, 3), or reduced reactivity to rewards and losses (4). Importantly, studies show that these alterations in reward reactivity are evident in children and adolescents at high risk for developing depression (5, 6) and prospectively predict worsening of depression symptoms (7–9). However, research on neural correlates of loss or reward sensitivity in adolescent depression is limited in that it has focused almost exclusively on reward sensitivity as well as striatal mechanisms that support it (1, 5). In contrast, the role of loss sensitivity and its contribution to future depression remains poorly understood (10, 11). This is a critical gap in the literature because loss sensitivity varies across development (11), contributes significantly to decision-making, perhaps more strongly than reward sensitivity (12), and is strongly linked to anxiety and mood disorders (5, 13, 14).
As part of a distributed network critical for valence processing and valuation (15, 16), the orbitofrontal cortex (OFC) plays an important role in processing salience and magnitude of losses as well as rewards (17–21). OFC represents the complete dimension of subjective valence ranging from unpleasant to pleasant (22, 23). Due to strong anatomical connectivity with sensory, striatal, limbic and insular regions, OFC plays a critical role in integrating loss- and reward-related information (19, 24). Furthermore, adolescence might be a developmentally sensitive period for studying OFC (25) and its relationship with future depression. Adults with history of or risk for depression show attenuated OFC activity for both rewards (7, 26, 27) and negative stimuli including losses (28, 29) (for review see (13)). Relatively less is known about functional and effective connectivity of OFC in adult depression, with studies showing greater connectivity of OFC with insula (30), amygdala (31), and a distributed network including prefrontal and cingulate cortices (32), and this enhanced connectivity is associated with more severe depression symptoms (30, 32). Despite prefrontal maturation during adolescence (33), adolescents and adults with and at risk for MDD show a similar pattern of prefrontal resting-state connectivity (34, 35). However, it is unclear whether attenuated OFC activity and/or enhanced OFC connectivity during rewards and losses represents a premorbid risk that precedes the onset of symptoms or is the result of depression onset or treatment exposure, indicating a need for longitudinal research on OFC functioning prior to depression onset.
Finally, depression is etiologically heterogeneous, with evidence suggesting differences in etiopathophysiological processes between more and less familial/genetic forms (36). Familial history of depression is one of the most important risk factors for depression and it is associated with altered reward sensitivity in adolescents (5, 6, 37). However, few studies have explored whether abnormalities in OFC functioning interacts with vulnerability imposed by familial history of depression to predict future depression. In the present longitudinal study, we used functional magnetic neuroimaging (fMRI) and a monetary gambling task to examine OFC blood-oxygen-level-dependent (BOLD) response, an indirect measure of neural activity (38), and OFC connectivity during loss and win feedback in a large cohort of adolescent females who were free of lifetime depressive disorders and who were assessed for depression again 9 months later. We hypothesized that 1) attenuated OFC activity and enhanced OFC functional connectivity with striatal, limbic and insular regions for losses and wins will be correlated with greater depression symptoms concurrently, and will predict depression symptoms 9 months later, even after controlling for concurrent depression, 2) parental history of depression will moderate the relationship between OFC activity and connectivity with future depression symptoms such that the association is stronger for groups with high versus low risk for depression, and 3) measures of OFC activity and connectivity will independently predict depression symptoms in both cross-sectional and prospective analyses.
Methods and Materials
Participants
261 adolescent females (mean age = 15 years, 3 months, SD = 7 months old) and their parents from the larger Adolescent Development of Emotions and Personality Traits (ADEPT) project participated in the present study. The ADEPT cohort consisted of adolescent females during a developmental stage marked by sharp increase in depression onset (39–41) (Supplement). Participants and their parents gave informed assent and consent respectively, and all families were financially compensated for participation. After the baseline assessment (wave1), participants were invited for follow-up clinical assessments every 9 months and fMRI at first follow-up (wave 2). See Supplement for details. Since we aimed to examine predictors of depression, participants with a lifetime history of MDD or dysthymia were excluded during initial screening. After exclusion of 32 participants due to data quality issues a final set of 229 subjects: 49 high-risk (HR) and 180 low-risk (LR), based on whether their participating parent met criteria for a lifetime history (Parental-History) of either MDD or dysthymia (Supplement). Additional analyses were conducted defining HR group based on family history reports of depression in non-participating parent (Supplement).
Clinical Measures
At baseline (wave1), lifetime history of major depressive or dysthymic disorder was determined based on Structured Clinical Interview for DSM-IV (SCID) (42) conducted with the participating biological parent (Supplement). Mood and anxiety symptoms in adolescents were measured using a new-generation factor-analytically derived Inventory of Depressive and Anxiety Symptoms (IDAS-II) (43). Relationship of OFC activity was examined with IDAS-II dysphoria scores at wave 2 (Dysw2) and wave 3 (Dysw3) because this scale captures core symptoms of depression (9, 43) as well as other IDAS-II anxiety and depression-related scales (Supplement).
Experiment Paradigm: the Doors task
Participants performed a modified version of a previously utilized forced choice monetary gambling task (44) to probe neural response to loss- and reward-related monetary feedback (Figure 1A). Participants chose between two doors presented simultaneously and received feedback of loss or win with 50/50 probability (Supplement). The losses were purposefully set at half as large as wins due to prior data suggesting a 2 times loss aversion ratio (45). Choice and RT was recorded. Although the outcome of each trial was predetermined and independent of performance, participants were informed that they could gain between $0 and $10 depending on success rate. All participants were paid $6 after the scan.
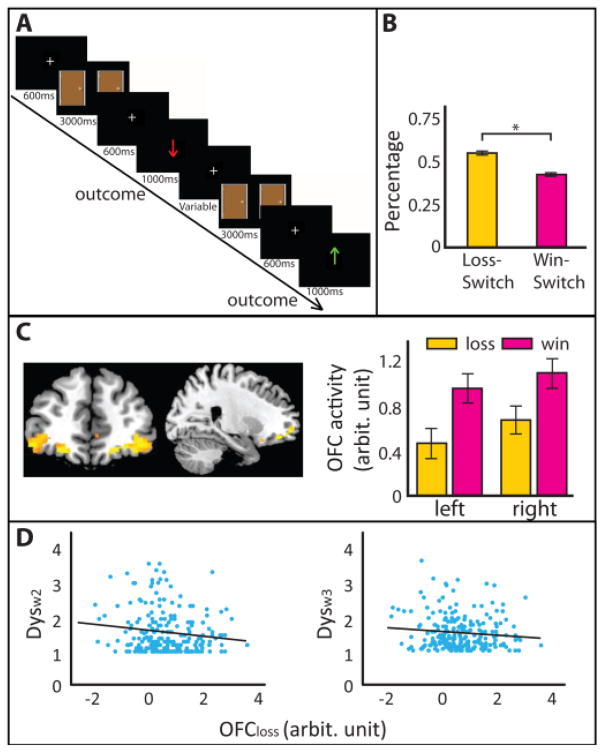
Figure 1. Monetary gambling task and OFC activity.
A. Experimental paradigm. Participants performed a gambling task in which each trial began with the presentation of two identical doors, on the left and right side of the screen simultaneously. Participants were asked to choose between the two doors by clicking one of two buttons corresponding to the left or right door on an MRI-compatible button box. After a short delay, a predetermined feedback of either loss (red downward arrow indicating −25¢) or win (green upward arrow indicating +50¢) was revealed B. Behavioral results. The percentage of Loss-Switch was significantly higher than that of Win-Switch. Error bars represent standard error of the mean, and * indicates, p < .001. C. OFC activity to losses and wins. Increased left OFC (peak coordinates: x = −22, y = 46, z = −14) and right OFC (peak coordinates: x = 24, y = 44, z = −14) activity was seen for loss and win conditions across all participants (p < .05, FWE corrected). The bar graphs show averaged contrast estimates for loss and win separately for left and right OFC. Error bars represent 95% confidence interval. D. Correlation between OFC activity to loss and depression symptoms. Decreased loss-related OFC activity (OFCloss) is associated with increased concurrent depression symptoms (Dysw2) in the left panel and increased depression symptoms 9 months later (Dysw3) in the right panel.
Behavioral data analyses
For each subject, we computed the percentage of times subjects switched the choice of door on the next trial (n+1) based on whether the door in the current trial (n) yielded a loss (Loss-Switch) or win feedback (Win-Switch). We compared Win-Switch and Loss-Switch using a paired sample t-test. We also examined choices across time (Supplement).
fMRI data acquisition and preprocessing
Whole brain functional and structural MRI images were acquired using a gradient echo T2*-weighted echoplanar (EPI) on a Siemens Trio 3T MRI whole body scanner. All functional images were spatially realigned, motion-corrected, normalized to standardized space, and spatially smoothed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/10spm/software/spm8/) software. See supplement for details regarding image parameters and preprocessing.
fMRI data analyses
Subject-level model
A general linear model (GLM) was estimated by convolving blood oxygenation level-dependent (BOLD) hemodynamic response function (HRF) with stick (delta) function aligned to the onset of feedback for the loss and win conditions (see Supplement for details). Contrast maps based on the subject-level per-voxel parameter estimate (β) maps representing the magnitude of BOLD response associated with each condition were subjected to group-level t-tests to obtain group-level per-voxel contrast maps of loss or win larger than baseline.
ROI analysis
We first identified OFC voxels that are sensitive to loss or win by examining results of the group-level t-test for loss- or win-related activity within an anatomically defined OFC mask (See Supplement for details) at the threshold of p < .05, family wise error (FWE) corrected. We confirmed that the OFC cluster also survived a more stringent whole-brain threshold of p < .05, FWE corrected (See Supplement for whole-brain results). The maximum probability atlas of Desai DD and Desai DKD maps in FreeSurfer (46, 47) was used to check the probability of each resultant cluster as belonging to OFC. For significant clusters with a high probability of belonging to OFC, we separately averaged across voxels in each cluster for loss- (OFCloss) and win- (OFCwin) related response to obtain a mean contrast value as the unbiased summary statistic that was used for examining the association with clinical measures in SPSS (SPSS Inc., Chicago, 17 Illinois, USA). Similar ROI analyses were conducted in striatum and a control region that correlated with concurrent depression (Supplement).
Functional connectivity analyses
Functional connectivity during loss and win conditions was examined using a generalized form of psychophysiological interaction (gPPI) analysis implemented in SPM8 package (48–50) (see Supplement for details). The right OFC seed region for connectivity analyses was identified functionally as the region that was most responsive to the task and depression symptoms above. PPI analysis generated the per-voxel parameter estimate (β) maps representing the magnitude of functional connectivity between the OFC seed region and voxel-wise activation in the brain as a function of loss and win conditions for each subject. These condition-related β maps were compared at the group-level using t-tests at a threshold of p < .05, whole-brain FWE corrected (voxel-wise) for multiple comparisons. We then extracted the averaged contrast values for connectivity of OFC during loss and win which were used for correlation and regression with self-report measures in SPSS. The significance of these correlations was determined if the r value survived Bonferroni correction for multiple clusters (α = .05/number of clusters).
Association of OFC activity and connectivity with depression symptoms
We examined our a priori hypothesis regarding the relationship between task-related OFC activity and connectivity with self-report symptom measures across all participants. First, OFCloss and OFCwin activity or connectivity summary measures derived from significant clusters as described above were correlated with participants’ self-reported depression symptoms from the wave concurrent with fMRI data acquisition (Dysw2), as well as the wave 9 months later (Dysw3). Next, we examined whether the relationship of OFCloss, OFCwin, or OFC connectivity with Dysw3 was moderated by parental history of depression (Parental-History), even after controlling for Dysw2. Accordingly, a hierarchical regression was conducted to predict Dysw3 using OFC activity or connectivity, Parental-History, participant’s age at the scan (Age), and Dysw2 at step one and the interaction between OFC response or connectivity and Parental-History at step two. After establishing the significant interaction effect, we conducted two regression analyses for the HR and LR groups separately, using OFC response to predict Dysw3 controlling for Age and Dysw2. Finally, because loss/punishment and reward share commonalities in terms of underlying psychological and neural mechanisms (51–53), we also examined whether OFCloss or OFC connectivity during loss uniquely predicted future depression even when controlling for OFCwin. Hence, another hierarchical regression analysis was conducted predicting Dysw3 using OFCloss, OFCwin, Parental-History, Dysw2, and Age at step one, and OFCloss × Parental-History as well as OFCwin × Parental-History at step two. We also examined relationship of OFC response with other IDAS-II depression and anxiety measures (Supplement).
Cross-validation tests
To test the robustness of the association between OFC response with Dysw3 for the HR group, we conducted a 1000 times 3-fold-cross-validation (3-fold-CV) analysis on the participants in the HR group. We first obtained the residuals of Dysw3 (resDysw3) regressing out Dysw2 so that later cross-validation results were not driven by high correlation between Dysw2 and Dysw3. See Supplement for details of the CV analysis.
Results
Loss feedback influences behavior differently than win feedback
There was a significantly higher percentage of switching choices following loss (Loss-Switch, M = 55.48%, SD = 18.02%) than win feedback (Win-Switch, M = 42.99%, SD = 18.43%) (t228 = 6.85, p < .001, Figure 1B). Hence, participants avoided choosing the same door that yielded loss feedback, an effect that grew stronger over the course (second half versus first half) of the experiment (Supplement). We did not see a difference in Loss-Switch (t227 = −.114, p = .909) and Win-Switch (t227 = −1.013, p = .191) between HR and LR groups.
OFC reactivity to loss and win feedback
Voxel-wise t-tests across all participants showed significant bilateral OFC activation to loss (OFCloss) and win (OFCwin) feedback (p < .05, FWE-corrected) (Figure 1C). Overall activity in this region showed no difference between HR and LR for OFCloss (mean difference = .03, t227 = .176, p = .861 for left OFC; mean difference = .05, t227 = .308, p = .759 for right OFC), or for OFCwin (mean difference = .097, t227 = .670, p = .505 for left OFC; mean difference = .14, t227 = 1.08, p = .284 for right OFC). See supplement for whole-brain task activation results.
OFC reactivity correlates with concurrent and future depression symptoms
In the full sample, reduced right OFCloss activity was associated with higher Dysw2 (r = −.14, p = .041) and Dysw3 (r = −.14, p = .031) symptoms (Figure 1D; Table 1). In contrast, OFCwin was not significantly correlated with either Dysw2 or Dysw3 symptoms (Table 1).
Table 1.
Correlations between OFC response to loss and depression measures
| Group | Correlation with Dysw2 | Correlation with Dysw3 | ||
|---|---|---|---|---|
|
| ||||
| Loss | Win | Loss | Win | |
| Full Sample (right) | −.14* | −.06 | −.14* | −.06 |
| Full Sample (left) | −.06 | .02 | −.09 | −.01 |
|
| ||||
| Right | ||||
| HR | −.26 | .24 | −.44* | −.22 |
| LR | −.10 | .03 | −.05 | −.03 |
| Left | ||||
| HR | −.01 | .13 | −.22 | −.15 |
| LR | −.07 | .06 | −.05 | −.02 |
Note. Dysw3 = IDAS-II dysphoria on wave3.
= p < .05.
HR = high risk group; LR = low risk group.
OFC reactivity to loss predicts future depression symptoms as a function of parental history of depression
Hierarchical regression revealed a significant OFCloss x Parental-History interaction (Table 2), indicating that reduced OFCloss predicted increased Dysw3, even after controlling for Age and Dysw2, a relationship that was stronger for HR than for LR adolescents (Figure 2A). Next, two regression analyses controlling for Age and Dysw2 for HR and LR separately confirmed a significant effect of OFCloss for the HR (β = −.275, p = .010) but not for LR (β = −.031, p = .635) group. Furthermore, the OFCloss × Parental-History interaction predicted Dysw3 even after controlling for OFCwin (Table 3). These results were confirmed with the HR group created using participating parents’ SCID and family history reports of depression in the non-participating parents (Supplement). Similar findings were not observed for striatum and a control region as well as other IDAS measures except for lassitude (Supplement).
Table 2.
OFC response to loss predicting future depression
| Predictors | R2 | F | β | p |
|---|---|---|---|---|
| Model at step one | ||||
|
| ||||
| Overall Model | .356 | 30.815 | --- | < .001 |
| OFCloss | --- | 2.868 | −.093 | .092 |
| Parental-History | --- | 1.975 | .077 | .161 |
| Age | --- | 2.721 | .091 | .101 |
| Dysw2 | --- | 109.940 | .577 | < .001 |
|
| ||||
| Model at step two | R2 change = .012, p = .041 | |||
|
| ||||
| Overall Model | .365 | 25.857 | --- | < .001 |
| OFCloss | --- | 6.226 | −.157 | .013 |
| Parental-History | --- | 1.929 | .076 | .166 |
| Age | --- | 2.606 | .088 | .108 |
| Dysw2 | --- | 108.151 | .570 | < .001 |
| OFCloss x Parental-History | --- | 4.222 | −.128 | .041 |
Note. OFCloss = OFC response to loss; Parental-History = Parental history of depression; and Dysw2 = IDAS-II dysphoria on wave2
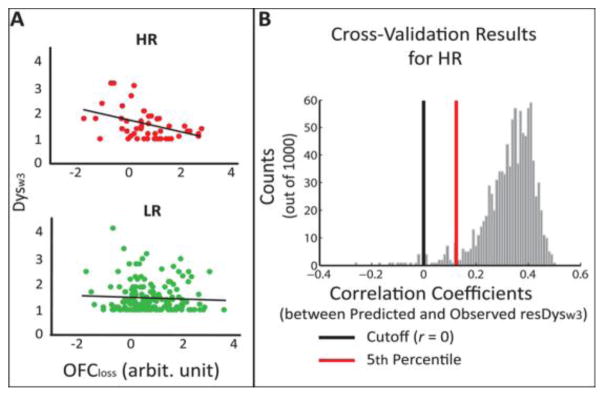
Figure 2. OFC activity to loss interacts with parental history of depression to predict future depression symptoms.
A. Parental history of depression moderates the relationship between OFC activity to loss and future depression symptoms. The left panel shows a significant correlation between OFC activity to loss (OFCloss) and depression symptoms 9 months later (Dysw3) for HR group; whereas, the right panel shows no correlation between the 24 two for LR group. B. Cross-validation analysis of OFC activity to loss predicting future depression for HR group. Distribution of the Pearson’s correlation coefficients between predicted and observed resDysw3 obtained from the 1000 times 3-fold-cross-validation analysis is shown. The distribution shows that > 95% (the red line marks the 5th percentile) of the correlations are > 0 (indicated by the black line).
Table 3.
OFC response to loss and win predicting future depression
| Predictors | R2 | F | β | p |
|---|---|---|---|---|
| Model at step one | ||||
|
| ||||
| Overall Model | .354 | 18.681 | --- | < .001 |
| OFCloss | --- | 6.446 | −.130 | .084 |
| OFCwin | --- | 1.342 | .054 | .473 |
| Parental-History | --- | 1.170 | .073 | .184 |
| Age | --- | 2.858 | .095 | .087 |
| Dysw2 | --- | 108.707 | .575 | < .001 |
|
| ||||
| Model at step two | R2 change = .016, p = .072 | |||
|
| ||||
| Overall Model | .364 | 18.681 | --- | < .001 |
| OFCloss | --- | 6.446 | −.214 | .012 |
| OFCwin | --- | 1.342 | .132 | .248 |
| Parental-History | --- | 1.170 | .060 | .281 |
| Age | --- | 2.858 | .093 | .092 |
| Dysw2 | --- | 108.707 | .573 | < .001 |
| OFCloss x Parental-History | --- | 5.202 | −.191 | .024 |
| OFCwin x Parental-History | --- | 1.508 | −.139 | .221 |
Note. OFCloss = OFC response to loss; OFCwin = OFC response to win; Parental-History = Parental history of depression; and Dysw2 = IDAS-II dysphoria on wave2
Cross-validation test of OFC predicting future depression symptoms
The predictive power of OFCloss for the HR group was supported with a stringent 1000 times 3-fold-CV test that showed a moderate correlation (mean r = .33) between the estimated and the observed Dysw3 scores for the HR group such that 99.6% of the r values generated over 1000 iterations were above zero. (Figure 2B). Similar results were seen with a 5-fold version of the analysis (Supplement).
Functional connectivity between OFC and insula during loss predicts future depression symptoms
Voxel-wise t-tests across all participants for loss or win conditions showed significant OFC connectivity with bilateral caudate and insula (p < .05, whole-brain FWE-corrected; Figure S4, Table 4). Only stronger OFC-posterior insula connectivity for loss was marginally associated with increased Dysw2 (r = .12, p = .083) and significantly associated with increased Dysw3 (r = .20, p = .003) (Table 4). Hierarchical regression showed a significant main effect of OFC-posterior insula connectivity for loss (β = .13, p = .020) but no main effect of Parental-History (β = .07, p = .206) or interaction between OFC-posterior insula connectivity for loss and Parental-History (β = −.08, p = .210) in predicting Dysw3 controlling Age and Dysw2. Furthermore, OFC-posterior insula connectivity for loss remained significant (β = .128, p = .022) even when controlling for win connectivity (β = .055, p = .319), demonstrating the unique contribution of OFC-posterior insula connectivity for loss to Dysw3.
Table 4.
Correlation between loss-related OFC functional connectivity with depression measures
| Region | Peak (x, y, z) | Correlation with Dysw3 | |
|---|---|---|---|
|
| |||
| Loss | Win | ||
| Caudate (right) | 12, −4, 16 | .06 | .01 |
| Caudate (left) | −12, −6, 16 | .00 | −.02 |
| Insula (right) | 30, 24, −2 | .10 | .08 |
| Insula (right) | 36, −8, 12 | .20* | .08 |
| Insula (left) | −34, −10, 8 | −.00 | −.01 |
Note. Dysw3 = IDAS-II dysphoria on wave3.
= p < .05, Bonferroni corrected for five clusters (α = .05/5)
A hierarchical regression model revealed that adding OFC-posterior insula connectivity for loss enhanced predictive power over and above OFCloss, Parental- History, Age, Dysw2, OFCloss x Parental-History in predicting Dysw3 (ΔR2 = .02, p = .009), with a significant effect of OFC-posterior insula connectivity for loss (β = .143, p = .009), while both the OFCloss (β = −.167, p = .008) and OFCloss x Parental-History (β = − .145, p = .020) remain significant. This result indicates that OFC activity and connectivity for loss account for unique variance in Dysw3.
Discussion
The role of OFC in the representation of primary and abstract reinforcers as well as adaptive decision-making is well-established (18, 19, 23, 24); however, its role in reward or loss related decision making in adolescent depression remains unknown. The present study provides the first evidence that alterations in OFC activity predict future depression symptoms in never-depressed adolescent females who are at high familial risk for developing depression, indicating that aberrant OFC activity and connectivity may represent a vulnerability factor for depression. Due to OFC’s role in the representation and integration of motivationally salient information (22, 23), flexible value-based computation (54–59) and adaptive decision-making (60–62), the present findings have the potential to clarify how complex representations of loss and reward, as well as their integration, contribute to the development of future depression.
Our finding showing that decreased OFC response to loss in adolescents is associated with increased depression symptoms is consistent with findings in adult depression showing that reduced superior frontal gyrus activity in response to loss is associated with increased depressive rumination symptoms (28). Reduced frontal activity in response to loss in depression has been interpreted as indicative of increased sensitivity to loss (28). However, the relationship between the direction of OFC activity and the direction of loss sensitivity can be studied only by manipulating levels or magnitude of loss, which few studies have done. In one study, OFC as well as other regions involved in loss or reward processing showed parametrically increasing activity with increasing gains and parametrically decreasing activity with increasing losses with a steeper negative slope for increasing losses compared to corresponding positive slope for increasing gains, consistent with the pattern of loss aversion (63). Another study found this pattern for the medial parts of the OFC but not for lateral OFC (18). Since greater sensitivity to loss has been linked to depressive symptoms (64), reductions in OFC activity that predict future depression in our study may be indicative of greater sensitivity to loss; however, further studies that parametrically modulate the magnitude of losses and examine its relationship with severity of depression symptoms are needed.
The role OFC plays in integrating information regarding negative and positive motivational values of stimuli and subsequent decision-making is attributed to its strong anatomical connectivity with higher sensory cortices of all modalities, and reciprocal connections with areas such as the amygdala, hippocampus and striatum (24, 65). In the present study we found that increased functional connectivity of OFC with posterior insula during loss was associated with concurrent and future depression, even when controlling for concurrent depression. Unlike OFC activity, the relationship between OFC connectivity and future depression was not moderated by a parental history of depression, indicating it may be a vulnerability factor independent of familial history of depression. Evidence suggests posterior insula receives sensory information across modalities (66), and supports the primary cortical representation of interoceptive signals (67), playing an important role in emotional awareness (68). In line with this role of posterior insula, the functional connectivity of OFC and posterior insula in response to loss may be indicative of integration of the value of loss with its emotional and interoceptive awareness, with a stronger integration predicting more severe future depression symptoms.
While the OFC was responsive to both loss and win in the present study, it is interesting that OFC activity and connectivity changes in response to loss, but not win, predicted concurrent and future depression symptoms. Exploring the role of both loss and reward sensitivity is critical to understanding how adolescents make choices to engage in or avoid real-life situations, why these choices may differ across development, and how they contribute to the development of internalizing and externalizing disorders. Economic models of decision-making have suggested that losses actually ‘loom larger’ than gains for most individuals, including adolescents (10), i.e. the aversiveness of a potential loss is greater than the desirability of an equal potential gain, referred to as loss aversion. Keeping in mind this difference, the loss magnitude in our task was half the reward magnitude in an attempt to make the subjective magnitude of the two conditions comparable. Despite this, we found that, behaviorally, participants show very different patterns of responding following win and loss feedback. Neurally, decreased OFC response to loss uniquely predicted future depression, over and above response to wins, which is consistent with previous finding in other regions (69), indicating the importance of this measure as a vulnerability factor in the development of depression. Overall, the stronger relationship between OFC and future depression symptoms may be because losses (compared to wins) have a significantly greater effect on decision-making which may contribute to future depression symptoms in adolescents.
Our study is limited because history of depression was thoroughly assessed only in the participating parent. To address this limitation, we confirmed that our results remained the same when the risk groups were defined based on a history of depression in participating and non-participating parents (see Supplement). Additionally, a follow-up duration longer than 9 months may be helpful in studying adolescents meeting criteria for depression diagnosis. While our study highlights how neural reactivity to reward or loss feedback predicts depression, future studies using more complex paradigms are needed to pinpoint which specific aspects of motivational processing are impaired. These include tasks examining neural reactivity during anticipation as well as changing magnitude of rewards and losses. Additionally, tasks involving more complex contingency learning and choice behavioral models (70–72), particularly with more trials would allow researchers to tease apart trial-by-trial effects (72).
Adolescence is a high-risk time for onset of depression, and this period also coincides with active development of OFC (73). Research shows that a relatively delayed developmental trajectory of OFC compared to striatum may underlie aberrant decision-making in adolescents (25). Additionally, compared to adults, adolescents, in general, show an increased response in subcortical regions to emotionally salient stimuli, paired with a decreased response in frontal regions when cognitive control or regulation is required (74). Furthermore, keeping in mind the complexity and abstractness of reinforcers that adolescents experience, like praise, peer acceptance and peer rejection, the OFC is an ideal candidate for investigation of loss and reward sensitivity in adolescent depression. Our study provides initial evidence for decreased OFC activity and increased OFC-insula connectivity during loss processing as possible mechanisms that are involved in the development of future depression. Further, decreased OFC activity interacts with risk due to parental history of depression to predict future depression symptoms. In a reward literature that has focused largely on subcortical mechanisms, the present findings underscore the need to include the role of prefrontal loss processing in existing developmental models of depression. The present findings also highlight the need for studies on the developmental progression of loss processing as well as its different components and their contribution to decision-making in real life situations and ultimately to the development of depressive disorders.
Supplementary Material
Acknowledgments
This study was supported by a National Institute of Mental Health Program Project R01 MH093479. We thank Molly Gromatsky (ADEPT coordinator) and Melissa Carr (fMRI coordinator), and the families who participated.
Footnotes
FINANCIAL DISCLOSURE
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 3.Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 5.Luking KR, Pagliaccio D, Luby JL, Barch DM. Reward Processing and Risk for Depression Across Development. Trends Cogn Sci. 2016 doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry. 2015;172:1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards prospectively predicts the development of depression in adolescent girls. The American Journal of Psychiatry in press. [Google Scholar]
- 10.Barkley-Levenson EE, Van Leijenhorst L, Galvan A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev Cogn Neurosci. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luking KR, Pagliaccio D, Luby JL, Barch DM. Do losses loom larger for children than adults? Emotion. 2016;16:338–348. doi: 10.1037/emo0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica: Journal of the Econometric Society. 1979:263–291. [Google Scholar]
- 13.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KA, Farris SG, Schmidt NB, Smits JA, Zvolensky MJ. Panic attack history and anxiety sensitivity in relation to cognitive-based smoking processes among treatment-seeking daily smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013;15:1–10. doi: 10.1093/ntr/ntr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 19.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 20.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 21.Kahnt T, Park SQ, Haynes JD, Tobler PN. Disentangling neural representations of value and salience in the human brain. Proc Natl Acad Sci U S A. 2014;111:5000–5005. doi: 10.1073/pnas.1320189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, Zelano C, Gottfried JA, Mohanty A. Human Amygdala Represents the Complete Spectrum of Subjective Valence. J Neurosci. 2015;35:15145–15156. doi: 10.1523/JNEUROSCI.2450-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chikazoe J, Lee DH, Kriegeskorte N, Anderson AK. Population coding of affect across stimuli, modalities and individuals. Nat Neurosci. 2014;17:1114–1122. doi: 10.1038/nn.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. J Neurosci. 2012;32:6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition & Emotion. 2000;14:711–724. [Google Scholar]
- 27.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 28.Schiller CE, Minkel J, Smoski MJ, Dichter GS. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord. 2013;151:756–762. doi: 10.1016/j.jad.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Q, Zou K, He Z, Sun X, Chen H. Causal connectivity alterations of cortical-subcortical circuit anchored on reduced hemodynamic response brain regions in first-episode drug-naive major depressive disorder. Sci Rep. 2016;6:21861. doi: 10.1038/srep21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 34.Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol Psychiatry. 2015;78:635–646. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. Altered Intrinsic Functional Brain Architecture in Children at Familial Risk of Major Depression. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 37.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 39.Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. Am Psychol. 1993;48:155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 43.Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, et al. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19:399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- 44.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Tversky A, Kahneman D. Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and uncertainty. 1992;5:297–323. [Google Scholar]
- 46.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 50.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 51.Low A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: emotional arousal in threat and reward. Psychol Sci. 2008;19:865–873. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 53.Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 55.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 56.Howard JD, Gottfried JA, Tobler PN, Kahnt T. Identity-specific coding of future rewards in the human orbitofrontal cortex. Proc Natl Acad Sci U S A. 2015;112:5195–5200. doi: 10.1073/pnas.1503550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J, Padoa-Schioppa C. Neuronal remapping and circuit persistence in economic decisions. Nat Neurosci. 2016;19:855–861. doi: 10.1038/nn.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe M. Neurobiology. Attraction is relative not absolute. Nature. 1999;398:661, 663. doi: 10.1038/19414. [DOI] [PubMed] [Google Scholar]
- 60.Mainen ZF, Kepecs A. Neural representation of behavioral outcomes in the orbitofrontal cortex. Curr Opin Neurobiol. 2009;19:84–91. doi: 10.1016/j.conb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Jones JL, Esber GR, McDannald MA, Gruber AJ, Hernandez A, Mirenzi A, et al. Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science. 2012;338:953–956. doi: 10.1126/science.1227489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- 63.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 64.Luking KR, Pagliaccio D, Luby JL, Barch DM. Child Gain Approach and Loss Avoidance Behavior: Relationships With Depression Risk, Negative Mood, and Anhedonia. J Am Acad Child Adolesc Psychiatry. 2015;54:643–651. doi: 10.1016/j.jaac.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 67.Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 69.Luking KR, Pagliaccio D, Luby JL, Barch DM. Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry. 2016;55:328–337. doi: 10.1016/j.jaac.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ho TC, Zhang S, Sacchet MD, Weng H, Connolly CG, Henje Blom E, et al. Fusiform Gyrus Dysfunction is Associated with Perceptual Processing Efficiency to Emotional Faces in Adolescent Depression: A Model-Based Approach. Front Psychol. 2016;7:40. doi: 10.3389/fpsyg.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.