Abstract
Atypical hemolytic uremic syndrome and C3 glomerulopathy (dense deposit disease and C3 glomerulonephritis) are characterized inappropriate activation of the alternative complement pathway (AP). Genetic mutations affecting the AP regulating proteins (complement factor H, I, membrane co-factor protein, complement factor H related proteins) and triggers (such as infection, surgery, pregnancy, and autoimmune disease flares) result in the clinical manifestation of these diseases. A decade ago prognosis of these disease states was quite poor with the majority of patients developing end-stage renal disease. Furthermore, renal transplantation in these conditions was associated with poor outcomes due to graft loss to recurrent disease. Recent advances in targeted complement inhibitor therapy resulted in significant improvement in disease remission, renal recovery, health-related quality of life and allograft survival.
Keywords: Alternative complement pathway dysregulation, atypical hemolytic uremic syndrome, C3 glomerulopathy, Eculizumab
INTRODUCTION
In this focused review of renal diseases caused by dysregulated alternative complement pathway (AP), we describe the essential components of AP, common sites of dysregulation, pathophysiology and clinical course related diseases, such as C3 glomerulopathy (dense deposit disease -DDD and C3 glomerulonephritis - C3GN) and atypical hemolytic uremic syndrome (aHUS). At the end of the review, we focus the discussion on recent advances in therapy.
ALTERNATIVE COMPLEMENT PATHWAY
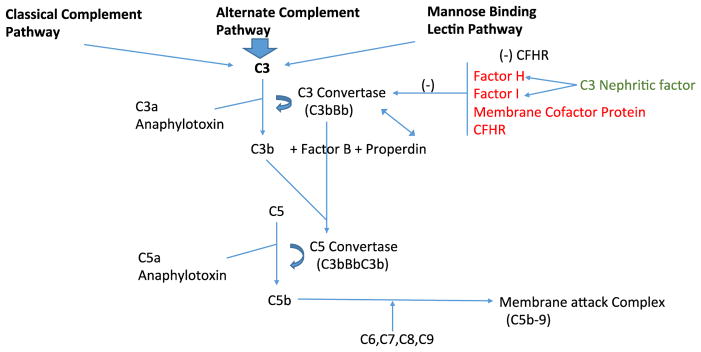
Complement system (CS) is an integral part of innate and adaptive immunity and plays a critical role in host defense against infections and non-microbial stressors. The functions of CS include removal of pathogens, mediating inflammation, recruitment of inflammatory cells and removal of damaged and apoptotic cells. It is composed of several proteins which are cleaved during the activation (Figure 1). There are three pathways of activation: classical, lectin and AP. All the paths converge at a crucial stage of CS activation which includes generation of C3 convertase. C3 convertase cleaves C3 to C3a and C3b. C3b in combination with C3 convertase acts as C5 convertase which cleaves C5 to C5a and C5b. This ultimately generates terminal complement complex termed as membrane attack complex (MAC, composed of C5 b-9). The effector functions of CS are mediated by C3a, C5a, C3b and membrane attack complex C5b-9. C3a and C5a are anaphylatoxins, induce vasodilatation, cytokine release, recruitment of leukocytes. C5a activates T cells and antigen presenting cells. C3b is fixed in nearby cells where it keeps AP activation on at a low level. MAC forms a pore in the membranes of target cells which lead to apoptosis and lysis of cells. 1,2
Figure 1.
Complement Cascade
AP is inherently active at baseline at a low level by spontaneous conversion of C3 to C3b, c3b binds with factor B to generate more C3 convertase. To keep the AP activation at low-level several soluble and membrane-bound proteins tightly control/inhibit C3 convertase activity including complement factor H (CFH), factor I (CFI), and membrane cofactor protein (MCP, also known as CD46). Dysfunction of these regulatory proteins leads conversion of the low-grade AP activation to uncontrolled levels leading to disease states such as C3 GN and aHUS.2,3
CFH is a soluble regulator of AP that is important in protecting the kidney. Factor H is comprised of 20 repeating structural domains (SCR). The complement inhibiting the function of CFH is performed by the N-terminus first four SCRs, whereas the other parts are involved in binding of factor H to the cell surface. The carboxyl terminus including SCR 19–20 binds to C3b and confers the ability to bind and protect endothelial cells. Five complement factor H related proteins (CFHR 1–5) were identified that share structural and functional similarities to CFH.4 These CFHR proteins compete with CFH for binding to C3b, the process known as CFH deregulation.
Susceptibility of Kidney to Complement injury
GBM entirely depends on soluble regulators of complement (fluid phase) such as CFH that lacks membrane-bound regulators. DDD characterized by localized complement injury within GBM results from uncontrolled activation of AP regulators in fluid-phase. However, aHUS results from AP dysregulation at the level of the cell membrane with impaired cell surface protection. The microvascular endothelium is targeted in aHUS. Hence C3GN mostly associated with fluid phase defect of AP manifests with low serum C3 levels, whereas aHUS with dysregulation at membrane level demonstrates with normal C3 levels. 5 Reasons, why kidney is more susceptible to complement injury, include the presence of fenestrae in endothelial cells always exposing subendothelial tissues to complement activators. Furthermore, there appears to be lower baseline expression of complement regulators.
Dysregulation of alternative complement pathway activation
The dysregulation of AP may be familial or triggered by external factors. A genetic or acquired defect in complement regulatory proteins if severe enough may lead to the disease process. On the other hand, a genetic mutation with incomplete penetrance might need an environmental trigger to initiate the disease process. The common environmental triggers are infections, vaccinations, immunosuppressive agents, antineoplastic agents, oral contraceptives, pregnancy and post-partum state.
Mutations in CFH are identified in 30% of cases of aHUS, the majority of these mutations affect the C-terminus membrane-binding region. Acquired defects in CFH are seen in the presence of autoantibody against CFH. These autoantibodies are identified in 5–10% of cases of aHUS. The majority of these antibodies attach the C-terminus leading to aHUS due to impaired binding to endothelial cells. There appears to be a predisposition to develop CFH antibodies in patients expressing deletion of CFHR 1/3 genes. Functional CFH deficiency by autoantibodies is seen monoclonal gammopathies, where the monoclonal light chains act as CFH inactivating autoantibody. 4,6
Mutations affecting CFHR proteins lead to the generation of CFHRs with increased avidity to CFH leading to inactivation. CFI mutations are seen in approximately 12% of cases of aHUS. Mutations in MCP are identified in 15% of cases of aHUS. Activating mutations in factor B leads to resistance of C3 convertase to CFH. Gain-of-function mutations of C3 result in resistance of C3 convertase to CFI and are noted in up to 10% of cases of aHUS. Identifying the underlying mutation will assist in the management and also in work up of available living related donors; however, these results are not readily available, and in approximately 30% of cases of aHUS a particular mutation cannot be identified. 7
C3 Nephritic Factor
C3 nephritic factor (C3Nef) includes Ig G and Ig M autoantibodies that bind to C3 and make it resistant to CFH and CFI-mediated degradation. Conditions associated with C3Nef are evidenced by low serum C3. C3Nef is associated with majority of DDD (80%) and C3 GN (40–50%).8
ATYPICAL HEMOLYTIC UREMIC SYNDROME (aHUS)
aHUS is a life-threatening systemic disease, which presents with a triad of acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia. Atypical HUS and thrombotic thrombocytopenic purpura (TTP) have common clinical manifestations. However, it is important for clinicians to distinguish between the two disorders since they differ entirely in pathophysiology and therapy. Another differential for aHUS is the classic HUS mediated by Shiga-toxin producing Escherichia coli infection (STEC-HUS).9
Dysregulated AP activation leads to endothelial injury (swelling and hypertrophy) and microvascular thrombosis. The resulting narrowing of the arterioles and increased shear stress leads to intravascular hemolysis. Endothelial injury activates platelets leading to thrombosis and thrombocytopenia due to consumption. Impaired tissue perfusion leads to end-organ manifestations. Increased vascular permeability is noticed as a consequence of the generation of C3a and C5a (anaphylatoxins) results in interstitial edema. Activation of juxtaglomerular apparatus in the glomeruli presents with uncontrolled HTN.10 Biopsy of the involved tissue reveals fibrin-rich thrombi with inflammatory infiltrate. Renal biopsy may be difficult in the setting of severe thrombocytopenia. EM shows endothelial swelling with micro thrombi.
aHUS affects wide age range of patients from childhood to octogenarians with a female preponderance in adults. Triggering factors are identified in 35–70% of the cases, with predominant factors being pregnancy, diarrhea or respiratory illness. Extrarenal manifestations can be seen in 20% of the patients including and not limited to cardiac, neurological or gastrointestinal/hepatobiliary involvement (abdominal pain, nausea, vomiting, elevated liver enzymes, pancreatitis, etc.). Also, there is a predilection for pulmonary, ocular and digital ischemia. The majority of patients present with severe renal impairment with approximately 50–80% needing renal replacement therapy at diagnosis, and before anti-complement therapy, more than 50% of the patients progressed to ESRD within one-year. Recurrence of aHUS is a very common post renal transplant and presents with unexplained anemia, uncontrolled hypertension, proteinuria or acute renal failure. 11
Laboratory data show evidence of hemolysis (elevated lactate dehydrogenase, anemia, low haptoglobin, schistocytes on peripheral smear), low platelet count (generally above 30,000/mL) in addition to elevated serum creatinine (generally above 1.7 mg/dl) at presentation.
Second pregnancy, especially in post-partum state, appears to be common triggers for aHUS. It is important to distinguish this condition from HELLP syndrome seen with severe preeclampsia. Autoimmune diseases such as lupus can trigger aHUS and need to be differentiated from thrombotic microangiopathy mediated by antiphospholipid antibody syndrome. Both solid organ and bone marrow transplant recipients are at risk for aHUS, mostly associated with the use of calcineurin inhibitors (CNI) in most cases (approximately 70%). Since the liver is the primary site of synthesis of complement regulatory proteins (CFH, CFI, Factor B and C3), mutations in these proteins could be acquired via liver transplant and hence increase the post-transplant risk of aHUS.12 Additional triggers include viral infections, vaccinations, and drugs. Among the drugs, more prominent ones are CNIs, sirolimus, and clopidogrel.7
aHUS as identified cause of ESRD accounts to approximately <1 % of kidney transplants performed in the United States. Tanriover et al., in an analysis of UNOS database studied the outcomes of patients with HUS who received kidney transplantation (KT). In this analysis, pediatric KT with HUS had comparable outcomes with matched controls with non-HUS etiologies of renal failure. However, adults with HUS were identified to have inferior graft and patient survival. Patients with identified recurrent HUS post-transplant were 4–5 fold higher risk of increased graft loss and mortality compared to the group without recurrence. In this study, acute rejection was associated with HUS recurrence. The traditional association between CNIs and HUS in transplant recipients was not validated by this study.13
Work up of a patient with AHUS involves identifying the underlying defect in AP. The workup includes genetic testing and autoantibody testing. Genetic tests include tests for mutations in genes that encode CFH, FI, MCP, CFHR 1–5 proteins. Checking for antibodies such as C3Nef, anti-FH and anti-FI should also be performed.
Outcome measures to be focused on while treating aHUS include improvement in thrombocytopenia (no decrease in platelet count >25% from baseline), continued the need for plasma therapy and renal function improvement. Kidney transplant (KT) alone in cases of aHUS leading to ESRD is associated with risk of graft loss to recurrent disease as high as 80% within first two years. Hence KT should be considered with additional prophylactic measures to prevent graft loss from recurrent disease. Recombinant factor H would overcome this problem in situations with inherited or acquired CFH deficiency; unfortunately, this therapy is not available in the U.S. at present. There are three other approaches to overcome the recurrence risk with variable success rates:
KT & Plasma exchange (PE) pre and post-operative and lifelong. Over time patients could become resistant and develop recurrence. This approach works in patients with low and functional deficiency of regulatory proteins (mainly in CFH) and the presence of CFH antibody; however it makes the clinical picture worse in cases with C3 and Factor B gain of function mutation, is ineffective in patients with MCP mutation.
KT alone & C5 convertase inhibitor, eculizumab (EZ). This approach is successful in most cases, but the expense of EZ may be limiting factor.
Combined liver-kidney transplantation. It may cure CFH deficiency, but the surgery is associated with very high morbidity and mortality in aHUS cases, heavily relies on the use of PE & EZ peri-operatively.
Replacement of deficient gene products with plasma therapy
Identifying the mutations involved in the pathogenesis of aHUS would determine which patients would benefit from plasma therapy. Patients with mutations involving deficiency of AP regulatory proteins benefit from replacement of factors with plasma therapy. Patients might need lifelong plasma therapy. In contrast, plasma therapy is ineffective in patients with MCP mutations since it is a membrane bound protein and replenishing plasma with this protein would not offer protection to the endothelial cells. In patients with gain of function mutations in complement activating proteins (such as Factor B or mutant C3 convertase resistant to CFH), plasma therapy may be detrimental since it provides additional culprit substance to activate AP. In contrast to TTP, plasma exchange is not definitive therapy for aHUS. It may correct the hematologic abnormalities but remains ineffective in reversing the end organ damage.
Liver transplantation
The majority of complement regulatory proteins are produced in the liver; hence simultaneous liver-kidney transplantation can correct several genetic abnormalities and missing complement proteins (CFH, CFI, C3, and CFB) in aHUS. Perioperative PE & EZ use is typically needed to overcome complement injury intraoperatively as well as to bridge the post-operative period till the newly transplanted liver establishes graft function. In experienced centers, the success rate of this approach is as high as 80%.
Elimination of autoantibodies and mutant proteins
PE replenishes plasma with deficient proteins, removes autoantibodies or mutant proteins that activate the AP. The response to PE remains short lived and rebound complement activation is noted within 48 hours hence ongoing plasma exchange is needed which poses risks with long-term central venous catheters, infection, and high-cost burden.
Immunosuppression
Theoretically, B cell suppressive therapy should reduce or eliminate autoantibody production. However, the effectiveness of this approach appears marginal. Traditional agents used are corticosteroids with or without cyclophosphamide and rituximab. This method is not supported by randomized controlled trials, and most of the evidence comes from single center case reports. Furthermore, the success rate of this approach appears to be marginal based on high recurrence rate of the original disease in allograft recipients who are already immunosuppressed. Before EZ, immunosuppression was reserved for patients with the rapidly progressing disease process.
Complement inhibition
Eculizumab (EZ) is a recombinant, completely humanized monoclonal antibody (Ig G) that binds with high affinity to the human C5 and effectively stops C5 cleavage and generation of MAC. EZ has revolutionized the treatment of AHUS with an effective role in preventing progression to ESRD. Earlier initiation of therapy is associated with better outcomes. Major side effect of EZ therapy is recurrent meningococcal infections (incidence less than 1%). Patients are mandated to receive the meningococcal vaccination in advance to therapy to reduce infection risk. Before subjecting a patient with aHUS to EZ therapy, it is recommended to rule out TTP with ADAMTS 13 level as well as STEC- HUS with stool culture / PCR testing. Identifying the genetic abnormality leading to aHUS is not mandatory before EZ therapy.
Zuber et al. have summarized the reports of initial 22 cases of aHUS treated with EZ, the majority of these patients had partial or no response to plasma therapy or were intolerant to plasma therapy. Results show complete improvement in hematologic and extrarenal manifestations. Four (out of six) of the dialysis dependent patients were weaned off dialysis. Other patients with renal involvement showed significant improvement in serum creatinine.14 In two open labeled, Phase 2 studies utilizing EZ therapy for a median of 62–64 weeks in patients with active PE dependent chronic aHUS, results show significant improvement in platelet counts, renal function and quality of life. 15 The appropriate duration of therapy is not clearly defined; early discontinuation is associated with recurrence (estimated 25–50%). Patients with identified genetic mutations and transplant recipients are at increased risk for recurrence due to ongoing endothelial injury mediated by drugs, immunologic insults, infections, etc. Long-term therapy with EZ is associated with immense cost burden. Withdrawal protocols need further studies.
EZ has indeed opened the path for kidney alone transplantation in aHUS. Deceased donor or living unrelated donor transplantation is preferably recommended since living related donors might be at risk to aHUS after donation. Living related donors could be considered in special circumstances when the recipient’s culprit mutation is clearly established, and the donor testing for the same mutation is negative. Post-transplant prophylactic EZ requires recipient risk assessment. Patients at low risk of recurrence (for example patients with mutations in MCP or anti-CFH antibody negative patients) need close monitoring to no initiation of therapy unless disease manifests. However, patients at high risk for recurrence (history of early recurrence in prior transplant or a family member, mutations in CFH or gain of function in C3 or CFB) should be planned for prophylactic EZ therapy with or without PE. Those patients also need consideration for combined liver-kidney transplantation. Use of EZ for a year is associated with an approximate cost of $600,000.16. However, these costs should be weighed against, the costs required with PE, central line, hospitalization, impaired renal recovery, ESRD, and reduced health-related quality of life. 14,17
C3 GLOMERULOPATHIES
1) Dense Deposit Disease
DDD is characterized by EM conversion of lamina densa of GBM into thick strand like electron dense material with characteristic C3 stain by immunofluorescence (IF) and no complex immune deposition. DDD predominantly affects Caucasians in childhood to adolescence, approximately 50% progress to ESRD within ten years of diagnosis. Nasr et al. in a North American cohort of 32 cases described natural course of DDD. The majority (80%), especially in children, demonstrated low C3 levels with the detectable C3 nephritic factor. Patients commonly presented with nephrotic range proteinuria, microscopic hematuria. MPGN (membranoproliferative glomerulonephritis) features on light microscopy were noted in approximately half of these patients. Funduscopic examination shows drusen bodies in some patients with DDD. This study was before the era of EZ, and hence therapy included predominantly RAS blockade with or without additional immunosuppressive therapy. 25% of these patients progressed to ESRD. Factors predictive of ESRD included older age, high serum creatinine at diagnosis and degree of arteriosclerosis on biopsy. 18 DDD is associated with almost universal recurrence post-KT with approximately 45% of patients losing allografts to recurrent disease within five years of transplantation.
2) C3 Glomerulitis (C3GN)
Differs from DDD regarding the location of C3 deposition within glomeruli, by the presence of subendothelial and mesangial deposits exclusively staining for C3 by IF with no immunoglobulins. EM shows no intramembranous deposits to differentiate from DDD. Pathogenesis is driven by fluid phase AP activation by a C3 Nephritic factor. The majority of C3GN cases show MPGN features with endocapillary proliferation by light microscopy. Approximately 30–50% of patients with C3GN slowly (in decades) progress to ESRD in reported series with poor renal prognosis.
Treatment of C3 Glomerulopathy
As with any GN, RAS blockade remains part of therapy along with lipid-lowering therapy. Plasma therapy can be used in patients with identified antibody activating AP. Corticosteroids, mycophenolate mofetil, and rituximab have been used with inconsistent success. 19 Bomback et al. report their original experience with the use of EZ in C3 glomerulopathy in the first six patients (three with DDD and three with C3GN) in an open labeled unblinded study. This is the first report of the use of EZ for C3GNs. The dosage of eculizumab was chosen based on prior regimens used in aHUS and continued for a total of 1-year duration. Two of the six patients showed improvement in renal function, one patient had partial improvement in proteinuria, and one patient showed stabilization of lab values with improvement in biopsy findings post therapy.20 Improvement in clinical parameters in this study was more profoundly noted in patients with elevated serum MAC. Again the duration of therapy with eculizumab remains undetermined in these conditions as well.
Compstatin is a C3 inhibitory peptide that blocks the interaction between C3 and its convertase and prevents complement activation via all the three pathways. CP 40, a compstatin analog, is a selective C3 inhibitor. In-vitro studies have shown CP40 to be effective in preventing complement-mediated hemolysis by sera of patients with C3GN and also strong inhibition of dysregulated AP activation by autoantibodies and genetic mutations. Hence CP 40 (early, C3 level, the blockade of complement activation) may offer promise in the treatment of C3GN and DDD (since C3d is the central complement fragment deposited), currently tested in patients with adult macular degeneration where complement degradation products occur. Several clinical trials utilizing targeted complement inhibition with novel agents are ongoing and may offer hope in treating these dreadful conditions.21
Conclusion
Targeted complement inhibition has considerably improved the prognosis of patients with aHUS and in some of the C3GN cases. It has been shown to prevent recurrence of these diseases posts renal transplantation as well. However, it is not clear how long targeted complement inhibition needs to be maintained, and long-term use of this drug which is required in these diseases (due to the inherent risk of recurrence) is not feasible due to cost burden. Future studies focusing on safe withdrawal protocols will be helpful. Several new complement inhibitory therapies are in the pipeline including monoclonal antibodies, small interfering RNAs which may offer promising results soon. 5
Acknowledgments
Funding:
This research is partly supported by the UT Southwestern O’Brien Kidney Research Core Center (Grant number: P30DK079328).
Abbreviations
- aHUS
Atypical hemolytic uremic syndrome
- AP
Alternative Complement Pathway
- CFHR
Complement Factor H related proteins
- C3GN
C3 glomerulopathy
- CFI
Complement Factor I
- CFH
Complement factor H
- CS
Complement system
- DDD
Dense deposit disease
- ESRD
End stage renal disease
- GBM
Glomerular basement membrane
- KT
Kidney Transplantation
- MAC
Membrane attack complex
- MCP
Membrane Cofactor Protein
- STEC
Shiga toxin-producing E. Coli
- TTP
Thrombotic thrombocytopenia purpura
Footnotes
Disclosure:
Authors describe no conflict of interest relevant to the manuscript, except Dr. Tanriover served as a speaker for Alexion Pharmaceutical LC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thurman JM, Nester CM. All Things Complement. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.01710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vriese AS, Sethi S, Van Praet J, Nath KA, Fervenza FC. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. J Am Soc Nephrol. 2015;26:2917–29. doi: 10.1681/ASN.2015020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medjeral-Thomas N, Pickering MC. The complement factor H-related proteins. Immunol Rev. 2016;274:191–201. doi: 10.1111/imr.12477. [DOI] [PubMed] [Google Scholar]
- 4.George JN, Nester CM, McIntosh JJ. Syndromes of thrombotic microangiopathy associated with pregnancy. Hematology Am Soc Hematol Educ Program. 2015;2015:644–8. doi: 10.1182/asheducation-2015.1.644. [DOI] [PubMed] [Google Scholar]
- 5.Thurman JM, Le Quintrec M. Targeting the complement cascade: novel treatments coming down the pike. Kidney Int. 2016;90:746–52. doi: 10.1016/j.kint.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15–20. doi: 10.1182/asheducation-2011.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Nester CM, Barbour T, de Cordoba SR, et al. Atypical aHUS: State of the art. Mol Immunol. 2015;67:31–42. doi: 10.1016/j.molimm.2015.03.246. [DOI] [PubMed] [Google Scholar]
- 8.Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8:634–42. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]
- 9.Nester CM. Managing atypical hemolytic uremic syndrome: chapter 2. Kidney Int. 2015;87:882–4. doi: 10.1038/ki.2015.60. [DOI] [PubMed] [Google Scholar]
- 10.Tsai HM. Thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: an update. Hematol Oncol Clin North Am. 2013;27:565–84. doi: 10.1016/j.hoc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Laurence J. Atypical hemolytic uremic syndrome (aHUS): making the diagnosis. Clin Adv Hematol Oncol. 2012;10:1–12. [PubMed] [Google Scholar]
- 12.Brown JH, Tellez J, Wilson V, et al. Postpartum aHUS secondary to a genetic abnormality in factor H acquired through liver transplantation. Am J Transplant. 2012;12:1632–6. doi: 10.1111/j.1600-6143.2012.03991.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanriover B, Lakhia R, Shen YM, et al. Characteristics and Outcomes of Renal Transplant Recipients with Hemolytic Uremic Syndrome in the United States. Transplant Direct. 2015:1. doi: 10.1097/TXD.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V French Study Group for a HCG. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–57. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 15.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura J, Yamamoto M, Hayashi S, et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370:632–9. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- 17.Zuber J, Le Quintrec M, Morris H, Fremeaux-Bacchi V, Loirat C, Legendre C. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando) 2013;27:117–25. doi: 10.1016/j.trre.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Nasr SH, Valeri AM, Appel GB, et al. Dense deposit disease: clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol. 2009;4:22–32. doi: 10.2215/CJN.03480708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22:231–7. doi: 10.1097/MNH.0b013e32835da24c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomback AS, Smith RJ, Barile GR, et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748–56. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Shao D, Ricklin D, et al. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993–8. doi: 10.1016/j.imbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]