Abstract
Purpose of Review
Brucellosis is a neglected, zoonotic disease of nearly worldwide distribution. Despite brucellosis being recognized as a reproductive disease in animals, it has been historically known as a flu-like illness in humans with little or no significant role in maternal or newborn health. This review focuses on what is currently known relative to the epidemiology of brucellosis in human pregnancy as well as new insights of placental immunology.
Recent Findings
New evidence suggests that maternal infection poses a significant risk factor for adverse pregnancy outcomes including increased risk for miscarriage during the first and second trimester of gestation, preterm delivery, and vertical transmission to the fetus. Adverse pregnancy outcomes were not associated with any specific clinical sign. However, prompt diagnosis and treatment significantly decreased the risk of miscarriage or any other adverse effect.
Summary
Brucellosis during pregnancy should be considered a significant risk factor for adverse pregnancy outcomes in humans. The identification of the mechanism behind bacterial tropism should prove powerful for the development of new countermeasures to prevent these detrimental effects. Increased awareness concerning brucellosis in pregnant women, its transmission, and prevention measures should be considered as a pressing need.
Keywords: Human, Abortion, Brucellosis, Zoonosis, Placenta
Introduction
Brucellosis is a neglected, under-recognized zoonosis of widespread geographic distribution. Among the different Brucella species, B. melitensis (goat and sheep), B. suis (pig), B. abortus (cattle), and B. canis (dog) are pathogenic and virulent not only for their target species but also for humans. In most cases, human infections occur through the consumption of unpasteurized milk and dairy products or exposure to infected body fluids and tissues (mainly placenta) from infected animals. Despite the bacterium being recognized as a cause of disease in humans for more than 130 years, little information is available describing the mechanism related to adverse pregnancy outcomes in humans. Brucellosis is still considered an emerging disease with tremendous economic and public health impact under resource-limited settings or within emerging economies [1]. This has led to the classification of brucellosis by the World Health Organization (WHO) as one of the “top 10” neglected zoonoses, a group of diseases that are simultaneously ongoing threats to human health and a source of perpetuation of poverty.
The Disease
In animals, brucellosis is recognized as a reproductive disease often leading to abortion in the middle to last trimester of gestation (sheep, goats, cattle, dogs, and pigs), following bacterial colonization of the placenta (Table 1). Other reproductive symptoms associated with infection include apparent failure to conceive or stillbirths. In males, Brucella canis targets the epididymis and prostate, a feature shared by Brucella ovis and infrequently by other Brucella species [2]. Less commonly reported clinical signs include arthritis, discospondylitis, and carpal hygromas [3–5]. The range of signs of infection can vary from asymptomatic to severe, despite ongoing systemic infection [6, 7]. Although Brucella-induced abortion is commonly associated with agricultural species and dogs, it is also been reported among other hosts including dolphins, pinnipeds, camels, and non-human primates [8–12].
Table 1.
Abortions in animals
| Susceptible species | Type of placenta | Average length of gestation (days) |
Most susceptible stage for abortion |
Brucella species | Reference |
|---|---|---|---|---|---|
| Baboon (Papio spp.) | Hemochorial | 180 | 117 days | Brucella spp. | [8]a |
| Bison | Epitheliochorial | 283 | 195–225 days | Brucella abortus | [78, 79] |
| Camel | Epitheliochorial | 396 | – | Brucella melitensis | [80]b |
| Canine | Endotheliochorial | 65 | 45–55 days | Brucella canis | [81, 82] |
| Cattle | Epitheliochorial | 283 | 226–242 days | Brucella abortus | [78] |
| Mid to late gestation | Brucella spp. | [83]c | |||
| 180–283 days | Brucella abortus | [84, 85] | |||
| Dolphin (Tursiops truncates) | Epitheliochorial | 365 | 270 days | Brucella delphini (ceti) | [86]d |
| Elk | Epitheliochorial | 250 | 35–84 days post challenge | Brucella abortus | [87, 88] |
| Goats | Epitheliochorial | 150 | 117–135 days | Brucella melitensis | [89–92] |
| Lama | Epitheliochorial | 345 | 240 days | Brucella abortus | [93] |
| Sheep | Epitheliochorial | 148 | 107–146 days | Brucella melitensis | [92, 94–96] |
| Swine | Epitheliochorial | 114 | May occur at anytime | Brucella suis | [51, 81, 97] |
Reported two cases of stillbirths in baboons with positive culture of a novel Brucella spp. isolate confirmed by PCR
Based on aborted camels tested by RBT and confirmed by CFT. Brucella melitensis was isolated from two fetuses
Based on 150 abortion cases positives to RBT and confirmed by PCR
Brucella spp. were isolated from two specimens of Tursiops truncates that aborted
Traditionally, clinical signs associated with human infection are different from those described in animals. Human brucellosis is referred to as a “flu-like illness” characterized by a non-specific clinical syndrome with relapsing fever and arthritis being the most commonly reported symptoms [7]. Brucella infection can also cause splenomegaly or hepatomegaly, as well as life-threatening conditions including endocarditis and various neuropathies [7, 13]. The lack of pathognomonic signs associated with infection, along with the absence of accurate point of care diagnostic assays, has led to the misassumption that presentation of fever without any other associated condition in endemic countries is indicative of malaria. This in many cases impedes accurate assessment of the overall spectrum of disease manifestation. The lack of accurate diagnostic tools, as well as non-specific symptomatology, has made it extremely challenging to prevent and control the disease, making brucellosis a classic example of an old zoonotic disease that is emerging and re-emerging worldwide [14].
Obstetric Outcomes in Pregnant Women with Brucellosis
In contrast to animal brucellosis, pregnancy-associated complications associated with human brucellosis are thought to be uncommon and are rarely described in the literature. However, early reports of human miscarriages date to 1908, when a case of abortion in a pregnant farmer’s wife was associated with Brucella infection [15]. Since this initial observation of human abortion associated with brucellosis more than 100 years ago, less than 40 reports of adverse pregnancy outcomes have been documented in the literature [16–20, 21•, 22–50]. The majority of these reports consist of individual case reports describing patients residing in low to middle-income countries, where the capacity to conduct appropriate diagnostic examinations is very limited, thus preventing recognition of any association between brucellosis and human pregnancy. More recently, larger retrospective studies investigating the outcomes in pregnant women with a confirmed Brucella infection have demonstrated a strong association between infection and an increased incidence of adverse obstetric outcomes [19, 21•, 22, 27, 41]. These larger case studies (Table 2) have been conducted in the Middle East (Turkey, Saudi Arabia), South America (Peru), and Africa (Rwanda), with spontaneous miscarriage rates ranging from 18.6 to 73.3 % [19, 21•, 22, 27, 41]. Interestingly, the majority of the cases are documented to occur during the first and second trimester of gestation (first trimester of pregnancy is defined as a gestational age of <12 weeks, second trimester is considered between 12 and 24 weeks, and third trimester is >24 weeks), and differs from the time of occurrence of abortion in animals, commonly manifested as a late gestational event [51].
Table 2.
Reported case series of adverse pregnancy-associated complications in pregnant women diagnosed with brucellosis
| Study year and country |
Pregnant womena |
Diagnostic criteria | Spontaneous abortion rate |
Preterm delivery rate |
Intrauterine fetal death rate |
Associated clinical signs |
Reference |
|---|---|---|---|---|---|---|---|
| 2015, Peru | 86 | SAT (>1/160) and blood culture | 16 (18.6 %) | 12 (13.9 %) | 7 (8.14 %) | Osteoarticular, haematological and congenital disorders | [22] |
| 2014, Africa | 15 | RBPT | 11 (73.3 %) | – | 4 (26.7 %) | – | [41] |
| 2011, Turkey | 39 | Coombs test (>1/160) and blood culture | 1 (2.56 %) | 7 (17.95 %) | – | Fever, arthralgia, myalgia, hepatospenomegaly | [32] |
| 2011, Iran | 19 | SAT (>1/160), 2ME titer >1/80 | 10 (53 %) | – | – | Fever, arthralgia, back pain, sweating | [31] |
| 2010, Turkey | 29 | SAT (>1/160) and blood culture | 7 (24.14 %) | 2 (6.9 %) | 1 (3.45 %) | Sacroiliitis with Joint pain, hepatosplenomegaly | [27] |
| 2008, Saudi Arabia and Kuwait | 55 | SAT (>1/160) | 19 (34.5 %) | 13 (23.6 %) | 11 (20 %) | Common symptoms and signs of brucellosis in 35 women | [25] |
| 2001, Saudi Arabia | 92 | SAT (1:2560) and blood culture | 40 (43.5 %) | – | 2 (2.17 %) | Vaginal bleeding, febrile illness, 24 patients with recurrent abortion | [21•] |
| 2001, Saudi Arabia | 30 | SAT and blood culture | 12 (41 %) | 9 (30 %) | 1 (3.4 %) | Fever, arthritis, headache, sweating, | [42] |
| 1998, Kuwait | 25 | SAT (1/160), ELISA and tissue culture | 2 (8 %) | 18 (72 %) | 5 (20 %) | Fever, arthralgia, myalgia backache, headache, anorexia | [43] |
| 1991, Lebanon | 6 | SAT (>1/160, direct >1/80) | 1 (17 %) | 1 (17 %) | – | Fever, back pain, sweating, fatigue and malaise, chills | [36] |
| 1988, Kuwait | 35 | SAT (>1/160) and ELISA: (IgG>1/1600, IgM>1/400, IgA≫1/200) | 11 (31.4 %) | – | – | Fever, arthralgia, back pain, hepatosplenomegaly, headache, sweating | [44] |
| 1974, Iran | 51 | SAT, urine, uterine and blood culture | 6 (11.6 %) | – | – | Undulant fever, arthralgia, sweating and muscle pain | [45] |
| 1954, Argentina | 200 | SAT | 52 (26 %) | – | – | Febrile and non-febrile | [38] |
Positive for Brucella. Fetal death before 24 weeks of gestation is considered spontaneous abortion and fetal death after 24 weeks of gestation is considered intrauterine fetal death. Delivery before 37 weeks is considered preterm delivery. SAT serum agglutination test, RBPT Rose Bengal plate test
The second most commonly documented adverse event occurring in humans is preterm delivery. Preterm birth, defined as the birth of an infant before 37 weeks of pregnancy, is the leading cause of death in children under the age of 5, with as many as 10–11 % of all births estimated to be preterm [52]. However, the incidence of preterm birth in low- to middle-income settings is considerably higher with estimates reaching 15 to 24 % with the highest reported rates occurring in sub Saharan Africa and Asia, in which preterm births associated with brucellosis ranged from 6.9 to 72 % (Table 2) [25, 27, 32, 36, 41–43]. This is a significant finding, not only because the majority of brucellosis occurs in sub Saharan Africa and Asia, but also because of the elevated incidence of child mortality in low-income settings, due to lack of feasible, cost-effective care [52]. In children that survive, preterm delivery is considered to be a major determinant of immediate as well as long-term morbidity and is associated with growth and developmental delay [52]. This clearly suggests the critical need for increased awareness of potential risks associated with brucellosis, especially in endemic countries, where, despite the known-association of preterm delivery with well-identified infectious agents (e.g., Plasmodium falciparum, Listeria monocytogenes, herpes simplex virus, and influenza), brucellosis has not been associated with this side-effect. Preterm birth has also been commonly reported for many years in animals, commonly referred to as preterm whelping in bitches and preterm farrowing in sows [53–55]. Another adverse pregnancy outcome reported in the Peruvian study, as well as in single case reports, is “congenital brucellosis.” This is not surprising, since vertical transmission to the fetus is a well-documented effect in animals [2, 3, 51]. In this human case series, Vilchez et al. confirmed that of 86 brucellosis patients, 4.6 % had vertical transmission to the fetus [22].
Clinical signs in pregnant women with brucellosis are nonspecific, ranging from asymptomatic to repeated episodes of excessive sweating and arthralgia, fever, and vaginal bleeding [32]. However, no correlation has been identified between any single clinical sign and pregnancy outcome. Furthermore, 90–95% of all the cases available in the literature have had a history of high-risk factors for Brucella infection, including ingestion of non-pasteurized dairy products and close proximity with animals. Interestingly, prompt diagnosis and treatment significantly decreased the risk of miscarriage and other adverse effects, clearly demonstrating the importance of a rapid and accurate diagnosis to improve pregnancy outcomes in endemic areas [19].
Pregnancy and the Immune System
Pregnancy poses a unique challenge for the maternal immune system [56, 57]. Infection during pregnancy differs from infection in non-pregnant individuals as the presence of the fetus and placenta alter maternal immunity and physiology in order to sustain pregnancy. Successful pregnancy requires the maternal host to effectively balance the opposing processes of maternal immune reactivity to the infectious agents while maintaining tolerance to the fetus [56]. For many years, the uterus and amniotic cavity were considered sterile environments. However, this concept has been reviewed in recent studies demonstrating the presence of a “placental microbiome” during healthy pregnancy [58]. These findings further suggest that mechanisms preventing or limiting invading microbial proliferation and pathological consequences are in place to sustain pregnancy [59]. For many years, it was assumed, that pregnancy was associated with a state of cell-mediated immune suppression that subsequently increased susceptibility to intracellular pathogens. As a result, a T helper 2 (Th2)-biased immune response was considered the main reason pregnant women were capable of controlling extracellular pathogens more efficiently [60–62]. This model was considered substantiated by the clinical observation that cell-mediated inflammatory disorders including the clinical signs associated with rheumatoid arthritis were ameliorated during pregnancy, whereas antibody-mediated disorders, such as those observed in systemic lupus erythematosus symptomatology, were exacerbated [62]. Today, increasing evidence suggests that the immune system during pregnancy is fully functional and that the placenta and the decidua (uterine lining during pregnancy) represent important immune modulators affecting the global immunological response [57]. Nevertheless, some pathogens are capable of breaching the maternal-fetal barrier which can lead to adverse obstetric outcomes such as abortion, preterm delivery, or congenital infections. Just how some pathogens are capable of evading immune mechanisms in place is only partially understood at this time.
Pregnancy and Placenta
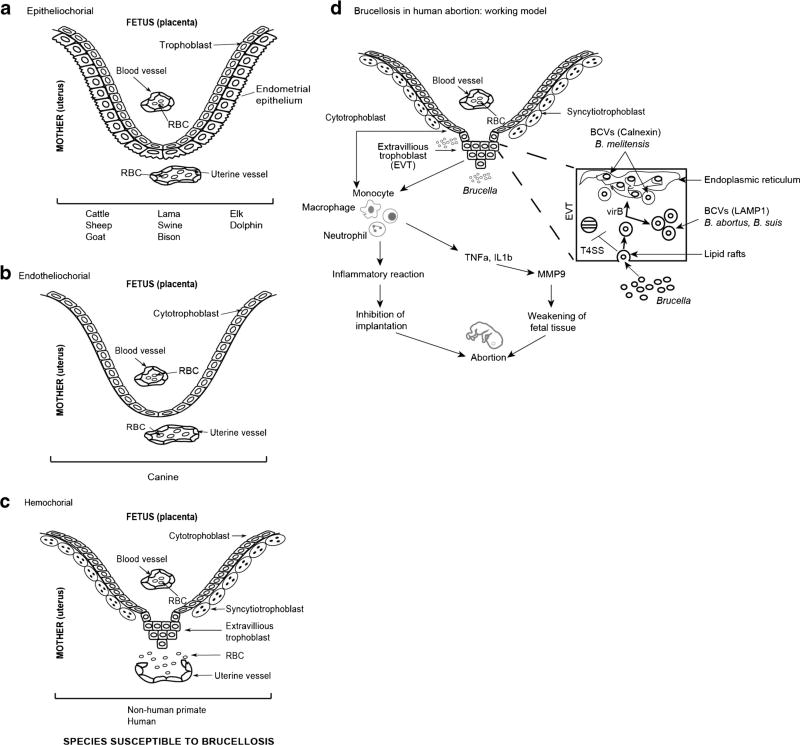
In an effort to understand the mechanisms leading to placental and fetal infection, it is necessary to consider the different components of the placenta and decidua. The placenta, composed of maternal and fetal tissues, performs a number of important functions throughout gestation including (i) anchoring of the developing fetus to the uterine wall, (ii) oxygen/carbon dioxide exchange, (iii) fetal nutrition, (iv) waste product removal, and (v) maternal immune tolerance [63]. Placentas of different mammals exhibit great differences at the maternal-fetal interface. This is an extremely important characteristic to consider when extrapolating physiological, immunological, or any other observation across species. In general, placentas are classified based on the histological structure of the maternal-fetal interface (epitheliochorial, endotheliochorial, hemochorial; Fig. 1a–c), the type of maternal-fetal interdigitation, and the gross aspect (diffuse, cotyledonary, discoid, or zonary) [63]. In humans, the placenta is composed of individual units termed chorionic villi. Each villus has a connective tissue core that contains (1) mesenchymal cells, (2) macrophages, termed Hofbauer cells, (3) fetal vascular cells, and (4) trophoblasts. Trophoblasts are fetal-derived cells that, depending on their differentiation, pose different roles. The trophoblast population within the placental villous surface is characterized by an inner layer of cytotrophoblasts that either fuse to form the overlying multinucleated syncytiotrophoblasts or assume invasive capabilities in anchoring villi (extravillous cytotrophoblast, EVT) that attaches the placenta to the uterus. Syncytiotrophoblast cover the entire surface of villous trees and is in direct contact with maternal blood, providing an abundant surface area for gas and nutrient exchange for the mother and fetus [64]. EVTs migrate through the decidua and enters the fetal spiral arterial walls, providing an anchoring capability. Previous studies have demonstrated that trophoblasts express pattern recognition receptors that function as “sensors” capable of recognizing the presence of bacteria, viruses, and parasites present in the surrounding environment, and are capable of secreting cytokines and chemokines able to act on cells of the innate immune system present in the decidua guiding them to work together in support of the growing fetus [65]. This supports the theory that the placenta plays an active role during immune regulation. Within the decidua, there are unique immune cell populations that actively contribute to the fetal tolerance and immunity of the placenta. This cell population consists of uterine natural killer (uNK) cells that in humans represent approximately 70 % of the total leukocyte population and are critical for the development of the placenta [66, 67]. uNK cells are also believed to play a role in facilitating invasion by fetal HLA-G+ extravillous trophoblasts (EVT) into maternal tissues for the establishment of healthy pregnancies. In addition, uNK contain cytotoxic granules, functioning in immunity to viral infections. Interaction of these cells with EVT leads to the acquisition of HLA-G. Thus, uNK cells provide tolerance as wells as anti-viral immunity [68]. Macrophages are the predominant subset of antigen-presenting cells (APC) and compromise about 20–25 % of the total decidua leukocytes, and are necessary for a wide range of gestational processes including implantation, ovarian function, placental development, and immunity [69].
Fig. 1.
Schematic representation illustrating the relationship between the fetal trophoblast cells and maternal blood of the three main types of placentation, susceptible to Brucella infection (a–c) and the working model regarding the pathogenesis of brucellosis in human abortion (d). a Epitheliochorial: Trophoblast cells are in direct apposition with the surface of the uterine epithelial cells with no trophoblast invasion beyond this layer. b Endotheliochorial: The uterine epithelium is breached and trophoblasts are in direct contact with endothelial cells of maternal uterine vessels. c Hemochorial: Maternal blood directly bathes the chorionic villi. d Brucella in human pregnancy target trophoblasts to survive and replicate. B. abortus and B. suis replicate inside LAMP1 positive acidic vesicles, whereas B. melitensis replicate inside vesicles positive for endoplasmic reticulum marker calnexin and inhibits implantation of trophoblasts. Brucella-infected cytotrophoblasts secrete cytokines and interleukins like IL8, IL6, MCP1 that cause infiltration of neutrophils, macrophages, and monocytes in the placenta. The inflammatory reaction might lead to implantation inhibition and abortion of the fetus. At the same time, macrophages and neutrophils secrete TNFα and IL1β that cause increase in MMP9 secretion potentially leading to weakening of fetal tissue and abortion
Mechanism of Brucella-Induced Abortion in Animals and Humans
Studies conducted over the last 30 years have demonstrated a correlation between an increased rate of adverse pregnancy outcomes with infection by certain microbial agents [70]. Historical, experimental, and epidemiological evidence supports the concept that Brucella infection in animals is a significant risk factor for abortion [19, 21•, 22]. Despite this evidence, very little is known about the mechanism of infection-induced abortion. Placental tissue tropism for Brucella was originally defined in ruminants based on (i) preference for erythritol as a carbon source, (ii) elevated erythritol levels in the ruminant placenta, and (iii) growth inhibition exhibited by erythritol sensitive B. abortus vaccine strain S19 [71]. However, genetic experimentation, in which restoration of the erythritol locus (ery) did not restore S19 to virulence and deletion of the ery locus from virulent B. abortus S2308 did not attenuate virulence, disproved this hypothesis [72]. Although genetic experimentation has disproved any relationship between virulence and erythritol utilization, placental tissue tropism remains a well-documented phenomenon in ruminants [73]. In an effort to identify cellular tropism and the mechanism of Brucella-induced abortion, the uterus and placenta from pregnant goats inoculated intravenously with a single dose of highly virulent B. abortus were evaluated via light and electron microscopy [74]. Placental infection was observed as early as 5 days post inoculation with the subsequent occurrence of abortion within 11 days. Interestingly, Brucella were observed within trophoblasts and chorionic villi [75]. More recently, Salcedo et al. investigated the ability of Brucella spp. to infect human trophoblasts using different cell lines and primary cultures representing the different trophoblast populations present in the placenta. Trophoblast colonization and replication was observed in the JEG-3 (EVT-like cells) when cells were infected using B. abortus or B. suis; however, replication was unusual since it was not completely dependent on virB type IV secretion system and replication was observed in large acidic LAMP-1 and CD63 positive compartments. B. melitensis, however, was able to replicate in a typical Brucella containing vacuole BCV compartment in a virB type IV-dependent manner. When other cell lines reflecting a syncytiotrophoblast phenotype were used, B. abortus was not found in acidic, LAMP-1 positive inclusions, but in ER-derived BCV [76•]. More recently, Fernandez et al. investigated the ability of B. abortus to infect a cytotrophoblast cell line Swan-71, demonstrating that B. abortus was capable of replicating inside these cells but survival was virB-type IV dependent. Infection elicited secretion of IL-8, MCP-1, and IL-6, and the authors suggested that trophoblasts may provide a local inflammatory environment that could potentially contribute to abortion [77•] (Fig. 1d).
Conclusions: Comment on Future Work
It is obvious that in order to address the important questions raised in this review pertaining to the precise details and/or mechanism of Brucella-induced abortion, there is the need to develop model systems capable of appropriate investigation. From an agricultural perspective, small ruminants represent one potential model. Reduction in human disease closely parallels reduction in animal disease. Thus, the ability to eliminate transmission may be expected to have a significant impact on public health in a relatively short period of time. Direct intervention to reduce human disease may follow the development of primate models based on similarities in placental structure. In either case, support for the development of in vivo systems is warranted based on the variability observed in tissue culture systems related to differences in experimental outcomes including novel trafficking, reduced virulence, and variable readouts associated with the use of different cell lines. Although work has focused on the capacity of Brucella to replicate in trophoblasts, it is unclear whether such replication reflects the prime function of these cells, i.e., to protect the placenta and intercept pathogens or the precipitating event leading to pathology. In contrast, examination of organism distribution in the infected placenta over time may be expected to provide improved insight with regard to abortion and intracellular invasion/replication.
Acknowledgments
Funding This study was funded by the National Institutes of Health (NIH), International Research Scientist Development Award-IRSDA/K01 (grant number 1K01 TW009981-01).
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol. 2014;5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen SC, Palmer MV. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. 2014;51(6):1076–89. doi: 10.1177/0300985814540545. [DOI] [PubMed] [Google Scholar]
- 3.Wanke MM. Canine brucellosis. Anim Reprod Sci. 2004;82–83:195–207. doi: 10.1016/j.anireprosci.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Johnson B, Mosier DA, Morton RJ, Confer AW. Experimental Brucella abortus strain 19 arthritis in young cattle. J Vet Diagn Investig. 1994;6(1):56–61. doi: 10.1177/104063879400600111. [DOI] [PubMed] [Google Scholar]
- 5.Coid CR, Vaughan LC. Incidence of carpal hygromas in dairy cattle infected with Br. abortus and maintained in an isolation compound. J Comp Pathol. 1957;67(1):53–6. doi: 10.1016/s0368-1742(57)80006-x. [DOI] [PubMed] [Google Scholar]
- 6.Pappas G, Papadimitriou P. Challenges in Brucella bacteraemia. Int J Antimicrob Agents. 2007;30(Suppl 1):S29–31. doi: 10.1016/j.ijantimicag.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–36. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 8.Schlabritz-Loutsevitch NE, Whatmore AM, Quance CR, Koylass MS, Cummins LB, Dick EJ, Jr, et al. A novel Brucella isolate in association with two cases of stillbirth in non-human primates - first report. J Med Primatol. 2009;38(1):70–3. doi: 10.1111/j.1600-0684.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernery U. Camelid brucellosis: a review. Rev Sci Tech. 2014;33(3):839–57. doi: 10.20506/rst.33.3.2322. [DOI] [PubMed] [Google Scholar]
- 10.Dagleish MP, Barley J, Finlayson J, Reid RJ, Foster G. Brucella ceti associated pathology in the testicle of a harbour porpoise (Phocoena phocoena) J Comp Pathol. 2008;139(1):54–9. doi: 10.1016/j.jcpa.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Guzman-Verri C, Gonzalez-Barrientos R, Hernandez-Mora G, Morales JA, Baquero-Calvo E, Chaves-Olarte E, et al. Brucella ceti and brucellosis in cetaceans. Front Cell Infect Microbiol. 2012;2:3. doi: 10.3389/fcimb.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne ET, Morton JK. Brucellosis in elk. II. Clinical effects and means of transmission as determined through artificial infections. J Wildl Dis. 1978;14(3):280–91. doi: 10.7589/0090-3558-14.3.280. [DOI] [PubMed] [Google Scholar]
- 13.Williams E. Brucellosis in humans: its diagnosis and treatment. APMIS. 1988;3:21–5. [PubMed] [Google Scholar]
- 14.Welburn SC, Beange I, Ducrotoy MJ, Okello AL. The neglected zoonoses—the case for integrated control and advocacy. Clin Microbiol Infect. 2015;21(5):433–43. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Eyre J. Melitensis septicemia. Lancet. 1908:1747–52. [Google Scholar]
- 16.Mohammad KI, El Ghazaly MM, Zaalouk TK, Morsy AT. Maternal brucellosis and human pregnancy. J Egypt Soc Parasitol. 2011;41(2):485–96. [PubMed] [Google Scholar]
- 17.Karcaaltincaba D, Sencan I, Kandemir O, Guvendag-Guven ES, Yalvac S. Does brucellosis in human pregnancy increase abortion risk? Presentation of two cases and review of literature. J Obstet Gynaecol Res. 2010;36(2):418–23. doi: 10.1111/j.1447-0756.2009.01156.x. [DOI] [PubMed] [Google Scholar]
- 18.Schreyer P, Caspi E, Leiba Y, Eshchar Y, Sompolinsky D. Brucella septicemia in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1980;10(2):99–107. doi: 10.1016/0028-2243(80)90087-8. [DOI] [PubMed] [Google Scholar]
- 19.Al-Tawfiq JA, Memish ZA. Pregnancy associated brucellosis. Recent Pat Antiinfect Drug Discov. 2013;8(1):47–50. doi: 10.2174/1574891x11308010009. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa Damian R, Rojas Rodriguez L, Marcano Tochon ES. Brucellosis in pregnancy: course and perinatal results. Ginecol Obstet Mex. 1995;63:190–5. [PubMed] [Google Scholar]
- 21•.Khan MY, Mah MW, Memish ZA. Brucellosis in pregnant women. Clin Infect Dis. 2001;32(8):1172–7. doi: 10.1086/319758. This is an interesting review article were they characterized 92 pregnant women with acute brucellosis and adverse pregnancy outcomes. [DOI] [PubMed] [Google Scholar]
- 22.Vilchez G, Espinoza M, D’Onadio G, Saona P, Gotuzzo E. Brucellosis in pregnancy: clinical aspects and obstetric outcomes. Int J Infect Dis. 2015;38:95–100. doi: 10.1016/j.ijid.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Cacace ML, Claros EA, Erazu KA, Escobar GI, Lucero NE. Congenital brucellosis in an infant. Vector Borne Zoonotic Dis. 2013;13(7):513–5. doi: 10.1089/vbz.2012.1165. [DOI] [PubMed] [Google Scholar]
- 24.Peker N, Turan V, Ergenoglu M, Yeniel O. Brucellosis in adolescent pregnancy—case report and review of literature. Ginekol Pol. 2011;82(3):226–9. [PubMed] [Google Scholar]
- 25.Elshamy M, Ahmed AI. The effects of maternal brucellosis on pregnancy outcome. J Infect Dev Ctries. 2008;2(3):230–4. doi: 10.3855/jidc.268. [DOI] [PubMed] [Google Scholar]
- 26.Fernihough TJ, Munoz WP, Mahadeyo I. The role of Brucella abortus in spontaneous abortion among the black population. S Afr Med J. 1985;68(6):379–80. [PubMed] [Google Scholar]
- 27.Kurdoglu M, Adali E, Kurdoglu Z, Karahocagil MK, Kolusari A, Yildizhan R, et al. Brucellosis in pregnancy: a 6-year clinical analysis. Arch Gynecol Obstet. 2010;281(2):201–6. doi: 10.1007/s00404-009-1106-0. [DOI] [PubMed] [Google Scholar]
- 28.Malone FD, Athanassiou A, Nores LA, Dalton ME. Poor perinatal outcome associated with maternal Brucella abortus infection. Obstet Gynecol. 1997;90(4 Pt 2):674–6. doi: 10.1016/s0029-7844(97)00345-1. [DOI] [PubMed] [Google Scholar]
- 29.Aydin B, Beken S, Akansel R, Dilli D, Okumus N, Zenciroglu A, et al. Prematurity due to maternal brucella infection and review of the literature. Turk J Pediatr. 2013;55(4):433–7. [PubMed] [Google Scholar]
- 30.Lubani MM, Dudin KI, Sharda DC, Abu Sinna NM, Al-Shab T, Al-Refe’ai AA, et al. Neonatal brucellosis. Eur J Pediatr. 1988;147(5):520–2. doi: 10.1007/BF00441980. [DOI] [PubMed] [Google Scholar]
- 31.Roushan MR, Baiani M, Asnafi N, Saedi F. Outcomes of 19 pregnant women with brucellosis in Babol, northern Iran. Trans R Soc Trop Med Hyg. 2011;105(9):540–2. doi: 10.1016/j.trstmh.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Gulsun S, Aslan S, Satici O, Gul T. Brucellosis in pregnancy. Trop Dr. 2011;41(2):82–4. doi: 10.1258/td.2011.100386. [DOI] [PubMed] [Google Scholar]
- 33.Ceylan A, Kostu M, Tuncer O, Peker E, Kirimi E. Neonatal brucellosis and breast milk. Indian J Pediatr. 2012;79(3):389–91. doi: 10.1007/s12098-011-0581-z. [DOI] [PubMed] [Google Scholar]
- 34.Porreco RP, Haverkamp AD. Brucellosis in pregnancy. Obstet Gynecol. 1974;44(4):597–602. [PubMed] [Google Scholar]
- 35.Poole PM, Whitehouse DB, Gilchrist MM. A case of abortion consequent upon infection with Brucella abortus biotype 2. J Clin Pathol. 1972;25(10):882–4. doi: 10.1136/jcp.25.10.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seoud M, Saade G, Awar G, Uwaydah M. Brucellosis in pregnancy. J Reprod Med. 1991;36(6):441–5. [PubMed] [Google Scholar]
- 37.Veznik Z. Brucellosis in gynecology and obstetrics. Lek List. 1954;9(23):548–9. [PubMed] [Google Scholar]
- 38.Criscuolo E, Di Carlo FC. Abortion and other gynecological and obstetrical disorders in brucellosis. Rev Fac Cienc Med Cordoba. 1954;12(3):321–30. [PubMed] [Google Scholar]
- 39.Schachter M. Congenital diseases in infants due to melitococcal infections in pregnancy. Acta Pediatr Esp. 1954;12(138):521–4. [PubMed] [Google Scholar]
- 40.Chheda S, Lopez SM, Sanderson EP. Congenital brucellosis in a premature infant. Pediatr Infect Dis J. 1997;16(1):81–3. doi: 10.1097/00006454-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Rujeni N, Mbanzamihigo L. Prevalence of Brucellosis among women presenting with abortion/stillbirth in Huye. Rwanda J Trop Med. 2014;2014:740479. doi: 10.1155/2014/740479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madkour MM. Pregnancy and Brucellosis. In: Madkour MM, editor. Madkour, s Brucellosis. 2. New York: Springer; 2001. pp. 197–202. [Google Scholar]
- 43.Makhseed M, Harouny A, Araj G, Moussa MA, Sharma P. Obstetric and gynecologic implication of brucellosis in Kuwait. J Perinatol. 1998;18(3):196–9. [PubMed] [Google Scholar]
- 44.Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. Human brucellosis in Kuwait: a prospective study of 400 cases. Q J Med. 1988;66(249):39–54. [PubMed] [Google Scholar]
- 45.Sarram M, Feiz J, Foruzandeh M, Gazanfarpour P. Intrauterine fetal infection with Brucella melitensis as a possible cause of second-trimester abortion. Am J Obstet Gynecol. 1974;119(5):657–60. doi: 10.1016/0002-9378(74)90128-8. [DOI] [PubMed] [Google Scholar]
- 46.Fallatah SM, Oduloju AJ, Al-Dusari SN, Fakunle YM. Human brucellosis in Northern Saudi Arabia. Saudi Med J. 2005;26(10):1562–6. [PubMed] [Google Scholar]
- 47.Hackmon R, Bar-David J, Bashiri A, Mazor M. Brucellosis in pregnancy. Harefuah. 1998;135(1–2):3–7. 88. [PubMed] [Google Scholar]
- 48.Young EJ. Human brucellosis. Rev Infect Dis. 1983;5(5):821–42. doi: 10.1093/clinids/5.5.821. [DOI] [PubMed] [Google Scholar]
- 49.Janbon M, de Kerleau C. Brucellose humanie et avortment: Isolement de Brucella melitensis dans le sangde la mere, les visceras, et le sang du foetus, le placenta, et les lochies. Presse Med. 1939;47:453. [Google Scholar]
- 50.Cornell EL, DeYoung CR. The incidence of undulant fever in pregnancy and abortion. Am J Obstet Gynecol. 1929;18:840–4. [Google Scholar]
- 51.Poester FP, Samartino LE, Santos RL. Pathogenesis and pathobiology of brucellosis in livestock. Rev Sci Tech. 2013;32(1):105–15. doi: 10.20506/rst.32.1.2193. [DOI] [PubMed] [Google Scholar]
- 52.Gladstone M, Oliver C, Van den Broek N. Survival, morbidity, growth and developmental delay for babies born preterm in low and middle income countries - a systematic review of outcomes measured. PLoS One. 2015;10(3):e0120566. doi: 10.1371/journal.pone.0120566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmichael LE, Shin SJ. Canine brucellosis: a diagnostician’s dilemma. Semin Vet Med Surg (Small Anim) 1996;11(3):161–5. doi: 10.1016/s1096-2867(96)80028-4. [DOI] [PubMed] [Google Scholar]
- 54.Carmichael LE, George LW. Canine brucellosis: newer knowledge. Dev Biol Stand. 1976;31:237–50. [PubMed] [Google Scholar]
- 55.Carmichael LE, Kenney RM. Canine brucellosis: the clinical disease, pathogenesis, and immune response. J Am Vet Med Assoc. 1970;156(12):1726–34. [PubMed] [Google Scholar]
- 56.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72(2):107–16. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16(4):328–34. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao B, Stout MJ, Lee I, Mysorekar IU. Placental microbiome and its role in preterm birth. Neoreviews. 2014;15(12):e537–45. doi: 10.1542/neo.15-12-e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witkin SS, Linhares IM, Bongiovanni AM, Herway C, Skupski D. Unique alterations in infection-induced immune activation during pregnancy. BJOG. 2011;118(2):145–53. doi: 10.1111/j.1471-0528.2010.02773.x. [DOI] [PubMed] [Google Scholar]
- 60.Kwak-Kim JY, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chem Immunol Allergy. 2005;88:64–79. doi: 10.1159/000087821. [DOI] [PubMed] [Google Scholar]
- 61.Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am J Reprod Immunol. 2002;48(2):77–86. doi: 10.1034/j.1600-0897.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan L, Nguyen T, McComb S. From mice to women: the conundrum of immunity to infection during pregnancy. J Reprod Immunol. 2013;97(1):62–73. doi: 10.1016/j.jri.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27(1):11–8. doi: 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantle SJ, Kaufmann P, Luckhardt M, Schweikhart G. Interpretation of syncytial sprouts and bridges in the human placenta. Placenta. 1987;8(3):221–34. doi: 10.1016/0143-4004(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 65.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63(6):434–44. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 67.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124(5):1872–9. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci U S A. 2015;112(23):7219–24. doi: 10.1073/pnas.1507977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63(6):460–71. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 70.Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–33. doi: 10.1093/humupd/dmv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith H, Williams AE, Pearce JH, Keppie J, Harris-Smith PW, Fitz-George RB, et al. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962;193:47–9. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- 72.Sangari FJ, Grillo MJ, Jimenez De Bagues MP, Gonzalez-Carrero MI, Garcia-Lobo JM, Blasco JM, et al. The defect in the metabolism of erythritol of the Brucella abortus B19 vaccine strain is unrelated with its attenuated virulence in mice. Vaccine. 1998;16(17):1640–5. doi: 10.1016/s0264-410x(98)00063-2. [DOI] [PubMed] [Google Scholar]
- 73.Bosseray N. Brucella infection and immunity in placenta. Ann Inst Pasteur Microbiol. 1987;138(1):110–3. doi: 10.1016/0769-2609(87)90087-1. [DOI] [PubMed] [Google Scholar]
- 74.Anderson TD, Cheville NF, Meador VP. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. II. Ultrastructural studies. Vet Pathol. 1986;23(3):227–39. doi: 10.1177/030098588602300302. [DOI] [PubMed] [Google Scholar]
- 75.Anderson TD, Meador VP, Cheville NF. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. I. Gross and histologic lesions. Vet Pathol. 1986;23(3):219–26. doi: 10.1177/030098588602300301. [DOI] [PubMed] [Google Scholar]
- 76•.Salcedo SP, Chevrier N, Lacerda TL, Ben Amara A, Gerart S, Gorvel VA, et al. Pathogenic brucellae replicate in human trophoblasts. J Infect Dis. 2013;207(7):1075–83. doi: 10.1093/infdis/jit007. First report describing the mechanism of Brucella infection in human trophoblast. [DOI] [PubMed] [Google Scholar]
- 77•.Fernandez AG, Ferrero MC, Hielpos MS, Fossati CA, Baldi PC. Proinflammatory Response of Human Trophoblastic Cells to Brucella abortus Infection and upon Interactions with Infected Phagocytes. Biol Reprod. 2016;94(2):48. doi: 10.1095/biolreprod.115.131706. This article nicely decribed pathophisiology of pregnancy complications in brucellosis using human trophoblastic cell line (Swan 71). [DOI] [PubMed] [Google Scholar]
- 78.Olsen SC, Johnson C. Comparison of abortion and infection after experimental challenge of pregnant bison and cattle with Brucella abortus strain 2308. Clin Vaccine Immunol. 2011;18(12):2075–8. doi: 10.1128/CVI.05383-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis DS, Templeton JW, Ficht TA, Huber JD, Angus RD, Adams LG. Brucella abortus in Bison. II. Evaluation of strain 19 vaccination of pregnant cows. J Wildl Dis. 1991;27(2):258–64. doi: 10.7589/0090-3558-27.2.258. [DOI] [PubMed] [Google Scholar]
- 80.Dawood HA. Brucellosis in Camels (Camelus dromedorius) in the south province of Jordan. Am J Agric Biol Sci. 2008;3:623–6. [Google Scholar]
- 81.Megid J, Mathias LA, Robles CA. Clinical manifestations of brucellosis in domestic animals and humans. Open Vet Sci J. 2010;4:119–26. [Google Scholar]
- 82.Carmichael LE, Kenney RM. Canine abortion caused by Brucella canis. J Am Vet Med Assoc. 1968;152(6):605–16. [PubMed] [Google Scholar]
- 83.Barkallah M, Gharbi Y, Hassena AB, Slima AB, Mallek Z, Gautier M, et al. Survey of infectious etiologies of bovine abortion during mid- to late gestation in dairy herds. PLoS One. 2014;9(3):e91549. doi: 10.1371/journal.pone.0091549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carvalho Neta AV, Mol JP, Xavier MN, Paixao TA, Lage AP, Santos RL. Pathogenesis of bovine brucellosis. Vet J. 2010;184(2):146–55. doi: 10.1016/j.tvjl.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Samartino LE, Enright FM. Pathogenesis of abortion of bovine brucellosis. Comp Immunol Microbiol Infect Dis. 1993;16(2):95–101. doi: 10.1016/0147-9571(93)90001-l. [DOI] [PubMed] [Google Scholar]
- 86.Miller WG, Adams LG, Ficht TA, Cheville NF, Payeur JP, Harley DR, et al. Brucella-induced abortions and infection in bottlenose dolphins (Tursiops truncatus) J Zoo Wildl Med. 1999;30(1):100–10. [PubMed] [Google Scholar]
- 87.Kreeger TJ, Cook WE, Edwards WH, Elzer PH, Olsen SC. Brucella abortus strain RB51 vaccination in elk. II. Failure of high dosage to prevent abortion. J Wildl Dis. 2002;38(1):27–31. doi: 10.7589/0090-3558-38.1.27. [DOI] [PubMed] [Google Scholar]
- 88.Kreeger TJ, Miller MW, Wild MA, Elzer PH, Olsen SC. Safety and efficacy of Brucella abortus strain RB51 vaccine in captive pregnant elk. J Wildl Dis. 2000;36(3):477–83. doi: 10.7589/0090-3558-36.3.477. [DOI] [PubMed] [Google Scholar]
- 89.Edmonds MD, Cloeckaert A, Hagius SD, Samartino LE, Fulton WT, Walker JV, et al. Pathogenicity and protective activity in pregnant goats of a Brucella melitensis Deltaomp25 deletion mutant. Res Vet Sci. 2002;72(3):235–9. doi: 10.1053/rvsc.2002.0555. [DOI] [PubMed] [Google Scholar]
- 90.Elzer PH, Enright FM, McQuiston JR, Boyle SM, Schurig GG. Evaluation of a rough mutant of Brucella melitensis in pregnant goats. Res Vet Sci. 1998;64(3):259–60. doi: 10.1016/s0034-5288(98)90135-7. [DOI] [PubMed] [Google Scholar]
- 91.Phillips RW, Elzer PH, Robertson GT, Hagius SD, Walker JV, Fatemi MB, et al. A Brucella melitensis high-temperature-requirement A (htrA) deletion mutant is attenuated in goats and protects against abortion. Res Vet Sci. 1997;63(2):165–7. doi: 10.1016/s0034-5288(97)90012-6. [DOI] [PubMed] [Google Scholar]
- 92.Zundel E, Verger JM, Grayon M, Michel R. Conjunctival vaccination of pregnant ewes and goats with Brucella melitensis Rev 1 vaccine: safety and serological responses. Ann Rech Vet. 1992;23(2):177–88. [PubMed] [Google Scholar]
- 93.Gidlewski T, Cheville NF, Rhyan JC, Miller LD, Gilsdorf MJ. Experimental Brucella abortus induced abortion in a llama: pathologic effects. Vet Pathol. 2000;37(1):77–82. doi: 10.1354/vp.37-1-77. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Sancho M, Duran-Ferrer M, Garcia-Seco T, Macias P, Garcia N, Martinez I, et al. Interferon-gamma responses in sheep exposed to virulent and attenuated Brucella melitensis strains. Vet Immunol Immunopathol. 2014;160(1–2):123–8. doi: 10.1016/j.vetimm.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 95.Duran-Ferrer M, Leon L, Nielsen K, Caporale V, Mendoza J, Osuna A, et al. Antibody response and antigen-specific gamma-interferon profiles of vaccinated and unvaccinated pregnant sheep experimentally infected with Brucella melitensis. Vet Microbiol. 2004;100(3–4):219–31. doi: 10.1016/j.vetmic.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Verger JM, Grayon M, Zundel E, Lechopier P, Olivier-Bernardin V. Comparison of the efficacy of Brucella suis strain 2 and Brucella melitensis Rev. 1 live vaccines against a Brucella melitensis experimental infection in pregnant ewes. Vaccine. 1995;13(2):191–6. doi: 10.1016/0264-410x(95)93135-v. [DOI] [PubMed] [Google Scholar]
- 97.OIE. Porcine Brucellosis. OIE Terrestrial Manual. 2012 [Google Scholar]