Abstract
Geraniin, a hydrolysable tannin, used in traditional medicine in Southeast Asia, is known to exhibit various biological activities. As an antioxidant it is known to up-regulate phase II enzyme Heme oxygenase-1 (HO-1). However its mechanism is not clearly understood. Nuclear factor erythroid-derived 2 related factor 2 (Nrf-2) is transcriptionally up-regulated by Extracellular signal-regulated kinase (ERK) 1/2 and retained in nucleus due to inactivated Glycogen synthase kinase 3 beta (GSK-3β). Geraniin additionally down-regulates expression of microRNA 217 and 377 (miR-217 and miR-377) which target HO-1 mRNA. Expression of BTB and CNC homolog 1 (BACH-1), another regulator of HO-1, is also down-regulated by up-regulating microRNA 98 (miR-98), a negative regulator of BACH-1. Thus, geraniin up-regulates HO-1 expression both through activating its positive regulator Nrf-2 and by down-regulating its negative regulator BACH-1. Up-regulation of HO-1 also confers protection to HepG2 cells from tertiary butyl hydroperoxide (TBH) induced cytotoxicity.
Keywords: Antioxidants, Geraniin, HO-1, miRNAs, Nrf-2
INTRODUCTION
Reactive oxygen species (ROS) are generated as byproducts of metabolism or due to extracellular stress. Though, low levels of ROS are necessary for cells as activators of signal transduction pathways, excessive generation of ROS leads to oxidative stress (OS) where biological molecules such as proteins, lipids, and DNA are damaged ultimately resulting in cell death via apoptosis or necrosis (1). OS also plays an important role in the pathogenesis and progression of different diseases like cancer, diabetes, liver cirrhosis, etc. (2, 3). To overcome damaging effects of ROS, under normal conditions, cells activate their defense mechanisms through endogenous antioxidant enzymes and molecules. However, to ensure sufficient levels of antioxidants, very often natural antioxidant molecules are used as dietary supplements (4, 5).
Geraniin is a hydrolyzable tannin which has a unique dehydrohexahydroxydiphenoyl moiety and is widely distributed amongst dicotyledonous plants. There have been several studies on geraniin, either as a plant extract or pure compound, where it has been shown to exhibit strong antioxidative potential (6, 7) as well as anticancer, antiviral, antihypertensive and antihyperglycaemic (8–12) activities.
Cellular antioxidant defense system consists of first line enzymes such as superoxide dismutase (SOD), catalase (CAT) and phase II detoxification enzymes such as glutathione S-transferases, glutamate-cysteine ligase, NAD(P)H: quinine oxidoreductase, glutathione peroxidase (GPx) and HO-1. Most of the supplementary antioxidants used are known to activate HO-1. Induction of HO-1 requires Nrf-2 activation, its translocation to nucleus and binding to cis-acting enhancer element in promoter of HO-1, known as ARE (13). Among other regulatory factors binding to ARE motif is BACH-1 which is a transcriptional repressor competing with Nrf-2 for binding to ARE (14, 15). HO-1 and its negative regulator BACH-1 are also regulated by specific microRNAs (miRNAs).
Geraniin is known to up-regulate the expression of HO-1 by activating PI3K/AKT and MAPK signaling pathways (16). In the present study, we demonstrate that up-regulation of HO-1 is by activation of upstream regulators of Nrf-2, namely, GSK-3β and ERK, and on levels of miRNAs involved in regulation of both BACH-1 and HO-1. We have also shown that this up-regulation of HO-1 by geraniin confers cytoprotection to HepG2 cells against TBH induced toxicity by using tin protoporphyrin (SnPP), specific inhibitor of HO-1.
RESULTS
Geraniin exhibits protective effect against TBH-induced cytotoxicity in HepG2 cells
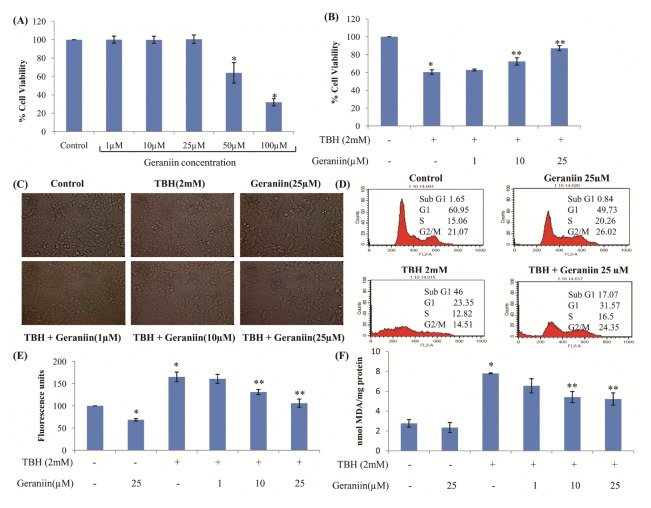
Viability of HepG2 cells exposed to different concentrations of geraniin for 24 hrs was checked. It did not change significantly between 1–25 μM whereas at 50 and 100 μM concentration it was significantly reduced (Fig. 1A). Therefore, the concentration range of 1–25 μM was used for further experiments. To check protective effect of geraniin against TBH-induced OS, cells were pre-treated with geraniin for 2 hrs and then exposed to TBH for 3 hrs. Pretreatment with 10 and 25 μM geraniin significantly protected cells against TBH-induced cytotoxicity (Fig. 1B). Morphological studies also confirmed the same. TBH treated cells were shrunk, rounded and stressed whereas incubation with 10 and 25 μM, but not 1 μM geraniin for 2 hrs restored cell morphology effectively (Fig. 1C). To further confirm these results, cell cycle analysis was performed. As seen in Fig. 1D, in cells treated with geraniin alone, the cell cycle profile remained unaltered, whereas in cells treated with 2 mM TBH for 3 hrs, 46% of cell population was apoptotic. In cells treated with geraniin followed by exposure to TBH, apoptosis was reduced significantly (P < 0.05) to 17%.
Fig. 1.
Geraniin exhibits protective effect against TBH-induced cytotoxicity in HepG2 cells. (A) HepG2 cells were treated with various concentrations of geraniin for 24 h, and the viability was measured by MTT assay. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. control. (B) Cells were pretreated with different concentrations of geraniin (0, 1, 10, and 25 μM) for 2 hrs, followed by exposure to TBH (2 mM). Cell viability was assessed by MTT assay. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. control. **P < 0.05 vs. TBH (2 mM). (C) Cell morphology was observed under inverted microscope. (D) Cell cycle analysis was done in presence and absence of geraniin (25 μM). (E) Inhibition of intracellular ROS generated and (F) lipid peroxidation on TBH treatment in presence of geraniin. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. control. **P < 0.05 vs. TBH (2 mM).
TBH is known to produce ROS and induce lipid peroxidation in cells. Therefore, amount of ROS generated in cells was evaluated by using non-fluorescent DCFH-DA dye. Significant increase in ROS in TBH treated cells was observed which in presence of geraniin was significantly reduced (Fig. 1E). Treatment of cells with TBH increased lipid peroxidation to 7.79 ± 0.04 nmols MDA/mg protein (Fig. 1F) and in cells treated with geraniin this was significantly reduced.
Altered antioxidant enzyme gene expression and GSH level in HepG2 cells treated with TBH is restored in presence of geraniin
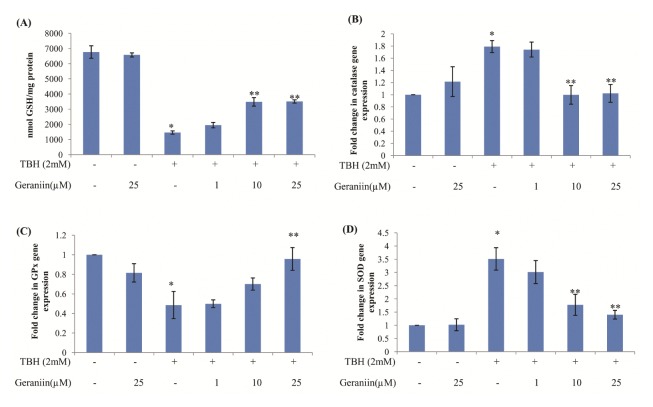
Glutathione, major antioxidant of the cell was found to be reduced significantly in cells exposed to TBH, which was effectively restored by up to 40% in presence of higher concentration of geraniin (Fig. 2A).
Fig. 2.
Altered antioxidant enzyme gene expression and GSH level in HepG2 cells treated with TBH is restored in presence of Geraniin. (A) Intracellular GSH level was determined in terms of nmol GSH/mg protein. (B) CAT (C) GPx and (D) SOD gene expression was measured in HepG2 cells pretreated with geraniin (0, 1, 10, and 25 μM) for 2 hrs and exposed to TBH (2 mM) for 3 hrs. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. control. **P < 0.05 vs. TBH (2 mM).
Expression of antioxidant enzymes such as CAT, GPx and SOD was measured using quantitative real-time PCR (qRT-PCR) and by biochemical assays. Exposure to TBH, significantly increased CAT and SOD expression to 1.79 ± 0.09 and 3.32 ± 0.58 fold respectively and decreased GPx expression to 0.48 ± 0.13 fold. Treatment with 25 μM geraniin significantly restored the level of all antioxidant enzymes. Geraniin by itself did not alter level of antioxidant enzymes (Fig. 2B–D).
Geraniin increases HO-1 expression through Nrf-2 nuclear translocation
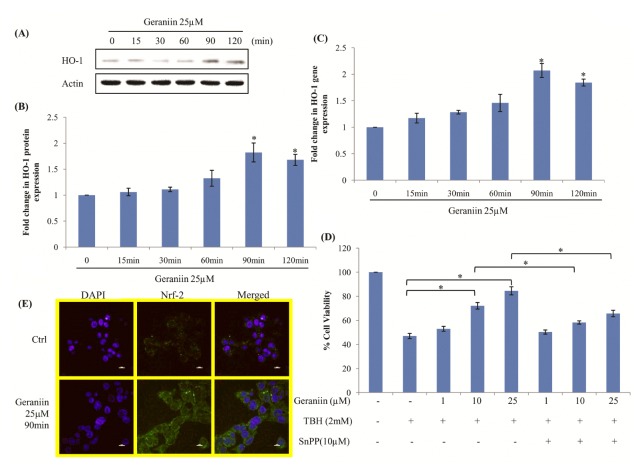
Activation of HO-1, by geraniin, was checked both by western blot and qRT-PCR. Cells were treated with 25 μM geraniin for different time intervals and expression of HO-1 was evaluated. HO-1 increased in a time-dependent manner with maximum expression observed at 90 min both by western blot analysis and qRT-PCR (Fig. 3A–C). To confirm that cytoprotective effect of geraniin against TBH is indeed due to HO-1 expression, cells were treated with SnPP which is a potent competitive inhibitor of HO-1. SnPP significantly suppressed cell protection exerted by geraniin indicating involvement of up-regulated HO-1 in conferring cytoprotection (Fig. 3D). Since Nrf-2 is a known potent regulator of HO-1, its localization was determined by immunostaining. We observed that treatment with geraniin significantly up-regulates Nrf-2 which translocates to nucleus (Fig. 3E).
Fig. 3.
Geraniin increases HO-1 expression by Nrf-2 nuclear translocation in cells. Cells were treated with 25 μM geraniin at different time points and HO-1 expression was determined by (A) Western blot analysis, (B) its densitogram analysis and (C) qRT-PCR. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. Control. Cells treated with Geraniin (D) To confirm involvement of HO-1 in protection caused by Geraniin, cells were treated with 1, 10 and 25 μM of geraniin and 10 μM SnPP, and were subsequently exposed to TBH (2 mM) for 3 hrs. Cell viability was determined by MTT. Data shown represent the mean values of three experiments ± SE. *P < 0.05. (E) Nrf-2 a positive regulator of HO-1 was detected by immunolocalisation in control and 25 μM geraniin treated cells for 90 mins.
Geraniin phosphorylates upstream regulators of Nrf-2 namely ERK1/2 and GSK-3β
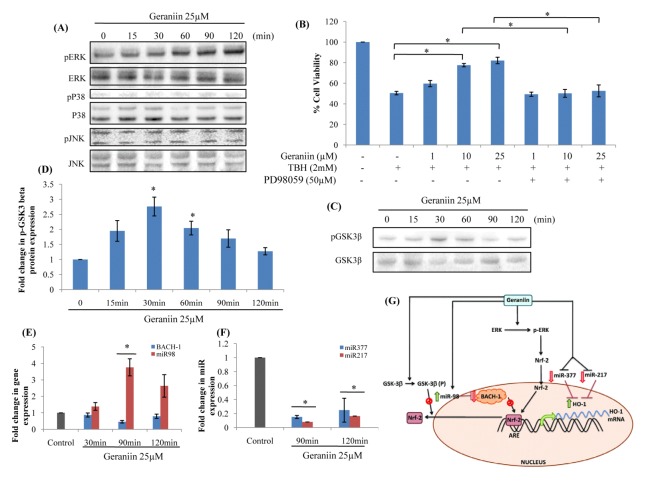
MAPKs such as ERK1/2, JNK and P38 are known to be involved in activation of Nrf-2 pathway (17). Under OS, these get phosphorylated and activate Nrf-2 cascade. Treatment with geraniin led to time-dependent induction of only ERK phosphorylation (Fig. 4A). To confirm the involvement of activated ERK in protection exhibited by geraniin, an inhibitor against ERK (PD98059, 2′-amino-3′-methoxyflavone) was used and cell protection was checked. Inhibition of ERK effectively repressed cytoprotection caused by geraniin confirming its involvement in induction of HO-1 through Nrf-2 (Fig. 4B).
Fig. 4.
Geraniin activates HO-1 by regulating upstream targets. (A) Western blot analysis of pERK, pJNK and pP38 MAPKs in HepG2 cells subjected to 25 μM geraniin at various time points. (B) To confirm the role of pERK MAPK in protection offered by geraniin, cells were treated with 0, 1, 10, 25 μM geraniin and 50 μM PD98059, an inhibitor of ERK1/2, and were subsequently exposed to TBH (2 mM) for 3 hrs. Cell viability was determined by MTT. Data shown represent the mean values of three experiments ± SE. (C) Western blot analysis of pGSK-3β and (D) its densitogram analysis, in HepG2 cells subjected to 25 μM geraniin at indicated time periods. (E) qRT-PCR analysis of BACH-1 and its negative regulator, miR-98 expression and (F) negative regulators of HO-1, miR-377 and miR-217 expression in HepG2 cells treated with 25 μM geraniin at indicated time periods. Data shown represent the mean values of three experiments ± SE. *P < 0.05 vs. Control. (G) Proposed molecular mechanism of cytoprotective role of geraniin in HepG2 cells.
GSK-3β is known negative regulator of Nrf-2. In presence of geraniin, increase in pGSK-3β (ser9) was observed leading to its inactivation (Fig. 4C and D). Inactivation of GSK-3β leads to retention of Nrf-2 in the nucleus favouring induction of HO-1.
Geraniin regulates miRNA targets of HO-1 and BACH-1
Another important negative regulator of HO-1 is BACH-1 which competes with Nrf-2 in binding to ARE motif. The expression of BACH-1 was significantly reduced at 90 min. Expression of miR-98 a known negative regulator of BACH-1 was checked. We indeed observed, increase in miR-98 expression at 90 mins, which led to decrease in BACH-1 expression (Fig. 4E).
miR-377 and miR-217 are negative regulators of HO-1 which affect gene expression post-transcriptionally by interacting with HO-1 mRNA and inhibiting translation. Significant decrease in expression of both miR-377 and miR-217 was observed in presence of geraniin (Fig. 4F).
DISCUSSION
Geraniin is a dehydroellagitannin, present in many medicinal herbs known to exhibit different biological activities among which antioxidant activity has been widely studied (6). Cytoprotective effects of geraniin against OS has been shown previously in different cell types (16, 18, 19). In a recent study geraniin has shown to exhibit cytoprotective effect by reducing H2O2-induced cell death in vitro by activating PI3K/AKT and ERK1/2 pathways and Nrf-2 signaling (16). We initially confirmed radical scavenging property of geraniin using standard radical scavenging assays (Supplementary Fig. 1) and observed that it reduced formation of lipid peroxides, restored protein sulphydryl groups and also inhibited formation of single/double strand breaks in plasmid DNA against OS in a concentration-dependent manner (Supplementary Fig. 2).
In cells treated with geraniin, TBH induced cytotoxicity, ROS production and lipid peroxidation was significantly reduced. TBH treatment led to increase in antioxidant defense enzymes namely SOD and CAT, while GPx was decreased. Transcripts of all these enzymes were restored on geraniin treatment. Geraniin by itself did not activate any one of these enzymes. GSH, a major redox buffer of cell was observed to be decreased on TBH treatment and this was prevented significantly in presence of Geraniin. All these results, demonstrate a strong antioxidant potential of geraniin.
HO-1, an important phase II antioxidant enzyme was strongly up-regulated in presence of geraniin and its involvement in cytoprotective effect against TBH was confirmed by using SnPP, competitive inhibitor of HO-1 activity. Induction of HO-1 was due to up-regulation of Nrf-2, a known positive regulator of HO-1. Since, MAP kinases are known to be involved in activation of Nrf-2, the expression of p38, JNK and ERK was checked and only ERK was found to be phosphorylated in presence of geraniin similar to a previous report (16). This was further reconfirmed by using ERK inhibitor (PD98059), in whose presence cytoprotection offered by geraniin was significantly reduced.
GSK-3β normally promotes degradation of Nrf-2. If GSK-3β is inactivated, Nrf-2 is not degraded and accumulates in the nucleus. We indeed observed increase in pGSK-3β (Ser 9) on geraniin treatment which inactivates the enzyme leading to retention of Nrf-2 in the nucleus which was confirmed by immunostaining. Thus, we have shown that both activation of ERK and inactivation of GSK-3β are important for up-regulation of Nrf-2 on geraniin treatment, which then leads to HO-1 activation.
miR-217 and miR-377 directly regulate HO-1 expression (20). Both these miRNAs were significantly reduced in presence of geraniin which helped in enhanced translation of HO-1 mRNA.
Another regulator of HO-1 is BACH-1, which competes with Nrf-2 in binding to ARE motif and represses downstream gene expression. On its release form ARE motif, it allows Nrf-2 to bind to its enhancer sequence and activate gene expression (15). We observed that in the cells treated with geraniin, BACH-1 expression was significantly reduced. BACH-1, itself is also known to be regulated negatively by a number of miRNAs namely, let-7b, Let-7c, miR-196, miR-98. Out of these we found only miR-98 to be significantly increased when BACH-1 expression was significantly reduced.
Present study thus confirms cytoprotective effect of geraniin against TBH induced cytotoxicity in HepG2 cells via HO-1 induction. HO-1 activation by geraniin is brought about both by up-regulating its positive regulator Nrf-2 and down-regulating its negative regulator BACH-1 and HO-1 specific microRNAs (miR-217 and miR-377) (Fig. 4G).
MATERIALS AND METHODS
Chemicals
Dulbecco’s modified essential media (DMEM), Foetal Bovine Serum (FBS), trypsin and Penicillin-streptomycin were purchased from Gibco. TBH, 2-thiobarbituric acid (TBA), 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Aldrich, USA. Reduced glutathione, potassium phosphate, sodium carbonate, trichloroacetic Acid (TCA) and 5,5′-dithio-bis (2 nitrobenzoic acid) (DTNB) were obtained from Sisco Research Laboratory, India. Other common chemicals used in our studies were of the highest quality, commercially available from local suppliers. Geraniin (purity of > 90%) was procured from AJ organic Pvt. Ltd., Pune, India.
Cell culture
HepG2 cell line was procured from National Centre for Cell Science, Pune, India. These cells were grown in DMEM supplemented with 10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, in humidified atmosphere with 5% CO2 at 37°C.
Cell viability assay and morphology examination
2 × 104 cells were seeded per well in 96 well plate and incubated at 37°C in CO2 incubator for 48 hrs. Cells were then exposed to different concentrations of geraniin (1, 10, 25, 50 and 100 μM) and TBH. Cell viability was determined using MTT assay (21). Morphological changes were observed under a microscope, and images were taken with an Olympus digital camera (Olympus, Japan).
Cell cycle analysis
Control and treated cells were washed with 1× phosphate buffered saline (1× PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) and fixed at 4°C for 2 hrs using 70% ethanol. The single cell suspension was then treated with RNase A (100 mg/ml) at 37°C for 30 mins followed by staining with 50 mg/ml propidium iodide (22). Stained cells were analyzed with FACSCanto, using cell quest software (BD, USA). Cell cycle profile was obtained by analyzing 10,000 cells per sample.
Measurement of TBARS
Lipid peroxidation was performed following the protocol described by Ohkawa et al., (23) with slight modification. In brief, 1.5 × 106 cells were seeded in 60 mm plates and incubated at 37°C for 48 hrs. Cells were then treated with geraniin (1, 10 and 25 μM) for 2 hrs, followed by TBH exposure for 3 hrs. These were washed with 1× PBS, collected and homogenized in 100 μl protein extraction buffer. 600 μl TBA reagent (0.8% (w/v) TBA in 20% TCA) was added to each tube and vortexed. The reaction mixture was incubated at 95°C for 20 mins and reaction was terminated by keeping tubes on ice. Reaction mixture was centrifuged at 10,000 rpm for 10 mins and the absorbance of the total thiobarbituric reactive substances (TBARS) in supernatant was measured fluorometerically at Ex/Em wave length of 535/585 nm and expressed as nmoles of malondialdehyde (MDA) equivalents formed/mg protein.
ROS measurement
1 × 104 cells were seeded per well in 96 well plate and after 48 hrs incubation, they were treated with geraniin (1, 10 and 25 μM) for 2 hrs. Cells were then stained with 10 mM DCFH-DA in serum free DMEM for 30 mins in the dark. Cells were washed twice with serum free DMEM and treated with 2 mM TBH for 3hrs. Fluorescence was recorded at an Ex/Em wavelength of 485/530 nm (24).
Glutathione assay
Glutathione assay was carried out using DTNB (25). 1.5 × 106 cells were seeded in 60 mm plates, incubated at 37°C for 48 hrs and treated with geraniin (1, 10 and 25 μM) for 2 hrs followed by exposure to TBH for 3 hrs. Cells were then harvested and lysed. Proteins were transferred to fresh tubes followed by sulfosalicycilc acid addition. To each sample DTNB was added and the rate of its conversion to colored product was measured spectrophotometrically at 412 nm using ELISA reader. A standard curve was plotted using different concentrations of GSH.
RNA Isolation and qRT-PCR amplification
1 × 106 cells were seeded in 6 well plate and incubated at 37°C for 48 hrs. They were then treated with geraniin for 2 hrs followed by TBH exposure for 3 hrs. Cells were then washed with ice-cold PBS and RNA was isolated using TRIzol reagent. It was precipitated using isopropanol and dissolved in Diethyl pyrocarbonate (DEPC) treated water. After quantification, 2 μg RNA was used for reverse transcription using cDNA synthesis kit (Thermo scientific, USA) according to manufacturers instruction. Resulting cDNA was used for qRT-PCR amplification (Step One Plus, Applied Biosystems, USA) using gene specific primers and actin was used as an internal control. From the same RNA using stem-loop primers, specific for microRNA, cDNA was synthesized and qRT-PCR was performed using miRNA-specific primers (26) (Supplementary Fig. 3).
Western blot analysis
HepG2 cells were harvested and washed with 1× PBS and lysed in 20 mM Tris-Cl buffer (pH 7.4) containing protease inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride (PMSF). Protein concentration was estimated using Lowry method and 40 μg protein from each sample was resolved on 12% SDS-PAGE, followed by transfer onto a polyvinylidene difluoride (PVDF) membrane. Membrane was blocked with 5% (w/v) bovine serum albumin or non-fat dry milk for 90 mins, incubated with the primary antibodies against p-JNK, p-p38, p-ERK and total ERK (Cell Signaling Technology, USA), total JNK, total p38 (Santa Cruz, USA), followed by incubation with peroxidase-conjugated secondary antibody for 1 hr and finally detection by Optiblot ECL detect kit (Abcam, UK) using ChemiDoc (BioRad, US).
Immunofluorescence assay
5 × 105 cells were cultured on cover slips in 6 well plate for 24 hrs and incubated in presence or absence of geraniin for 90 mins. Cells were then washed with 1× PBS, fixed with Methanol:Acetone (3:1) and blocked with blocking buffer (3% BSA, 0.3% Triton X-100 in 1× PBS) over night. Cells were incubated with rabbit anti-Nrf-2 antibody for 1 hr followed by incubation with Alexa Fluor 488-labeled goat anti-rabbit IgG secondary antibody. Finally they were washed thoroughly, stained with 4′,6-diamidino-2-phenylindole (DAPI) and cover slips were mounted with Vectashield (Vector Laboratories, USA).
Statistical analysis
Throughout the text, data are expressed as the mean ± SE of at least three independent experiments. To determine statistical significance among different groups, one-way analysis of variance (ANOVA), followed by post-hoc test was performed using SPSS 19.0 software. P < 0.05 was considered as statistically significant.
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by University Grant Commission-Centre for Advance Studies (UGC-CAS) [F-5-2/2005(SAP-II)], Government of India, Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) [GOI-A-670], Government of India, India. HA was recipient of fellowship from Indian Council for Cultural Relations (ICCR).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 2.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E. Basic mechanisms of antioxidant activity. Biofactors. 1997;6:391–397. doi: 10.1002/biof.5520060404. [DOI] [PubMed] [Google Scholar]
- 6.Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules. 2010;15:1453–1465. doi: 10.3390/molecules15031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Londhe JS, Devasagayam TP, Foo LY, Ghaskadbi SS. Antioxidant activity of some polyphenol constituents of the medicinal plant Phyllanthus amarus Linn. Redox Rep. 2008;13:199–207. doi: 10.1179/135100008X308984. [DOI] [PubMed] [Google Scholar]
- 8.Wongnoppavich A, Kanjana J, Seewaboon S. Triphala: The Thai traditional herbal formulation for cancer treatment. Songklanakarin J Sci Technol. 2009;31:139–149. [Google Scholar]
- 9.Notka F, Meier GR, Wagner R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antiviral Res. 2003;58:175–186. doi: 10.1016/S0166-3542(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-beta-D-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J Ethnopharmacol. 2007;110:555–558. doi: 10.1016/j.jep.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Lin SY, Wang CC, Lu YL, Wu WC, Hou WC. Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem Toxicol. 2008;46:2485–2492. doi: 10.1016/j.fct.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Palanisamy UD, Ling LT, Thamilvaani M, David A. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011;127:21–27. doi: 10.1016/j.foodchem.2010.12.070. [DOI] [Google Scholar]
- 13.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 15.Oyake T, Itoh K, Motohashi H, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/MCB.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Peng X, Wei ZF, et al. Geraniin exerts cytoprotective effect against cellular oxidative stress by upregulation of Nrf2-mediated antioxidant enzyme expression via PI3K/AKT and ERK1/2 pathway. Biochim Biophys Acta. 2015;1850:1751–1761. doi: 10.1016/j.bbagen.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling LT, Saito Y, Palanisamy UD, Cheng HM, Noriko N. Cytoprotective effects of geraniin against peroxynitrite- and peroxyl radical-induced cell death via free radical scavenging activity. Food Chem. 2012;132:1899–1907. doi: 10.1016/j.foodchem.2011.12.024. [DOI] [Google Scholar]
- 19.Kang KA, Lee IK, Zhang R, et al. Radioprotective effect of geraniin via the inhibition of apoptosis triggered by gamma-radiation-induced oxidative stress. Cell Biol Toxicol. 2011;27:83–94. doi: 10.1007/s10565-010-9172-4. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JD, Chen C, Nguyen J, et al. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem. 2011;286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Mittal SPK, Kulkarni AP, Mathai J, Chattopadhyay S, Pal JK. Dose-dependent differential response of mammalian cells to cytoplasmic stress is mediated through the heme-regulated eIF2α kinase. Int J Biochem Cell Biol. 2014;5:186–197. doi: 10.1016/j.biocel.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Sohn JH, Han KL, Choo JH, Hwang JK. Macelignan protects HepG2 cells against tert-butylhydroperoxide-induced oxidative damage. Biofactors. 2007;29:1–10. doi: 10.1002/biof.5520290101. [DOI] [PubMed] [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Kramer MF. Stem-loop RT-qPCR for miRNAs. Curr Protoc Mol Biol. 2011;Chapter 15(Unit 15):10. doi: 10.1002/0471142727.mb1510s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.