Abstract
Intercellular adhesion molecule-1 (ICAM-1), which is induced by tumor necrosis factor (TNF)-α, contributes to the entry of immune cells into the site of inflammation in the skin. Here, we show that protein tyrosine phosphatase non-receptor type 21 (PTPN21) negatively regulates ICAM-1 expression in human keratinocytes. PTPN21 expression was transiently induced after stimulation with TNF-α. When overexpressed, PTPN21 inhibited the expression of ICAM-1 in HaCaT cells but PTPN21 C1108S, a phosphatase activity-inactive mutant, failed to inhibit ICAM-1 expression. Nuclear factor-κB (NF-κB), a key transcription factor of ICAM-1 gene expression, was inhibited by PTPN21, but not by PTPN21 C1108S. PTPN21 directly dephosphorylated phospho-inhibitor of κB (IκB)-kinase β (IKKβ) at Ser177/181. This dephosphorylation led to the stabilization of IκBα and inhibition of NF-κB activity. Taken together, our results suggest that PTPN21 could be a valuable molecular target for regulation of inflammation in the skin by dephosphorylating p-IKKβ and inhibiting NF-κB signaling.
Keywords: Inhibitor of κB kinase, Intercellular adhesion molecule-1, Nuclear factor kappaB, Protein tyrosine phosphatase non-receptor type 21, Skin inflammation
INTRODUCTION
A critical step in the development of inflammatory skin diseases is the infiltration of various immune cells, such as monocytes, neutrophils, and activated T cells, from the blood into the skin (1). Upon stimulation with proinflammatory agents, including tumor necrosis factor (TNF)-α and interferon (IFN)-γ, keratinocytes in the skin express proinflammatory cytokines, chemokines, and adhesion molecules, like intercellular adhesion molecule-1 (ICAM-1), which contribute to the entry of immune cells from the blood into the site of inflammation in the skin (2, 3).
TNF-α is a major proinflammatory cytokine produced by a number of cells, including keratinocytes (4). Stimulation of keratinocytes with TNF-α leads to activation of nuclear factor-κB (NF-κB) and subsequently increases the expression of adhesion molecules and proinflammatory genes (5, 6). NF-κB activation by TNF-α is due to the activation of the inhibitor of κB (IκB)-kinases (IKKs) complex, an upstream kinase complex of IκBα, which phosphorylates IκBα at Ser32/36, resulting in its ubiquitination and subsequent proteasomal degradation (7). Degradation of IκBα leads to translocation of cytosolic NF-κB complexes into the nucleus, where they activate the transcription of proinflammatory target genes (7).
Protein tyrosine phosphatase non-receptor type 21 (PTPN21), also known as PTPD1, is localized in the cytoplasm and phosphorylated by the proto-oncogene tyrosine-protein kinase (Src) (8, 9). It also upregulates Src by dephosphorylating Src at Tyr527 in response to stimulation by growth factors (10). PTPN21 forms a complex with actin, Src, and focal adhesion kinase (FAK), localizes at focal adhesion sites, and exerts major effects on cell adhesion, scattering, and migration (11). Through the formation of complex with A-kinase anchor protein 121 (AKAP121), PTPN21 plays diverse roles in cellular processes. Efficient maintenance of mitochondrial membrane potential and ATP oxidative synthesis occur via the PTPN21-Src-AKAP121 complex (12). When not in complex with AKAP121, PTPN21 activates extracellular signal-regulated kinases 1/2- and Elk1-dependent gene transcription by directing epidermal growth factor/Src signaling to the nucleus (10). PTPN21 activates Etk, a member of the Tec family kinases, by specific interaction with Etk, resulting in the activation of Janus kinase 2-independent signal transducer and activator of transcription 3 (13). However, other substrates of PTPN21 involved in the regulation of cellular signal transduction pathways remain to be identified.
Previous studies on the regulation of ICAM-1 expression and skin inflammation focused on the findings of chemical or plant-extracted components and the elucidation of their mechanisms of action. In this study, we investigated PTP candidates that can regulate the expression of ICAM-1 and identified PTPN21 as a potential candidate. We examined the effect of PTPN21 on TNF-α-induced ICAM-1 expression and the role of PTPN21 in the regulation of NF-κB signaling in a human keratinocyte cell line, HaCaT. Our study may provide a basis for the therapeutic use of PTPN21 in inflammatory skin diseases.
RESULTS
TNF-α regulates ICAM-1 and PTPN21 expression in HaCaT cells
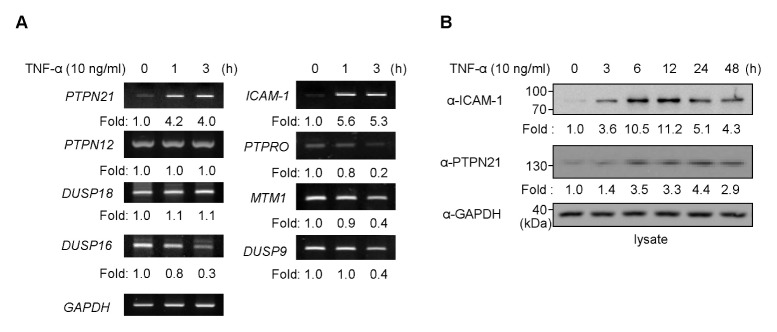
TNF-α is one of the most critical cytokines that induce the expression of ICAM-1, which is an important adhesion molecule for the recruitment of various immune cells to inflammatory sites. To identify PTPs that are regulated by TNF-α in keratinocytes, we investigated how PTP gene expression changes upon TNF-α treatment. ICAM-1 mRNA expression was induced 1 h after TNF-α treatment and remained constant until 3 h. Of the forty-nine PTPs tested (Supplementary table 1), PTPN21 expression was induced by TNF-α treatment, whereas other PTPs showed unchanged or decreased expression (Fig. 1A). This implies that the PTPN21 gene is induced by TNF-α and might be involved in the regulation of ICAM-1 expression. We then measured the expression profiles of PTPN21 and ICAM-1 proteins at different time points of TNF-α treatment to investigate the relationship between PTPN21 and ICAM-1 expression (Fig. 1B). ICAM-1 expression started to increase at 3 h, showed maximal expression at 12 h, and decreased from 24 h after TNF-α treatment, whereas PTPN21 expression increased until 24 h after TNF-α treatment and thereafter decreased.
Fig. 1.
TNF-α treatment regulates PTPN21 expression. (A) After stimulation of HaCaT cells with TNF-α (10 ng/ml) for the indicated time periods (0, 1, and 3 h), total RNA was extracted and cDNA was synthesized. Expression levels of PTPs in each sample were determined by RT-PCR. Expression levels of each PTP, relative to GAPDH levels, were represented as fold changes in comparison with the untreated group. (B) Total cell lysates were prepared after TNF-α (10 ng/m) stimulation for the indicated time periods (0, 3, 6, 12, 24, and 48 h). Immunoblotting analysis was performed using anti-ICAM-1 and anti-PTPN21 antibodies. Each protein level was normalized to endogenous GAPDH levels and the relative values compared to the untreated group were represented as fold changes.
PTPN21 inhibits TNF-α-induced ICAM-1 expression in HaCaT cells
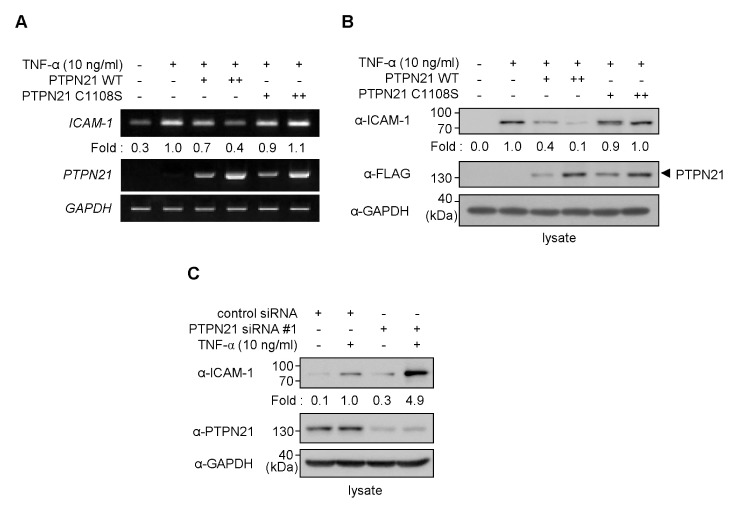
As shown in Fig. 1B, a correlation between PTPN21 and ICAM-1 expression upon TNF-α treatment was observed. We, therefore, investigated the effect of PTPN21 on TNF-α-induced ICAM-1 expression in HaCaT cells. HaCaT cells were transfected with either PTPN21 wild type (WT) or a catalytically inactive C1108S mutant expression plasmid, and stimulated with TNF-α prior to mRNA extraction (Fig. 2A). TNF-α treatment increased ICAM-1 mRNA expression in HaCaT cells. While overexpression of PTPN21 WT inhibited the TNF-α-induced expression of ICAM-1, PTPN21 C1108S overexpression failed to do so. Similarly, TNF-α-induced ICAM-1 protein levels were tightly regulated by PTPN21 WT, but not by PTPN21 C1108S (Fig. 2B). These results suggest that PTPN21 WT regulates ICAM-1 gene expression and its phosphatase activity is required for gene regulation.
Fig. 2.
PTPN21 inhibits ICAM-1 production. (A and B) HaCaT cells were transfected with FLAG-PTPN21 plasmids (WT or C1108S; 1 μg for + or 2 μg for ++) for 48 h. (A) After stimulation with TNF-α (10 ng/ml) for 1 h, total RNA was extracted and cDNA was synthesized. Expression levels of ICAM-1 and PTPN21 in each sample were measured by RT-PCR. ICAM-1 expression levels were normalized to GAPDH levels and the relative values compared to the TNF-α-treated group were represented as fold changes. (B) Total cell lysates were prepared after TNF-α (10 ng/ml) stimulation for 12 h. Immunoblotting analysis was performed using appropriate antibodies. ICAM-1 protein levels were normalized to endogenous GAPDH levels and the relative values compared to the TNF-α-treated group were represented as fold changes. (C) HaCaT cells were transfected with control siRNA (50 nM) or PTPN21 siRNA#1 (100 nM) for 45 h and were stimulated with TNF-α (10 ng/ml) for 3 h. ICAM-1 protein levels were determined by immunoblotting analysis with appropriate antibodies. ICAM-1 protein levels were normalized to endogenous GAPDH levels and the relative values compared to control siRNA-transfected group were represented as fold changes.
To confirm that PTPN21 affects the expression levels of ICAM-1, knockdown of PTPN21 gene expression was performed by transfecting HaCaT cells with PTPN21-targeting siRNAs. By performing siRNA transfection followed by immunoblotting analysis with anti-PTPN21 antibody, siRNA#1 was selected to inhibit the expression of PTPN21 in HaCaT cells throughout this study (Supplementary Fig. 1). PTPN21 knockdown enhanced the TNF-α-induced expression of ICAM-1, compared to transfection with non-targeting control siRNA (Fig. 2C). This result suggests that PTPN21 negatively regulates the TNF-α-induced ICAM-1 expression in HaCaT cells.
PTPN21 inhibits TNF-α-induced NF-κB activation in HaCaT cells
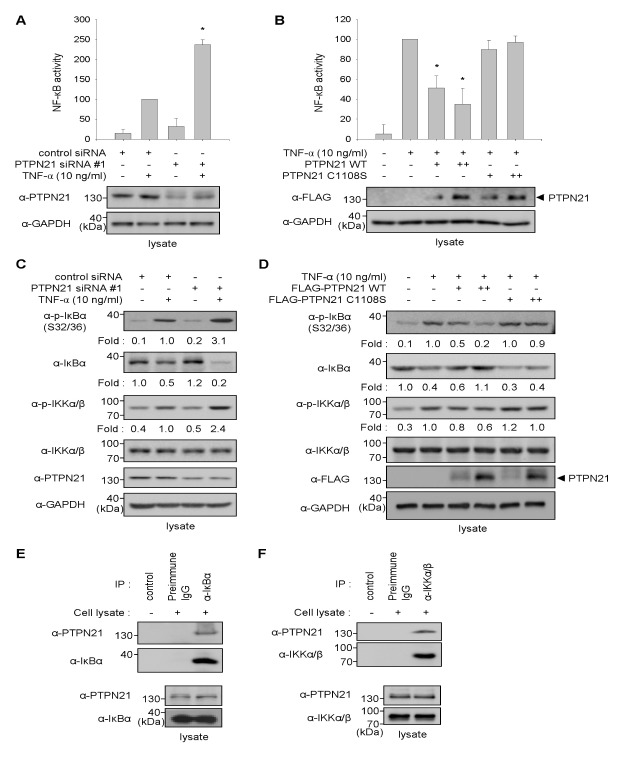
TNF-α activates NF-κB signaling to induce ICAM-1 expression (14). To verify that PTPN21-mediated decrease in ICAM-1 expression is a result of NF-κB inhibition, knockdown of endogenous PTPN21 expression in HaCaT cells was performed by transfection with PTPN21 siRNA#1. NF-κB transcriptional activities were then measured using a NF-κB reporter system. As shown in Fig. 3A, in the absence of TNF-α treatment, NF-κB transcriptional activities, which are represented as luciferase activities, were not distinguishable between control siRNA- and PTPN21 siRNA#1-transfected cells. However, PTPN21 knockdown cells showed a significant increase in NF-κB activity compared to control siRNA-transfected cells upon exposure to TNF-α.
Fig. 3.
PTPN21 inhibits NF-κB activation. (A) HaCaT cells were transfected with pNF-κB-luc reporter plasmid containing a luciferase gene and a gWIZ-GFP plasmid, and subsequently transfected with control siRNA (50 nM) or PTPN21 siRNA#1 (100 nM). After 24 h of incubation, the cells were treated with TNF-α (10 ng/ml) and further incubated for 24 h. The luciferase activities of each group were calculated using GFP fluorescence values as an internal control for transfection efficiency. Luciferase activities relative to control siRNA-transfected group were represented as a bar graph. *P < 0.05 relative to the TNF-α-treated group. (B) HaCaT cells were transfected with pNF-κB-luc reporter plasmid containing a luciferase gene and a gWIZ-GFP plasmid, and subsequently transfected with FLAG-PTPN21 WT or C1108S plasmids (1 μg for + or 2 μg for ++). After 24 h of incubation, the cells were treated with TNF-α (10 ng/ml) and further incubated for 24 h. The luciferase activities of each group were calculated using GFP fluorescence values as an internal control for transfection efficiency. Luciferase activities relative to the TNF-α-treated group were represented as a bar graph. *P < 0.05 relative to the TNF-α-treated group. (C) HaCaT cells were transfected with control siRNA (50 nM) or PTPN21 siRNA#1 (100 nM) for 48 h and were stimulated with TNF-α (10 ng/ml) for 10 min. The levels of p-IκBα, IκBα, and p-IKKα/β were determined by immunoblotting analysis with appropriate antibodies. p-IκBα and IκBα levels were normalized to endogenous GAPDH levels, and p-IKKα/β levels were normalized to total IKK levels. The relative values of each protein were represented as fold changes compared to control siRNA-transfected group. (D) HaCaT cells were transfected with FLAG-PTPN21 plasmids (WT or C1108S; 1 μg for + or 2 μg for ++) for 48 h. Total cell lysates were prepared after TNF-α (10 ng/ml) stimulation for 15 min. Immunoblotting analysis was performed using appropriate antibodies. Relative levels of p-IκBα and IκBα were normalized to endogenous GAPDH levels, and that of p-IKKα/β (p-Ser176/180 of IKKα and p-Ser177/181 of IKKβ) was normalized to total IKKα/β levels. The values relative to the TNF-α-treated group were represented as fold changes. (E and F) Total cell lysates from HaCaT cells were immunoprecipitated with anti-IκBα (E) or anti-IKKα/β (F) antibody. The immunoprecipitates were subjected to immunoblotting analysis with anti-PTPN21 antibody to detect interaction. Endogenous expression of PTPN21, IκBα, and IKKα/β in total cell lysates was confirmed by immunoblotting analysis with each antibody.
Next, we investigated the effect of PTPN21 WT or C1108S overexpression on TNF-α-induced NF-κB transcriptional activity in HaCaT cells. As shown in Fig. 3B, TNF-α-induced NF-κB activity was reduced by PTPN21 WT, whereas PTPN21 C1108S did not exert any inhibitory effect on NF-κB activity. These results imply that PTPN21 inhibits NF-κB transcriptional activity in a phosphatase activity-dependent manner.
PTPN21 inhibits IκB and IKKs phosphorylation in HaCaT cells by its phosphatase activity
The reporter assay results prompted us to investigate the alterations in NF-κB activation by silencing PTPN21 in HaCaT cells. PTPN21 knockdown was achieved by transfecting HaCaT cells with PTPN21-targeting siRNA#1. As shown in Fig. 3C, TNF-α-induced phosphorylation and degradation of IκBα significantly increased in PTPN21 siRNA-transfected cells compared to control siRNA-transfected cells. In addition to the effect on IκBα, IKK phosphorylation was also notably increased in PTPN21 knockdown cells. These results indicate that PTPN21 inhibits NF-κB transcriptional activity by regulating the phosphorylation of IκBα and IKK.
To elucidate a more concise mechanism of action of PTPN21 in the regulation of NF-κB signaling, the effects of PTPN21 on the phosphorylation of IκBα at Ser32/36 and IKKs (IKKα at Ser176/180 and IKKβ at Ser177/181) were determined by overexpressing PTPN21 WT and C1108S since phosphorylation at those sites is critical for NF-κB activation. Phosphorylation of IκBα and IKKs were induced by TNF-α stimulation in HaCaT cells. TNF-α-induced phosphorylation and subsequent degradation of IκBα were decreased by PTPN21 WT in a dose-dependent manner, whereas PTPN21 C1108S did not influence IκBα phosphorylation and degradation (Fig. 3D). Similar to the result of IκBα phosphorylation, PTPN21 WT led to dephosphorylation of p-IKKα/β, whereas PTPN21 C1108S did not. These results indicate that the regulatory effects of PTPN21 on NF-κB signaling are due to the dephosphorylation of p-IκBα and p-IKKα/β in a phosphatase activity-dependent manner.
To gain more insight into the direct target of PTPN21 for the regulation of NF-κB signaling, we examined the association between PTPN21 and the NF-κB signaling axis, including IκBα and IKKs, which are the key molecules for NF-κB activation and are tightly regulated by phosphorylation in HaCaT cells. Immunoprecipitation of endogenous IκBα or IKKα/β by specific antibodies and subsequent immunoblotting analysis with anti-PTPN21 antibody showed the association of IκBα and IKKα/β with endogenous PTPN21 in HaCaT cells (Fig. 3E and F). These data indicate that PTPN21 associates with endogenous IκBα and IKKα/β in HaCaT cells.
PTPN21 directly interacts with IKKβ and dephosphorylates p-IKKβ
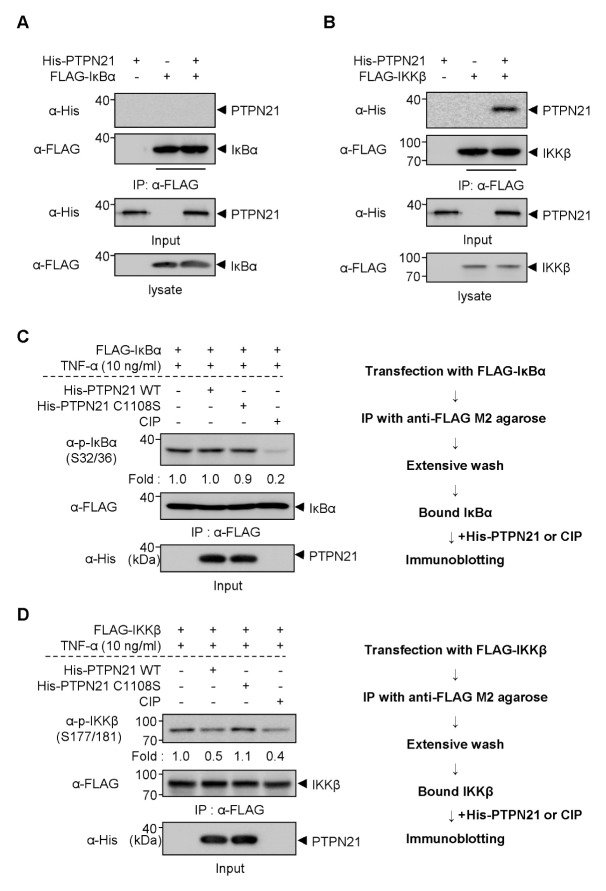
The results of Fig. 3D–F provoke the question as to what is the direct dephosphorylating target of PTPN21 in the regulation of NF-κB signaling. IKKβ is activated through phosphorylation at Ser177/181 and it subsequently catalyzes the phosphorylation of IκBα at Ser32/36 (15). Therefore, we performed in vitro binding assays using recombinant His-PTPN21 protein expressed in and purified from bacteria to find out the direct association of PTPN21 with IκBα and IKKβ. When immunoprecipitated FLAG-IκBα was incubated with recombinant His-PTPN21, no direct interaction between IκBα and PTPN21 WT was detected (Fig. 4A). However, immunoprecipitated FLAG-IKKβ showed an association with His-PTPN21 protein (Fig. 4B). These results indicate that IKKβ might be the direct target of PTPN21 in the regulation of NF-κB signaling.
Fig. 4.
PTPN21 directly dephosphorylates p-IKKβ. (A and B) After transfection with FLAG-tagged IκBα (A) or IKKβ (B), cell lysates were immunoprecipitated with anti-FLAG M2 agarose and then mixed with purified His-PTPN21 WT proteins. After binding, the immunoprecipitates were subjected to immunoblotting analyses with indicated antibodies to detect direct interaction between the immunoprecipitated FLAG-tagged proteins and purified His-PTPN21. (C and D) After transfection with FLAG-tagged IκBα (C) or IKKβ (D), cell lysates were immunoprecipitated with anti-FLAG M2 agarose and extensively washed. The immunoprecipitates were then reacted with purified His-PTPN21 WT or C1108S proteins in PTP reaction buffer. The reaction was stopped by adding sample buffer and the samples were subjected to immunoblotting analyses with indicated antibodies. CIP-treated group was used to detect the disappearance of p-IκBα and p-IKKβ in immunoprecipitates.
To clarify whether PTPN21 directly dephosphorylates p-IKKβ, but not p-IκBα, we performed an in vitro phosphatase assay using recombinant His-PTPN21 WT or C1108S. Immunoprecipitates from FLAG-tagged IκBα- or IKKβ-transfected HEK 293 cells were extensively washed and then incubated with His-PTPN21 proteins followed by immunoblotting with phospho-specific antibodies. As shown in Fig. 4C, both His-PTPN21 WT and C1108 proteins did not dephosphorylate p-IκBα in vitro. However, His-PTPN21 WT decreased the levels of p-IKKβ at Ser177/181, whereas His-PTPN21 C1108S did not (Fig. 4D). These results suggest that PTPN21 directly dephosphorylates p-IKKβ in a phosphatase activity-dependent manner, leading to dephosphorylation of p-IκBα in the cells.
DISCUSSION
Growing evidence indicates that adhesion molecules, such as ICAM-1 on the surface of epidermal keratinocytes, play important roles in the initiation and maintenance of skin inflammation (3, 16). Therefore, elucidating the signaling molecules that regulate ICAM-1 expression as well as the discovery of pharmacological agents inhibiting ICAM-1 expression in keratinocytes are considered crucial for the treatment of inflammatory skin diseases. In this study, we showed that PTPN21 significantly inhibited the expression of ICAM-1 in TNF-α-stimulated HaCaT cells, indicating that PTPN21 could be a potential cellular target for the treatment of inflammatory skin diseases. Further investigations, such as alleviation of TNF-α-induced adhesion of monocytes to HaCaT cells by PTPN21, may be required to verify the consequences of PTPN21-mediated ICAM-1 inhibition.
A major signaling pathway activated by TNF-α is the NF-κB signal transduction pathway, which plays a central role in skin inflammatory responses by inducing the production of adhesion molecules, including ICAM-1 and basal-cell adhesion molecule in epidermal keratinocytes (17). Upon cell stimulation with TNF-α, the cytosolic NF-κB heterodimer is freed from IκBα and translocates into the nucleus, where it activates the transcription of proinflammatory genes (7). Our data and the molecular basis for regulation of NF-κB and ICAM-1 provide a clue that PTPN21 can regulate ICAM-1 expression in HaCaT cells by inhibiting NF-κB signaling.
PTPN21 is one of the class I PTPs, which consist of receptor type and non-receptor type PTPs, and has been reported to exhibit substrate specificity for phosphorylation at Tyr residues (9). However, in this study, PTPN21 dephosphorylated p-IKKβ at Ser177/181. This suggests that PTPN21 has a phosphatase activity towards p-Ser as well as p-Tyr. Protein tyrosine phosphatase receptor type O, which is known as a Tyr-specific class I PTP, also has an ability to dephosphorylate p-Akt at Ser473 and p-glycogen synthase kinase 3β (GSK3β) at Ser9 (18). Furthermore, phosphatase and tensin homolog (PTEN), a Tyr- and lipid-specific PTP (19), dephosphorylates p-Akt at Ser473 and p-GSK3α/β at Ser21/9 in insulin-like growth factor-II-overexpressing rhabdomyosarcomas cells (20). Our results as well as these reports imply that some receptor type and non-receptor type PTPs have phosphatase activity toward p-Ser as well as p-Tyr.
Using siRNAs targeting various phosphatases, Li et al. reported that a variety of phosphatases, including PTPN21, inhibit NF-κB transcriptional activity in mouse astrocytes (19). The inhibitory effects of some identified phosphatases and their underlying mechanisms of action on NF-κB activation were further elucidated. However, the involvement of PTPN21 in the regulation of NF-κB was not investigated at a molecular level.
In addition, there are several reports that protein phosphatases regulate NF-κB signaling. IKKβ phosphorylation and activation were observed when PP2A was inhibited by ultraviolet-B (UVB) radiation, leading to UVB-induced apoptosis (21, 22). PTEN reduced NF-κB DNA binding to the FAK promoter by inhibiting the PI3K/NF-κB pathway, and subsequently inhibited invasion and metastasis of gastric cancer (23). Furthermore, profilin, a tumor suppressor protein, interacts with PTEN and suppresses NF-κB activity via inhibition of IKK phosphorylation (24). Unlike these phosphatases, no further investigations were available although PTPN21 seemed to be involved in the regulation of NF-κB activity (19). Therefore, the results of our study, which suggest that PTPN21 is a phosphatase activity-dependent regulator of p-IKKα/β, provide valuable insights for understanding PTPN21-mediated NF-κB regulation.
PTPN21 inhibits ICAM-1 expression by inhibiting the NF-κB signal transduction pathway in HaCaT cells (Fig. 2 and 3). PTPN21 also activates the Src signaling pathway, which has been known to induce ICAM-1 expression and phosphorylation, by dephosphorylating its inhibitory phosphorylation site at Tyr527 (10). These controversial effects of PTPN21 on the NF-κB and Src signaling pathways give rise to a hypothesis that the activation of NF-κB signaling is more dominant for ICAM-1 expression than that for Src in HaCaT cells. This hypothesis can be supported by a previous study, which reported that low-molecular-weight protein tyrosine phosphatase (LMW-PTP) is oncogenic or anti-oncogenic depending on its interaction with different substrates (25). LMW-PTP was revealed to induce cell migration, suggesting that its oncogenic properties are more dominant than its anti-oncogenic properties (25). Similarly, we assume that PTPN21-mediated activation of the NF-κB signaling pathway might be more crucial for regulating ICAM-1 expression compared to PTPN21-mediated activation of the Src signaling pathway.
NF-κB is usually considered as an anti-apoptotic factor since it promotes cell proliferation. The inhibitory effect of PTPN21 on the NF-κB signaling pathway seems controversial when compared to PTPN21-mediated activation of the Src signaling pathway and its promoting effects on cell motility and migration (10, 11). However, NF-κB activation by interleukin-1 leads to NF-κB-dependent secretion of TNF-α, which activates TNF-R1 in an autocrine fashion to enhance UVB-induced apoptosis (26). These data suggest that PTPN21-mediated inhibition of TNF-α-induced NF-κB signaling could have the same cancer-promoting effects as PTPN21-mediated Src activation. Further studies are needed to clarify the consequence of PTPN21-mediated NF-κB inhibition with regard to cancer promotion.
MATERIALS AND METHODS
See the supplementary informations.
Supplementary Information
ACKNOWLEDGEMENTS
We thank Dr. A. Feliciello for kindly providing us with FLAG-PTPN21 WT plasmid and anti-PTPN21 antibodies. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1A2A2A11001446 and 2015R1A 5A1008958) and by the Ministry of Education, Science and Technology (NRF-2016R1A6A3A11931134).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Grone A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/S0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 2.Dustin ML, Singer KH, Tuck DT, Springer TA. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1) J Exp Med. 1988;167:1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trefzer U, Brockhaus M, Loetscher H, et al. 55-kd tumor necrosis factor receptor is expressed by human keratinocytes and plays a pivotal role in regulation of human keratinocyte ICAM-1 expression. J Invest Dermatol. 1991;97:911–916. doi: 10.1111/1523-1747.ep12491668. [DOI] [PubMed] [Google Scholar]
- 4.Kock A, Schwarz T, Kirnbauer R, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222:729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- 6.Seo WY, Youn GS, Choi SY, Park J. Butein, a tetrahydroxychalcone, suppresses pro-inflammatory responses in HaCaT keratinocytes. BMB Rep. 2015;48:495–500. doi: 10.5483/BMBRep.2015.48.9.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Youn GS, An SY, Kwon HY, Choi SY, Park J. 2,3-Dimethoxy-2′-hydroxychalcone ameliorates TNF-α-induced ICAM-1 expression and subsequent monocyte adhesiveness via NF-kappaB inhibition and HO-1 induction in HaCaT cells. BMB Rep. 2015;49:57–62. doi: 10.5483/BMBRep.2016.49.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr AJ, Ugochukwu E, Lee WH, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moller NP, Moller KB, Lammers R, Kharitonenkov A, Sures I, Ullrich A. Src kinase associates with a member of a distinct subfamily of protein-tyrosine phosphatases containing an ezrin-like domain. Proc Natl Acad Sci U S A. 1994;91:7477–7481. doi: 10.1073/pnas.91.16.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardone L, Carlucci A, Affaitati A, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24:4613–4626. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlucci A, Gedressi C, Lignitto L, et al. Protein-tyrosine phosphatase PTPD1 regulates focal adhesion kinase autophosphorylation and cell migration. J Biol Chem. 2008;283:10919–10929. doi: 10.1074/jbc.M707248200. [DOI] [PubMed] [Google Scholar]
- 12.Livigni A, Scorziello A, Agnese S, et al. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell. 2006;17:263–271. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jui HY, Tseng RJ, Wen X, et al. Protein-tyrosine phosphatase D1, a potential regulator and effector for Tec family kinases. J Biol Chem. 2000;275:41124–41132. doi: 10.1074/jbc.M007772200. [DOI] [PubMed] [Google Scholar]
- 14.Thommesen L, Sjursen W, Gasvik K, et al. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- 15.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 16.Krutmann J, Czech W, Parlow F, et al. Ultraviolet radiation effects on human keratinocyte ICAM-1 expression: UV-induced inhibition of cytokine-induced ICAM-1 mRNA expression is transient, differentially restored for IFN gamma versus TNF alpha, and followed by ICAM-1 induction via a TNF alpha-like pathway. J Invest Dermatol. 1992;98:923–928. doi: 10.1111/1523-1747.ep12460737. [DOI] [PubMed] [Google Scholar]
- 17.Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- 18.Jiang R, Chen D, Hou J, et al. Survival and inflammation promotion effect of PTPRO in fulminant hepatitis is associated with NF-kappaB activation. J Immunol. 2014;193:5161–5170. doi: 10.4049/jimmunol.1303354. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Wang L, Berman MA, Zhang Y, Dorf ME. RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling. Mol Cell. 2006;24:497–509. doi: 10.1016/j.molcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22:8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- 21.Barisic S, Strozyk E, Peters N, Walczak H, Kulms D. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ. 2008;15:1681–1690. doi: 10.1038/cdd.2008.98. [DOI] [PubMed] [Google Scholar]
- 22.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LL, Liu J, Lei S, Zhang J, Zhou W, Yu HG. PTEN inhibits the invasion and metastasis of gastric cancer via downregulation of FAK expression. Cell Signal. 2014;26:1011–1020. doi: 10.1016/j.cellsig.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi AH, Manna SK. Profilin-PTEN interaction suppresses NF-kappaB activation via inhibition of IKK phosphorylation. Biochem J. 2016;473:859–872. doi: 10.1042/BJ20150624. [DOI] [PubMed] [Google Scholar]
- 25.Alho I, Costa L, Bicho M, Coelho C. The role of low-molecular-weight protein tyrosine phosphatase (LMW-PTP ACP1) in oncogenesis. Tumour Biol. 2013;34:1979–1989. doi: 10.1007/s13277-013-0784-1. [DOI] [PubMed] [Google Scholar]
- 26.Poppelmann B, Klimmek K, Strozyk E, Voss R, Schwarz T, Kulms D. NF kappaB-dependent downregulation of tumor necrosis factor receptor-associated proteins contributes to interleukin-1-mediated enhancement of ultraviolet B-induced apoptosis. J Biol Chem. 2005;280:15635–15643. doi: 10.1074/jbc.M413006200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.