Abstract
Nanomedicine is a relatively new and rapidly evolving field combining nanotechnology with the biomedical and pharmaceutical sciences.1–3 Nanoparticles (NPs) can impart many pharmacokinetic, efficacy, safety, and targeting benefits when they are included in drug formulations.1–5 Many nanodrugs have entered clinical practice, and even more are being investigated in clinical trials for a wide variety of indications.2 However, nanopharmaceuticals also face challenges, such as the need for better characterization, possible toxicity issues, a lack of specific regulatory guidelines, cost–benefit considerations, and waning enthusiasm among some health care professionals. 4,5 For these reasons, expectations regarding nanodrugs that are in early stages of development or clinical trials need to remain realistic.4
INTRODUCTION
Nanomedicine is a relatively new and rapidly evolving field combining nanotechnology with the biomedical and pharmaceutical sciences.1–3 Nanoparticles (NPs) can impart many pharmacokinetic, efficacy, safety, and targeting benefits when they are included in drug formulations.1–5 Many nanodrugs have entered clinical practice, and even more are being investigated in clinical trials for a wide variety of indications.2 However, nanopharmaceuticals also face challenges, such as the need for better characterization, possible toxicity issues, a lack of specific regulatory guidelines, cost–benefit considerations, and waning enthusiasm among some health care professionals. 4,5 For these reasons, expectations regarding nanodrugs that are in early stages of development or clinical trials need to remain realistic.4
POTENTIAL BENEFITS OF NANODRUGS
What Are Nanomedicines and Nanodrugs?
Nanomedicine is a relatively new and rapidly evolving field combining nanotechnology, biomedical, and pharmaceutical sciences.1–3 Nanomedicine encompasses nanopharmaceuticals, nanoimaging agents, and theranostics.1,6 This article will discuss only nanopharmaceuticals (i.e., “nanodrugs”).
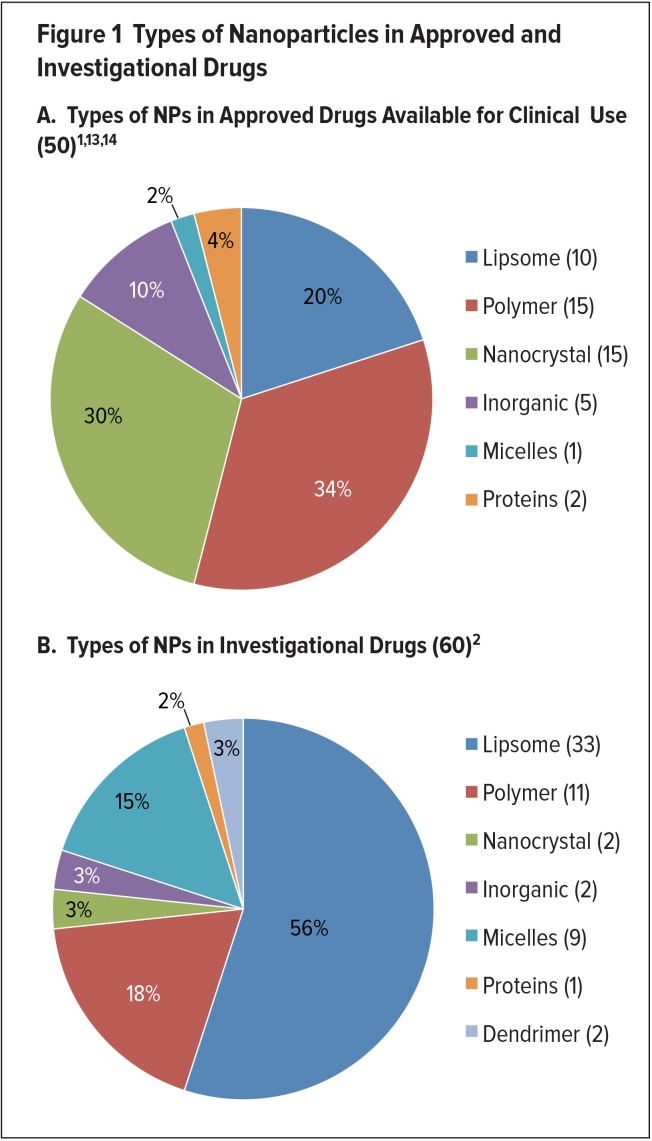
Nanodrug formulations can impart many physical and biological advantages, such as improved solubility and pharmacokinetics (PK), enhanced efficacy, reduced toxicity, and increased tissue selectivity, compared with conventional medicines.1–6 Some controversy exists regarding the definition of nanodrugs. However, the Food and Drug Administration (FDA) considers the products it regulates (including drugs and biologics) to incorporate nanotechnology if they contain or are manufactured with: NPs that range from 1 to 100 nanometers (nm), which due to their small size and high surface area exhibit key differences in comparison to bulk materials; or materials outside of this range that exhibit related dimension-dependent properties or phenomena.1,3,6 NPs can change the biochemical, electronic, magnetic, and/or optical properties of a drug formulation in a way that can then be applied for therapeutic purposes.1,3,7 NPs used in nanodrug formulations currently include liposomes, polymers, micelles, nanocrystals, metals/metal oxides and other inorganic materials, and proteins, although research is being conducted with other types of NPs, such as carbon nanotubes (Figure 1).1–3
Figure 1.
Types of Nanoparticles in Approved and Investigational Drugs
The particle shape, size, and surface chemistry of NPs are significant in determining important PK criteria, such as adsorption, cellular uptake, accumulation and biodistribution patterns, and clearance mechanisms.1,3 In addition, NPs can be combined to form a multistage vector, which can survive various in vivo conditions or compartments that it encounters and sequentially release its cargo.3,7 To date, 50 nanopharmaceuticals have been approved and are available for use in clinical practice, and even more are being studied in clinical trials for a wide range of indications.4,5
Potential Pharmacokinetic Benefits
A common practice in the development of nanodrugs is to conjugate or encapsulate a therapeutically active agent to an NP to alter its PK.3,5 Nanopharmaceuticals can overcome some of the limitations of conventional medicines by promoting more desirable PK and distribution, independent of the molecular structure of the active ingredient.5 They can be designed to enable a medicine to reach previously impervious areas, circulate for longer times to allow greater accumulation, or be targeted toward a disease site.1,3,5,7 The incorporation of NPs in a pharmaceutical formulation can also alter the concentration-time profile of a drug, enabling its release (and exposure to diseased and/or healthy tissues) in a controlled and sustained manner.5
Currently, most nanodrugs are previously existing drugs conjugated to NPs to improve PK and/or pharmacodynamic (PD) properties.1 In the majority of cases, these drug–NP conjugates use “passive targeting,” which involves nonspecific accumulation in diseased tissue, often tumors.1 However, “active targeting” can be achieved by attaching ligands (e.g., proteins, antibodies, or small molecules) to the surface of the drug–NP conjugate that are designed to attach to receptors on specific cells.1,3 Active targeting can result in an increase in intracellular drug accumulation and uptake by the cells of the targeted tissue.1
Preclinical and clinical studies are necessary to characterize the PK, PD, biodistribution, efficacy, and toxicity of nanopharmaceuticals to understand how they differ from conventional dosage forms.5 These studies are needed because drugs formulated with NPs can dramatically alter PK.5 For example, administration of a 50 mg/m2 dose of liposomal doxorubicin in humans was found to increase the area under the curve (AUC) by 300-fold and reduce clearance 250-fold compared to free drug.5
Potential Efficacy Benefits
When formulating nanodrugs, diverse strategies can be applied to improve drug efficacy. These include: exploiting the small size of NPs to circumvent important physiological barriers (the immune system, renal clearance, enzymatic and mechanical degradation, and others); using NPs to entrap drug molecules to protect them from physiologically hostile environments; and/or using surface conjugation to target drugs to specific tissues, enabling higher therapeutic levels at a target site even with the use of lower doses.6,7
Nanomaterials also have immunomodulatory effects that might potentially promote or shape the adaptive immune response.8 Some (e.g., polymeric NPs, liposomes, nanoemulsions, and virus-like NPs) are capable of entering antigen-presenting cells.9,10 This ability may potentially permit them to regulate the immune response, for example, by inducing a Th1-type response against intracellular pathogens.9,10 Poly-D,L-lactic acid-co-glycolic acid copolymer (PLGA) NPs have also been shown to deliver antigens to dendritic cells.11 Due to efficient delivery and potential immune regulating properties, the use of NP adjuvants may therefore increase the efficacy of vaccines.8 Nanodrugs may also play a role in improving the efficacy of cancer immunotherapies because they can be designed to deliver timed, targeted signals to maximize a coordinated immune response against specific cells.2
Many nanodrugs are being developed for the treatment of cancer.5 In most instances, the NPs used in these formulations either passively or actively target a tumor site, or they operate via a combination of both mechanisms.5 Passive targeting is often used to target solid tumors because the increased permeability of blood vessels and poor lymphatic drainage (called the enhanced permeability and retention [EPR] effect) enable the preferential accumulation of drug within the tumor microenvironment.1,2,5,12 In contrast, active targeting relies on ligands conjugated to the NPs that bind with tumor biomarkers.2,5,6 This mechanism potentially enhances the accumulation of NPs at the tumor site and increases uptake by cells expressing the target receptor.2,5 Many preclinical and clinical studies have shown that nanoformulations can passively enhance tumor accumulation, decreasing normal tissue exposure.2 However, the clinical validation of active NP targeting is more limited and not as easily achieved.5
Another way nanopharmaceutical formulations can benefit cancer treatment is through the incorporation of drugs into long-circulating NPs that remain active for an extended period of time.5 Consequently, tumor sites experience longer exposure to the drugs due to the slow rate of drug release from the NP and the retention of the drug-loaded NPs in the vascular compartment.5
Potential Safety Benefits
The increased drug accumulation in diseased tissue provided by nanoformulations may allow the effective dose of a drug to be reduced, diminishing side effects.7 It has been observed that typically less than 0.01% of an injected dose of angstromsized agents accumulates in a target region, compared to 1% to 5% for NPs.7 Better accumulation, as well as targeted release, can enable dose reduction, which decreases side effects.6,7 In fact, the earliest nanodrugs were granted approval by the FDA based on lower toxicity compared with conventional formulation counterparts.7 Doxil (doxorubicin hydrochloride, Janssen), the first nanoformulated drug to gain FDA approval, received an indication for the treatment of Kaposi’s sarcoma in patients with human immunodeficiency virus (HIV) in 1995.1,2,6,7 Although equally effective, its main advantage in comparison to conventionally formulated doxorubicin is considered by many to be reduced cardiotoxicy; however, Doxil has been associated with adverse events related to the nanoformulation, such as palmar-plantar erythrodysesthesia and complement activation-related pseudoallergy-like infusion reactions.6,7 More than 20 years after its approval, Doxil is still widely used for its original indication, as well as to treat ovarian and metastatic breast cancer and multiple myeloma.6,7
Nanoformulations can also help manage the dose-limiting toxicities associated with conventional chemotherapeutic agents.1,2,5 Many cancer chemotherapies are hydrophobic and relatively insoluble in aqueous solutions.2,7 Therefore, they often require toxic solubilizing agents for parenteral administration (such as polyethoxylated castor oil [Kolliphor EL, BASF Corp.] for paclitaxel).2,7 Consequently, these drugs often require dose reduction to manage systemic toxicity, limiting efficacy.1,2 There has long been interest in developing delivery systems for these therapies that do not require toxic solubilizing agents, and nanoformulation is viewed as a viable solution to the problems associated with administering poorly water-soluble drugs.7
For these reasons, nanoformulations of many chemotherapies have been approved and more are in clinical development. 2 Perhaps most notable is Abraxane (nab-paclitaxel, Celgene), a formulation of paclitaxel bound to albumin NPs.4,5,7 Abraxane was approved by the FDA in 2005 for previously treated metastatic breast cancer and has since been granted indications for other cancers.4,5,7 Abraxane is considered to be more tolerable than conventional paclitaxel, which is formulated with Kolliphor EL.2,5,7 The increased tolerance (which is attributed in part to the absence of toxic solvent) allows Abraxane to be administered to patients at a considerably higher dose, potentially achieving greater efficacy.2,5,7
TRENDS IN APPROVAL AND DEVELOPMENT
Approved Nanodrugs
Since 1995, 50 nanopharmaceuticals have received FDA approval and are currently available for clinical use.1,2,13,14 Nanodrugs are typically administered orally or intravenously, and less frequently transdermally.2 Polymeric, liposomal, and nanocrystal formulations are heavily represented among approved nanodrugs.1,2,6 A diverse array of drug-delivery platforms based on other NPs has also been used in approved nanodrugs, including micelles and inorganic NPs (metals/metal oxides and other inorganic nanomaterials).1,2 Nanodrugs have been approved for a variety of indications, including cancer.5,6 Approvals of nanodrugs by the FDA peaked between 2001 and 2005, followed by a significant drop after 2006, possibly because of lower investment due to the 2008 financial crisis.4 A listing of approved nanodrugs appears in Table 1.2,5
Table 1.
| Trade Name (Manufacturer) | Generic Name | Indication(s)* | Benefit of NP** |
|---|---|---|---|
| Liposome NPs | |||
| Curosurf (Chiesi USA) | Poractant alfa | Respiratory distress syndrome | Increased delivery with smaller volume, decreased toxicity |
| Doxil (Janssen) | Doxorubicin HCl liposome injection | Karposi’s sarcoma, ovarian cancer, multiple myeloma | Increased delivery to disease site, decreased systemic toxicity of free drug |
| Abelcet (Sigma-Tau) | Liposomal amphotericin B lipid complex | Fungal infections | Decreased toxicity |
| AmBIsome (Gilead Sciences) | Liposomal amphotericin B | Fungal/protozoal infections | Decreased nephrotoxicity |
| DepoDur (Pacira Pharmaceuticals) | Liposomal morphine sulphate | Postoperative analgesia | Extended release |
| DepoCyt (Sigma-Tau) | Liposomal cytarabine | Lymphomatous meningitis | Increased delivery to tumor site, decreased systemic toxicity |
| Marqibo (Spectrum Pharmaceuticals) | Liposomal vincristine | ALL | Increased delivery to tumor site, decreased systemic toxicity |
| Onivyde (Ipsen Biopharmaceuticals) | Liposomal irinotecan | Pancreatic cancer | Increased delivery to tumor site, decreased systemic toxicity |
| Visudyne (Bausch and Lomb) | Liposomal verteporfin | Wet AMD, ocular histoplasmosis, myopia | Increased delivery to site of diseased vessels, photosensitive release |
| Vyxeos (Jazz Pharmaceuticals) | Liposomal daunorubicin and cytarabine | AML, AML with myelodysplasiarelated changes | Increased efficacy through synergistic delivery of co-encapsulated agents |
| Polymer NPs | |||
| Adagen (Leadiant Biosciences) | Pegademase bovine | SCID | Longer circulation time, decreased immunogenicity |
| Adynovate (Shire) | Antihemophilic factor (recombinant), pegylated | Hemophilia | Greater protein stability, longer half-life |
| Cimzia (UCB) | Certolizumab pegol | Crohn’s disease, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis | Longer circulation time, greater stability in vivo |
| Copaxone (Teva) | Glatimer acetate | Multiple sclerosis | Controlled clearance |
| Eligard (Tolmar) | Leuprolide acetate and polymer | Prostate cancer | Longer circulation time, controlled payload delivery |
| Krystexxa (Horizon) | Pegloticase | Chronic gout | Greater protein stability |
| Macugen (Bausch and Lomb) | Pegaptinib | Neovascular AMD | Greater aptamer stability |
| Mircera (Vifor) | Methoxy polyethylene glycol-epoetin beta | Anemia associated with CKD | Greater aptamer stability |
| Neulasta (Amgen) | Pegfilgrastim | Chemotherapy-induced neutropenia | Greater protein stability |
| Oncaspar (Baxalta U.S.) | Pegaspargase | ALL | Greater protein stability |
| Pegasys (Genentech) | Pegylated IFN alpha-2a | Hepatitis B, hepatitis C | Greater protein stability |
| PegIntron (Merck) | Pegylated IFN alpha-2b | Hepatitis C | Greater protein stability |
| Plegridy (Biogen) | Pegylated IFN beta-1a | Multiple sclerosis | Greater protein stability |
| Rebinyn (Novo Nordisk) (available in 2018) | Coagulation factor IX (recombinant), glycopegylated | Hemophilia B | Longer half-life, greater drug levels between infusions |
| Renvela (Genzyme); and Renagel (Genzyme) | Sevelamer carbonate; and Sevelamer HCl | CKD | Longer circulation time and therapeutic delivery |
| Somavert (Pfizer) | Pegvisomant | Acromegaly | Greater protein stability |
| Zilretta (Flexion Therapeutics) | Triamcinolone acetonide ER injectable suspension | Osteoarthritis knee pain | Extended release |
| Micelle NPs | |||
| Estrasorb (Novavax) | Micellar estradiol | Vasomotor symptoms in menopause | Controlled delivery |
| Nanocrystal NPs | |||
| Avinza (Pfizer) | Morphine sulfate | Psychostimulant | Greater drug loading and bioavailability, ER |
| EquivaBone (Zimmer Biomet) | Hydroxyapatite | Bone substitute | Mimics bone structure |
| Emend (Merck) | Aprepitant | Antiemetic | Greater absorption and bioavailability |
| Focalin (Novartis) | Dexamethylphenidate HCl | Psychostimulant | Greater drug loading and bioavailability |
| Invega Sustenna (Janssen) | Paliperidone palmitate | Schizophrenia, schizoaffective disorder | Slow release of injectable low-solubility drug |
| Megace ES (Par Pharmaceuticals) | Megestrol acetate | Antianorexic | Lower dosing |
| NanOss (RTI Surgical) | Hydroxyapatite | Bone substitute | Mimics bone structure |
| Ostim (Heraeus Kulzer) | Hydroxyapatite | Bone substitute | Mimics bone structure |
| OsSatura (IsoTis Orthobiologics) | Hydroxyapatite | Bone substitute | Mimics bone structure |
| Rapamune (Wyeth Pharmaceuticals) | Sirolimus | Immunosuppressant | Greater bioavailability |
| Ritalin LA (Novartis) | Methylphenidate HCl | Psychostimulant | Greater drug loading and bioavailability |
| Ryanodex (Eagle Pharmaceuticals) | Dantrolene sodium | Malignant hypothermia | More rapid rate of dministration at higher doses |
| Tricor (AbbVie) | Fenofibrate | Hyperlipidemia | Greater bioavailability simplifies administration |
| Vitoss (Stryker) | Calcium phosphate | Bone substitute | Mimics bone structure |
| Zanaflex (Acorda) | Tizanidine HCl | Muscle relaxant | Greater drug loading and bioavailability |
| Inorganic NPs | |||
| Dexferrum (American Regent) | Iron dextran | Iron deficiency in CKD | Increased dose |
| Feraheme (AMAG Pharmaceuticals) | Ferumoxytol | Iron deficiency in CKD | Prolonged, steady release with less frequent dosing |
| Ferrlecit (Sanofi-Aventis) | Sodium ferric gluconate complex in sucrose injection | Iron deficiency in CKD | Increased dose |
| Infed (Actavis Pharma) | Iron dextran | Iron deficiency in CKD | Increased dose |
| Venofer (American Regent) | Iron sucrose | Iron deficiency in CKD | Increased dose |
| Protein NPs | |||
| Abraxane (Celgene) | Albumin-bound paclitaxel | Breast cancer, NSCLC, pancreatic cancer | Greater solubility, increased delivery to tumor |
| Ontak (Eisai) | Denileukin diftitox | Cutaneous T-cell lymphoma | Targeted T-cell specificity, lysosomal escape |
Refer to complete prescribing information.
Compared with conventional formulations.
ALL = acute lymphoblastic leukemia; AMD = age-related macular degeneration; AML = acute myeloid leukemia; CKD = chronic kidney disease; ER = extended release; HCl = hydrochloride; IFN = interferon; NP = nanoparticle; NSCLC = non–small-cell lung cancer; SCID = severe combined immunodeficiency disease.
Most of the nanodrugs approved to date have demonstrated reduced toxicity rather than improved efficacy compared to conventional formulations.2 In fact, many nanodrugs have not survived clinical development because they were unable to demonstrate a significant improvement in efficacy and because improved toxicity could be achieved with other drugs or nanoformulations.2 However, nanoformulated versions of existing drugs that are undergoing clinical development have shown promising results with respect to improved efficacy and, therefore, appear likely to gain regulatory approval.2
Nanodrugs in the Pipeline
New nanodrugs enter clinical investigation every year, but most are nanoformulations of previously approved drugs.2 As of October 2017, 56 clinical trials including the term “nano” were listed as “recruiting” or “active” on ClinicalTrials.gov.15 There are also many nanodrugs in the very early stages of development; details are unknown because this information is proprietary.4 The number of nanodrugs that have received investigational new drug (IND) approval from the FDA to undergo clinical trials has steadily increased since 2007.1 The years from 2013 to 2015 had the highest number of nanoformulations entering clinical trials; this suggests an increase in the availability of FDA-approved nanodrugs in the future.1
There are currently more anticancer and antimicrobial nanodrugs in clinical trials than any other drug classes.2 However, there are also many formulations being developed for other indications, including autoimmune conditions, anesthesia, metabolic disorders, ophthalmic conditions, neurological and psychiatric diseases, and others.2 Of the products in development, the majority incorporate NPs that have already proven successful, such as liposomes and polymers.2 The remainder of investigational nanodrugs demonstrate a trend toward agents using micelles, as well as the introduction of formulations using dendrimers.1,4 Polymer-based nanoformulations are less prevalent in investigational nanodrugs than approved nanodrugs.1 However, many new micelle-, liposome-, and protein-based nanoformulations still incorporate a synthetic polymer component.1 Furthermore, nanoformulations based on other types of NPs (nanocrystals, inorganic NPs, and others) almost universally use surface coatings composed of antifouling polymers.1
Another clear trend is the movement away from relatively simple NPs to complex, multicomponent drug delivery plat-forms.1 Modern approaches in protein engineering, as well as advances in polymer and inorganic chemistry, have also resulted in an expansion of novel nanomaterials that blur the boundaries between the categories of traditional materials.1 This has allowed the initial goals for nanopharmaceuticals (improved PK, efficacy, and safety) to evolve into system designs that allow for more complex functions, such as controlled release and active targeting.1 Although the majority of FDA-approved nanodrugs rely on passive targeting via the EPR effect, some next-generation drugs in clinical trials employ active targeting approaches.1 One example of a targeted nanodrug that is being investigated is SGT-53 (SynerGene Therapeutics), which contains an antitransferrin antibody fragment that binds with a transferring glycoprotein receptor on cancer cells.1 This agent is in phase 1 and 2 trials for the treatment of solid tumors, glioblastoma, and metastatic pancreatic cancer.15
APPROVED AND INVESTIGATIONAL NANODRUGS
Selected approved and investigational nanodrugs are discussed in the following section, categorized by the type of NP they incorporate. Table 1 also lists approved nanodrugs by NP type, along with their indications and benefits.
Liposomal NPs
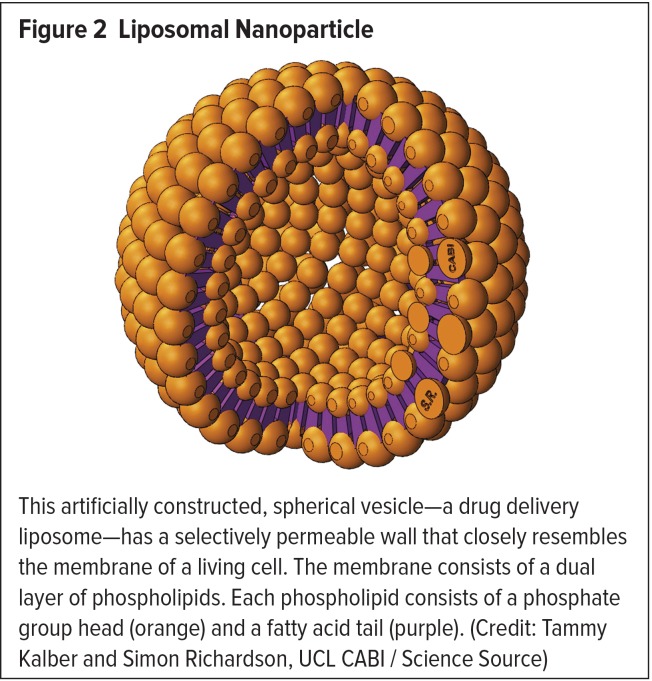
Due to their many unique properties, liposomes are frequently used in nanodrug formulations.3 A liposome is a spherical vesicle composed of a lipid bilayer membrane arranged around an empty core (Figure 2).1,3 Liposomes were initially described in 1965 and were first proposed as a simple drug delivery system in the 1970s.1 They are usually 90 to 150 nm in diameter, and thus are sometimes slightly larger than conventional NPs.2 Liposomes are self-assembling and can carry and deliver either hydrophilic or hydrophobic therapies, which can be stored in their empty cores.1,2 However, they are also often designed to carry biomolecules (e.g., monoclonal antibodies, antigens) that are conjugated to their surfaces as ligands.3 Compared with nonliposomal drugs, liposome-based nanodrugs can circulate in the bloodstream for an extended time, providing a longer treatment effect.2,3 Liposomes can also accumulate at the site of a tumor or infection, naturally locating and delivering higher drug levels to these targets.3 By using lipids of different fatty-acid-chain lengths, liposomes can also be constructed to be temperature- or pH-sensitive, thereby permitting the controlled release of their contents only when they are exposed to specific environmental conditions.2,3
Figure 2.
Liposomal Nanoparticle
This artificially constructed, spherical vesicle—a drug delivery liposome—has a selectively permeable wall that closely resembles the membrane of a living cell. The membrane consists of a dual layer of phospholipids. Each phospholipid consists of a phosphate group head (orange) and a fatty acid tail (purple). (Credit: Tammy Kalber and Simon Richardson, UCL CABI/Science Source)
The use of liposomal nanoformulations for drug delivery has significantly impacted pharmacology.1 Nanoformulations of existing drugs with low bioavailability or high toxicity have benefitted from the stability and improved biodistribution that liposomes provide.1,4 When delivered intravenously, conventional liposomes have short circulating half-lives due to rapid clearance.1 This is because the lipid bilayer structure of the liposome is recognized by the immune system and cleared from circulation by macrophages.1 However, in liposomal nanoformulations, this clearance has been minimized by pegylation (attachment of polyethylene glycol [PEG, Figure 3] chains to a molecule) of the liposome surface.1 Compared with free doxorubicin, pegylated liposomal doxorubicin has been reported to result in fourfold to 16-fold enhancement of drug levels in malignancies.16 In addition, many approved liposomal nanoformulations rely on passive targeting, which successfully increases distribution to diseased tissue.1 However, liposomes are easily synthesized and can integrate different targeting ligands into liposomal drug carriers to create new, actively targeted combinations for drug delivery.1
Figure 3.
Polyethylene Glycol (PEG) Crystals
PEG is often coupled to drug molecules to enhance tolerability, reduce clearance, and lengthen circulation time, especially in cancer therapy. (Credit: Antonio Romero/Science Source)
Co-encapsulation of drugs in NPs can also provide a novel means of drug delivery.2 More specifically, NPs can be formulated to deliver drugs sequentially and at specific molar ratios within the tumor microenvironment, allowing for maximal synergy that isn’t possible with conventional drug delivery methods.2 For example, Vyxeos, a liposomal formulation for the treatment of acute myeloid leukemia (AML), codelivers cytarabine and daunorubicin in a 5:1 fixed molar ratio.5 Vyxeos demonstrated improved efficacy in two phase 2 clinical trials, compared with a standard cytarabine and daunorubicin regimen.17,18 In August 2017, the FDA approved Vyxeos for the treatment of AML based on data from five clinical trials, including a pivotal phase 3 trial for which the primary endpoint was met.
Many nanodrugs incorporating liposomes have been approved, including antifungals, anticancer drugs, and an analgesic.1,2,4 Liposomes were the first nanodrugs to be granted IND status by the FDA to be studied in clinical trials.1 Starting with approval of Doxil in 1995, an increasing number of nanoformulations using liposomal delivery have been approved or are being investigated.1,4
Many liposomal nanoformulations of drugs are under clinical investigation. Arikayce (Insmed, Inc.) is an inhaled liposomal formulation of amikacin for the treatment of serious chronic lung infections.2 Arikayce, which significantly improves the drug half-life compared with conventionally formulated amikacin, has completed phase 1, 2, and 3 trials in patients with chronic lung infections.2,15 Despite the association of aminoglycosides with renal and neurological toxicity, one phase 2 trial found no notable difference in adverse effects between liposomal amikacin and placebo.19 Lipoquin (Aradigm Corp.) is a liposomal formulation of ciprofloxacin that permits prolonged drug release.2 Pulmaquin (Aradigm Corp.) combines liposomal and aqueous-phase ciprofloxacin to alter the amount of drug that undergoes delayed and rapid release.2 Both of these formulations have completed company-sponsored phase 2 studies in patients with either cystic fibrosis (CF) or non-CF bronchiectasis. 2 Mitomycin C is a useful but toxic drug, approved for the treatment of anal squamous cell carcinoma.2 A pegylated liposomal formulation of a mitomycin C prodrug, Promitil (LipoMedix Pharmaceutical, Inc.), is being investigated in preclinical studies; a phase 1 study in advanced solid tumors is expected to complete enrollment soon.2,15
Two novel liposomal nanoformulations of doxorubicin are being studied in clinical trials.2 One is HER2-targeted MM-302 (Merrimack Pharmaceuticals, Inc.).2 HER2 targeting is expected to improve efficacy compared to untargeted liposomal doxorubicin; a phase 1 trial of this drug in patients with HER2-positive breast cancer is ongoing.2,15
There are also many advanced clinical trials being conducted with nanodrugs that incorporate complex targeted liposomal NPs.1 Thermodox (Celsion Corp.) contains liposome-bound doxorubicin formulated with thermally sensitive lipids that degrade when exposed to high heat, disrupting the lipid bilayer and releasing the drug.1,2 The coupling of this nanodrug with radiofrequency thermal ablation allows the drug to be released in a site-specific manner at the tumor.1,2 Several phase 3 trials combining Thermodox and radiofrequency ablation in the treatment of hepatobiliary tumors (including solitary liver metastases and hepatocellular carcinoma) have been completed or are ongoing.2,15
Hepatocyte-directed vesicular (HDV) insulin is a nanoformulation of liposomal insulin that provides prolonged delivery of the drug directly to the liver.2 Several phase 1 and 2 trials demonstrated slightly improved peripheral glucose control with subcutaneous HDV compared with regular insulin.20,21 An oral formulation of HDV insulin is also undergoing evaluation in phase 2 and 3 clinical trials.2,15 HDV insulin is an example of a nanoformulated drug that can simultaneously improve the mechanism of action, pharmacokinetics, and routes of administration of a drug.2
Polymer NPs
Polymer NPs are easily synthesized and large amounts of data regarding their efficacy and safety exist; as a result, they are widely used in nanomedical research.1,3 Polymers can be natural, synthetic, or pseudosynthetic.4 The most well-established polymer is PEG.1 Polymer NPs can be fabricated in a wide range of varieties and sizes from 10 nm to 1 mcm.3 They can range in size from a single polymer chain— used directly as a therapeutic or as a modifying agent for a drug or diagnostic agent—to large aggregates within the nanoscale.1
Some polymer NPs can facilitate drug release for weeks without accumulating in the body.3 Therefore, polymeric NPs are considered promising carriers for numerous medications, including treatments for cancer, cardiovascular disease, and diabetes; bone-healing therapies; and vaccinations.3 Contrast agents can also be conjugated to the surface of polymer NPs, allowing them to be used in diagnostic imaging.3 Biodegradable polymers are of particular interest because they can be fully metabolized and removed from the body.3 PLGA is an especially intriguing example of a biodegradable polymer because relative proportions of polylactic acid (PLA) and polyglycolic acid can be used to fine-tune the biodegradability of PLGA.3
The utility of polymers in improving conventional diagnostic and therapeutic medicines is evident by their prevalence among approved and investigational nanodrugs.1 Polymer nanodrugs are usually: 1) degradable polymer forms for controlled release applications, and 2) polymer–drug conjugates that increase circulation time and drug half-life or improve biocompatibility/solubility.1 However, because they can be designed and controlled easily through organic synthesis methods, polymers are also being incorporated with other types of NPs.1
Many FDA-approved nanodrugs incorporate polymers.1 Two of the top 10 best-selling drugs in the U.S. in 2013 were polymeric drugs—Copaxone (glatiramer acetate injection, Teva Pharmaceuticals), approved in 1996 for the treatment of relapsing–remitting multiple sclerosis (MS), and Neulasta (pegfilgrastim, Amgen), approved in 2002 for chemotherapy-induced neutropenia.1 Additional pegylated biologic drugs have been approved more recently. Plegridy (peginterferon beta-1a, Biogen) was approved in 2014 for the treatment of relapsing forms of MS.1 In this nanodrug, pegylation of interferon gamma beta-1a improved drug half-life and exposure, compared with interferon alone.22 Because of a longer half-life, Plegridy can be administered once every two to four weeks, compared with other MS treatments that often need to be administered daily.1 Adynovate (antihemophilic factor [recombinant], pegylated, Baxalta U.S., Inc.) was approved in 2015 for bleeding prophylaxis and the treatment of acute bleeding in hemophilia A.1 Adynovate can be administered less frequently (compared to nonpegylated formulations of factor VIII) because of an increased half-life due to its pegylated nanoformulation; this may reduce anti-factor VIII antibody generation that reduces drug efficacy.1 Rebinyn (coagulation factor IX [recombinant], glycopegylated, Novo Nordisk), was approved in 2017 for on-demand treatment and control of bleeding episodes, and perioperative bleeding management in patients with hemophilia B.13,14 Glycopegylation increases the circulating half-life of recombinant factor IX, which allows for less frequent intravenous dosing and lower bleeding frequency. 13 Zilretta (triamcinolone acetonide extended-release injectable suspension, Flexion Therapeutics) was approved in October 2017 for the treatment of osteoarthritis knee pain.13,14 Zilretta is formulated as a suspension of microspheres within which small crystals of triamcinolone acetonide are embedded in a PLGA copolymer matrix.23 Nanochannels that form on the microsphere surface limit the release of triamcinolone acetonide, prolonging drug release.23
Many polymer-containing nanodrugs are being investigated in clinical trials.2 In addition to increasing half-life, polymer conjugation can improve passive tumor targeting by increasing the size of a drug.2 Opaxio (Cell Therapeutics, Inc.) is a nanodrug that contains polyglutamic acid-conjugated (poliglumex) paclitaxel.2 Although results in the treatment of non–small-cell lung cancer (NSCLC) were disappointing, early stage trials in the treatment of ovarian and fallopian tube cancers have shown promise.24–26 Opaxio is also being investigated as a maintenance therapy for patients with ovarian cancer who achieved a complete response after platinum and taxane therapy.2 NKTR-102 (Nektar Therapeutics) is a pegylated etirinotecan drug undergoing phase 3 clinical trials.27 Extended exposure of tumor cells to this topoisomerase I inhibitor showed enhanced therapeutic response, which can be attributed to the longer circulation of pegylated etirinotecan.27
Paclitaxel conjugated with poliglumex is also being investigated in clinical trials for use as a radiosensitizer.2 Chemotherapy treatment with 5-fluorouracil and cisplatin is frequently given to sensitize tumor cells to radiation treatment.2 Unfortunately, with this treatment, normal tissues are also sensitized to the toxic effects of radiation.2 Nanoformulation can potentially improve chemoradiotherapy treatment through tumor-specific delivery of the drugs, which increases efficacy while decreasing toxicity in normal tissues.2 Opaxio has shown potential as a radiosensitizer combined with cisplatin for esophageal cancer and with temozolomide for high-grade gliomas.28–30 In 2012, the FDA granted orphan drug status to Opaxio for the treatment of glioblastoma based on favorable overall survival (OS) and progression-free survival in this disease.2
Camptothecin is a topoisomerase I inhibitor that has potent antineoplastic activity; however, its clinical use is rare because of significant systemic side effects.2 A nanoformulation of camptothecin is expected to improve the safety profile of this drug. CRLX101, a drug–conjugate formulation of camptothecin and a cyclodextran-PEG polymer, is being studied alone and in combination with other drugs in numerous phase 1 and 2 clinical trials in the treatment of lung cancers (SCLC and NSCLC), gynecological malignancies, and solid tumors.2,15 Clinical studies of CRLX101 in renal cell carcinoma and gastrointestinal cancers have been completed.15 CRLX101 has shown promising early clinical results.31 A polymer conjugate of docetaxel named CRLX301 is also being studied in a phase 1/2a clinical trial in patients with advanced solid tumors.15
Polymer NPs with antibacterial properties are also being investigated in the treatment of active infections.2 Quaternary ammonium polyethyleneimine-based polymers have potent activity that makes them particularly promising.32 Their highly charged nature can disrupt bacterial membranes in a number of gram-positive and gram-negative bacteria.32 Polymeric formulations of doxycycline have also been developed to improve the treatment of chronic periodontitis.33 These nanoformulations have demonstrated a more sustained release and improved efficacy compared with free drug in treating this condition.2 Polymeric doxycycline is also being studied in phase 2 trials examining the potential benefits of the use of this nanodrug following mechanical debridement.2
Two polymeric nanoformulations of antiretroviral agents are being investigated in HIV treatment.2 Efavirenz, a nonnucleoside reverse transcriptase inhibitor, is often used as a preferential first-line treatment for HIV infection.2 Lopinavir, a protease inhibitor, is commonly used in combination therapy in HIV.2 NANOefavirenz and NANOlopinavir are nanoformulations of these antiretroviral agents that have been developed with the aim of reducing total dosage while maintaining clinical efficacy, thereby improving patient tolerability and decreasing treatment costs.2 Preclinical studies have demonstrated bioequivalent efficacy in suppressing HIV-1 replication and slower emergence of drug resistance with HIV-IIIB and subtype A virus.2
Micelle NPs
Micelles are self-assembling polymeric amphiphile NPs that can be customized for the slow, controlled delivery of the hydrophobic drugs they may carry.2 The composition and structure of a micellar NP can be finely tuned to achieve different particle size, drug loading, and release characteristics. 2 Micelles have a hydrophobic internal core, which can be used to encapsulate drugs that have poor aqueous solubility.1 However, the exterior surface of a micelle has enough polarity to allow dissolution in aqueous solutions.1
Estrasorb (estradiol hemihydrate, Novavax, Inc.) is an FDA-approved micellar formulation that is indicated for moderate-to-severe vasomotor symptoms associated with menopause.1 Transdermal delivery of estradiol avoids first-pass metabolism and leads to stable serum levels for eight to 14 days.1 This route of delivery also avoids gastrointestinal side effects.1 Paclical (Oasmia Pharmaceutical), a micellar formulation of paclitaxel encapsulated in a proprietary retinoid compound (XR-17), was granted orphan drug status in 2009 by the FDA for the treatment of ovarian cancer.2 This approval was based on preclinical data that suggested it was less toxic than Kolliphor-based paclitaxel.2 Because of the broad applicability of micellar-based nanoformulations, new products are expected in the near future.1
Micellar formulations of other cancer treatments are being investigated in clinical trials.1,2 Oxaliplatin and cisplatin are frequently used platinum chemotherapies that have well-known dose-limiting nephrotoxicities and neurotoxicities.2 Nanoplatin (NC-6004, NanoCarrier Co., Ltd.), a micellar formulation of cisplatin, is being investigated in several phase 1 and 2 clinical trials studying its use alone and in combination with other chemotherapies (e.g., gemcitabine).2,15 A micellar nanoformulation of SN-38, an active metabolite of the topoisomerase inhibitor irinotecan, is also being studied.2 Two phase 1 trials have been completed, as well as phase 2 trials in solid tumors, NSCLC, and triple-negative breast cancer.15 Genexol-PM (Samyang Biopharm) is an mPEG-block-D,L-PLA micellar formulation of paclitaxel that is being developed as an alternative to Kolliphor-based paclitaxel.2 Genexol-PM has been approved for the treatment of metastatic breast cancer and advanced lung cancer in South Korea and is being investigated in phase 1 and 2 clinical trials elsewhere.2,4,15 Several phase 1–2 trials completed in patients with metastatic breast cancer or NSCLC have demonstrated fairly low toxicity rates and favorable overall response rates ranging from 40% to 68%.34–37
Nanocrystal NPs
Nanocrystals are versatile NPs that are used to improve the PK/PD properties of poorly soluble organic or inorganic materials by increasing their bioavailability and solubility.4,38,39 Nanocrystals possess a narrow, tunable, symmetric emission spectrum and are photochemically stable.40 They are composed of an optically active core surrounded by a shell that provides a physical barrier against the external environment, making them less sensitive to photo-oxidation or medium changes.40
Nanocrystal-based drugs are unique because they are composed entirely of drug compound.1 Increased surface area on the nanoscale promotes enhanced dissolution speed and saturation solubility.1,4 Saturation solubility increases the forces that drive diffusion-based mass transfer through biologic structures, such as the walls of the gastrointestinal tract.1 However, the oral absorption mechanism for nanocrystal formulations is not fully understood, and their behavior after subcutaneous injection is not fully predictable.4
Solubility issues for a number of drug compounds have been resolved through conversion into nanocrystals, which are marketed for a wide range of indications.1 Rapamune, granted FDA approval in 2000, was the first milled organic nanocrystal drug.1 Its active ingredient is sirolimus, a bacterial-derived macrocyclic immunosuppressant used to prevent rejection after transplantation of an organ (particularly a kidney).1 Rapamune’s nanocrystal-based formulation provides poorly soluble sirolimus with a continuous extended-release profile that is well suited for its indication.1
The milling technology developed by Elan Nanosystems (now Alkermes) that produced Rapamune nanocrystals has proven to be flexible for application to other types of formulations, including oral suspensions, tablets, and intramuscular injections.1 After the FDA approved Rapamune, the same milling technique was used to produce several other approved nanocrystal drug formulations, such as Tricor (fenofibrate, AbbVie) and Emend (aprepitant, Merck).1,4 This milling approach is expected to be applied as a potential solution for the wide range of solubility issues that occur with an estimated 70% to 90% of drug compounds.1,4 Inorganic nanocrystal formulations approved by the FDA are limited and include only nanocrystal forms of hydroxyapatite and calcium phosphate for use as bone-graft substitutes.1
Matinas Biopharma is developing two lipid nanocrystal formulations of antimicrobial agents.2 MAT2203, a nanoformulation of the antifungal amphotericin B, is undergoing phase 2 trials in patients with chronic candidiasis who are intolerant or refractory to standard nonintravenous treatments.15 MAT2501 is a lipid nanocrystal formulation of amikacin.2 Conventional formulations of amikacin are associated with neurotoxicity and nephrotoxicity and require careful monitoring.2 The targeted delivery of amikacin to designated areas by MAT2501 results in a reduction in total dose and an improved safety profile.2 Matinas Biopharma is waiting for an IND designation from the FDA prior to commencing phase 1 trials with MAT2501.2
Inorganic NPs
A large number of inorganic materials, such as metal oxide, metal, or silica, can be used to create NPs.1 In particular, metal and metal oxide NPs are being investigated intensely for therapeutic and imaging applications.4
Iron oxide NPs have been studied in numerous clinical trials investigating their use as contrast enhancement reagents for magnetic resonance imaging (MRI).1 However, the majority of FDA-approved iron oxide nanodrugs are indicated as iron replacement therapies.1 These include Venofer (iron sucrose injection, American Regent, Inc.), Ferrlecit (sodium ferric gluconate complex in sucrose injection, Sanofi-Aventis U.S.), Infed (iron dextran injection, Actavis Pharma), and Dexferrum (iron dextran injection, American Regent, Inc.), which are all indicated for the treatment of anemia associated with chronic kidney disease (CKD).1 These nanoformulations contain an iron oxide core, coated with hydrophilic polymers (e.g., dextran, sucrose), that allow the iron to dissolve slowly after intravenous injection.1 Using this formulation allows large doses to be administered rapidly without an increase in free iron levels in the blood, avoiding toxicity.1
Superparamagnetic iron oxide nanoparticles (SPIONs) have low toxicity, remain in circulation for a long time, and are usually biodegradable.3 SPIONs (particularly iron oxide and magnetite) have long been used as nontargeted contrast agents for MRI.3 They also respond strongly when exposed to a magnetic field and therefore can be “functionalized” to target specific tumors.2,3 As a consequence, SPIONs are being used increasingly in the development of targeted MRI contrast agents and drug-delivery systems.2 Three SPION drug formulations have received FDA approval—Feraheme (ferumoxytol, AMAG Pharmaceuticals), Feridex, and GastroMARK; however, the latter two have been withdrawn from the market.1 Feraheme is indicated for the treatment of anemia associated with CKD and is still available.1 This nanoformulation is also being studied as an imaging agent in numerous clinical trials.15
Another application for SPIONs involves the energy that they release in a magnetic field, which permits them to be used as hyperthermia agents.2 Several SPIONS have demonstrated promising preclinical and early clinical results when used as a hyperthermia treatment against tumors.2 Nanotherm (MagForce AG) utilizes aminosilane-coated SPIONS for local hyperthermia treatment of glioblastoma tumors.1,2 After injection of Nanotherm directly into the tumor, an alternating magnetic field is applied to selectively heat the particles.1 This results in the local heating of the tumor microenvironment to 40–45° C, causing programmed and nonprogrammed cell death.1 During clinical trials, treatment of glioblastoma tumors with Nanotherm demonstrated an OS increase of up to 12 months.41 Nanotherm is currently awaiting FDA approval.1
Gold NPs have also shown promise as antineoplastic agents when used alone or as a drug delivery vector.2 Gold has a unique combination of thermal and optical properties and can be tuned by varying size, shape, and/or surface chemistry.1 Excitement of electrons in the gold NP by electromagnetic radiation can generate a substantial amount of energy.2 Colloidal gold NPs that are smaller than 5 nm are excellent radiosensitizers when used with high-energy electromagnetic radiation produced by a linear accelerator, as well as with lower-energy laser-based therapies.2
Gold NPs have been studied as a drug delivery vector for the extremely toxic antitumor agent tumor necrosis factoralpha (TNFα), which can cause profound cardiovascular compromise.2 Formulations of gold NPs studied as a drug delivery platform for TNFα in preclinical studies demonstrated decreased toxicity; however, because they were rapidly cleared by the reticuloendothelial system, they were determined to be of little clinical value.2 However, a nanoformulation of gold NPs conjugated with PEG is being developed; this has been found to significantly decrease clearance rates.2 In Aurimune (CytImmune), recombinant human TNF is attached to gold NPs using a PEG linker that also acts as a biocompatible antifouling layer.4 In a phase 1 study, Aurimune was shown to be well tolerated in patients with advanced cancer.42 The PEG layer was also determined to decrease uptake by the mononuclear phagocyte system, which aided in the accumulation of drug in tumor masses via the EPR effect.42 To date, the FDA has not yet approved any gold-based nanodrugs.1 Several metals, including silver, are known to be potent antimicrobials.5 Metal ions can easily penetrate bacterial cells and induce toxic effects.2 The size of silver NPs can also be tuned to establish and augment its plasmonic effects.1
Cornell dots are inorganic silica NPs that are being developed at Cornell University as a diagnostic and therapeutic tool in cancer treatment.1 Although designed for lymph-node mapping in cancer patients, these NPs have also been found to induce cancer cell death in vitro and reduce the size of tumors after multiple high-dose injections were administered to mice.1,43 They are composed of an internal silica core labeled with a near-infrared fluorescent dye, a targeting moiety, and an antifouling polymer layer.1 This design has created an NP that is more stable and 20 to 30 times brighter than a conventional solution of the constituent dye.1 A recent “first in human” trial (N = 5) demonstrated a favorable PK/distribution and safety profile when used as a tumor imaging agent, allowing investigation in additional trials with humans in the near future.43
Protein NPs
Protein-based NPs include drugs conjugated to protein carriers, formulations where the protein itself is the active therapeutic, and complex combined platforms that use proteins for targeted delivery.1 Early protein-based NPs exploited the properties of proteins in blood serum, which allow the transport and dissolution of drugs during circulation.1 Natural proteins were combined with conventional drugs in these agents to reduce toxicity.1
During the past 10 years, albumin has gained attention as a drug carrier, leading to the investigation of numerous albumin-based NPs in clinical trials.44 Similar to other NPs, albumin particles alter the PK of a free drug, increasing passive accumulation in solid tumors via the EPR effect.44 In addition, after the protein NP dissociates into individual drug-loaded albumin molecules, cellular uptake mechanisms mediated by albumin-receptors are enabled.44
Abraxane, which incorporates 130-nm albumin NPs conjugated with paclitaxel, is an early example of an albuminbased nanodrug.44 Approved in 2005, Abraxane was designed to eliminate the toxic solvent Kolliphor, which was necessary to solubilize paclitaxel.1 Albumin-bound paclitaxel NPs improved infusion time and eliminated the need to concomitantly administer antihistamines and dexamethasone to prevent an immune reaction to Kolliphor.1 In addition to improving toxicity, Abraxane improved drug PK and efficacy compared to treatment with a conventional formulation of paclitaxel.45 After the success of Abraxane, several additional albuminbound NPs (NABs) have entered clinical trials for the purpose of improving the efficacy and safety of other drugs.1 Among these are NAB-docetaxel, NAB-heat shock protein inhibitor, and NAB-rapamycin.1
After the approval of Abraxane, a shift occurred from the use of unmodified proteins to engineered particle complexes designed to enable active targeting.1 Ontak (denileukin diftitox, Eisai, Inc.), approved in 2008, is an example of an engineered fusion protein that combines targeting proteins with cytotoxic molecules.1 It is an interleukin (IL)-2 receptor antagonist that was initially designed to treat an aggressive form of non-Hodgkin’s peripheral T-cell lymphomas (PTCL) by targeting the cytocidal action of diphtheria toxin toward cells that over-express the IL-2 receptor on T cells.1 In clinical trials, combination therapy with Ontak and cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP), the first-line chemotherapy for PTCL, achieved an OS of 63.3%, compared with an OS of 32% to 35% with CHOP alone.46 Ontak was also not observed to be myelosuppressive, nor was it associated with significant organ toxicity.47 Ontak, representing the first actively targeted proteinaceous NP, may be effective for a range of hematological malignancies, many of which overexpress IL-2.4,48
RSV-F (Novavax) is a protein-based NP containing a respiratory syncytial virus (RSV) fusion protein that was developed to treat RSV in infants.2 Passive immunization enhances maternal RSV antibody transfer, offering a viable vaccination option.2 Preclinical data have shown that RSV-F and palivizumab (Synagis, MedImmune) demonstrate potential clinical significance when given in combination.49 A phase 2 trial is assessing the safety and immunogenicity of different formulations of RSV-F NPs in healthy women of childbearing age.15
Dendrimer NPs
Dendrimers are expected to be one of the more useful nanodrug platforms to date.2 They are composed of iterative monomers arranged concentrically around a central core.2 This configuration allows pharmacologically active substances to be encased within the interior cavity or to be connected to the NP surface.50 Precise control of many important NP properties is possible when forming dendrimers, including shape, size, charge, surface properties, and composition.2 Dendrimers can also consist of functional subunits that act as delivery vehicles or drugs.2
There is a wealth of information in the preclinical literature to support the use of dendrimeric platforms in nanodrugs.2 Dendrimers have been successfully used for nanodrug formulations administered via many routes, including cutaneously, intravenously, orally, rectally, and vaginally.2 Many dendrimer-based drug compounds are expected to enter early phase clinical trials within the next few years.2 The many possible routes of administration for dendrimer-based nanodrugs make it likely that some of these agents will receive FDA approval.2
A dendrimer-based cancer treatment, DTXSPL8783, is being investigated in clinical trials; a phase 1 study of this agent is under way in patients with advanced cancer.2 A dendrimeric antiviral/antibiotic compound, Vivagel (Starpharma), is in phase 3 clinical trials for bacterial vaginosis (BV).15 This unique nanodrug incorporates naphthalene disulphate groups on the surface of dendrimers.2 Phase 2 data have indicated high rates of clinical and pathologic cure of BV, as evidenced by symptomatic improvement and clear laboratory results, respectively.2 However, phase 3 data have been equivocal, with high rates of symptomatic improvement but lower rates of clinical laboratory cure being observed.2 Vivagel has also exhibited potent in vitro activity against HIV and herpes simplex virus.2 Phase 1 studies have indicated that vaginal use of this nanoformulation is well tolerated and that antiviral activity is retained by cervicovaginal fluids in most patients up to 24 hours after administration.51–53 Vivagel is available in Australia as a condom lubricant.2,4
CHALLENGES FOR NANODRUG DEVELOPMENT
Nanodrugs have been a major focus of pharmaceutical research in the last decade, creating new challenges for scientists, industry, and regulators.6 A discussion of some of the difficult issues faced by these stakeholders follows.
NPs Need to Be Categorized Further
A significant challenge for the development of nanodrugs is the characterization of new nanomaterials with respect to safety and toxicity.1 A large amount of data has accumulated regarding liposomes, polymers, and micelles; however, the numerous possibilities with respect to formulations and applications requires the toxicity of each novel or complex product to be characterized.5,6 To avoid the development of unpredictable side effects, the properties of these products need to be well understood prior to being marketed.6 However, to achieve this goal, significant research still needs to be conducted to be able to understand and predict how NPs will affect biological systems, including the development of new assays that the presence of NPs will not interfere with.1,54
The unknown properties of nanodrugs have raised important questions.6 Researchers are still learning how structure–function relationships regarding NPs and their characteristics (e.g., charge, composition, size, shape, surface coatings, complex architectures) affect biological systems.1 It is certain that tissue cells readily take up NPs via passive and active mechanisms.1,3 However, nanodrugs can potentially interact with many types of cells, organs, and tissues on the way from the site of administration to the intended target.4 They may influence coagulation effects, complement activation, immune system compatibility, phagocyte activation, and other unwanted responses.5 Some nanodrug formulations also present a challenge with respect to precise control of drug release and biodistribution.4 In addition, concerns exist regarding the physiological effects of NPs that do not readily biodegrade.4 Because of these concerns, nanodrugs need to be tested to demonstrate a favorable risk–benefit ratio and gain regulatory approval.5 In addition to general toxicity studies, it may be useful for individual NP components to be characterized, using in vitro assays to measure biological activity and toxicity.5
Attempts have been made to overcome the challenge of characterizing NPs by completing preclinical studies in numerous biological models and carefully selecting indications for clinical development.5 However, specific protocols to characterize nanodrugs at the physicochemical and physiological/biological levels are still lacking.55 In fact, for some agents, the lack of standard protocols for toxicity testing in early stages of investigation has been said to contribute to failure in late-stage clinical trials.6 This lack of standard protocols has frustrated and complicated researchers’ efforts in determining the potential toxicity of nanodrugs.1,6 In the meantime, efforts have been made to measure the PD activity of NP disease-site targeting through imaging studies; such efforts in early clinical studies can potentially reduce the risks involved in later-stage development.5
Standards for characterization have not been defined despite several attempts.1,6 Closer collaboration between regulatory agencies is still needed, but major efforts have been made.1,6 Perhaps most notably, the FDA has published guidelines regarding the importance of nanomaterial characterization that include emerging standards for this purpose.1 These efforts will likely lead to the availability of better data regarding the toxicity of nanomaterials.1 Data demonstrating the physicochemical properties, efficacy, and toxicity of a nanodrug can then be compiled into an IND application for FDA review and approval.1 The Nanotechnology Characterization Laboratory (NCL), established by the National Cancer Institute, has also published documents about innovative platforms for the development of nanodrugs for cancer treatment.6 Researchers can send nanomaterials to the NCL to have them tested and validated according to a series of emerging protocols.1
Safety Issues
It is impossible to make a generalized statement about the safety of nanodrugs because they incorporate a variety of NPs and materials.7 However, reports have been published regarding the tendency of some NPs to display toxicity.7 Toxic effects have been observed at the molecular, cellular, or tissue level.7 As an NP moves through the body, it can be exposed to different biological environments, including the cytoplasm, extracellular matrix, cellular organelles, and blood.7 Depending on size and physicochemical properties, NPs have been known to adsorb plasma proteins and to interact with immune cells.6 The free radical/oxidative activity of some NPs may also cause genotoxicity.7
Interactions at the NP–biological interface may even affect organ function.7 Several studies have reported inflammation in the liver, lung, and brain due to NP-induced oxidative stress.56–58 Some NPs have also been observed in in vitro and in vivo studies to cross the blood–brain barrier after IV administration, causing neurotoxicity.59 The organs that may be most affected are those that experience the highest levels of NP accumulation.7 Lung inflammation has been observed following the administration of carbon nanotubes via intratracheal installation.7 Positively charged lipid NPs in the blood have also been found to cause hepatotoxicity after intravenous administration, which was evident based on increased hematologic liver enzyme levels in the blood.7 Evidence also suggests that NP structures can have strong immunomodulating activity.60 NPs have been observed to induce both immunostimulation and immunosuppression.60 Controlling the immunological properties of NPs is one of the most important aspects necessary for their safe use.
Toxic effects have been reported more frequently for certain categories of NPs than for others.7 For example, carbon-based NPs have demonstrated toxicity in several in vitro and in vivo studies; however, results conflict.7 The mechanisms thought to be responsible for carbon nanotube toxicity include oxidative stress, inflammatory responses, interstitial fibrosis, granuloma formation, malignant transformation, and DNA mutations (errors in chromosome numbers and disruption of the mitotic spindle).61,62 Carbon nanotubes have also been observed to induce mesothelioma, a condition that is associated with asbestos, a carcinogenic, naturally occurring mineral fiber.7 It has been suggested that the toxic effects of carbon nanotubes may be a consequence of shape rather than material, demonstrating that NP toxicity may depend on particle morphology.7
Biocompatibility has also been found to depend on NP size.7 Gold NPs with a diameter of 1.4 nm were found to be toxic, while those having a diameter of 15 nm were not.7 However, other research showed that 10-nm and 60-nm glycol-coated gold NPs were highly toxic in animal models (causing elevated aspartate and alanine transaminase levels), while those measuring 5 nm and 30 nm were deemed sufficiently safe for medical applications.63 Gold NPs may also be toxic when used in high doses or over a long period because they tend to accumulate in the blood and tissues due to a low clearance rate.64 Gold NPs may also affect cell function through their affinity for DNA.4 Acute and chronic exposure to gold NPs has been found to alter gene expression.1
Other metallic NPs have also exhibited cytotoxicity, such as iron oxide and silver NPs.7 Iron oxide NPs have demonstrated harmful effects in vitro and in vivo due to the generation of reactive oxygen species.7 Inorganic, nonbiodegradable NPs, such as metallic or magnetic NPs, may persist in the environment for long periods, causing prolonged exposure of humans and animals to these materials with unknown consequences.65 Because they are included in many cleaning products, the National Institute of Environmental Health Sciences is investigating the effects of silver NPs on the immune response, the lungs, and their absorption into the bloodstream due to concerns about consumer safety.2
Despite toxic properties that have been associated with some NPs, this does not necessarily prevent them from being used in medical applications.7 The toxic properties of NPs may be harnessed for positive purposes, such as using tissue toxicity to ablate diseased tissue or immune induction for potentially beneficial use in cancer immunotherapy.7 In addition, the harmful effects of NPs can often be reduced through surface modification; for example, coating iron oxide NPs with a polymer has been shown to improve cell viability dramatically.7
Although NPs can clearly help reduce or eliminate many challenges observed with conventionally formulated drugs, they have their own limitations and causes for concern.1 In fact, after receiving FDA approval, some nanodrugs have been withdrawn from the market due to safety concerns (e.g., Feruglose and Resovist).1 Therefore, following FDA approval, phase 4 post-marketing studies should be conducted to further assess the risks associated with nanodrugs.1
Lack of Specific Regulatory Guidelines
The regulatory environment for nanodrugs has been challenging because of several key issues.6 Currently, the FDA approval process for nanodrugs is essentially the same as that for any other drug or biologic.1,5,6 Preclinical testing of nanodrugs usually involves animal studies to demonstrate efficacy, safety, and dose ranges.1 Following FDA approval of an IND, clinical trials are initiated to determine safety and efficacy in humans.1 These trials are separated into phase 1 (dosing, toxicity, and excretion in healthy subjects), phase 2 (safety and efficacy in subjects with the target illness), and phase 3 (randomized, placebo-controlled, multicenter trials).1 Once these trials are completed, a new drug application can be filed with the FDA to request approval of the nanodrug.1 After approval, phase 4 studies may be undertaken at the request of the FDA, health care professionals, or other groups.1
During June 2014, the FDA issued four (one draft and three final) guidance documents for industry regarding the use of nanotechnology in FDA-regulated products, including nanodrugs. 5 The FDA is expected to release another guidance document that specifically addresses nanodrugs and nanobiologics. 5 The guidance documents that have been released encourage manufacturers to consult the FDA early in the product development process regarding the specific regulatory and scientific issues considered relevant to the nanotechnology product.5 This consultation is also encouraged to address questions regarding the efficacy, safety, impact on the public, and/or regulatory status of the product.5 Therefore, unless specific findings warrant special consideration for a particular product, the development of nanodrugs follows the typical drug-development process.5 However, this approach to nanodrug regulation has been questioned.54 Critics say that rather than adapting and applying existing regulations, the FDA should establish regulatory guidelines that specifically apply to nanomedical products, particularly because the safety and toxicity of many nanomaterials have not been fully characterized.1,54
Cost–Benefit Considerations
The worldwide investment and interest in nanomedicine accelerated in the early 2000s and has continued.4 Because of these efforts, innovative nanodrugs are already available on the market.6 These sophisticated, effective agents were developed because of an increase in financial investment and partnership among industry, academia, and governments to integrate multiple technologies.6 The value of nanodrugs expected to be developed by 2019 has been estimated at $178 billion.4 Sales of Abraxane alone for several oncology indications were estimated to be $967 million, making it one of the best-selling nanodrugs in the world.4
However, despite the sales success of some nanopharmaceuticals, financial challenges still impede the development of these drugs.4 Demonstrating sufficient efficacy and safety to be granted regulatory approval is not easy, particularly when other products are on the market for the same target indication.2,4 In fact, a common feature among most (about 77%) approved nanodrugs is that a conventional formulation of the drug had already been approved.4 Because the efficacy and safety of the active ingredient had already been established, the decision to develop these nanodrugs involved reduced financial risk compared with a novel new chemical entity (NCE).4
More complex economic considerations are involved when developing a nanodrug that contains an NCE, such as the level of investment required to support chemistry, manufacturing, and controls development and production.5 Customized instrumentation, manufacturing equipment, and/or facilities may be cost prohibitive for some companies or may require stepwise investment strategies that depend on reaching incremental clinical development goals.5 The investment needed for the development of a nanodrug involving an NCE also must be evaluated with respect to overall risk–return in comparison to other drug-development opportunities.5 For the 23% of approved nanodrugs that did involve an NCE, nanoformulation was likely necessary because conventional medications were unsuccessful due to poor aqueous solubility.4
Unless a nanoformulation demonstrates improvements compared with available formulations with the same active ingredient, it is questionable whether the drug will obtain regulatory appoval.2 Nanoformulations of existing conventional drugs do not always meet this criteria.2 Liposomal nanoformulations of cisplatin (Li-PlaCls, lipoplatin, L-NDDP, and SPI-77) demonstrated less toxicity than free cisplatin but failed to demonstrate increased efficacy.2 Because other platinum alternatives exist that are less nephrotoxic than cisplatin (such as carboplatin), further development of liposomal cisplatin is unlikely.2 Several liposomal formulations of paclitaxel (EndoTAG-1 and LEP-ETU) were also abandoned after polymeric and albuminbound formulations of paclitaxel were successfully introduced to the market.2
Therefore, during the early stages of development, the potential efficacy and safety benefits that can be achieved realistically by a nanoformulation need to be carefully considered. 2 Comparison with competitor products and a cost–benefit analysis must occur to avoid investing resources in developing a drug that is unlikely to gain approval.2 Pharmacoeconomic studies need to be performed to determine the economic and social value added for nanoformulated products when compared with established treatments.6 Measures such as improvement in quality-adjusted life-expectancy years or cost reductions associated with future consecutive hospitalizations also have to be evaluated prior to developing nanodrugs.6 In addition, the success of a nanodrug may be challenged by the fact that expenses involved in development and regulatory approval may not be compensated by the limited sales for drugs that are approved for niche indications.6 This may be especially true for increasingly complex nanodrugs, which are associated with higher costs.1 The issuance of surprisingly broad patents by the Patent and Trademark Office for multiple NPs has been cause for additional concern.12 This has created confusion because competing interests are unsure of the validity and enforceability of the patents.12
Waning Enthusiasm Among Health Care Professionals
Waning enthusiasm among health care professionals is another challenge faced by nanodrug developers.2 This decrease is often attributed to the fact that most nanodrugs achieve improved safety rather than increased efficacy.2 While improvements in oncology drugs have been clinically meaningful, nanoformulations of chemotherapies have not dramatically altered the course of late-stage solid tumors.5 From a biological standpoint, this is not surprising because improved PK in a chemotherapy nanoformulation is not likely to be sufficient to overcome the development of resistance by the tumor.2 For nanodrugs to deliver on the promise of being potentially revolutionary, they will need to improve efficacy significantly.2
Views have also been expressed in the medical literature that the number of nanodrugs that have been approved is disproportionately small in comparison to the large investment made in this field.4,66 However, in the past, it has taken several decades before a medical discovery has been translated into a commercial product (e.g., biologics).4 Because information and knowledge is now exchanged more rapidly, a shorter timeframe may be possible; however, the translation of academic research into clinically available products will still take time.4 Better coordinated efforts in funding critical issues in nanomedicine by the National Institutes of Health and the National Science Foundation may also help to accelerate progress in this field.4
CONCLUSION
Nanodrug development has advanced significantly during the past decade.2,6 Dozens of nanodrugs have received FDA approval, and many more are in early stages of development or in clinical trials.2 Most of the currently approved nanodrugs are based on conventional drugs that had already been approved and are composed of simple NPs.1 However, nanodrug platforms are incorporating a broadening range of NP types and becoming more complex.1 Even though few nanodrugs that are in early stages of development will ultimately receive regulatory approval, the amount of work that is occurring in this field predicts that many new nanodrugs will eventually be available for clinical use.1,2 While many challenges face nanodrug development, it may only be a matter of time until these agents provide unique solutions for unmet clinical needs and greatly alter clinical practice.1,2,6
REFERENCES
- 1.Bobo D, Robinson KJ, Islam J, et al. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 2.Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1) doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 3.Ventola CL. The nanomedicine revolution: part 1: emerging concepts. P T. 2012;37(9):512–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Havel HA. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016;18(6):1351–1353. doi: 10.1208/s12248-016-9970-6. [DOI] [PubMed] [Google Scholar]
- 5.Havel H, Finch G, Strode P, et al. Nanomedicines: from bench to bedside and beyond. AAPS J. 2016;18(6):1373–1378. doi: 10.1208/s12248-016-9961-7. [DOI] [PubMed] [Google Scholar]
- 6.Sainz V, Conniot J, Matos AI, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Wolfram J, Zhu M, Yang Y, et al. Safety of nanoparticles in medicine. Curr Drug Targets. 2015;16(14):1671–1681. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, Wang R, Nie G. Applications of nanomaterials as vaccine adjuvants. Hum Vaccin Immunother. 2014;10:2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanberry LR, Simon JK, Johnson C, et al. Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine. 2012;30:307–316. doi: 10.1016/j.vaccine.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 11.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Ventola CL. The nanomedicine revolution: part 2: current and future clinical applications. P T. 2012;37(10):582–591. [PMC free article] [PubMed] [Google Scholar]
- 13.Centerwatch. 2017 FDA approved drugs. [Accessed October 25, 2017]. Available at: www.center-watch.com/drug-information/fda-approved-drugs.
- 14.Food and Drug Administration. Novel drug approvals for 2017. Oct 20, 2017. [Accessed October 25, 2017]. Available at: www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm537040.
- 15.ClinicalTrials.gov. [Accessed October 25, 2017]. Available at: https://clinicaltrials.gov.
- 16.Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethyleneglycol-coated liposomes. Cancer Res. 1994;54(4):987–992. [PubMed] [Google Scholar]
- 17.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs. cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121:234–242. doi: 10.1002/cncr.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy JP, Dupont L, Konstan MW, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68:818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geho WB, Geho HC, Lau JR, Gana TJ. Hepatic directed vesicle insulin: a review of formulation development and preclinical evaluation. J Diabetes Sci Technol. 2009;3:1451–1459. doi: 10.1177/193229680900300627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geho WB, Rosenberg LN, Schwartz SL, et al. A single-blind, placebo-controlled, dose ranging trial of oral hepatic-directed vesicle insulin add-on to oral antidiabetic treatment in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2014;8:551–559. doi: 10.1177/1932296814524871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Miller L, Richman S, et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol. 2012;52(6):798–808. doi: 10.1177/0091270011407068. [DOI] [PubMed] [Google Scholar]
- 23.Flexion Therapeutics, Inc. Our product: Zilretta (triamcinolone acetonide extended release formulation) [Accessed October 25, 2017]. Available at: https://flexiontherapeutics.com/our-product.
- 24.Verschraegen CF, Skubitz K, Daud A, et al. A phase 1 and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63:903–910. doi: 10.1007/s00280-008-0813-8. [DOI] [PubMed] [Google Scholar]
- 25.Morgan MA, Darcy KM, Rose PG, et al. Paclitaxel poliglumex and carboplatin as first-line therapy in ovarian, peritoneal, or fallopian tube cancer: a phase 1 and feasibility trial of the Gynecologic Oncology Group. Gynecol Oncol. 2008;110:329–335. doi: 10.1016/j.ygyno.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabbatini P, Sill MW, O’Malley D, et al. A phase II trial of paclitaxel poliglumex in recurrent or persistent ovarian or primary peritoneal cancer (EOC): a Gynecologic Oncology Group Study. Gynecol Oncol. 2008;111(3):455–460. doi: 10.1016/j.ygyno.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Awada A, Garcia AA, Chan S, et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol. 2013;14(12):1216–1225. doi: 10.1016/S1470-2045(13)70429-7. [DOI] [PubMed] [Google Scholar]
- 28.Dipetrillo T, Milas L, Evans D, et al. Paclitaxel poliglumex (PPXXyotax) and concurrent radiation for esophageal and gastric cancer: a phase 1 study. Am J Clin Oncol. 2006;29:376–379. doi: 10.1097/01.coc.0000224494.07907.4e. [DOI] [PubMed] [Google Scholar]
- 29.Dipetrillo T, Suntharalingam M, Ng T, et al. Neoadjuvant paclitaxel poliglumex, cisplatin, and radiation for esophageal cancer: a phase 2 trial. Am J Clin Oncol. 2012;35:64–67. doi: 10.1097/COC.0b013e318201a126. [DOI] [PubMed] [Google Scholar]
- 30.Jeyapalan S, Boxerman J, Donahue J, et al. Paclitaxel poliglumex, temozolomide, and radiation for newly diagnosed high-grade glioma: a Brown University Oncology Group Study. Am J Clin Oncol. 2014;37:444–449. doi: 10.1097/COC.0b013e31827de92b. [DOI] [PubMed] [Google Scholar]
- 31.Weiss GJ, Chao J, Neidhart JD, et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymercamptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest New Drugs. 2013;31:986–1000. doi: 10.1007/s10637-012-9921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega A, Farah S, Tranque P, et al. Antimicrobial evaluation of quaternary ammonium polyethyleneimine nanoparticles against clinical isolates of pathogenic bacteria. IET Nanobiotechnol. 2015;9:342–348. doi: 10.1049/iet-nbt.2014.0078. [DOI] [PubMed] [Google Scholar]
- 33.Valle JW, Armstrong A, Newman C, et al. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Invest New Drugs. 2011;29:1029–1037. doi: 10.1007/s10637-010-9399-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim TY, Kim DW, Chung JY, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Chung HC, Im SA, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 36.Kim DW, Kim SY, Kim HK, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non–small-cell lung cancer. Ann Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 37.Ahn HK, Jung M, Sym SJ, et al. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non–small-cell lung cancer. Cancer Chemother Pharmacol. 2014;74:277–282. doi: 10.1007/s00280-014-2498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bansal S, Bansal M, Kumria R. Nanocrystals: current strategies and trends. Int J Res Pharm Biomed Sci. 2012;3:406–419. [Google Scholar]
- 39.Gao L, Liu G, Ma J, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30:307–324. doi: 10.1007/s11095-012-0889-z. [DOI] [PubMed] [Google Scholar]
- 40.Bruchez M, Jr, Moronne M, Gin P, et al. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 41.Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neuro-Oncol. 2011;103(2):317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleischman T. Cancer killers: C dots show ability to induce cell death in tumors. Cornell Chronicle. Sep 26, 2016. [Accessed August 9, 2017]. Available at: http://news.cornell.edu/stories/2016/09/cancer-killers-c-dots-show-ability-induce-cell-death-tumors.
- 44.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine. 2015;10:1245–1257. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai N, Trieu V, Yao ZW, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 46.Fuentes AC, Szwed E, Spears CD, et al. Denileukin diftitox (Ontak) as maintenance therapy for peripheral T-cell lymphomas: three cases with sustained remission. Case Pep Oncol Med. 2015;2015:123756. doi: 10.1155/2015/123756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foss FM, Sjak-Shie N, Goy A, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine, and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma. 2013;54(7):1373–1379. doi: 10.3109/10428194.2012.742521. [DOI] [PubMed] [Google Scholar]
- 48.Foss F. Clinical experience with denileukin diftitox (Ontak) Semin Oncol. 2006;33(1 suppl 3):S11–S16. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Glenn GM, Fries LF, Smith G, et al. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine. 2015;33:6488–6492. doi: 10.1016/j.vaccine.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Svenson S, Tomalia DA. Dendrimers in biomedical applications— reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 51.O’Loughlin J, Millwood IY, McDonald HM, et al. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): a dose ranging, phase 1 study. Sex Transm Dis. 2010;37:100–104. doi: 10.1097/OLQ.0b013e3181bc0aac. [DOI] [PubMed] [Google Scholar]
- 52.Price CF, Tyssen D, Sonza S, et al. SPL7013 Gel (VivaGel) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6(9):e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen CR, Brown J, Moscicki AB, et al. A phase 1 randomized placebo controlled trial of the safety of 3% SPL7013 Gel (VivaGel) in healthy young women administered twice daily for 14 days. PLoS One. 2011;6(1):e16258. doi: 10.1371/journal.pone.0016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ventola CL. The nanomedicine revolution: part 3: regulatory and safety challenges. P T. 2012;37(11):631–639. [PMC free article] [PubMed] [Google Scholar]
- 55.Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64:129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003;15:39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- 57.Stone V, Brown DM, Watt N, et al. Ultrafine particle-mediated activation of macrophages: intracellular calcium signaling and oxidative stress. Inhal Toxicol. 2001;12:345–351. doi: 10.1080/08958378.2000.11463244. [DOI] [PubMed] [Google Scholar]
- 58.Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- 59.Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood–brain barrier disruption, edema formation, and brain pathology. Prog Brain Res. 2007;162:245–273. doi: 10.1016/S0079-6123(06)62013-X. [DOI] [PubMed] [Google Scholar]
- 60.Di Gioacchino M, Petrarca C, Lazzarin F, et al. Immunotoxicity of nanoparticles. Int J Immunopathol Pharmacol. 2011;24(1 suppl):S65–S71. [PubMed] [Google Scholar]
- 61.Kolosnjaj J, Szwarc H, Moussa F. Toxicity studies of carbon nanotubes. Adv Exp Med Biol. 2007;620:181–204. doi: 10.1007/978-0-387-76713-0_14. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 63.Zhang XD, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine. 2011;6:2071–2081. doi: 10.2147/IJN.S21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X. Gold nanoparticles: recent advances in the biomedical applications. Cell Biochem Biophys. 2015;7:771–775. doi: 10.1007/s12013-015-0529-4. [DOI] [PubMed] [Google Scholar]
- 65.Radomska A, Leszczyszyn J, Radomski MW. The nanopharmacology and nanotoxicology of nanomaterials: new opportunities and challenges. Adv Clin Exp Med. 2016;25(1):151–162. doi: 10.17219/acem/60879. [DOI] [PubMed] [Google Scholar]
- 66.Weissig V, Guzman-Villanueva D. Nanopharmaceuticals (part 2): products in the pipeline. Int J Nanomedicine. 2015;10:1245–1257. doi: 10.2147/IJN.S65526. [DOI] [PMC free article] [PubMed] [Google Scholar]