The immune system is one of the three main consumers of energy in the human body; brain, muscle, and the immune system use similar amounts (approximately 500 kcal/day) [1]. Rapid bursts of cellular proliferative, biosynthetic, and secretory activities by leukocytes require considerable metabolic resources that are especially important during periods of infection and inflammation [2, 3]. Because immune cells have negligible intracellular nutrient stores and rely on aerobic glycolysis for activation and proliferation, they are particularly dependent on the uptake of metabolic substrates [2–6]. Indeed, glucose uptake is the primary limiting factor in T-cell activation [5, 6]. T-cell activation and proliferation are decreased in low-glucose states along with reduced production of cytokine effectors of the immune response, including interferon γ (IFN-γ) [6–8]. Susceptibility to infections by individuals with metabolic diseases further underscores the significant impact of metabolic disruption on the functions of innate and adaptive immunity [9, 10].
Pathogens that are well adapted to their hosts have developed an extraordinarily wide range of mechanisms to modulate host immunity in order to facilitate and prolong infection and transmission. Several of these mechanisms are relatively well characterized and appear to act directly on host target tissues. Traditionally, the effects that many pathogens have on host metabolism have been assumed to be downstream consequences of pathogenesis. However, increasing evidence suggests that these pathogen-induced metabolic disturbances may instead reflect aspects of the pathogens’ modulation of the immune response to enhance and/or prolong the period of infection and transmissibility (Fig 1). Here, we examine three diverse but highly prevalent global pathogens that disrupt host metabolism during infection and may thereby alter the host immune response: Trypanosoma cruzi, Plasmodium falciparum, and Bordetella pertussis, responsible for Chagas disease, malaria, and whooping cough, respectively. Considering how metabolic changes that pathogens induce in the host can affect the immune response may reveal commonalities that can contribute to understanding, controlling, and treating a wide range of diseases.
Fig 1. Model of pathogen interactions between host metabolism and immune response.
We propose a generalizable model demonstrating the means by which pathogens manipulate host metabolism to facilitate infection. Diverse pathogens, including P. falciparum and B. pertussis, decrease host blood glucose levels. This results in an impaired host immune response that consequently exacerbates and/or prolongs infection by providing the pathogen with improved opportunities to increase load and/or persistence and enhance transmission to other hosts.
T. cruzi
T. cruzi disrupts host glucose homeostasis within 30 days post infection (DPI) [11, 12]. Parasites are detected as early as 15 DPI within insulin-secreting pancreatic beta cells. T. cruzi’s actions in the pancreas are quite selective. Pancreatic islet architecture is significantly disrupted such that beta cells are preferentially targeted by the parasite, and other islet cell types such as glucagon-secreting alpha cells are spared [12].
Acute and subacute T. cruzi infection produce a complex pattern of changes in host insulin secretion characterized by hypoinsulinemia with accompanying hyperglycemia [13]. During acute infection, T. cruzi impairs hepatic gluconeogenesis and induces a strong inflammatory response within the host capable of triggering a systemic “cytokine storm.” This buildup of cytokines leads to decreased feeding by the host and increased glucose uptake by the parasites [14]. In chronic infection, there is impaired insulin secretion from pancreatic beta cells secondary to the ability of T. cruzi to modify insulin granule fusion, resulting in failure to properly release insulin rather than a defect in hormone production [11, 12]. Additionally, other contributors to hypoinsulinemia during infection include pathogen-induced autonomic disruption of the parasympathetic innervation of the pancreas through denervation [15, 16]. Because pancreatic secretion of insulin relies on parasympathetic neuronal inputs [17], parasympathetic denervation may contribute to the diminished insulin secretion in Chagas disease [13]. Infection also elevates glucagon levels, which further disrupts glucose homeostasis and leads to host hyperglycemia [13]. However, given that most of the above studies were conducted in animal models, it remains unclear whether T. cruzi-induced metabolic phenomena are also evident in humans. Clinical studies demonstrating both hyperglycemia and hypoinsulinemia in patients with Chagas disease have been inconclusive or variable [18]. Nevertheless, an examination of subgroups of Chagas patients reveals significant abnormalities in glucose metabolism, including hypoinsulinemia, hyperglycemia, and glucose intolerance [18–20]. This suggests that some patients may be especially vulnerable to the metabolic consequences of T. cruzi infection. Future work is needed to clarify the factors responsible for such selective clinical vulnerability to metabolic disruption in Chagas patients and the specific mechanisms employed by T. cruzi to target these affected patients.
P. falciparum
P. falciparum induces hyperinsulinemia and hypoglycemia during infection [21–24]. These metabolic sequelae are associated with more severe morbidity and increased mortality in malaria [25, 26]. However, the mechanisms for malaria-induced hypoglycemia remain poorly understood.
Diminished hepatic gluconeogenesis coupled with increased metabolic demands arising from infection have been proposed as important contributors to host hypoglycemia [27, 28]. Intriguingly, P. falciparum appears to act directly on pancreatic beta cells to cause insulin hypersecretion and, ultimately, hypoglycemia. Treatment of cultured pancreatic beta cells with plasma from patients with malaria-induced hypoglycemia resulted in a significant increase in insulin secretion [21]. Significantly, these metabolic effects were attenuated in diabetic animals whose pancreatic beta cells were depleted via the beta cell toxin streptozotocin [23, 24], further implicating P. falciparum’s effects on these beta cells in metabolic disruption. Overall, these studies suggest that P. falciparum secretes factors and activates the host immune system to increase pancreatic beta cell insulin secretion, which contributes to hypoglycemia.
B. pertussis
B. pertussis’ expression of virulence factors plays a large role in pertussis illness. While these factors promote bacterial adhesion and invasion locally, they also act more globally as immunomodulators that subvert host innate and adaptive immunity [29–31]. Consequently, even though B. pertussis is best known for its actions on the respiratory tract, this pathogen also acts at several other host sites, including spleen and blood; both sites play direct roles in mobilizing the host immune response during different phases of infection [31]. Significantly, B. pertussis also has a profound effect on host metabolism.
As early as the 1930s, clinical reports described hyperinsulinemia and resultant long-lasting hypoglycemic states during B. pertussis infection [32]. Furthermore, B. pertussis-induced hyperinsulinemia and hypoglycemia were shown to significantly increase susceptibility to inflammatory and anaphylactoid reactions [33, 34]. Beginning in the 1960s, mouse models recapitulated B. pertussis-induced hypoglycemia and hyperinsulinemia [32, 35, 36]. Moreover, in vivo studies demonstrated that selective destruction of beta cells using the drug alloxan attenuated this B. pertussis-induced hyperinsulinemia and hypoglycemia [32]. These data thus suggest that, as with P. falciparum, beta cells within the pancreatic islet are specifically targeted to stimulate hyperinsulinemia during infection.
It was discovered that these metabolic effects were primarily caused by a virulence factor secreted by B. pertussis originally named islet-activating protein (IAP). Though subsequently renamed pertussis toxin (PTX), this toxin was initially isolated and studied based on its direct actions on pancreatic beta cells to stimulate insulin secretion [37, 38]. PTX was later found in the circulating serum of B. pertussis-infected animals, providing a route for its systemic actions [39].
A crucial clue in elucidating the mechanisms by which B. pertussis and other pathogens exert metabolic effects within the host may lie in the actions of secreted virulence factors such as PTX and adenylate cyclase toxin (ACT). Secretion of these factors is part of a common strategy used by pathogens to facilitate infection and pathogenesis. Mycobacterium tuberculosis, Bacillus anthracis, Salmonella enterica, and Listeria monocytogenes rely on multiple, complex secretion mechanisms for adhesion, evasion of host defenses, and virulence, among a plethora of other functions [40, 41], but the potential impact on metabolism is somewhat more complex and has been less well considered. Understanding how B. pertussis-secreted factors disrupt metabolism to affect pathogenesis and immunity may, therefore, shed light more generally on this common virulence strategy and provide an example relevant to other diseases.
PTX
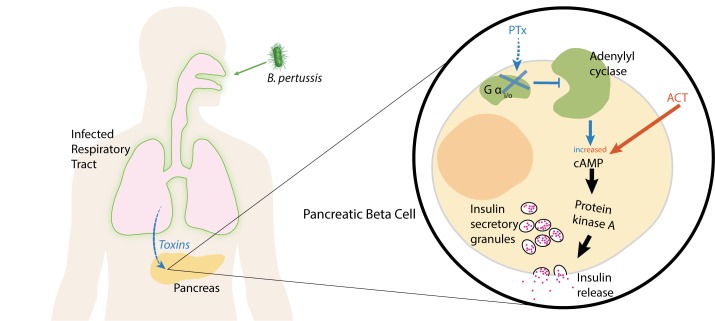
PTX is one of the most important virulence factors associated with B. pertussis pathogenesis [42]. PTX targets G-protein–coupled receptors (GPCRs) and inactivates alpha subunits of the heterotrimeric Gαi/o (Gi/o) protein family immediately downstream of these receptors by adenosine diphosphate (ADP) ribosylation [37]. GPCRs expressed in pancreatic beta cells, including α2 adrenergic and dopamine D2 receptors, play important roles in modulating insulin secretion. Because these GPCRs are Gi/o coupled, they are directly susceptible to PTX action. Under normal circumstances, GPCR stimulation results in an autocrine negative feedback circuit in which subsequent insulin release is diminished [43, 44]. The mechanism for this negative feedback is based on Gi/o’s ability to reduce levels of a key mediator of insulin secretion, cyclic AMP (cAMP). Gi/o inhibits adenylate cyclase, the enzyme responsible for cAMP synthesis, which thus prevents activation of the cAMP-dependent protein kinase A (PKA), a powerful stimulator of insulin secretion (Fig 2) [43]. This PTX-sensitive, Gi/o-mediated signaling mechanism prevents oversecretion of insulin during glucose stimulation and is important for maintaining metabolic homeostasis [44, 45]. Thus, PTX’s inhibitory actions on GPCR and Gi/o signaling provide a mechanism for the toxin’s stimulation of insulin release [37].
Fig 2. Model for PTX- and ACT-induced hyperinsulinemia.
Following infection, B. pertussis produces PTX, which acts not only within the respiratory tract but also directly on insulin-secreting pancreatic beta cells. Within these cells, PTX inhibits Gαi/o signaling that ordinarily inhibits adenylate cyclase, the enzyme responsible for cAMP synthesis. This leads to increases in cAMP and activates PKA, a key stimulator of insulin release. In parallel, B. pertussis also secretes ACT, which directly increases cAMP levels to also produce hyperinsulinemia and subsequent hypoglycemia in the host. ACT, adenylate cyclase toxin; PKA, protein kinase A; PTX, pertussis toxin.
In addition to stimulating insulin secretion, PTX acts on other aspects of host metabolism, including glucose transport. Acute PTX treatment diminishes insulin-stimulated glucose transport activity in key tissue targets of insulin action—myocytes and adipocytes—via the inhibition of Gi/o-mediated signaling independently of its effects on cAMP biosynthesis [46–48]. PTX also reduces insulin receptor affinity to insulin, further potentiating the toxin’s inhibitory effects on insulin-stimulated glucose transport [49]. Moreover, prolonged PTX action produces hypoglycemia by changing glucose transporter (GLUT) expression [50]. PTX increases the expression of GLUT-4 in muscle, resulting in increased transport of blood glucose into muscle, and lowers overall circulating glucose [50].
ACT action
In concert with PTX secretion, B. pertussis produces the toxin ACT, which is responsible for several aspects of B. pertussis’s virulence, including the impairment of T-cell activation and chemotaxis to undermine the host adaptive immune response [51, 52]. ACT is a bifunctional protein composed of an amino terminal adenylate cyclase domain and a repeat in toxin (RTX) domain that forms pores in membranes to facilitate the toxin’s entry into host cells [51]. Notably, the adenylate cyclase domain of ACT bypasses endogenous host adenylate cyclase by exhibiting its own very high catalytic activity [51]. This renders the toxin capable of rapidly generating superphysiological increases in intracellular cAMP levels within seconds of entry into host cells [53]. Therefore, analogous to PTX action in pancreatic beta cells, it is possible that ACT also acts on beta cells to increase cAMP, activate PKA, and thus significantly elevate insulin secretion.
Effects of host immune response on metabolism during infection
In addition to direct actions of pathogen-secreted toxins on host tissues to induce hypoglycemia, pathogens may also manipulate the immune response to disrupt host metabolism. For example, infection can trigger host immune cell production of cytokines, including interleukin-1 (IL-1). IL-1 activates the sympathetic nervous system and causes a drop in blood glucose [54]. Other cytokines, including IL-10, have been implicated in P. falciparum-induced hypoglycemia [55]. Likewise, Plasmodium spp. elicit the production of host immunomodulators such as tumor necrosis factor α (TNFα) during infection [56, 57], which also produces hypoglycemia [58]. Consequently, given the significant correlation between TNFα levels and hypoglycemia in severe malaria and cerebral malaria, it has been suggested that TNFα be used as a potential prognostic indicator for disease severity [59]. Given TNFα’s role as a proinflammatory cytokine, this hypoglycemia may result either through direct or indirect cytokine actions on host metabolic and immune functions or, more likely, some combination of both [60]. Future work is clearly needed to disentangle the mechanisms of TNFα’s metabolic effects during infection.
Like P. falciparum, B. pertussis infection increases host IL-1 production as well as raises levels of IL-17, which is also associated with hypoglycemia [30, 61]. Moreover, ACT action has been implicated in boosting host IL-10 production; IL-10 not only fosters hypoglycemia but also impedes the development of host adaptive immunity [62]. Unlike P. falciparum, however, B. pertussis toxins PTX and ACT inhibit TNFα production through their stimulation of cAMP synthesis in monocyte-derived dendritic cells [63].
Clinical implications
Despite numerous differences between B. pertussis and P. falciparum, they share the ability to cause profound disturbances in host metabolism during infection. Because hypoglycemia is often associated as a marker for disease severity, we propose that P. falciparum and B. pertussis induction of hypoglycemia serve as model systems to answer fundamental questions concerning how pathogen manipulation of metabolism can affect infection, pathogenesis, and the host immune response.
These experimental systems can address several important questions relevant to these and other diseases, such as the following:
Do pathogen-induced hyperinsulinemia and hypoglycemia modify the host immune response to enhance and/or prolong pathogenesis and infection?
Does correction of hypoglycemia during infection impact pathogen growth, persistence, and pathogenesis?
Do secreted toxins such as ACT and PTX act directly on insulin-secreting pancreatic beta cells to cause hyperinsulinemia? If so, what are the individual and collective contributions of the toxins and G-protein–mediated signaling to these metabolic disruptions?
Host immune cells depend on circulating blood glucose to supply adequate substrates for their metabolic requirements, particularly for their rapid activation and expansion during infection. Therefore, we propose that pathogen-induced hypoglycemia deprives the immune system of the energy required to mount an effective inflammatory and/or adaptive immune response. In contrast, unlike host lymphocytes, B. pertussis is largely resistant to the hypoglycemic state it induces because it is incapable of utilizing glucose as its main carbon source for generating energy owing to an incomplete citric acid cycle [64]. On the other hand, P. falciparum relies on glycolysis and the tricarboxylic acid cycle, making it likely more sensitive to changes in host blood glucose levels than B. pertussis [65].
An important implication of our reasoning is the prediction that correcting hypoglycemia will limit the duration and/or severity of infection. Such an approach may, therefore, constitute a new avenue of therapeutic interventions for the metabolic sequelae of infection. To date, therapeutic interventions to correct pathogen-induced metabolic disturbances have not been directly tested clinically. Nevertheless, there is encouraging evidence in animal models of diabetes that diabetic hyperglycemia can moderate sequelae of P. falciparum infection. Parasitemia during malaria was significantly lower in moderately diabetic animals compared with normal mice [23]. These findings suggested that raising blood glucose to counteract P. falciparum’s attempts to induce host hypoglycemia may limit pathogenesis. Indeed, some have argued that metabolic diseases such as diabetes may confer a selective advantage in some populations, in part because altered metabolic signaling pathways that are targeted by pathogens may protect the host from aspects of infectious disease [23, 66]. In contrast, in the setting of diabetic hyperglycemia, T. cruzi infection parasitemia and mortality were significantly increased [67], suggesting that, for this pathogen, diabetic hyperglycemia further erodes the capacity of the immune system to control infection. Taken together, these data support both the concept that the disruption of glucose homeostasis by pathogens impairs the host’s capability to effectively control infection and the prospect of simple clinical interventions that can modulate these effects.
Conclusions and future directions
The manipulation of the host’s metabolic state may not only affect the immune response to the respective causative pathogen but also to additional opportunistic infections, further compounding morbidity and mortality associated with infection. On the other hand, treating the metabolic manifestations of infection, including hyperinsulinemia and hypoglycemia, may potentially blunt or ameliorate the disease course of these pathogens and could be implemented relatively quickly, safely, and inexpensively to make a difference in the lives of many affected people globally.
Acknowledgments
We thank Drs. Valerie Ryman, Beth Stronach, David Lewis, and Robert Sweet for helpful comments during the development of the work. We thank Stephanie Harvill for production of the illustrations.
Funding Statement
This work is supported by a Department of Defense PRMRP Investigator Initiated Award PR141292 (Z.F.), the John F. and Nancy A. Emmerling Fund of The Pittsburgh Foundation (Z.F.), National Institutes of Health GM113681 (E.T.H.), AI116186 (E.T.H.), and AI122753 (E.T.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Straub RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis research & therapy. 2014;16(1):203 Epub 2014/02/15. doi: 10.1186/ar4484 ; PubMed Central PMCID: PMCPMC3978663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasinski F, Gregnani MF, Ornellas FH, Bacurau AV, Camara NO, Araujo RC, et al. Lymphocyte glucose and glutamine metabolism as targets of the anti-inflammatory and immunomodulatory effects of exercise. Mediators of inflammation. 2014;2014:326803 Epub 2014/07/06. doi: 10.1155/2014/326803 ; PubMed Central PMCID: PMCPMC4060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calder PC, Dimitriadis G, Newsholme P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Current opinion in clinical nutrition and metabolic care. 2007;10(4):531–40. Epub 2007/06/15. doi: 10.1097/MCO.0b013e3281e72ad4 . [DOI] [PubMed] [Google Scholar]
- 4.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annual review of immunology. 2014;32:609–34. Epub 2014/03/25. doi: 10.1146/annurev-immunol-032713-120236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. Journal of leukocyte biology. 2008;84(4):949–57. Epub 2008/06/26. doi: 10.1189/jlb.0108024 ; PubMed Central PMCID: PMCPMC2638731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. Journal of immunology (Baltimore, Md: 1950). 2008;180(7):4476–86. Epub 2008/03/21. ; PubMed Central PMCID: PMCPMC2593791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proud CG. Regulation of mammalian translation factors by nutrients. European journal of biochemistry. 2002;269(22):5338–49. Epub 2002/11/09. . [DOI] [PubMed] [Google Scholar]
- 8.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Molecular and cellular biology. 2003;23(20):7315–28. Epub 2003/10/01. doi: 10.1128/MCB.23.20.7315-7328.2003 ; PubMed Central PMCID: PMCPMC230333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu-Ashour W, Twells L, Valcour J, Randell A, Donnan J, Howse P, et al. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ open diabetes research & care. 2017;5(1):e000336 Epub 2017/08/02. doi: 10.1136/bmjdrc-2016-000336 ; PubMed Central PMCID: PMCPMC5530269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafar N, Edriss H, Nugent K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. The American journal of the medical sciences. 2016;351(2):201–11. Epub 2016/02/22. doi: 10.1016/j.amjms.2015.11.011 . [DOI] [PubMed] [Google Scholar]
- 11.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280(25):24085–94. Epub 2005/04/22. doi: 10.1074/jbc.M412802200 . [DOI] [PubMed] [Google Scholar]
- 12.Nagajyothi F, Kuliawat R, Kusminski CM, Machado FS, Desruisseaux MS, Zhao D, et al. Alterations in glucose homeostasis in a murine model of Chagas disease. Am J Pathol. 2013;182(3):886–94. Epub 2013/01/17. doi: 10.1016/j.ajpath.2012.11.027 ; PubMed Central PMCID: PMCPMC3586686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufurrena Q, Amjad FM, Scherer PE, Weiss LM, Nagajyothi J, Roth J, et al. Alterations in pancreatic β cell function and Trypanosoma cruzi infection: evidence from human and animal studies. Parasitology Research. 2017;116(3):827–38. doi: 10.1007/s00436-016-5350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holscher C, Mohrs M, Dai WJ, Kohler G, Ryffel B, Schaub GA, et al. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infection and immunity. 2000;68(7):4075–83. Epub 2000/06/17. ; PubMed Central PMCID: PMCPMC101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.dos Santos VM, de Lima MA, Cabrine-Santos M, de Stefani Marquez D, de Araujo Pereira G, Lages-Silva E, et al. Functional and histopathological study of the pancreas in hamsters (Mesocricetus auratus) infected and reinfected with Trypanosoma cruzi. Parasitol Res. 2004;94(2):125–33. Epub 2004/08/24. doi: 10.1007/s00436-004-1183-8 . [DOI] [PubMed] [Google Scholar]
- 16.dos Santos VM, Teixeira Vde P, da Cunha DF, da Cunha SF, Monteiro JP, dos Santos JA, et al. [Pancreatic anatomopathologic changes in chronic chagasic women. Preliminary data]. Arquivos de gastroenterologia. 1999;36(3):127–32. Epub 2001/02/07. . [PubMed] [Google Scholar]
- 17.Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best practice & research Clinical endocrinology & metabolism. 2014;28(5):745–56. Epub 2014/09/27. doi: 10.1016/j.beem.2014.05.002 . [DOI] [PubMed] [Google Scholar]
- 18.Oliveira LC, Juliano Y, Novo NF, Neves MM. Blood glucose and insulin response to intravenous glucose by patients with chronic Chagas' disease and alcoholism. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 1993;26(11):1187–90. Epub 1993/11/01. . [PubMed] [Google Scholar]
- 19.Guariento ME, Olga E, Muscelli A, Gontijo JA. Chronotropic and blood pressure response to oral glucose load in Chagas' disease. Sao Paulo medical journal = Revista paulista de medicina. 1994;112(3):602–6. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 20.Guariento ME, Saad MJ, Muscelli EO, Gontijo JA. Heterogenous insulin response to an oral glucose load by patients with the indeterminate clinical form of Chagas' disease. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 1993;26(5):491–5. Epub 1993/05/01. . [PubMed] [Google Scholar]
- 21.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309(2):61–6. Epub 1983/07/14. doi: 10.1056/NEJM198307143090201 . [DOI] [PubMed] [Google Scholar]
- 22.Phillips RE. Hypoglycaemia is an important complication of falciparum malaria. The Quarterly journal of medicine. 1989;71(266):477–83. Epub 1989/06/01. . [PubMed] [Google Scholar]
- 23.Elased K, De Souza JB, Playfair JH. Blood-stage malaria infection in diabetic mice. Clinical and experimental immunology. 1995;99(3):440–4. Epub 1995/03/01. ; PubMed Central PMCID: PMCPMC1534208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elased K, Playfair JH. Hypoglycemia and hyperinsulinemia in rodent models of severe malaria infection. Infection and immunity. 1994;62(11):5157–60. Epub 1994/11/01. ; PubMed Central PMCID: PMCPMC303239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planche T, Dzeing A, Ngou-Milama E, Kombila M, Stacpoole PW. Metabolic complications of severe malaria. Current topics in microbiology and immunology. 2005;295:105–36. Epub 2005/11/04. . [DOI] [PubMed] [Google Scholar]
- 26.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. Epub 2010/05/15. doi: 10.1016/S0140-6736(10)60549-1 . [DOI] [PubMed] [Google Scholar]
- 27.Osonuga OA, Osonuga AA, Osonuga IO, Osonuga A, Derkyi KL. Prevalence of hypoglycemia among severe malaria children in a rural African population. Asian Pacific Journal of Tropical Disease. 2011;1(3):192–4. doi: http://dx.doi.org/10.1016/S2222-1808(11)60026-1 [Google Scholar]
- 28.English M, Wale S, Binns G, Mwangi I, Sauerwein H, Marsh K. Hypoglycaemia on and after admission in Kenyan children with severe malaria. QJM: monthly journal of the Association of Physicians. 1998;91(3):191–7. Epub 1998/05/30. . [DOI] [PubMed] [Google Scholar]
- 29.Fedele G, Bianco M, Ausiello CM. The virulence factors of Bordetella pertussis: talented modulators of host immune response. Archivum immunologiae et therapiae experimentalis. 2013;61(6):445–57. Epub 2013/08/21. doi: 10.1007/s00005-013-0242-1 . [DOI] [PubMed] [Google Scholar]
- 30.Eby JC, Hoffman CL, Gonyar LA, Hewlett EL. Review of the neutrophil response to Bordetella pertussis infection. Pathogens and disease. 2015;73(9):ftv081 Epub 2015/10/04. doi: 10.1093/femspd/ftv081 ; PubMed Central PMCID: PMCPMC4626575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raeven RH, Brummelman J, Pennings JL, Nijst OE, Kuipers B, Blok LE, et al. Molecular signatures of the evolving immune response in mice following a Bordetella pertussis infection. PLoS ONE. 2014;9(8):e104548 Epub 2014/08/20. doi: 10.1371/journal.pone.0104548 ; PubMed Central PMCID: PMCPMC4138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furman BL, Sidey FM, Wardlaw AC. Role of insulin in the hypoglycaemic effect of sublethal Bordetella pertussis infection in mice. British journal of experimental pathology. 1986;67(2):305–12. Epub 1986/04/01. ; PubMed Central PMCID: PMCPMC2013161. [PMC free article] [PubMed] [Google Scholar]
- 33.Pieroni RE, Levine L. Enhancing effect of insulin on endotoxin lethality. Experientia. 1969;25(5):507–8. Epub 1969/05/15. . [DOI] [PubMed] [Google Scholar]
- 34.Pieroni RE, Levine L. Insulin-induced enhancement of anaphylactoid reaction to a non-carbohydrate antigen. Experientia. 1969;25(2):170–1. Epub 1969/02/15. . [DOI] [PubMed] [Google Scholar]
- 35.Keller KF, Fishel CW. In vivo and in vitro manifestations of adrenergic blockade in Bordetella pertussis-vaccinated mice. Journal of bacteriology. 1967;94(4):804–11. Epub 1967/10/01. ; PubMed Central PMCID: PMCPMC276735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furman BL, Wardlaw AC, Stevenson LQ. Bordetella pertussis-induced hyperinsulinaemia without marked hypoglycaemia: a paradox explained. British journal of experimental pathology. 1981;62(5):504–11. Epub 1981/10/01. ; PubMed Central PMCID: PMCPMC2041714. [PMC free article] [PubMed] [Google Scholar]
- 37.Katada T. The inhibitory G protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation. Biological & pharmaceutical bulletin. 2012;35(12):2103–11. Epub 2012/12/05. . [DOI] [PubMed] [Google Scholar]
- 38.Yajima M, Hosoda K, Kanbayashi Y, Nakamura T, Nogimori K, Mizushima Y, et al. Islets-activating protein (IAP) in Bordetella pertussis that potentiates insulin secretory responses of rats. Purification and characterization. J Biochem. 1978;83(1):295–303. Epub 1978/01/01. . [DOI] [PubMed] [Google Scholar]
- 39.Elahi S, Brownlie R, Korzeniowski J, Buchanan R, O'Connor B, Peppler MS, et al. Infection of newborn piglets with Bordetella pertussis: a new model for pertussis. Infection and immunity. 2005;73(6):3636–45. Epub 2005/05/24. doi: 10.1128/IAI.73.6.3636-3645.2005 ; PubMed Central PMCID: PMCPMC1111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma AK, Dhasmana N, Dubey N, Kumar N, Gangwal A, Gupta M, et al. Bacterial Virulence Factors: Secreted for Survival. Indian journal of microbiology. 2017;57(1):1–10. Epub 2017/02/06. doi: 10.1007/s12088-016-0625-1 ; PubMed Central PMCID: PMCPMC5243249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhuri S, Gantner BN, Ye RD, Cianciotto NP, Freitag NE. The Listeria monocytogenes ChiA chitinase enhances virulence through suppression of host innate immunity. mBio. 2013;4(2):e00617–12. Epub 2013/03/21. doi: 10.1128/mBio.00617-12 ; PubMed Central PMCID: PMCPMC3604766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbonetti NH. Contribution of pertussis toxin to the pathogenesis of pertussis disease. Pathogens and disease. 2015;73(8):ftv073 Epub 2015/09/24. doi: 10.1093/femspd/ftv073 ; PubMed Central PMCID: PMCPMC4626579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26(10):1757–72. Epub 2012/08/24. doi: me.2012-1101 [pii] doi: 10.1210/me.2012-1101 ; PubMed Central PMCID: PMCPMC3458225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ustione A, Piston DW, Harris PE. Minireview: Dopaminergic regulation of insulin secretion from the pancreatic islet. Mol Endocrinol. 2013;27(8):1198–207. Epub 2013/06/08. doi: 10.1210/me.2013-1083 ; PubMed Central PMCID: PMCPMC3725340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Tornadu I, Perez-Millan MI, Recouvreux V, Ramirez MC, Luque G, Risso GS, et al. New insights into the endocrine and metabolic roles of dopamine D2 receptors gained from the Drd2 mouse. Neuroendocrinology. 2010;92(4):207–14. Epub 2010/10/27. doi: 10.1159/000321395 . [DOI] [PubMed] [Google Scholar]
- 46.Moises RS, Heidenreich KA. Pertussis toxin catalyzed ADP-ribosylation of a 41 kDa G-protein impairs insulin-stimulated glucose metabolism in BC3H-1 myocytes. Journal of cellular physiology. 1990;144(3):538–45. Epub 1990/09/01. doi: 10.1002/jcp.1041440323 . [DOI] [PubMed] [Google Scholar]
- 47.Honnor RC, Naghshineh S, Cushman SW, Wolff J, Simpson IA, Londos C. Cholera and pertussis toxins modify regulation of glucose transport activity in rat adipose cells: evidence for mediation of a cAMP-independent process by G-proteins. Cell Signal. 1992;4(1):87–98. Epub 1992/01/01. . [DOI] [PubMed] [Google Scholar]
- 48.Joost HG, Goke R. Effects of islet-activating protein on insulin- and isoprenaline-stimulated glucose transport in isolated rat adipocytes. FEBS letters. 1984;167(1):5–9. Epub 1984/02/13. . [DOI] [PubMed] [Google Scholar]
- 49.Ciaraldi TP, Maisel A. Role of guanine nucleotide regulatory proteins in insulin stimulation of glucose transport in rat adipocytes. Influence of bacterial toxins. The Biochemical journal. 1989;264(2):389–96. Epub 1989/12/01. ; PubMed Central PMCID: PMCPMC1133593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Lee JK, et al. Pertussis toxin administered spinally induces a hypoglycemic effect on normal and diabetic mice. Pharmacology. 2014;94(1–2):29–40. Epub 2014/08/30. doi: 10.1159/000363578 . [DOI] [PubMed] [Google Scholar]
- 51.Carbonetti NH. Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools. Future microbiology. 2010;5(3):455–69. Epub 2010/03/10. doi: 10.2217/fmb.09.133 ; PubMed Central PMCID: PMCPMC2851156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paccani SR, Dal Molin F, Benagiano M, Ladant D, D'Elios MM, Montecucco C, et al. Suppression of T-lymphocyte activation and chemotaxis by the adenylate cyclase toxin of Bordetella pertussis. Infection and immunity. 2008;76(7):2822–32. Epub 2008/04/23. doi: 10.1128/IAI.00200-08 ; PubMed Central PMCID: PMCPMC2446734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ladant D, Ullmann A. Bordatella pertussis adenylate cyclase: a toxin with multiple talents. Trends in microbiology. 1999;7(4):172–6. Epub 1999/04/28. . [DOI] [PubMed] [Google Scholar]
- 54.Saito M, Akiyoshi M, Shimizu Y. Possible role of the sympathetic nervous system in responses to interleukin-1. Brain research bulletin. 1991;27(3–4):305–8. Epub 1991/09/01. . [DOI] [PubMed] [Google Scholar]
- 55.Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. International journal for parasitology. 2012;42(6):549–55. Epub 2012/05/03. doi: 10.1016/j.ijpara.2012.03.010 . [DOI] [PubMed] [Google Scholar]
- 56.Taylor K, Bate CA, Carr RE, Butcher GA, Taverne J, Playfair JH. Phospholipid-containing toxic malaria antigens induce hypoglycaemia. Clinical and experimental immunology. 1992;90(1):1–5. Epub 1992/10/01. ; PubMed Central PMCID: PMCPMC1554551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richards AL. Tumour necrosis factor and associated cytokines in the host's response to malaria. International journal for parasitology. 1997;27(10):1251–63. Epub 1997/12/12. . [DOI] [PubMed] [Google Scholar]
- 58.Clark IA, MacMicking JD, Gray KM, Rockett KA, Cowden WB. Malaria mimicry with tumor necrosis factor. Contrasts between species of murine malaria and Plasmodium falciparum. Am J Pathol. 1992;140(2):325–36. Epub 1992/02/01. ; PubMed Central PMCID: PMCPMC1886435. [PMC free article] [PubMed] [Google Scholar]
- 59.Kinra P, Dutta V. Serum TNF alpha levels: a prognostic marker for assessment of severity of malaria. Tropical biomedicine. 2013;30(4):645–53. Epub 2014/02/14. . [PubMed] [Google Scholar]
- 60.Ratter JM, Rooijackers HM, Tack CJ, Hijmans AG, Netea MG, de Galan BE, et al. Proinflammatory Effects of Hypoglycemia in Humans With or Without Diabetes. Diabetes. 2017;66(4):1052–61. Epub 2017/01/25. doi: 10.2337/db16-1091 . [DOI] [PubMed] [Google Scholar]
- 61.Eik WF, Marcon SS, Krupek T, Previdelli IT, Pereira OC, Silva MA, et al. Blood levels of pro-inflammatory and anti-inflammatory cytokines during an oral glucose tolerance test in patients with symptoms suggesting reactive hypoglycemia. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2016;49(8). Epub 2016/07/14. doi: 10.1590/1414-431x20165195 ; PubMed Central PMCID: PMCPMC4954733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sebo P, Osicka R, Masin J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert review of vaccines. 2014;13(10):1215–27. Epub 2014/08/05. doi: 10.1586/14760584.2014.944900 . [DOI] [PubMed] [Google Scholar]
- 63.Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. Journal of leukocyte biology. 2002;72(5):962–9. Epub 2002/11/14. . [PubMed] [Google Scholar]
- 64.Bogdan JA, Nazario-Larrieu J, Sarwar J, Alexander P, Blake MS. Bordetella pertussis autoregulates pertussis toxin production through the metabolism of cysteine. Infection and immunity. 2001;69(11):6823–30. Epub 2001/10/13. doi: 10.1128/IAI.69.11.6823-6830.2001 ; PubMed Central PMCID: PMCPMC100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olszewski KL, Llinas M. Central carbon metabolism of Plasmodium parasites. Molecular and biochemical parasitology. 2011;175(2):95–103. Epub 2010/09/21. doi: 10.1016/j.molbiopara.2010.09.001 ; PubMed Central PMCID: PMCPMC3004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brima W, Eden DJ, Mehdi SF, Bravo M, Wiese MM, Stein J, et al. The brighter (and evolutionarily older) face of the metabolic syndrome: evidence from Trypanosoma cruzi infection in CD-1 mice. Diabetes/metabolism research and reviews. 2015;31(4):346–59. Epub 2015/01/24. doi: 10.1002/dmrr.2636 ; PubMed Central PMCID: PMCPMC4427523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanowitz HB, Amole B, Hewlett D, Wittner M. Trypanosoma cruzi infection in diabetic mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(1):90–3. Epub 1988/01/01. . [PubMed] [Google Scholar]