Abstract
Novel targeted anti-cancer therapies have resulted in improvement in patient survival compared to standard chemotherapy. Renal toxicities of targeted agents are increasingly being recognized. The incidence, severity, and pattern of renal toxicities may vary according to the respective target of the drug. Here we review the adverse renal effects associated with a selection of currently approved targeted cancer therapies, directed to EGFR, HER2, BRAF, MEK, ALK, PD1/PDL1, CTLA-4, and novel agents targeted to VEGF/R and TKIs. In summary, electrolyte disorders, renal impairment and hypertension are the most commonly reported events. Of the novel targeted agents, ipilumumab and cetuximab have the most nephrotoxic events reported. The early diagnosis and prompt recognition of these renal adverse events are essential for the general nephrologist taking care of these patients.
Keywords: AKI, chemotherapy, hypokalemia, hyponatremia, nephrotoxicity, onconephrology, renal failure, targeted therapy
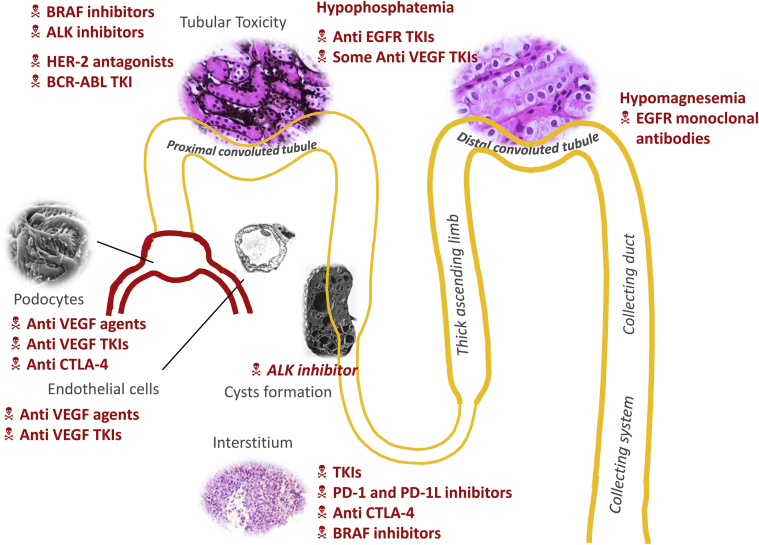
In the past decade, advances in cell biology have led to development of anticancer agents that target specific molecular pathways. The National Cancer Institute (NCI) defines targeted therapies as “drugs or substances that block the growth and spread of cancer by interfering with specific molecules involved in tumor growth and progression.”1 Targeted therapies are commonly used in cancer treatment, and it is vital that their renal toxicities be recognized and investigated. Adverse renal effects of targeted therapies occur through several complex mechanisms. Wide ranges of toxicities affecting various parts of the nephron have been reported with the novel targeted therapies. Recognition of adverse renal effects of these agents in a timely manner is extremely important for optimal patient care. Table 1 summarizes the oncological indications for the major targeted therapies discussed in this review. Figure 1 summarizes the renal toxicities that are associated with novel classes of targeted therapies and their effects on various parts of the nephron.
Table 1.
Approved hematology and oncology indications for targeted therapies along with dosing in CKD and ESRD
| Generic name of targeted therapy (trade name) | Target | Cancer | Renal excretion | Dose adjustment for GFR 30–90 ml/min/1.73 m2 | Dialysis dose adjustment |
|---|---|---|---|---|---|
| Afatineb (Gilotrif) | EGFR TKI | Metastatic NSCLC | <5% | No | No data |
| Axitinib (Inlyta) | Multi target TKI | Pancreatic cancer, RCC, CML | <25% | No | No |
| Aflibercept (Eylea or Zaltrap) | VEGF | Colorectal cancer | No | No | No |
| Bevacizumab (Avastin) | VEGF | Colorectal cancer, NSCLC, RCC, breast cancer, epithelial ovarian cancer, GBM | No | No | No |
| Bosutinib (Bosulif) | BCR-ABL TKI | CML | No | Reduce dose to 300 mg once daily | No data |
| Cetuximab (Erbitux) | EGFR | Colorectal cancer, head and neck SCC | No | No | No |
| Crizotinib (Xalkori) | ALK | NSCLC | No | No | No |
| Dabrafenib (Tafinlar) | BRAF | Melanoma | <25% | No | No data |
| Dasatinib (Sprycel) | BCR-ABL TKI | CML | <5% | No | No data |
| Erlotinib (Tarceva) | EGFR TKI | NSCLC, pancreatic cancer | <10% | No | No |
| Gefitinib (Iressa) | EGFR TKI | NSCLC | <5% | No | No |
| Ibrutinib (Imbruvica) | Bruton kinase TKI | CLL, mantle cell lymphoma | No | No data | No data |
| Imatinib (Gleevac) | BCR-ABL TKI | Gastrointestinal stromal tumors, CML | <15% | No | No |
| Ipilimumab (Yervoy) | CTLA4 | Melanoma | No | No | No data |
| Lapatinib (Tykerb) | ERBB2 | Breast cancer | <5% | No | No |
| Nivolumab (Opdivo) | PD-1 | Melanoma, NSCLC, Hodgkin lymphoma, RCC | No | No | No data |
| Nilotinib (Tasigna) | BCR-ABL TKI | CML | No | No | No data |
| Panitumumab (Vectibix) | EGFR | Colorectal cancer | No | No | No |
| Pazopanib (Votrient) | Multitarget TKI | RCC, soft tissue sarcoma | <4% | No | No |
| Pembrolizumab (Keytruda) | PD-L1 | Melanoma, NSCLC, Hodgkin lymphoma | No data | No | No |
| Pertuzumab (Perjeta) | ERBB2 | Breast cancer | No | No | No data |
| Ponatanib (Iclusig) | BCR-ABL TKI | CML, ALL | No | No | No data |
| Regorafenib (Stivarga) | Multitarget TKI | Colorectal cancer, gastrointestinal stromal tumors | <20% | No | No |
| Sorafenib (Nexavar) | Multitarget TKI | RCC, hepatocellular carcinoma, thyroid carcinoma | <20% | No | No |
| Sunitinib (Sutent) | Multitarget TKI | RCC, gastrointestinal stromal tumors, pancreatic neuroendocrine tumors | <20% | No | No |
| Trametinib (Mekinist) | MEK | Melanoma | <20% | No | No data |
| Trastuzumab (Herceptin) | ERBB2 | Breast cancer | No | No | No |
| Vandetanib (Caprelsa) | Multitarget TKI | Medullary thyroid cancer | <25% | No | No data |
| Vemurafenib (Zelboraf) | BRAF | Melanoma, thyroid cancer, colorectal cancer | <5% | No | No data |
ALK, anaplastic lymphoma kinase; ALL, acute lymphocytic leukemia; BCR-ABL, breakpoint cluster region–abelson; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CTLA, cytotoxic T lymphocyte antigen−4; EGFR, epidermal growth factor receptor; GBM, gliobastoma multiforme; MEK, mitogen-activated protein kinase; NSCLC, non−small-cell lung cancer; PD, programmed cell death; RCC, renal cell carcinoma; SCC, squamous cell cancer; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Information obtained from package inserts of agents, clinical trials, and published case reports.
No data available for most agents for dose adjustments for GFR < 30 ml/min/1.73 m2 except vandetanib, which requires dose adjustment.
Figure 1.
Summary of renal adverse events noted with targeted therapies. ALK, anaplastic lymphoma kinase; BCR-ABL, breakpoint cluster region–abelson; CTLA, cytotoxic T lymphocyte antigen−4; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor−2; PD, programmed cell death; TKI, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor.
FDA Adverse Reporting System Review
A recent study by our group had reviewed the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) data from 2011 to 2015 and found a high number of renal adverse events with novel targeted therapies.2 The total number of renal adverse events reported was 2943. Of the 3 categories of events, 1390 (47.3%) were metabolic disturbances, 1243 (42.2%) were renal impairment, and 310 (10.5%) were hypertension (HTN). Ipilumumab and cetuximab, with 508 and 467 events respectively, were the most common targeted therapies associated with reported nephrotoxicities. The rate of adverse events were similar between men (n = 1369) and women (n = 1305).2 Renal impairment was reported in 636 men and 522 women (P < 0.001). Metabolic disturbances were reported in 620 men and 639 women (P = 0.5). HTN was reported with 113 men and 144 women (P = 0.053).2 The most common electrolyte abnormality was hypokalemia (n = 539). This analysis indicates that electrolyte abnormalities are the most common renal or metabolic toxicity. Overall, for all renal events, there was no difference in the incidence between men and women. However, men seem to have a higher risk of developing renal impairment with targeted therapies compared to women. No such gender difference exists in standard chemotherapy-related acute kidney injury (AKI). There was no difference in gender for electrolyte disorders and HTN associated with targeted therapies.2 Hypokalemia, hypophosphatemia, hypomagnesemia, and hyponatremia are concerns in patients receiving targeted therapies.
There are important limitations that one must keep in mind when using the FAERS database. The events are reported by providers and/or patients and therefore could have a reporting bias. In addition, not all demographic and comorbidity information is available to help identify whether other nephrotoxic risk factors are present, such as use of nonsteroidal anti-inflammatory agents, history of HTN or diabetes mellitus, known chronic kidney disease (CKD), recent use of contrast agent, or recent use of standard chemotherapy that could be nephrotoxic. Most importantly, it is not possible to determine whether an event is truly caused by the drug as opposed to the underlying disease or concomitant medications or by prior chemotherapies administered to these patients. In addition, we cannot get an accurate assessment of incidence rate, as we do not have complete information on the total number of patients who have actually received these agents.
In this narrative review, we discuss the renal adverse events of a few novel anti−vascular endothelial growth factor (VEGF) and tyrosine kinase inhibitors (TKI) but focus more on the other novel targeted therapies used in cancer patients. We also compare the published renal adverse data to the FDA-reported events, providing a comprehensive overview on the toxicities.
VEGF and VEGF Receptor Blockade
A hypothesis that tumor growth is angiogenesis dependent led to the development of antiangiogenic drugs.3 These drugs target the VEGF or VEGF receptor (VEGFR). The VEGF ligand inhibitors (bevacizumab and aflibercept) bind to the VEGF molecule, preventing it from binding to the receptor and inhibiting endothelial cell proliferation and vessel formation. Bevacizumab and aflibercept can produce asymptomatic albuminuria, nephrotic syndrome, and thrombotic microangiopathy (TMA).4 Eremina et al. showed that podocyte-specific knockout of the VEGF gene resulted in renal limited TMA.5 Most drugs that target the VEGF pathway can present with 1 of the known renal toxicities. HTN frequently accompanies proteinuria. Although proteinuria appears to be an effect common to all agents targeted at the VEGF pathway, the factors associated with the occurrence and severity of the proteinuria are unknown.4 Pre-existing renal disease (including higher baseline urinary protein levels and hypertension) and renal cell carcinoma (as compared to other malignant diseases) may be predisposing factors.4 Table 2 summarizes the known renal toxicities of VEGF-inhibitory agents. The HTN associated with anti-VEGF agents is mediated via several mechanisms. Decreased nitrous oxide leading to endothelial dysfunction and capillary rarefaction, pressure natriuresis, and decreased lymph-angiogenesis leading to volume overload are proposed mechanisms that cause the HTN,6 but sometimes additional classes of agents are required for management. Choice of antihypertensive agents should be individualized, with angiotensin-converting enzyme inhibitor (ACEI) or angiotensive receptor blocker (ARB) inhibition as first-line options and calcium channel blockers as a reasonable second choice. Centrally acting antihypertensive or diuretic agents may be added to adequately control blood pressure. Close follow-up is critical for appropriate titration, and if the blood pressure cannot be maintained below 140/90 mm Hg (or 130/89 mm Hg in certain high-risk groups), then prompt referral to a hypertension specialist is indicated. If patients develop hypertensive crisis or encephalopathy, the cancer therapy needs to be discontinued. The proteinuria associated with these agents is due to disruption of the glomerular filtration barrier.7, 8 A kidney biopsy sample usually shows renal limited TMA and in some cases minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS).9 Treatment can be continued in most cases involving non−nephrotic-range proteinuria, and HTN and proteinuria can be aggressively managed with ACEIs or ARBs. Since treatment options and prognosis might be influenced by kidney histological findings, a kidney biopsy is usually recommended whenever feasible. The decision to stop anti-VEGF therapy or to switch to alternative agents should be made in the setting of significant proteinuria in a multidisciplinary setting. Nephrotic-range proteinuria and TMA are generally considered reasons to discontinue the offending agent.10 Recent in-depth reviews on anti-VEGF agents that induced kidney disease4, 10 exist in the literature, and this review will not focus on those agents.
Table 2.
Tyrosine kinase inhibitors and VEGF inhibitory drug−related renal toxicities
| Agent | Reported nephrotoxicity | References |
|---|---|---|
| VEGF/R antibodies | ||
| Bevacizumab | HTN, proteinuria, preeclampsia-like syndrome, renal limited TMA | 4, 5, 6, 7, 8, 9, 10 |
| Aflibercept | HTN, proteinuria | 4, 5, 6, 7, 8, 9, 10 |
| Receptor TKIs, VEGF family | ||
| Sunitinib | HTN, proteinuria, MCD/FSGS, AIN, chronic interstitial nephritis | 7, 9, 13 |
| Pazopanib | HTN, proteinuria | 7, 9 |
| Axitinib | HTN, proteinuria | 7, 9 |
| Sorafenib | HTN, proteinuria, MCD/FSGS, AIN, chronic interstitial nephritis, hypophosphatemia | 7, 9, 13, 14 |
| Regorafenib | HTN, hypophosphatemia, hypocalcemia, proteinuria, AKI | 2, 17, 18 |
| Vandetanib | HTN, hypokalemia, hypocalcemia | 2, 19 |
| Cellular TKIs, BCR-ABL | ||
| Imatinib | ATN, Rhabdomyolysis, hypophosphatemia | 2, 26, 27, 28, 29, 32, 33, 34 |
| Nilotinib | HTN | 7, 9, 37 |
| Ponatinib | HTN | 7, 9 |
| Dasatinib | Rhabdomyolysis, ATN, proteinuria, TMA | 40, 41, 42, 43, 44, 45, 46 |
| Bosutinib | Hypophosphatemia | 47 |
AIN, acute interstitial nephritis; AKI, acute kidney injury; FSGS, focal segmental glomerulosclerosis; HTN, hypertension; MCD, minimal change disease; TKI, tyrosine kinase inhibitor; TMA, thrombotic microangiopathy; VEGF, vascular endothelial growth factor; VEGF/R, vascular endothelial growth factor/receptor.
Tyrosine Kinase Inhibitors
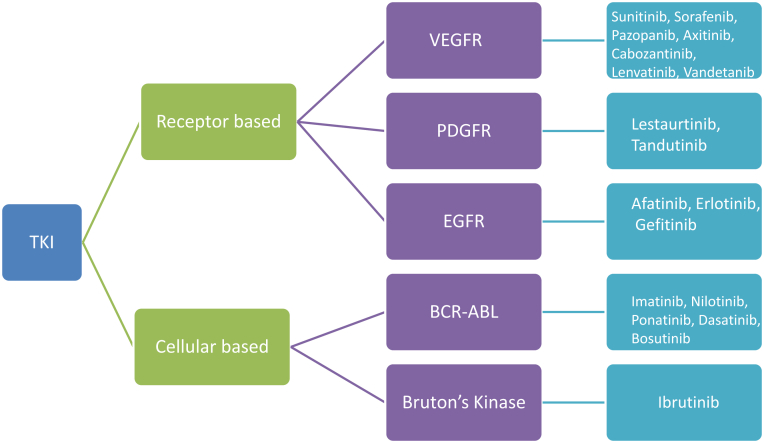
An important mechanism in signal transduction pathways in cells is protein phosphorylation, which is carried out by protein kinases. These kinases regulate the proliferation, differentiation, migration, metabolism, and antiapoptotic signaling of cells. The most important kinases are the serine/threonine and tyrosine kinases, which are characterized by their ability to catalyze the phosphorylation of serine/threonine or tyrosine amino acid residues in proteins respectively. There are 2 types of tyrosine kinases: cellular and receptor tyrosine kinases.11 Imatinib is a cellular tyrosine kinase inhibitor (TKI) that is designed to target the fusion protein breakpoint cluster region–abelson (BCR-ABL) and members of the SRC tyrosine kinase family. Receptor TKIs are designed to target the epidermal growth factor (EGFR), platelet-derived growth factor (PDGFR), and the VEGFR tyrosine kinase families. These could target single receptors such as EGFR (gefitinib) or could be multikinase or multitarget TKIs and target many receptors such as sunitinib, which targets VEGFR,1, 2, 3 PDGFR, kit, Flt3 and RET.11 Figure 2 is a simplified version of the different types of TKIs used in cancer treatment.
Figure 2.
Simplistic view of various tyrosine kinases available for treatment of cancer. There are 2 types of tyrosine kinases: cellular and receptor tyrosine kinases. Receptor tyrosine kinase inhibitors (TKIs) are designed to target the epidermal growth factor (EGFR), platelet-derived growth factor (PDGFR), and vascular endothelial growth factor receptor (VEGFR) tyrosine kinase families. These could target single receptors such as EGFR (gefitinib) or could be multikinase or multitarget TKIs and target many receptors such as sunitinib, which targets VEGFR,1, 2, 3 PDGFR, kit, Flt3, and RET. The figure represents the predominant receptor involved as the target.
Receptor TKIs: TKIs of the PDGFR Family
PDGFR inhibitors are in clinical development for cancer therapy; most are directed against several tyrosine kinases. Examples of these agents are lestaurtinib and tandutinib, which are used for treatment of leukemia and pancreatic cancer, respectively. Since they are in phase I and II trials, they will not be discussed here.12
Receptor TKIs: TKIs of the VEGFR Family
Sunitinib and sorafenib inhibit angiogenesis and cell proliferation by targeting multiple receptor kinases, including VEGFRs, PDGFRs, c-KIT, and others.13 Similar to anti-VEGF agents, these agents are potent inhibitors of angiogenesis and lead to adverse effects similar to them. TKIs (sunitinib, sorafenib, pazopanib, axitinib, cabozantinib, lenvatinib, and vandetanib) block the intracellular domain of the VEGFR. Sunitinib, sorafenib, pazopanib, and axitinib have known effects of HTN, proteinuria, TMA, and chronic, and acute interstitial nephritis.4, 13 Sorafenib also is known to cause hypophosphatemia and hypocalcemia. This effect has been linked to pancreatic dysfunction from the drug, leading to vitamin D malabsorption and secondary hyperparathyroidism.14 Hence, patients on this agent should be screened for vitamin D, phosphorous, and calcium levels. Since the advent of antiangiogenic agents, newer agents with other molecular targets1 have been approved. The renal toxicities VEGFR-related TKIs are well described in the nephrology and oncology literature and are not discussed in further detail in this review; however, the renal toxicities of the newer targeted therapies are limited to case reports or series. In this review, we discuss 2 novel VEGF-receptor−based TKI agents with emerging data, namely, regorafenib and vandetanib.
Regorafenib
Regorafenib is associated with several electrolyte abnormalities, including hypophosphatemia, hypocalcemia, hyponatremia, and hypokalemia.2, 15, 16 These abnormalities are usually mild to moderate and do not require dose reductions or treatment interruptions. Initial trials reported the incidence of HTN to be around 28%, of which 7% were listed as grade 3.15 The incidence of proteinuria was lower at 7%, and 1% were grade 3.15 A recent trial found HTN in 11% of patients and hypophosphataemia in 7% of patients.16 According to Yilmaz et al., the HTN associated with regorafenib may not be a dose-dependent effect.17 A systematic review by Wang et al. evaluated 1069 patients (regorafenib, n = 750; controls, n = 319) from 5 clinical trials. The overall incidence of all-grade HTN was 44.4% (95% confidence interval [CI] 30.8%–59.0%).18 The use of regorafenib in cancer patients was associated with a significantly increased risk of all-grade HTN (relative risk [RR] = 3.76, 95% CI = 2.35–5.99).18 Our analysis of FAERS data found 125 cases of regorafenib-related renal toxicity. Similar to the published literature, HTN was the most common adverse event (57 cases), followed by AKI (40 cases) and hypophosphatemia (8 cases). It is interesting that AKI has not been described in prior studies with this agent.2 Given the lack of published biopsy-proven cases, pathophysiology is not easy to elicit in regorafenib-induced AKI. The mechanism of hypophosphatemia might be similar to that of sorafenib, as discussed above.
Vandetanib
Vandetanib is a receptor TKI that inhibits many targets including VEGFR2, EGFR, and RET. According to initial studies, it has been associated with a number of electrolyte disturbances, such as hypocalcemia, hypokalemia, hyponatremia, and hypercalcemia.19, 20 HTN has also been reported in close to 10% of patients.19, 21 Data from a phase 2 trial of vandetanib in locally advanced or metastatic differentiated thyroid cancer confirmed that HTN is frequently seen in nearly 34% of cases.22 Electrolyte disturbances, namely hypokalemia and hypocalcemia, were seen at a lower rate (4%).20 Vandetanib also been shown to have an inhibitory activity on several human renal transporters, such as MATE-1 and MATE-2, which are responsible for the clearance of multiple drugs and toxins. Inhibition of MATE-1 and MATE-223 at the apical membrane of the tubular cells might lead to increased concentrations of the drug within renal cells, resulting in worsening of renal toxic effects of other drugs; especially cisplatin.24 Despite this, a Medline search did not reveal any published case reports or series of any specific postmarketing renal adverse events. In the FAERS analysis, a total of 57 cases were found, with a majority of AKI (30 cases), followed by HTN (21 cases), and the remainder electrolyte disorders.2
TKIs of the EGFR Family
Afatinib, erlotinib, and gefitinib are small-molecule inhibitors of the tyrosine kinase domain of the EGFR, which are used in the treatment of non−small-cell lung cancer. Monoclonal antibodies targeting the EGFR (cetuximab, panitumumab) are also used as targeted therapies. Given their widely known association with electrolyte disorders, both variants of EGFR inhibitors are discussed below in a different section.
Cellular TKIs
Imatinib
Imatinib is a potent inhibitor of BCR-ABL fusion protein, and platelet-derived growth factors. It is a TKI that is classically used in chronic myelogenous leukemia (CML) and gastric stromal tumors. In 1 study of patients with CML, AKI was seen in 7% of patients and CKD in 12%.25 Potential mechanisms of injury appear to be tumor lysis syndrome, biopsy-proven acute tubular damage, and or Fanconi syndrome.26, 27, 28, 29, 30 Renal injury appears to be dose dependent, as seen with renal cell cancer treatment,31 in which higher doses are associated with higher incidence of tubular damage. Interestingly, rhabdomyolysis has been reported with this agent.32, 33
A common electrolyte abnormality reported with imatinib is hypophosphatemia. In 1 case series, hypophosphatemia was associated with low calcium and 25-OH vitamin D levels,34 with an incidence close to 10%. This may be related to inhibition of renal tubular reabsorption of phosphorus.35 The mechanism underlying phosphaturia is unclear. The FAERS report2 found 44 cases of imatinib-related renal toxicity, and 25 events were AKIs, in line with published literature. We found only 1 reported case of hypophosphatemia reported to the FDA. In addition, there were 5 cases of HTN, 3 of hypokalemia, and 4 of hyponatremia that are not described in the literature. Interestingly, based on rat models, imatinib may have renal-protective effects. Reduced expression of transforming growth factor−β1 (TGF-β1) in the renal cortex leads to suppression of proteinuria, improved renal function, attenuation of glomerulosclerosis and tubule-interstitial injury.36
Nilotinib
Nilotinib is an inhibitor of BCR-ABL kinase, c-KIT, and PDGFR. It has been associated with HTN in 10% of cases.37 Interestingly, in animal studies, nilotinib significantly decreased renal cortical expression of profibrogenic genes, such as IL-1β and monocyte chemotactic protein−1, which correlated closely with tubulointerstitial damage; in addition, nilotinib treatment significantly prolonged survival of the rats.38
Ponatinib
Ponatinib is a BCR-ABL TKI, along with other targets such as PDGFR, SRC kinases, and VEGFR. Given its activity on VEGFR, the toxicities of anti-VEGF agents can be applied to this agent. HTN might be common finding with this agent.
Dasatinib
Dasatinib is a second-generation TKI used in imatinib-resistant CML.39 It has effects on the BCR-ABL target and other targets such as the PDGFR and c-KIT. There are 3 published case reports of AKI with this drug.40, 41, 42 One case report of TMA43 and 1 case report causing rhabdomyolysis44 similar to that with imatinib have been reported. A possible mechanism of action could be direct tubular toxicity, as noted in 1 of the published cases with a kidney biopsy.40
There is a 5% incidence of proteinuria with this agent.39 A case of biopsy-proven renal limited TMA and another case with clinical TMA has been reported with this agent.45, 46 A possible mechanism for this injury is through inhibition of the Src family kinases that are required for VEGF signaling. It is possible that the kidney injury could be similar to that with other VEGF inhibitors. Of the TKIs that affect the BCR-ABL pathway, this is the only agent associated with proteinuric disease. Switching to imatinib or nilotinib might be optimal for patients who develop proteinuria on dasatinib.
Bosutinib
Bosutinib is a TKI approved for treatment of refractory CML. Hypophosphatemia and a decline in GFR have been reported during therapy with bosutinib.47 No cases of renal toxicities with this agent have been reported in the literature.
Other TKIs
Ibrutinib
Ibrutinib is a TKI that irreversibly binds to and inhibits the Bruton tyrosine kinase. It is currently approved for the treatment of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. In 1 study, 23% of patients developed elevated serum creatinine and edema.48 Of the patients, 18% developed HTN as well.48 In another study of patients with mantle cell leukemia, 35% had increases in serum creatinine from baseline. There were 4 cases of grade 3 AKI, but all were confounded by pre-existing HTN and renal dysfunction, and concurrent dehydration as well.49 Four patients also developed tumor lysis syndrome, leading to electrolyte abnormalities and AKI in this study.49 Patients receiving ibrutinib should undergo periodic creatinine level monitoring and should maintain hydration.49 The mechanism of this injury is unclear at this point, but tumor lysis syndrome could be a possible mechanism. No biopsy-proven cases have been reported in the literature. In unpublished data on cardiac effects of ibrutinib, 23% of the patients developed new-onset systolic HTN with increases in systolic blood pressure >20 mm Hg. The mechanism of the HTN is also unclear.
Epidermal Growth Factor Receptor−1 Target Inhibitors
These therapies target the epidermal growth factor receptor−1 (EGFR). They include 2 monoclonal antibodies, namely, cetuximab and panitumumab. Other EGFR therapies include 3 small-molecule TKI, namely, erlotinib, gefitinib, and afatinib. Table 3 summarizes data reported in the literature and prior clinical trials investigating these 5 agents.
Table 3.
Summary of renal toxic events with EGFR targeting agents
| Drug | Renal adverse events reported | References |
|---|---|---|
| Cetuximab (monoclonal antibody) | Hypomagnesaemia, hypokalemia, AKI, hyponatremia, glomerulonephritis | 2, 52, 53, 54, 55, 56, 57, 58 |
| Panitumumab (monoclonal antibody) | Hypomagnesaemia, AKI, hypokalemia | 2, 53, 65, 66 |
| Erlotinib (anti-EGFR TKI) | AKI, hypomagnesemia, hypophosphatemia | 2, 53, 68, 69 |
| Afatinib (anti-EGFR TKI) | AKI, hypokalemia, hyponatremia | 2, 53 |
| Gefitinib (anti-EGFR TKI) | AKI, hypokalemia, fluid retention, minimal change disease, proteinuria | 2, 53, 72, 73, 74 |
AKI, acute kidney injury; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Cetuximab
This monoclonal antibody against EGFR 1 is known to cause hypomagnesemia.50 This happens as a result of reduction in the transport of transient receptor potential melastatin (TRPM) 6/7 ion channels to the apical membrane of the distal renal tubule.51 Filtered magnesium (Mg) is reabsorbed mainly in the thick ascending limb of Henle and the distal tubule, where most of the EGFR is located in the kidney. Epidermal growth factor is an autocrine paracrine hormone that regulates renal Mg reabsorption by regulating the activity and transport of TRPM6.51 Blocking EGFR with cetuximab blunts the movement of TRPM6/7 channel, which leads to renal Mg wasting and hypomagnesemia. The main risk factors for developing hypomagnesemia are duration of treatment, age, and baseline Mg values.52 Low Mg levels may lead to hypokalemia, hypocalcemia, and cardiac arrhythmias.53 The literature review revealed multiple studies that have reported hypomagnesemia as an adverse effect of this agent.54, 55 Severe grade 3 (<0.9–0.7 mg/dl) or 4 (<0.7 mg/dl, <0.3 mmol/l, life-threatening consequences) hypomagnesemia has been seen in 36% and 27% of patients treated with cetuximab.56 Another study showed the incidence of hypomagnesemia to be close to 10% to 15%.30 A more recent study revealed an incidence rate of hypomagnesemia with cetuximab to be close to 30%. Of the cases, 22% were grade 1 (i.e., below the lower limit of normal to 1.2 mg/dl).57 There was no statistically significant correlation between Mg level and patient age, duration of treatment, localization of primary tumor or metastases, and number of metastases. However, there was an upward trend in a logistic regression model showing that the risk of developing hypomagnesemia increases with age.57 Management of EGFR inhibitor−induced hypomagnesemia is summarized by Fakih in a review.58 One has to ensure that the patient is not on any other offending medication, such as thiazide diuretics or proton pump inhibitors. For patients with grade 1 or 2 hypomagnesemia, oral Mg may be sufficient after discontinuation of cetuximab. Testing for Mg levels should be performed every 2 to 3 weeks. For patients who cannot tolerate oral Mg, i.v. Mg up to 4 g can be given. Amiloride (Mg-sparing diuretic) has been tried in unpublished case reports, with success in treating the hypomagnsemia.59 For grades 3 and 4 hypomagnesemia, patients should receive 6 to 10 g of magnesium sulfate daily to twice weekly if possible. An initial strategy of i.v. replacement and every other day monitoring of magnesium levels can help guide frequency. An alternative strategy for patients requiring frequent magnesium infusions may be to consider a 2-month stop-and-go approach to cetuximab treatment.58
In our review of the literature, we also found 1 clinical trial that reported renal failure in 2% of patients,60 1 published case report of a diffuse proliferative glomerulonephritis,61 and another case report of nephrotic syndrome.62 In our review of the FAERS, we found 467 individuals who had experienced renal events.2 This is the second highest number of events for any of the targeted therapies. Although the literature search revealed hypomagnesaemia as the most commonly reported toxicity,54, 55 FAERS data suggest a high degree of renal impairment with cetuximab.2 A total of 172 cases of AKI with this agent were reported to FAERS.2 This was followed by hypokalemia in 113 cases; hyponatremia, 78 cases; hypomagnesaemia, 58 cases; and HTN, 24 cases. AKI might be an important finding that needs to be studied further, as prior reviews do not discuss this in detail.53 The cause of AKI is not reported in the FAERS database. EGFR, which is mainly expressed in the distal and collecting tubules, is involved in maintaining tubular integrity. EGFR activation leads to growth and generation of tubular epithelial cells after acute tubular necrosis.63 In patients prone to experiencing renal injury, treatment with anti-EGFR agents might be a “second hit” for development of AKI.
Panitumumab
Panitumumab is a monoclonal antibody similar to cetuximab. In the original trials, this agent was not associated with any nephrotoxic effects.64 However, in a recent phase 3 trial, for head and neck cancer, there was a high incidence of hypomagnesemia (12%) and hypokalemia (10%).65 Hypomagnesemia was reported in 36% of patients who were treated with panitumumab in a phase 3 trial for colorectal cancer.66 The FAERS review found 337 renal adverse events with this agent. The majority of the cases were of hypomagnesemia (103 cases), followed by hypokalemia (85 cases) and AKI (82 cases). Overall, there should be close Mg-monitoring strategies in patients receiving EGFR agents.67 Treatment strategies are similar to that for cetuximab-induced hypomagnesemia.
Erlotinib
Compared to cetuximab and pantumumab, the next few agents discussed here (erlotinib, gefitinib, and afatinib) are TKIs that affect the EGFR. Erlotinib is a small-molecule TKI that blocks the activity of the EGFR tyrosine kinase, a key regulator of intracellular signaling pathways crucial for cancer cell survival. Our literature search revealed no data on nephrotoxicity with this agent. Erlotinib can influence magnesium handling, but its effect on the systemic magnesium concentration seems less potent than that observed with antibody-based EGFR inhibitors. Animal data suggest that typical human dosages of erlotinib are unlikely to severely affect serum magnesium concentrations.68 Although erlotinib can cause hypomagnesemia, the magnesium stearate supplementation present as part of the erlotinib tablet might alleviate the deficiency. Counter to data reported in the literature, our FAERS analysis found 63 cases of AKI and only 8 cases of hypomagnesemia. Further research is needed to clarify the association between erlotinib and AKI.2 At this time, the mechanism of AKI is unclear.
The FAERS analysis also revealed 3 cases of hypophosphatemia with erlotinib.2 A small phase 1 trial of 17 patients who used sorafenib and erlotinib for solid tumors found a 76% incidence (13 of 17 patients) of hypophosphatemia.69 A more recent, small phase 1 study of 25 patients using erlotinib in gliomas found a 30% incidence of hypophosphatemia.70 The mechanism of erlotinib-induced hypophosphatemia is unclear, but might involve sodium phosphate cotransporters in the proximal tubule.53
Gefitinib
Similar to erlotinib, gefitinib is also a small-molecule TKI. A literature review revealed fluid retention in 6.6% of patients.71 In addition, 1 case of AKI and another case of nephrotic syndrome (due to an autoimmune response to the agent) have also been reported.72, 73 More recently, a biopsy-proven case of MCD was reported that responded to drug withdrawal and a switch to erlotinib.74 Fifteen cases of renal adverse events were noted in the FAERS review,2 including 7 cases of AKI7, 4 cases of hypokalemia, and 2 cases of hyponatremia, but no cases of hypomagnesemia.
Afatinib
In the initial trials of afatinib, there was a 34% incidence of hypokalemia.75 No published cases of any nephrotoxicities with this agent are reported in the literature. In the FAERS, there were 26 cases of AKI, followed by 6 cases of hypokalemia and 5 cases of hyponatremia.2
Human Epidermal Growth Factor−2 (HER-2) Target Inhibitors
HER-2 is a membrane receptor that is overexpressed in many forms of breast and gastric cancers. This receptor is part of the EGFR family.
Trastuzumab
Trastuzumab is a recombinant humanized monoclonal antibody that binds to the receptor tyrosine-kinase erbB-2 (ERBB-2) on tumor cells and induces antibody-mediated cellular toxicity in cells that overexpress this protein. The drug alone does not cause renal toxicity, but it can lead to cardiac toxicity and subsequently cardio-renal syndrome.76 One study illustrated that GFR < 78 ml/min/1.73 m2 was the strongest predictor of cardiac toxicity.76 In addition, in the TANDEM study, the combination of trastuzumab and anastrozole was associated with a higher incidence of HTN compared to anastrozole alone.77 In the TOGA study, the arm that had trastuzumab with standard chemotherapy had a higher incidence of renal toxicity compared to the arm with standard chemotherapy alone.78 More recently, a case of tumor lysis syndrome has been reported with this agent.79 In addition, there have now been a few cases of fetal nephrotoxicity with renal impairment noted with oligohydramnios and anhydramnios. In these cases, there was spontaneous improvement of renal function in the fetus after stopping traztuzumab.80, 81, 82 Additional review of the literature found no published cases of hypokalemia or hypomagnesemia, but there are 3 published cases of hyponatremia. In each of the 3 cases, other potentially hyponatremia-causing chemotherapies were also involved.83, 84, 85 The FDA analysis indicated a large number of cases reported of renal toxicity in the FAERS database.2 Reports included 124 cases of AKI, 112 cases of hypokalemia, 52 cases of HTN, 24 cases of hypomagnesemia, 28 cases of hyponatremia, and 11 cases of hypophosphatemia. Most of the cases were females, as cancers treated relate mainly to women.2 It is important to monitor renal function, electrolytes, and blood pressure in these patients, as AKI was reported to the FAERS database in a number of cases. Given its known cardiac toxicity, it is possible that the cases of AKI might be related to a cardio-renal syndrome physiology and the electrolyte disorders related to diuretic use.
Pertuzumab
Pertuzumab is a recombinant humanized monoclonal antibody that targets the extracellular dimerization domain of the ERBB-2. No published cases or reports of renal toxicity are reported in the literature or from clinical trials. Our FAERS analysis found a total of 100 cases; AKI (46 cases) and hypokalemia (26 cases) were the top 2 causes.2
Lapatinib
Lapatinib is a dual tyrosine kinase inhibitor that interrupts both the EGFR and ERBB-2 pathways. In a phase 2 trial with this agent, 7 patients experienced treatment-related grade 3 toxicity, and 2 of them developed hyponatremia.86 Our literature search revealed no published reports of this agent leading to AKI or any electrolyte disorders. Our analysis demonstrated a total of 171 cases reported in the FAERS database. Most cases were of hypokalemia (61 cases) and AKI (48 cases). There were a few cases of HTN, hypomagnesemia, and hyponatremia as well.2
BRAF Target Inhibitors
Advanced melanoma has traditionally been unresponsive to standard chemotherapy agents and formerly had a dismal prognosis. Genetically targeted small-molecule inhibitors of the oncogenic BRAF V600 mutation or a downstream signaling partner (mitogen-activating protein kinase [MEK]) are effective treatment options for the 40% to 50% of patients with melanoma who harbor mutations in BRAF. Selective BRAF and MEK inhibitors induce frequent and dramatic objective responses and markedly improve survival compared with cytotoxic chemotherapy.87, 88, 89 In the last decade after discovery of this mutation, drugs such as vemurafenib and dabrafenib have been approved by the FDA and the European Medicines Agency for the treatment of V-600 mutated melanomas. Although the initial trials did not signal any renal toxicities with the BRAF inhibitors, recent case reports, case series, and the FDA adverse reporting systems have uncovered significant nephrotoxicities with these agents.87, 88, 89
Vemurafenib
In the initial phase III trial, 51 patients developed edema, but there were no reports of proteinuria. There were also no reported cases of AKI.87 In 2013, in a letter to the editor, Uthurriague et al. were the first to report AKI. They reviewed 16 patients who were on this agent for more than 8 months.88 Fifteen patients experienced significant decline in GFR at 1 month, with a mean reduction of 29 ml/min, and this decline persisted for 3 months. Since then, multiple publications have highlighted renal toxicities in various forms with this agent, but mostly AKI and biopsy-proven ATN.89, 90, 91, 92, 93, 94 Although biopsy-proven tubular toxicity93 has been the mechanism of injury likely causing the AKI, a recent study showed that vemurafenib induces a dual mechanism of increase in plasma creatinine with both an inhibition of creatinine tubular secretion and slight renal function impairment. However, this adverse effect is mostly reversible when vemurafenib is discontinued, and should not lead physicians to discontinue the treatment if it is effective.95 Table 4 summarizes all known toxicities with this agent. Our analysis of the FAERS database was reported separately.94 In that publication, 132 cases of AKI were reported in association with vemurafenib during the timeframe reviewed. The toxicity was more common in men.2, 93, 94
Table 4.
| Allergic interstitial disease |
| Acute tubular necrosis |
| Proximal tubular damage (Fanconi syndrome) |
| Hypophosphatemia |
| Hyponatremia |
| Hypokalemia |
| Sub−nephrotic range proteinuria |
Dabrafenib
The European summary of product characteristics of the drug reports that renal failure has been identified in less than 1% of patients treated with dabrafenib.96 Recently, 1 case of biopsy-proven acute interstitial nephritis was reported with this agent,97 which responded to steroid treatment. The FDA data that we analyzed were reported separately.94 Most of the cases reported to the FAERS in that timeframe were AKI.94 Contrary to prior publications that had shown a 7% incidence of hypophosphatemia,96 no cases of hypophosphatemia were found in the FAERS database.94 Our group recently summarized the toxicities of the BRAF agents, and that review provides more details.98
Mitogen-Activated Protein Kinase Enzymes MEK1 and/or MEK2 (MEK) Inhibitors
Trametinib
There have been no published cases of nephrotoxicity with trametinib. Monotherapy with this agent can lead to hypertension, but cases of AKI and hyponatremia were noted more frequently in patients treated with combined trametinib and dabrafenib.99 Our analysis found similar results of a small number of cases of AKI (28 cases) and hyponatremia (11 cases) with this agent. Any renal toxicity may result from a combination effect with the BRAF inhibitors rather than a sole effect of an MEK inhibitor.97
Anaplastic Lymphoma Kinase (ALK) Target Inhibitors
Crizotinib
Crizitonib is an inhibitor of the anaplastic lymphoma kinase (ALK) indicated in patients with non−small-cell lung cancer (NSCLC) who have ALK fusions. No renal adverse events were reported in earlier clinical trials.100 However, a recent study did report AKI in a patient treated with this agent.101 A biopsy-proven case of ATN was published from France that was attributed to crizotinib.102
A recent analysis from the University of Colorado looked at 38 patients with NSCLC who were treated with this agent. The mean GFR decreased by 23.9% compared to baseline, and this was seen in the first 2 weeks of therapy. Close to 84% of the patients recovered renal function after cessation of therapy. The investigators thought that the rapid reversibility raised the possibility that the drug led to a defect in tubular secretion of creatinine rather than a true drop in GFR. The study did not report biopsy data.103 In addition, renal cysts have been reported with crizotinib. The cysts tend to be reversible and occur in 4% of patients.104, 105 A series from Taiwan found changes in renal cysts in 22% of patients who received this agent. Crizotinib not only appears to be associated with formation of new cysts but can also lead to progression of prior existing cysts.106 The molecular mechanism by which crizotinib increases risk of developing renal cysts in currently unknown. In the FAERS analysis,2 consistent with the published reports, 88 cases of renal impairment were noted. In addition, we found evidence of electrolyte disorders as well (hyponatremia, 24 cases; hypokalemia, 13 cases). There is no evidence of those disorders reported in the existing literature. In summary, besides AKI, renal cyst formation and progression, hyponatremia and hypokalemia also need to be considered with crizotinib. A recent review on this topic summarizes the renal injury in depth.107
Immune Checkpoint Inhibitor: Programmed Cell Death−1 and Ligand Target (PD-1 and PDL-1)
Cancer immunotherapy has revolutionized the treatment of cancer by targeting the immune system rather than the cancer. This pathway includes 2 proteins called programmed death−1 (PD-1), which is expressed on the surface of immune cells, and programmed death ligand−1 (PD-L1), which is expressed on cancer cells. The PD-1 molecule is expressed in activated T cells, B cells, natural killer T cells, monocytes, and dendritic cells. It has 2 ligands, PD-ligand 1 (L1) and PD-ligand 2 (L2). When PD-1 and PD-L1 bind to each other, a biochemical “shield” is formed, which leads to T-cell inactivation and protection of the tumor cells from being destroyed by the immune system. Monoclonal antibodies directed against PD-1 constitute a class of drugs that prevent engagement of PD-1 to its ligands,108, 109 rescuing the activated T-cell from apoptotic death and thus allowing it to continue its attack on tumor cells. Nivolumab and pembrolizumab are 2 monoclonal antibody therapies designed to directly block the interaction between PD-1 and its ligands in the treatment of melanoma, lung cancer, renal cancer, and hematological malignancies, and have been used since 2014.110 The objective of blocking program cell death is to restore the activation of the immune system, directed to tumor cells. These agents are referred to as immune checkpoint inhibitors.108, 109, 110
Nivolumab
Nivolumab was the first anti−PD-1 antibody, tested initially in melanoma. In December 2014, the FDA granted an accelerated approval to nivolumab for the treatment of patients with unresectable or metastatic melanoma.111 Since then, this agent has also been approved for use in renal cell cancer.112 In 1 trial,111 there was an increased incidence of elevated creatinine noted in the nivolumab-treated group as compared to the chemotherapy-treated group (13% vs. 9%). Steroids helped to resolve the renal dysfunction in 50% of the cases. The FDA label113 has guidelines to start steroids if the creatinine rises rapidly. Recently, there were 4 biopsy-proven cases of acute interstitial nephritis (AIN) related to nivolumab described in a case series.114 All patients in this series were also taking other drugs (proton pump inhibitors, nonsteroidal anti-inflammatory drugs) linked to AIN, but in most cases, the use of these drugs long preceded anti−PD-1 antibody therapy. Treatment with steroids resolved the AIN in most cases, and rechallenge with nivolumab in 1 case resulted in recurrence of AIN. Cortazar et al. reported an additional case of diffuse AIN with this agent.115 Steroids were administered, with no response. The authors think that the PD-1 inhibitor therapy may release suppression of T-cell immunity that normally permits renal tolerance of drugs known to be associated with AIN.114, 115 The AKI appears late, usually in the 6- to 12-month period. Hyponatremia has also been reported with this agent, in 25% of the cases. The underlying mechanism remains unclear.2, 113 In the FAERS analysis,2 we found 20 cases of AKI, 14 of hyponatremia, and 5 of hypokalemia associated with nivolumab.
Pembrolizumab
Pembrolizumab (MK-3475) is another monoclonal antibody therapy designed to directly block the interaction between PD-1 and its ligands. This drug has been used in melanoma and hematological malignancies since 2014. In the initial trials, acute interstitial nephritis was confirmed by kidney biopsy in 2 of 3 patients with AKI. All 3 patients fully recovered renal function with treatment with high-dose corticosteroids (≥40 mg prednisone or equivalent per day) followed by a corticosteroid taper.116, 117 The Keynote-2 study118 reported grade 3 to 4 adverse events that included generalized edema and myalgia (each in 2 patients [1%]) in those given pembrolizumab 2 mg/kg, and hypopituitarism and hyponatremia (each in 2 patients [1%]) in those given pembrolizumab 10 mg/kg. The literature is scant, but recently, Shirali et al. reported 2 biopsy-proven cases of AIN with this agent.114 One case of rhabdomyolysis and AKI has been reported,119 and renal failure occurred in 2% of the patients in a recent review.115 Cortazar et al.115 reported 2 additional cases of AIN, 1 case that presented at 3 weeks and another at 33 weeks after drug initiation. Both patients received steroids; 1 patient responded partially and required dialysis, and the other patient responded with complete recovery. Overall, the 4 cases of biopsy-proven AIN reported with this agent were in the timeframe of 1 month to 12 months. Of the case patients, 75% responded to steroids with complete remission, and 1 patient required dialysis and had partial remission with steroids. The steroids used were variable, from i.v. forms to oral steroids for a 1- to 3-month timeframe. Compared to cases reported in the literature, the FAERS analysis found 19 cases of hyponatremia and 16 cases of AKI. There were also 4 reports of hypokalemia and hyperkalemia. It is possible that the electrolyte disorders are related to hypopituitarism. Given the immune-mediated mechanism of action of this drug, the kidney can be a potential target and can lead to AIN.
Immune Checkpoint Inhibitor: Cytotoxic T Lymphocyte Antigen−4 (CTLA-4) as a Target
Ipilimumab
Ipilimumab is a monoclonal antibody that has antitumor activity by targeting CTLA-4 and activating the immune system. This agent is also considered an immune checkpoint inhibitor. A member of the Ig family, CTLA4 is expressed on CD4+ T-helper cell surface and transmits an inhibitory signal to T cells. Blocking of CTLA-4 prevents this signal and improves antineoplastic response. Ipilimumab is an antibody that binds to human CD152 and enhances T-cell response, especially against tumor cells. Migration of activated T cells into the kidney leads to AKI. Cell-mediated immune response leading to inflammatory cell infiltrates with and without granulomas have been reported.120, 121 At least 12 cases of AIN in the initial trials, arising 2 to 12 weeks after drug administration, have been reported, and 1 of them demonstrated granulomas.115, 121, 122, 123, 124 The largest series of 6 cases of AIN was reported by Cortazar et al.115 Pathology revealed AIN in most cases, with varying degrees of foot process effacement. Cortazar et al. also reported 1 additional case of thrombotic microangiopathy (TMA) related to ipilumumab.115 In a phase III study conducted in patients with melanoma recently,125 no renal toxic effects were reported. Based on our review of the literature, 2 cases of nephrotic syndrome126, 127 have also been reported, 1 patient with a lupus-like nephritis with a membranous pattern of injury126 and the other with minimal change in disease.127 The electrolyte disorders are not well reported in the literature with this agent. Two cases of hyponatremia have been published.128 A possible cause of hyponatremia is a syndrome of inappropriate antidiuretic hormone from hypophysitis. This complication related to the agent has now been well reported.129, 130 In the FAERS analysis,2 ipilumimub, with 508 events, had the highest number of reports of renal events of all recently used targeted therapies. Most were AKI, followed by hyponatremia and hypokalemia. Hyponatremia related to this agent should prompt consideration of a pituitary-related disorder. In summary, ipilimumab is clearly nephrotoxic, with many cases of AKI and electrolyte disorders reported in the literature and FAERs reports. Most of the AKI occurred at 6- to 12-weeks’ time following the start of treatment with this agent. Steroids remitted most cases of AIN with this agent. Some patients had partial remission, and a few required dialysis as well. AKI likely falls under an immune-mediated mechanism of injury, as noted with other immune checkpoint inhibitors. Hence, corticosteroids remain the mainstay of treatment for AIN related to ipilumumab therapy. In the literature of cases reviewed, steroids were administered in various form; some patients received oral prednisone 60 mg daily and tapered over 3 months, and some received i.v. solumedrol ranging from 250 mg to 500 mg for 3 to 4 days and then tapered to oral steroids.
Table 5 compares the renal toxicities of both immune checkpoint inhibitor classes (CTLA-4 antagonists and PD-1 inhibitors). For both immune checkpoint inhibitors (anti−PD-1 and CTLA-4), if AKI is confirmed on a kidney biopsy as AIN or a podocytopathy, we would recommend checkpoint inhibitor discontinuation and a course of corticosteroids. However, we cannot recommend a definitive dose and duration of steroid therapy. Based on cases reported, prednisone 1 mg/kg with a 1- to 2-month taper may be sufficient. We additionally recommend weekly creatinine monitoring for 1 month after completing steroids. Rechallenge with immune checkpoint therapy may be reasonable if other potentially offending agents are withdrawn and AIN has resolved. With this approach, serum creatinine should be monitored regularly, with reinstitution of steroids at the first sign of AKI from AIN without another cause. For patients on ipilimumab, renal function monitoring should be more frequent in the first 3 months, as the injury appears to occur earlier in the treatment course. For the PD-1 inhibitors, the injury usually happens later, so monitoring serum chemistries on a monthly basis might be more prudent.
Table 5.
Comparison of renal toxicities of CTLA-4 antagonists and PD-1 inhibitors2, 114, 115, 121, 122, 124, 126, 127, 128
| Toxicity | CTLA-4 antagonists (ipilimumab) | PD-1 inhibitors (nivolumab and pembrolizumab) |
|---|---|---|
| Onset of AIN | AIN appears 6–12 weeks after initiation of therapy | AIN appears 3–12 mo after initiation of therapy |
| Glomerular findings | Podocytopathy reported | No cases of podocytopathy reported |
| Electrolyte disorders | Hyponatremia cases related to hypophysitis | Hyponatremia is rare |
AIN, acute interstitial nephritis; CTLA-4, cytotoxic T lymphocyte antigen−4; PD-1, programmed cell death–1.
Dosing of Targeted Therapies In CKD and Dialysis
Because the majority of the targeted therapies are associated with renal toxicity, dosing in CKD and patients on dialysis is of great importance. As noted in prior chemotherapy trials, most trials excluded patients on dialysis or with severe CKD (GFR < 30). This limits understanding of dosing in CKD and dialysis. In Table 1, we summarize the existing published literature on dosing of these agents in CKD and dialysis (wherever data are available).
Conclusions
The use of novel targeted therapies has led to significant improvement in survival and overall prognosis with many malignancies. However, there is evolving knowledge on renal adverse events with these agents. Timely recognition of these toxicities can aid in the proper management of cancer patients. In this study and review, we recognized that there are multiple ways in which targeted therapies can have an impact on renal function. Table 6 summarizes our recommended monitoring strategy for patients receiving these agents. In addition, newer targeted agents are entering clinical trials. It is exceedingly difficult to keep up with all the new drugs along with their associated renal toxicities. Therefore, it is important that the medical community advocates for an international database registry for targeted therapies and their renal adverse effects.
Table 6.
Recommended routine physical examination, imaging, and serum and urine monitoring with various targeted therapies
| Blood pressure monitoringa |
| Peripheral edema examinationb |
| Serum creatinine |
| Serum potassium |
| Serum phosphorus |
| Serum sodium |
| Serum calcium |
| Serum magnesiumc |
| Complete blood count |
| Urinalysis evaluation for cells and casts |
| Urine protein/creatinine ratioa |
| LDHa |
| Haptoglobina |
| CKd |
| Renal sonogram for cyst evaluationb |
BCR-ABL, breakpoint cluster region–abelson; CK, creatinine kinase; LDH, lactate dehydrogenase.
Most targeted therapies require all the tests listed below, and a few require extra tests that are labeled with the legend below.
Anti−vascular endothelial growth factor (VEGF) agents, tyrosine kinase inhibitors, cytotoxic T lymphocyte antigen−4 (CTLA-4) inhibitor.
Crizitonib.
Epidermal growth factor (EGFR) inhibitors.
BCR-ABL tyrosine kinase inhibitors.
Disclosure
KDJ serves on the American Society of Nephrology (ASN) Onconephrology Forum. KDJ and RW are expert members of the Cancer and Kidney International Network, and KDJ serves on the Governing Board of Cancer and Kidney International Network. All other authors declare no competing interests.
Acknowledgments
We thank Drs. Mark Perazella, Valerie Barta, and Mr. Adriel Sanchez for creation of figures.
References
- 1.National Cancer Institute. Targeted therapy. Available at: http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies. Accessed January 1, 2016.
- 2.Jhaveri K.D., Sakhiya V.B., Wanchoo R. Renal effects of novel anti cancer targeted therapies: a review of the FDA Adverse Event Reporting System. Kidney Int. 2016;90:705–707. doi: 10.1016/j.kint.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Gurevich F., Perazella M.A. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Eremina V., Jefferson J.A., Kowalewska J. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pande A., Lombardo J., Spangenthal E., Javle M. Hypertension secondary to anti-angiogenic therapy: experience with bevacizumab. Anticancer Res. 2007;27:3465–3470. [PubMed] [Google Scholar]
- 7.Izzedine H., Sène D., Hadoux J. Thrombotic microangiopathy related to anti-VEGF agents: intensive versus conservative treatment? Ann Oncol. 2011;22:487–490. doi: 10.1093/annonc/mdq743. [DOI] [PubMed] [Google Scholar]
- 8.Izzedine H., Massard C., Spano J.P. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer [Oxford, England: 1990] 2010;46:439–448. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H., Escudier B., Lhomme C. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine. 2014;93:333–339. doi: 10.1097/MD.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzedine H., Rixe O., Billemont B. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Broekman F., Giovannetti E., Peters G.J. Tyrosine kinase inhibitors: multi-targeted or single-targeted? World J Clin Oncol. 2011;2:80–93. doi: 10.5306/wjco.v2.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y. Platelet-derived growth factor receptor tyrosine kinase inhibitors: a review of the recent patent literature. Expert Opin Ther Patents. 2010;20:885–897. doi: 10.1517/13543776.2010.493559. [DOI] [PubMed] [Google Scholar]
- 13.Jhaveri K.D., Flombaum C.D., Croog K., Glezerman I.G. Nephrotoxicities associated with the use of tyrosine kinase inhibitors: a single-center experience and review of the literature. Nephron Clin Pract. 2011;117:c312–c319. doi: 10.1159/000319885. [DOI] [PubMed] [Google Scholar]
- 14.Bellini E., Pia A., Brizzi M.P. Sorafenib may induce hypophosphatemia through a fibroblast growth factor-23 (FGF23)-independent mechanism. Ann Oncol. 2011;22:988–990. doi: 10.1093/annonc/mdr010. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A., Van Cutsem E., Sobrero A. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Qin S., Xu R. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz B., Kemal Y., Teker F. Single dose regorafenib-induced hypertensive crisis. Exp Oncol. 2014;36:134–135. [PubMed] [Google Scholar]
- 18.Wang Z., Xu J., Nie W. Risk of hypertension with regorafenib in cancer patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2014;70:225–231. doi: 10.1007/s00228-013-1598-1. [DOI] [PubMed] [Google Scholar]
- 19.Wells S.A., Jr., Robinson B.G., Gagel R.F. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.S., Hirsh V., Park K. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30:1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 21.Leboulleux S., Bastholt L., Krause T. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 22.Chrisoulidou A., Mandanas S., Margaritidou E. Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Oncol Targets Ther. 2015;8:2435–2442. doi: 10.2147/OTT.S86322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrissey K.M., Stocker S.L., Wittwer M.B. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 24.Shen H., Yang Z., Zhao W. Assessment of vandetanib as an inhibitor of various human renal transporters: inhibition of multidrug and toxin extrusion as a possible mechanism leading to decreased cisplatin and creatinine clearance. Drug Metab Dispos. 2013;41:2095–2103. doi: 10.1124/dmd.113.053215. [DOI] [PubMed] [Google Scholar]
- 25.Marcolino M.S., Boersma E., Clementino N.C. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol. 2011;22:2073–2079. doi: 10.1093/annonc/mdq715. [DOI] [PubMed] [Google Scholar]
- 26.Gafter-Gvili A., Ram R., Gafter U. Renal failure associated with tyrosine kinase inhibitors—case report and review of the literature. Leuk Res. 2010;34:123–127. doi: 10.1016/j.leukres.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Pou M., Saval N., Vera M. Acute renal failure secondary to imatinib mesylate treatment in chronic myeloid leukemia. Leuk Lymphoma. 2003;44:1239–1241. doi: 10.1080/1042819031000079140. [DOI] [PubMed] [Google Scholar]
- 28.Foringer J.R., Verani R.R., Tjia V.M. Acute renal failure secondary to imatinib mesylate treatment in prostate cancer. Ann Pharmacother. 2005;39:2136–2138. doi: 10.1345/aph.1G131. [DOI] [PubMed] [Google Scholar]
- 29.Francois H., Coppo P., Hayman J.P. Partial Fanconi syndrome induced by imatinib therapy: a novel cause of urinary phosphate loss. Am J Kidney Dis. 2008;51:298–301. doi: 10.1053/j.ajkd.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Takikita-Suzuki M., Haneda M., Sasahara M. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuky J., Isacson C., Fotoohi M. Phase II trial of imatinib (Gleevec) in patients with metastatic renal cell carcinoma. Investig New Drugs. 2003;24:85–88. doi: 10.1007/s10637-005-4543-z. [DOI] [PubMed] [Google Scholar]
- 32.Penel N., Blay J.Y., Adenis A. Imatinib as a possible cause of severe rhabdomyolysis. N Engl J Med. 2008;358:2746–2747. doi: 10.1056/NEJMc0708896. [DOI] [PubMed] [Google Scholar]
- 33.Gordon J.K., Magid S.K., Maki R.G. Elevations of creatine kinase in patients treated with imatinib mesylate (GleevecTM) Leuk Res. 2010;34:827–829. doi: 10.1016/j.leukres.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Berman E., Nicolaides M., Maki R.G. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan S., Horne A., Wattie D. Decreased bone turnover despite persistent secondary hyperparathyroidism during prolonged treatment with imatinib. J Clin Endocrinol Metab. 2009;94:1131–1136. doi: 10.1210/jc.2008-2324. [DOI] [PubMed] [Google Scholar]
- 36.Iyoda M., Shibata T., Wada Y. Long- and short-term treatment with imatinib attenuates the development of chronic kidney disease in experimental anti-glomerular basement membrane nephritis. Nephrol Dial Transplant. 2013;28:576–584. doi: 10.1093/ndt/gfs414. [DOI] [PubMed] [Google Scholar]
- 37.Bondon-Guitton E., Combret S., Pérault-Pochat M.C. Cardiovascular risk profile of patients with peripheral arterial occlusive disease during nilotinib therapy. Target Oncol. 2016;11:549–552. doi: 10.1007/s11523-016-0417-x. [DOI] [PubMed] [Google Scholar]
- 38.Iyoda M., Shibata T., Hirai Y., Kuno Y., Akizawa T. Nilotinib attenuates renal injury and prolongs survival in chronic kidney disease. J Am Soc Nephrol. 2011;22:1486–1496. doi: 10.1681/ASN.2010111158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;11:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Ozkurt S., Temiz G., Acikalin M.F., Soydan M. Acute renal failure under dasatinib therapy. Ren Fail. 2010;1:147–149. doi: 10.3109/08860220903391226. [DOI] [PubMed] [Google Scholar]
- 41.Kaiafa G., Kakaletsis N., Savopoulos C. Simultaneous manifestation of pleural effusion and acute renal failure associated with dasatinib: a case report. J Clin Pharm Ther. 2013;1:102–105. doi: 10.1111/jcpt.12107. [DOI] [PubMed] [Google Scholar]
- 42.Holstein S.A., Stokes J.B., Hohl R.J. Renal failure and recovery associated with second-generation Bcr-Abl kinase inhibitors in imatinib-resistant chronic myelogenous leukemia. Leuk Res. 2008;2:344–347. doi: 10.1016/j.leukres.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Martino S., Daguindau E., Ferrand C. A successful renal transplantation for renal failure after dasatinib-induced thrombotic thrombocytopenic purpura in a patient with imatinib-resistant chronic myelogenous leukaemia on nilotinib. Leuk Res Rep. 2013;1:29–31. doi: 10.1016/j.lrr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uz B., Dolasik I. An unexpected and devastating adverse event of dasatinib: rhabdomyolysis. Leuk Res Rep. 2015;5:1–2. doi: 10.1016/j.lrr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace E., Lyndon W., Chumley P. Dasatinib-induced nephrotic-range proteinuria. Am J Kidney Dis. 2013;6:1026–1031. doi: 10.1053/j.ajkd.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Hirano T., Hashimoto M., Korogi Y. Dasatinib-induced nephrotic syndrome. Leuk Lymphoma. 2015;3:726–727. doi: 10.3109/10428194.2015.1075020. [DOI] [PubMed] [Google Scholar]
- 47.Pfizer Labs. Bosulif (bosutinib) [prescribing information]. New York, NY: Pfizer Labs; 2015.
- 48.Byrd J.C., Furman R.R., Coutre S.E. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M.L., Blum K.A., Martin P. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–745. doi: 10.1182/blood-2015-03-635326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrelli F., Borgonovo K., Cabiddu M. Risk of anti EGFR monoclonal antibody related hypomagnesemia: systematic review and pooled analysis of randomized studies. Exp Opin Drug Saf. 2012;11(suppl):S9–S19. doi: 10.1517/14740338.2011.606213. [DOI] [PubMed] [Google Scholar]
- 51.Voets T., Nilius B., Hoefs S. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 52.Fakih M.G., Wilding G., Lombardo J. Ceuximab induced hypomagnesaemia in patients with colorectal cancer. Clin Colorectal Cancer. 2006;6:152–156. doi: 10.3816/CCC.2006.n.033. [DOI] [PubMed] [Google Scholar]
- 53.Izzedine H., Bahleda R., Khayat D. Electrolyte disorders related to EGFR-targeting drugs. Crit Rev Oncol Hematol. 2010;73:213–219. doi: 10.1016/j.critrevonc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Chen P., Wang L., Li H. Incidence and risk of hypomagnesemia in advanced cancer patients treated with cetuximab: a meta-analysis. Oncol Lett. 2013;5:1915–1920. doi: 10.3892/ol.2013.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao Y., Liao C., Tan A. Meta-analysis of incidence and risk of hypomagnesemia with cetuximab for advanced cancer. Chemotherapy. 2010;56:459–465. doi: 10.1159/000321011. [DOI] [PubMed] [Google Scholar]
- 56.Schrag D., Chung K.Y., Flombaum C.D., Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97:1221–1224. doi: 10.1093/jnci/dji242. [DOI] [PubMed] [Google Scholar]
- 57.Streb J., Püsküllüoğlu M., Glanowska I. Assessment of frequency and severity of hypomagnesemia in patients with metastatic colorectal cancer treated with cetuximab, with a review of the literature. Oncol Lett. 2015;10:3749–3755. doi: 10.3892/ol.2015.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fakih M. Management of anti-EGFR targeting monoclonal antibody induced hypomagnesemia. Oncology [Williston Park] 2008;22:74–76. [PubMed] [Google Scholar]
- 59.Vij R., Sachdeva M. Severe electrolyte abnormalities after cetuximab—management challenge. Am J Kidney Dis. 2010;55(4):617–772. [Google Scholar]
- 60.Harari P.M. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689–708. doi: 10.1677/erc.1.00600. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki K., Anderson E., Shankland S.J., Nicosia R.F. Diffuse proliferative glomerulonephritis associated with cetuximab, an epidermal growth factor receptor inhibitor. Am J Kidney Dis. 2013;61:988–991. doi: 10.1053/j.ajkd.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Ito C., Fujii H., Ogura M. Cetuximab-induced nephrotic syndrome in a case of metastatic rectal cancer. J Oncol Pharm Pract. 2013;19:265–268. doi: 10.1177/1078155212459668. [DOI] [PubMed] [Google Scholar]
- 63.Nakapoulou L., Stefanaki K., Boletis J. Immunohistochemial study of epidermal growth factor receptor in various types of renal injury. Nephrol Dial Transplant. 1994;9:764–769. [PubMed] [Google Scholar]
- 64.Rowinsky E.K., Schwartz G.H., Gollob J.A. Safety, pharmacokinetics and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004;22:3003–3015. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 65.Vermorken J.B., Stohlmacher-Williams J., Davidenko I. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SPECTRUM): an open label phase 3 randomized trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 66.Van Cutsem E., Peeters M., Siena S. Open label phase III trial on panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 67.do Pazo-Oubiña F., Estefanell-Tejero A., Riu-Viladoms G. Magnesium monitoring practice in monoclonal anti-epidermal growth factor receptor antibodies therapy. J Clin Pharm Ther. 2013;38:101–103. doi: 10.1111/jcpt.12028. [DOI] [PubMed] [Google Scholar]
- 68.Dimke H., Van der Wijst J., Alexander T.R. Effects of the EGFR inhibitor erlotinib on magnesium handling. J Am Soc Nephrol. 2010;21:1309–1316. doi: 10.1681/ASN.2009111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duran I., Hotté S.J., Hirte H. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:4849–4857. doi: 10.1158/1078-0432.CCR-07-0382. [DOI] [PubMed] [Google Scholar]
- 70.Broniscer A., Baker S.J., Stewart C.F. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15:701–707. doi: 10.1158/1078-0432.CCR-08-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E.S., Hirsh V., Mok T. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 72.Wan H.L., Yao N.S. Acute renal failure associated with gefitinib therapy. Lung. 2006;184:249–250. doi: 10.1007/s00408-005-2581-0. [DOI] [PubMed] [Google Scholar]
- 73.Kumasaka R., Nakamura N., Shirato K. Side effects of therapy: case 1. Nephrotic syndrome associated with gefitinib therapy. J Clin Oncol. 2004;22:2504–2505. doi: 10.1200/JCO.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 74.Maruyama K., Chinda J., Kuroshima T. Minimal change nephrotic syndrome associated with gefitinib and a successful switch to erlotinib. Intern Med. 2015;54:823–826. doi: 10.2169/internalmedicine.54.3661. [DOI] [PubMed] [Google Scholar]
- 75.Miller V.A., Hirsh V., Cadranel J. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomized trial. Lancet Oncol. 2012;13:528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 76.Russo G., Cioffi G., Di Lenarda A. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med. 2012;7:439–446. doi: 10.1007/s11739-012-0794-9. [DOI] [PubMed] [Google Scholar]
- 77.Kaufman B., Mackey J.R., Clemens M.R. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 78.Bang Y.J., Van Cutsem E., Feyereislova A. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 79.Taira F., Horimoto Y., Saito M. Tumor lysis syndrome following trastuzumab for breast cancer: a case report and review of the literature. Breast Cancer. 2015;22:664–668. doi: 10.1007/s12282-013-0448-4. [DOI] [PubMed] [Google Scholar]
- 80.Gottschalk I., Berg C., Harbeck N. Fetal renal insufficiency following trastuzumab treatment for breast cancer in pregnancy: case report and review of the current literature. Breast Care (Basel) 2011;6:475–478. doi: 10.1159/000335202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bader A.A., Schlembach D., Tamussino K.F. Anhydramnios associated with administration of trastuzumab and paclitaxel for metastatic breast cancer during pregnancy. Lancet Oncol. 2007;8:79–81. doi: 10.1016/S1470-2045(06)71014-2. [DOI] [PubMed] [Google Scholar]
- 82.Watson W.J. Herceptin (trastuzumab) therapy during pregnancy: association with reversible an-hydramnions. Obstet Gynecol. 2005;105:642–651. doi: 10.1097/01.AOG.0000141570.31218.2b. [DOI] [PubMed] [Google Scholar]
- 83.Yasuhara H., Imagawa A., Koike N. A case of renal salt-wasting syndrome during chemotherapy for advanced gastric cancer. Gan To Kagaku Ryoho. 2015;42:225–227. [PubMed] [Google Scholar]
- 84.Kolarich A.R., Reynolds B.A., Heldermon C.D. Ado-trastuzamab emtansine associated hyponatremia and intracranial hemorrhage. Acta Oncol. 2014;53:1434–1436. doi: 10.3109/0284186X.2014.920959. [DOI] [PubMed] [Google Scholar]
- 85.Turner N., Stewart J., Barnett F., White S. Syndrome of inappropriate anti-diuretic hormone secretion secondary to carboplatin after docetaxel-carboplatin-trastuzumab combination for early stage HER-2 positive breast cancer. Asia Pac J Clin Oncol. 2012;8:e9–e11. doi: 10.1111/j.1743-7563.2012.01526.x. [DOI] [PubMed] [Google Scholar]
- 86.de Souza J.A., Davis D.W., Zhang Y. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:2336–2343. doi: 10.1158/1078-0432.CCR-11-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.US Food and Drug Administration. Zolboraf. Highlights of prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202429s000lbl.pdf. Accessed July 2016.
- 88.Uthurriague C., Thellier S., Ribes D. Vemurafenib significantly decreases glomerular filtration rate. J Eur Acad Dermatol Venerol. 2014;28:978–979. doi: 10.1111/jdv.12322. [DOI] [PubMed] [Google Scholar]
- 89.Regnier-Rosencher E., Lazareth H., Gressier L. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol. 2013;169:934–938. doi: 10.1111/bjd.12555. [DOI] [PubMed] [Google Scholar]
- 90.Launay-Vacher V., Zimner-Rapuch S., Poulalhon N. Acute renal failure associated with the new BRAF inhibitor vemurafenib: a case series of 8 patients. Cancer. 2014;120:2158–2163. doi: 10.1002/cncr.28709. [DOI] [PubMed] [Google Scholar]
- 91.Yorio J.T., Mays S.R., Ciurea A.M. Case of vemurafenib-induced Sweet’s syndrome. J Dermatol. 2014;41:817–820. doi: 10.1111/1346-8138.12430. [DOI] [PubMed] [Google Scholar]
- 92.Denis D., Franck N., Fichel F. Fanconi syndrome induced by vemurafenib: a new renal adverse event. JAMA Dermatol. 2015;151:453–454. doi: 10.1001/jamadermatol.2014.4529. [DOI] [PubMed] [Google Scholar]
- 93.Teuma C., Perier-Muzet M., Pelletier S. New insights into renal toxicity of the B-RAF inhibitor, vemurafenib, in patients with metastatic melanoma. Cancer Chemother Pharmacol. 2016 doi: 10.1007/s00280-016-3086-7. [DOI] [PubMed] [Google Scholar]
- 94.Jhaveri K.D., Sakhiya V., Fishbane S. BRAF inhibitor related nephrotoxicity. JAMA Oncol. 2015;1:1133–1134. doi: 10.1001/jamaoncol.2015.1713. [DOI] [PubMed] [Google Scholar]
- 95.Hurabielle C., Pillebout E., Stehlé T. Mechanisms underpinning increased plasma creatinine levels in patients receiving vemurafenib for advanced melanoma. PLoS One. 2016;11:e0149873. doi: 10.1371/journal.pone.0149873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.US Food and Drug Administration. Tefinlar. Highlights of prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202806s000lbl.pdf. Accessed July 2016.
- 97.Jansen Y.J., Janssens P., Hoorens Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated withdabrafenib and trametinib. Melanoma Res. 2015;25:550–554. doi: 10.1097/CMR.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 98.Wanchoo R., Jhaveri K.D., Geray D., Launay-Vacher V. Renal effects of BRAF inhibitors. A review by the Cancer and the Kidney International Network (C-KIN) Clin Kidney J. 2016 doi: 10.1093/ckj/sfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flaherty K.T., Infante J.R., Daud A. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Camidge D.R., Bang Y.J., Kwak E.L. Activity and safety of crizotinib in patients with ALK-positive non small cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martín Martorell P., Huerta Alvaro M., Solís Salguero M.A., Insa Molla A. Crizotinib and renal insufficiency: a case report and review of the literature. Lung Cancer. 2014;84:310–313. doi: 10.1016/j.lungcan.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Gastaud L., Ambrosetti D., Otto J. Acute kidney injury following crizotinib administration for non small cell lung carcinoma. Lung Cancer. 2013;82:362–364. doi: 10.1016/j.lungcan.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Brosnan E.M., Weickhardt A.J., Lu X. Drug induced reduction in estimated glomerular filtration rate in patients with ALK-positive non-small cell lung cancer treated with the ALK inhibitor crizotinib. Cancer. 2014;120:664–674. doi: 10.1002/cncr.28478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin Y.T., Wang Y.F., Yang J.C. Development of renal cysts after crizotinib treatment in advanced ALK-positive non small cell lung cancer. J Thorac Oncol. 2014;9:1720–1725. doi: 10.1097/JTO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 105.Klempner S.J., Aubin G., Dash A., Ou S.H. Spontaneous regression of crizotinib associated complex renal cysts during continuous crizotinib treatment. Oncologist. 2014;19:1008–1010. doi: 10.1634/theoncologist.2014-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schnell P., Barlett C.H., Solomon B.J. Complex renal cysts associated with crizotinib treatment. Cancer Med. 2015;4:887–896. doi: 10.1002/cam4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Izzedine H., El-fekih R.K., Perazella M.A. The renal effects of ALK inhibitors. Invest New Drugs. 2016;34:643–649. doi: 10.1007/s10637-016-0379-y. [DOI] [PubMed] [Google Scholar]
- 108.Luke J.J., Ott P.A. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 2015;6:3479–3492. doi: 10.18632/oncotarget.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terme M., Ullrich E., Aymeric L. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2001;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 110.Tang P.A., Heng D.Y. Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep. 2015;15:98–104. doi: 10.1007/s11912-012-0284-2. [DOI] [PubMed] [Google Scholar]
- 111.Robert C., Long G.V., Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 112.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bristol-Myers Squibb. Nivolumab package insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125554lbl.pdf. Accessed January 1, 2016.
- 114.Shirali A., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death (PD)-1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.02.057. pii: S0272-6386(16)30025-7. [DOI] [PubMed] [Google Scholar]
- 115.Cortazar F.B., Marrone K.A., Troxell M.L. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016 doi: 10.1016/j.kint.2016.04.008. pii: S0085-2538(16)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merck Sharp & Dohme. Pembrolizumab package insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125514lbl.pdf. Accessed January 1, 2016.
- 117.Robert C., Schachter J., Long G.V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 118.Ribas A., Puzanov I., Dummer R. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Min L., Hodi F.S. Anti PD-1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–18. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voskens C.J., Goldinger S.M., Loquai C. The price of tumor control: an analysis of rare side effects of anti-CTLA4 therapy in metastatic melanoma from ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Izzedine H., Gueutin V., Gharbi C. Kidney injuries related to ipilimumab. Invest New Drugs. 2014;32:769–773. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 122.Thajudeen B., Madhrira M., Bracamonte E. Ipilimumab granulomatous interstitial nephritis. Am J Ther. 2015;22:e84–e87. doi: 10.1097/MJT.0b013e3182a32ddc. [DOI] [PubMed] [Google Scholar]
- 123.O'Day S.J., Maio M., Chiarion-Sileni V. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 124.Forde P.M., Rock K., Wilson G., O'Byrne K.J. Ipilimumab-induced immune-related renal failure—a case report. Anticancer Res. 2012;32:4607–4608. [PubMed] [Google Scholar]
- 125.Robert C., Thomas L., Bondarenko I. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 126.Fadel F., El Karoui K., Knebelmann B. Anti CTLA-4 antibody induced lupus nephritis. N Engl J Med. 2009;361:211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 127.Kidd J., Gizaw A. Ipilimumab associated minimal change disease. Kidney Int. 2016;89:720. doi: 10.1016/j.kint.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 128.Barnard Z.R., Walcott B.P., Kahle K.T. Hyponatremia associated with ipilimumab-induced hypophysitis. Med Oncol. 2012;29:374–377. doi: 10.1007/s12032-010-9794-7. [DOI] [PubMed] [Google Scholar]
- 129.Min L., Hodi F.S., Giobbie-Hurder A. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749–755. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chodakiewitz Y., Brown S., Boxerman J.L. Ipilimumab treatment associated pituitary hypophysitis: clinical presentation and imaging diagnosis. Clin Neurol Neurosurg. 2014;125:125–130. doi: 10.1016/j.clineuro.2014.06.011. [DOI] [PubMed] [Google Scholar]