Introduction
Male gender is associated with a higher morbidity and mortality in both children and adolescents with chronic kidney disease (CKD).1 Testosterone has been implicated in the difference of progression of CKD, by gender.2 Children with renal dysplasia often reach end-stage kidney disease during puberty.3, 4 There is evidence for a significantly steeper deterioration of renal function during puberty, independent of changes in body composition.3 In adults, there is also evidence for gender disparity of progression of CKD,5 with a higher rate of end-stage kidney disease in men.5 The direct influence of testosterone upon renal function, and in particular renal hemodynamics, has not been established in humans.
We recently had the opportunity to observe substantial worsening of renal function in a 14-year-old boy with hypergonadotropic hypogonadism who had repeatedly exhibited reduction in renal function following administration of testosterone. We used a novel computed tomography (CT) perfusion imaging technique. This was combined with a state-of-the-art 256-slice CT scanner, to provide whole organ coverage without the challenges of image registration associated with the limited scan slice thickness of commonly available instruments. This allowed an in vivo assessment of renal blood flow, patterns of renal perfusion, and other functional parameters of the kidneys in previously unobtainable detail. Our method has not been published, but the principles of measuring renal blood flow and blood volume are comparable to those presented by Ehling et al.6
Case Presentation
A 14-year-old boy with Townes Brock syndrome (Online Mendelian Inheritance in Man #107480) and CKD stage 3 (estimated glomerular filtration rate [eGFR], 33 ml/min per 1.73 m2 based on cystatin C,7 [pediatric cystatin C eGFR reference interval, 90–135 mL/min per 1.73m2]) and cryptorchidism was treated with 3 monthly 50-mg depot testosterone i.m. injections to promote pubertal development. Before these injections, his luteinizing hormone was pubertal at 13.4 IU/l (reference range, 1.7–8.6 IU/l) and follicle-stimulating hormone was 8.0 IU/l (reference range, 1.5–12.4 IU/l]. His testosterone concentration was inappropriately low at 3.4 nmol/l (reference interval, 8.6–29.0 nmol/l), consistent with hypergonadotropic hypogonadism. Full informed written consent was obtained from the consenting minor and his caregiver.
Following the injections, the patient’s serum creatinine levels increased from 133 to 211 μmol/l (reference interval, 62–120 μmol/l) and cystatin C increased from 2.5 to 3.5 mg/l (reference interval, 0.27–1.20 mg/l). The cystatin C eGFR7 dropped to 22 ml/min per 1.73 m2. For consistency, all eGFR measurements reported here are based on the cystatin C eGFR. The boy’s height was 169.2 cm (50th percentile) and his weight was 113.8 kg (> 99th percentile). His medication at that time included 10 mg ramipril twice daily, 25 mg carvedilol twice daily, 20 mg hydralazine thrice daily, 12.5 mg hydrochlorothiazide once daily, 1000 U vitamin D and 25 μg alfacalcidol at bedtime, 4000 mg sodium bicarbonate thrice daily, and ferrous sulfate 300 mg at lunchtime and 20 μg darbepoetin alfa every 4 weeks subcutaneously. Blood pressure was 131/71 mm Hg. His medication remained unchanged except for a need for a higher darbepoietin dosage, which was changed to every fortnight. Blood pressure improved slightly to 122/68 mm Hg.
After cessation of testosterone, the patient’s serum creatinine level dropped to 140 μmol/l and his cystatin C level dropped to 2.3 mg/l. He remained stable at this level of kidney function for 6 months, when he was rechallenged with a single dose of 50 mg testosterone i.m. for continued cryptorchidism and hypogonadism. Again, his serum creatinine level rose to 206 μmol/l and cystatin C to 2.95 mg/l, which equated to an eGFR of 27 ml/min per 1.73 m2. The changes induced by the testosterone challenge are given in Table 1. Magnesium, glucose, white blood cell count, platelets, thyroid stimulating hormone, and vitamin D metabolite levels were unchanged.
Table 1.
Clinical laboratory markers before and after the testosterone rechallenge
| Parameter | Reference interval | Pretestosterone | 1 month posttestosterone |
|---|---|---|---|
| Sodium (mmol/l) | 135-145 | 139 | 140 |
| Potassium (mmol/l) | 3.5-5.0 | 4.7 | 4.2 |
| Chloride (mmol/l) | 98-107 | 104 | 101 |
| Bicarbonate (mmol/l) | 22-29 | 20 | 25 |
| Urea (mmol/l) | < 8.0 | 10.7 | 13.6 |
| Creatinine (μmol/l) | 62-120 | 122 | 206 |
| Calcium (mmol/l) | 2.15-2.55 | 2.24 | 2.31 |
| Phosphate (mmol/l) | 0.8-1.33 | 1.14 | 1.37 |
| Hemoglobin (g/l) | 125-160 | 139 | 116 |
| Parathyroid hormone (pmol/l) | 1.6-6.9 | 8.0 | 12.0 |
| Aldosterone (pmol/l) | < 729 | < 83 | < 83 |
| Microalbumin/creatinine (mg/mmol) | 0.0-2.0 | 50.9 | 17.6 |
To further establish a potential direct causal link between testosterone exposure and to inform clinical choices over future sex hormone supplementation in this patient, CT perfusion imaging was performed before and after rechallenging with testosterone. Imaging was done on a GE Revolution 256-slice CT scanner (Boston, MA, USA) at St Joseph’s Health Care London, Ontario, Canada. Following low-volume iodinated contrast agent injection, dynamic contrast enhanced CT scanning of a 16-cm section of the abdomen fully encompassing both kidneys was performed without breath-hold. The section was divided into 32 slices of 5-mm thickness each and was scanned 42 times at 2.8-second intervals using 120 kV and 22.4 mA for a duration of approximately 2 minutes. Following the scan, images were reconstructed using adaptive statistical iterative reconstruction (GE Health Care Canada Inc, Mississauga, ON) to reduce image noise.
CT images were registered using nonrigid registration to minimize the effects of breathing motion during scanning. Registered images were analyzed using prototype CT Perfusion 4D software (GE Healthcare). Following artery region of interest selection, functional maps were computed for blood flow (BF), blood volume, mean transit time (a marker of blood flow over time), permeability surface area product, and contrast appearance time. Next, regions of interest were drawn over the kidney in the BF maps to encompass medullar and cortical areas. Then, BF values were averaged over the selected transverse slices to determine mean kidney BF values.
The testosterone re-exposure (measured 2 weeks after the re-exposure), and reduction in renal function, was associated with an 11% reduction in cortical BF. Average cortical BF dropped from 242 mL/min per 100 g to 216 mL/min per 100 g (P = 0.01, paired t test) (Table 2). Mean transit time decreased from 7.8 to 9.6 seconds (P = 0.00028). Blood volume and permeability surface area product remained unchanged. Medullary BF was relatively preserved compared with cortical BF (see Figure 1). Furthermore, the reduction in tissue perfusion was markedly heterogeneous (see Table 2), consistent with microcirculatory dysfunction.8 The patient received only 1 dose of testosterone but the cystatin C and serum creatinine did not fully recover after the re-exposure to testosterone. With the re-exposure, the patient’s serum creatinine level increased to 473 μmol/l 1 month after the injection and only recovered to 254 μmol/l, and cystatin C peaked at 5.35 mg/l and dropped only down to 4.16 mg/l. He subsequently underwent bilateral inguinal orchidopexy.
Table 2.
Renal perfusion before and after testosterone exposure
| Exposure | Blood flow | Blood volume | Mean transit time | Permeability surface area product | Contrast appearance time |
|---|---|---|---|---|---|
| Before testosterone | |||||
| Slice 1 | 252.67 | 27.458 | 7.69 | 50.822 | 0.653 |
| Slice 2 | 261.96 | 26.976 | 7.12 | 52.875 | 0.842 |
| Slice 3 | 244.34 | 27.352 | 7.62 | 53.836 | 0.66 |
| Slice 4 | 246 | 27.661 | 7.47 | 58.322 | 0.636 |
| Slice 5 | 263.56 | 27.188 | 7.06 | 57.231 | 0.72 |
| Slice 6 | 241 | 28.803 | 7.84 | 57.483 | 0.551 |
| Slice 7 | 221.89 | 27.866 | 7.86 | 54.375 | 0.575 |
| Slice 8 | 221.9 | 29.533 | 8.43 | 52.172 | 0.518 |
| Slice 9 | 227.18 | 32.276 | 9.02 | 52.724 | 0.394 |
| Mean | 242.28 | 28.35 | 7.79 | 54.43 | 0.62 |
| SD | 15.92 | 1.69 | 0.62 | 2.65 | 0.13 |
| After testosterone | |||||
| Slice 1 | 201.47 | 30.229 | 10.265 | 80.764 | 0.218 |
| Slice 2 | 206.09 | 28.802 | 9.75 | 68.982 | 0.397 |
| Slice 3 | 211.77 | 26.746 | 8.31 | 67.002 | 0.459 |
| Slice 4 | 231.15 | 27.097 | 8.75 | 64.737 | 0.45 |
| Slice 5 | 252.31 | 27.583 | 8.31 | 67.618 | 0.524 |
| Slice 6 | 253.44 | 27.824 | 8.36 | 62.308 | 0.585 |
| Slice 7 | 226.71 | 29.91 | 9.97 | 51.273 | 0.454 |
| Slice 8 | 184.47 | 34.209 | 11.239 | 42.549 | 0.24 |
| Slice 9 | 181.56 | 34.426 | 11.506 | 41.177 | 0.204 |
| Mean | 216.55 | 29.65 | 9.61 | 60.71 | 0.39 |
| SD | 26.38 | 2.90 | 1.25 | 13.12 | 0.14 |
| Paired t test | 0.01395 | 0.07131 | 0.00028 | 0.18923 | 0.00196 |
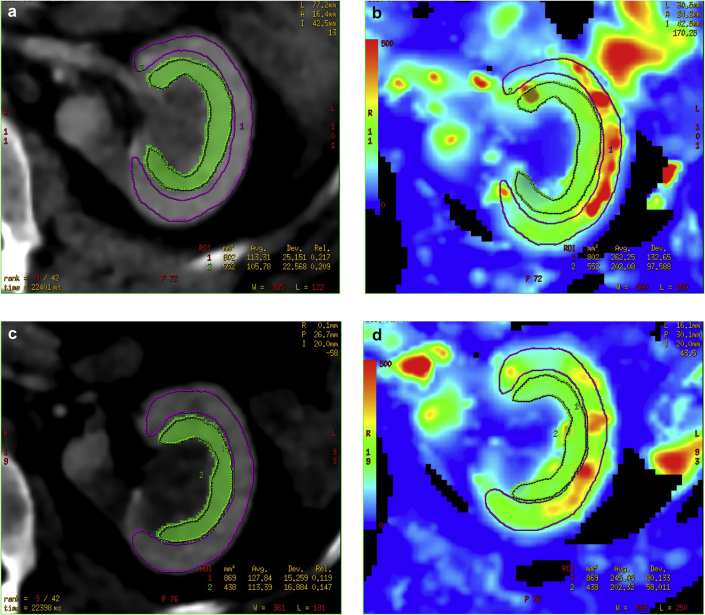
Figure 1.
Representative figures from computed tomography perfusion imaging (a and c) and corresponding blood flow maps (b and d) with regions of interest drawn over the medulla (green) and cortex (magenta) before (a and b) and after (c and d) testosterone.
Discussion
We present a case of acute testosterone-induced deterioration of renal function in a 14-year-old boy with Townes Brock syndrome and hypergonadotropic hypogonadism. Further reduction of renal function after subsequent reintroduction of testosterone was associated with the same response, inferring an association. The pathophysiology of this effect appears to be attributable to testosterone-mediated changes in intrarenal hemodynamic parameters. This was confirmed on direct dynamic imaging and strongly supported by the observed reduction in proteinuria.
The key questions raised by this potentially highly instructive case are, Why did this happen? and, Why was it reversible? There are several reports about a differential effect of sex hormones on renal tissues, including differential effects on mesangial cell proliferation,9 collagen synthesis,9, 10 and apoptosis.11, 12 Animal data suggest that podocytes are a target for testosterone. Testosterone has been shown to induce podocyte damage and apoptosis, whereas 17-β estradiol was protective.10 However, low-dose testosterone has been found to be protective against renal ischemia-reperfusion injury,13, 14 and a recent study found an association with low testosterone levels and lower eGFR in a cross-sectional study of men aged 40 to 80 years.15 In our case, the effect of testosterone was reversible because renal function returned to normal following cessation of testosterone replacement therapy, at least the first time. However, renal function did not return to baseline after re-exposure. There is evidence for the presence of testosterone receptors on the afferent arteriole of the kidneys and a specific dose-dependent effect of testosterone in an arteriole culture model.16 Afferent arterioles are the major resistance vessels in the kidney, and play an important role in the development of renal injury and hypertension. The reversible worsening of renal function, decrease of renal blood flow, and reduction of proteinuria suggest that changes in the renal perfusion rather than permanent damage to the nephrons were responsible for the observed effect. Furthermore, this implies that testosterone may be a key contributor to the observed gender differences in CKD progression and the development of acute kidney injury. Detailed intrarenal perfusion studies in animal models of acute kidney injury consistently identify the importance of the heterogeneity of perfusion within the organ.17, 18, 19 This pattern of perfusion was present within the kidneys of this patient after testosterone challenge. Such detailed study of these effects have not been previously possible to examine in humans, due to imaging technology limitations.
A strength of our case report is the fact that renal function was measured using both cystatin C and creatinine, thereby excluding the likelihood that changes in the tubular secretion of creatinine were responsible for the observed increase in renal retention parameters.20 The weight of the patient did not change, making changes in muscle mass unlikely. Another strength lies is the objective confirmation of renal perfusion changes by taking advantage of cutting-edge developments in both imaging technology and sequence development. There are several limitations, mostly due to the fact that a single case is presented without confirming the effects in other subjects, and the fact that a therapeutic testosterone dose was administered for the purpose of inducing puberty in a single dose rather than gradually increasing testosterone concentrations. Physiological changes of testosterone during puberty may have a much more blunted effect. It is also unclear if a sustained exposure to testosterone may have irreversible effects. It was fortuitous that with the particular circumstances in this patient, and the availability of renal perfusion measurement, we were able to establish an association and a possible pathomechanism for the observed testosterone-induced renal dysfunction. However, although the amount of contrast was very small, contrast-related acute kidney injury could have confounded the effect of testosterone, although serum creatinine and cystatin C levels were unaffected by the first contrast study.
This patient represents an index case to establish the importance of sex hormone status within the setting of CKD. This study provides the first direct human experimental evidence that testosterone directly modulates renal perfusion. This may be of crucial importance in understanding the observed sex differences in vulnerability to CKD and suggests that this may be an important dimension to be considered in future research into the development and progression of CKD.
Disclosure
All the authors declared no competing interests.
References
- 1.McDonald S.P., Craig J.C. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 2.Silbiger S.R. Raging hormones: gender and renal disease. Kidney Int. 2011;79:382–384. doi: 10.1038/ki.2010.474. [DOI] [PubMed] [Google Scholar]
- 3.Ardissino G., Testa S., Dacco V. Puberty is associated with increased deterioration of renal function in patients with CKD: data from the ItalKid Project. Arch Dis Child. 2012;97:885–888. doi: 10.1136/archdischild-2011-300685. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez Celedon C., Bitsori M., Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol. 2007;22:1014–1020. doi: 10.1007/s00467-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 5.Carrero J.J. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33:383–392. doi: 10.1159/000320389. [DOI] [PubMed] [Google Scholar]
- 6.Ehling J., Babickova J., Gremse F. Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol. 2016;27:520–532. doi: 10.1681/ASN.2015020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filler G., Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 8.Scully C.G., Mitrou N., Braam B. Detecting physiological systems with laser speckle perfusion imaging of the renal cortex. Am J Physiol Regul Integ Comp Physiol. 2013;304:R929–R939. doi: 10.1152/ajpregu.00002.2013. [DOI] [PubMed] [Google Scholar]
- 9.Kwan G., Neugarten J., Sherman M. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–1179. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 10.Doublier S., Lupia E., Catanuto P. Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011;79:404–413. doi: 10.1038/ki.2010.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metcalfe P.D., Leslie J.A., Campbell M.T. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab. 2008;294:E435–E443. doi: 10.1152/ajpendo.00704.2006. [DOI] [PubMed] [Google Scholar]
- 12.Verzola D., Gandolfo M.T., Salvatore F. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004;65:1252–1261. doi: 10.1111/j.1523-1755.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 13.Patil CN, Wallace K, LaMarca BD, et al. Low dose testosterone protects against renal ischemia-reperfusion injury by increasing renal IL-10:TNF-alpha ratio and attenuating T cell infiltration [e-pub ahead of print]. Am J Physiol Renal Physiol.http://dx.doi.org/10.1152/ajprenal.00454.2015, accessed June 30, 2016. [DOI] [PMC free article] [PubMed]
- 14.Soljancic A., Ruiz A.L., Chandrashekar K. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integ Comp Physiol. 2013;304:R951–R958. doi: 10.1152/ajpregu.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurita N., Horie S., Yamazaki S. Low testosterone levels and reduced kidney function in Japanese adult men: the Locomotive Syndrome and Health Outcome in Aizu Cohort Study. J Am Med Dir Assoc. 2016;17:371.e1–371.e6. doi: 10.1016/j.jamda.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y., Fu Y., Ge Y. The vasodilatory effect of testosterone on renal afferent arterioles. Gender Med. 2012;9:103–111. doi: 10.1016/j.genm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer K., Meral F.C., Zhang Y. High-resolution renal perfusion mapping using contrast-enhanced ultrasonography in ischemia-reperfusion injury monitors changes in renal microperfusion. Kidney Int. 2016;89:1388–1398. doi: 10.1016/j.kint.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calzavacca P., Evans R.G., Bailey M. Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med. 2015;43:e431–e439. doi: 10.1097/CCM.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 19.Hu L., Chen J., Yang X. Assessing intrarenal nonperfusion and vascular leakage in acute kidney injury with multinuclear (1) H/(19) F MRI and perfluorocarbon nanoparticles. Magnetic Res Med. 2014;71:2186–2196. doi: 10.1002/mrm.24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filler G., Yasin A., Medeiros M. Methods of assessing renal function. Pediatr Nephrol. 2014;29:183–192. doi: 10.1007/s00467-013-2426-7. [DOI] [PubMed] [Google Scholar]