To the Editor:
Metformin-associated lactic acidosis (MALA) following metformin overdose or reduced metformin clearance in the setting of acute kidney injury is associated with high mortality.1 For most severe cases, current guidelines recommend hemodialysis (HD) to correct acidosis and associated electrolyte abnormalities, although its effect on metformin removal is considered uncertain.2 We report a case of severe MALA treated with HD.
Case Report
A 66-year-old, 77.5-kg woman with a history of type 2 diabetes, hypertension, chronic obstructive pulmonary disease, and Child−Pugh A alcoholic cirrhosis presented at the emergency department in Verdun Hospital (Montreal, PQ, Canada) because of recent deterioration in her general state. The police found her on the floor and incoherent after she had called 911, and they were unable to obtain a history from her. The regular medication regimen of the patient included pregabalin, maxeran, pantoprazole, calcium/vitamin D, and metformin 850 mg 3 times daily.
On admission, vital signs showed hypotension (93/56 mm Hg), sinus bradycardia (25/min), hypothermia (30.4 °C), and agonal breathing. Initial laboratory tests revealed the following: sodium 135 mmol/l, potassium 6.8 mmol/l, creatinine 766 μmol/l, glucose 8.9 mmol/l, and lactate 22 mmol/l (normal range, 0.5−2.2); the venous blood gas showed a pH of 6.67, HCO3 of 2 mmol/l, and pCO2 of 15 mm Hg. The osmol gap was 19 and the anion gap was 20 mmol/l. Electrocardiography (ECG) revealed sinus bradycardia and heightened T-waves. Acetaminophen, ethanol, and salicylate concentrations were below the detection limits.
Because of the severe acidemia, high lactate concentration, and impaired kidney function, MALA due to metformin accumulation was suspected. The patient’s blood pressure continued to decrease, and atropine (1 mg) and epinephrine (1 mg) were administered. Cardiac massage for 1 minute was performed, and the patient was intubated. Sodium bicarbonate, norepinephrine, and vasopressin perfusion were also administered to correct hypotension.
Six hours after admission, the patient remained hypotensive (75/40) and hypothermic (31.8 °C); her lactate concentration had increased to 24 mmol/l, and, despite bicarbonate infusion, the pH decreased to 6.66. HD treatment was therefore performed to enhance metformin removal and to correct the associated electrolyte abnormalities. HD was initiated 8 hours after admission and performed for 5.9 hours using an FX 1000 filter (Helixone membrane, UF coefficient 75 ml/h mm Hg, surface area 2.2 m2; Fresenius Medical Care, Bad Homburg, Germany) via a temporary femoral catheter. Blood flow was 300 ml/min and the dialysate flow 750 ml/min. No ultrafiltration was prescribed, and no heparin was administered. Dialysate composition was the following: bicarbonate 39 mmol/l, potassium 3.0 mmol/l, sodium 140 mmol/l, and calcium 1.5 mmol/l.
Methods
Metformin Sampling
Metformin samples were simultaneously drawn before, during, and after hemodialysis from the arterial line, the venous blood line, and the outgoing dialysate line at regular intervals, when applicable. Metformin concentrations were determined by high-performance liquid chromatography coupled with triple-quad tandem mass spectrometry (HPLC-MS/MS; Agilent 6410 mass spectrometer and Agilent 1200 series HPLC, Agilent Technologies, Montreal, PQ, Canada) following protein precipitation. This HPLC-MS/MS method was linear for metformin between 0.01 and 40 mg/l and showed good accuracy and precision (102.5% ± 2.3%, intra-assay, n = 15).
The following calculations were used:
-
(i)
metformin half-life (T1/2) during HD was measured as: T1/2 = 0.693/Ke,
-
(ii)
estimated total body content of metformin (TBC) was measured as: TBC = [metformin]plasma × VD × W,
-
(iii)
instantaneous plasma clearance of metformin by HD at various time points (CLDIAL INST) by dialysate method was calculated as: CLDIAL INST = [metformin]dialysate × QD / [metformin]plasma,
-
(iv)
instantaneous plasma clearance of metformin by HD at various time points (CLAV INST) by AV method was calculated as: and = ([metformin]inflow − [metformin]outflow) × QB × (1-HcT), and
-
(v)
removal of metformin during HD was measured from the recovered dialysate.
In these calculations, VD = volume of distribution of metformin (1−5 l/kg); [metformin]plasma = plasma metformin concentration (mg/l); QB = blood flow rate (ml/min); QD = dialysate outflow rate (ml/min); [metformin]inflow = metformin concentration in the inflow/arterial line; [metformin]outflow = metformin concentration in the outflow/venous line; [metformin]dialysate = metformin concentration in dialysate (mg/l); T = time (min); W = body weight (kg); HcT = hematocrit; and Ke = elimination rate constant (represents the slope from the equation derived by best fit using linear regression log graph).
Results
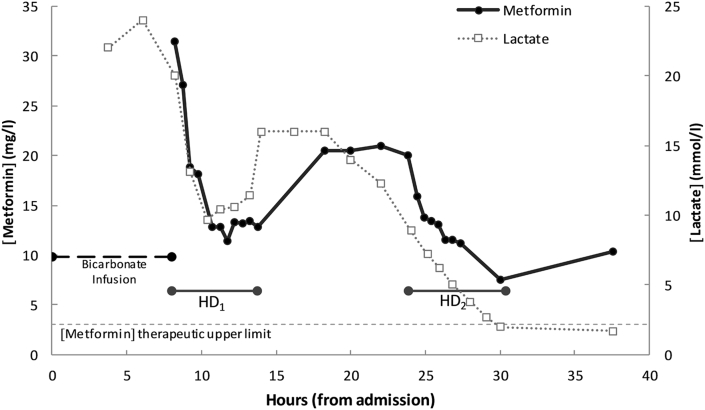
The serum metformin concentration at the start of dialysis was 31.4 mg/l (therapeutic range 0.5−3.0 mg/l). Within 90 minutes of HD initiation, the patient’s pH and blood pressure normalized, and both the norepinephrine and vasopressin were completely weaned off. Metformin and lactate concentrations readily decreased during dialysis (Figure 1). After the first HD session (HD1), hypotension recurred, and there was a rebound in the concentration of both metformin (increase from 12.8 to 21.9 mg/l) and lactate (increase from 11.4 to 16.0 mmol/l). Ten hours after the end of the first HD session, another HD session was performed (HD2) for 6.6 hours with the same parameters. During HD2, the patient again regained hemodynamic stability, and remained stable for the rest of her admission. The metformin concentration again rebounded after cessation of HD2 (from 7.5 to 10.3 mg/l), although lactate remained normal. There were no complications during either dialysis. The patient was extubated 2 days after admission and was discharged home without sequalae 3 days later.
Figure 1.
Metformin and lactate concentrations in relationship with time of arrival since admission. HD, hemodialysis.
A total of 1039 mg of metformin was removed in 5.5 hours during HD1 and another 463 mg of metformin was removed in 6.2 hours during HD2. Because metformin apparent volume of distribution has been reported to be anywhere between 1 and 5 l/kg, total body content at the start of HD1 may have been as little as 2.4 g or as much as 12.1 g; therefore, anywhere between 9% and 48% of total body content of metformin was removed during HD1. The measured metformin apparent half-life was 4.7 hours (R2 = 0.68) during HD1 and 5.5 hours (R2 = 0.98) during HD2. Instantaneous plasma clearance of metformin by both dialysate measurement and AV difference was, respectively, 170.3 ml/min and 178.2 ml/min during HD1 and 98.1 ml/min and 89.3 ml/min during HD2 (clearance decreased during the second session because of decreased achievable blood flow).
Discussion
This case was that of a patient with chronic metformin toxicity with features of severe clinical compromise who responded well to hemodialysis. Metformin is the most commonly prescribed oral antidiabetic medication. Metformin is mostly eliminated by the kidneys and follows a multiphasic elimination3, 4, 5: in patients with normal renal function, the half-life is initially 4 to 8 hours, and the terminal half-life is approximately 20 hours.2, 4, 5
Metformin toxicity and metformin-associated lactic acidosis (MALA)2, 6 may cause severe morbidity and mortality (30%−50%).1, 7, 8, 9, 10, 11 Toxicity can result either from an acute ingestion or, more commonly, from an unexpected rapid decline in kidney function, with or without aggravating conditions. MALA is diagnosed when blood lactate concentration is >5 mmol/l and pH blood level is <7.35.3, 8 Treatment for mild cases of MALA usually includes supportive care, gastrointestinal decontamination if pertinent, and bicarbonate infusion.
Although metformin is a small molecule (129 Da) and unbound to protein, it has a relatively large volume of distribution (1−5 l/kg), so its dialyzability is considered uncertain. In 2015, the Extracorporeal Treatments In Poisoning (EXTRIP) Workgroup provided recommendations for the use of extracorporeal treatments in metformin toxicity.12 The rationale for the recommendation included a correction of acidemia and electrolyte abnormalities, improvement of hyperlactatemia, and support of impaired kidney function. The workgroup acknowledged that metformin’s dialyzability was imprecise because of the limited toxicokinetic data.
Most published reports present incomplete data: high-efficiency hemodialysis provides metformin clearance that surpasses 120 ml/min and a metformin half-life of approximately 4 hours.13, 14, 15, 16, 17, 18, 19, 20, 21 In comparison, metformin clearance with continuous renal replacement therapy is usually about one-fourth of that obtained with HD, and the half-life is at least 3 times as long.21, 22, 23, 24, 25, 26, 27 However, because of metformin’s large volume of distribution, the significance of these data is debatable without quantifying metformin removal.
Only 2 publications have presented data concerning metformin removal by extracorporeal treatment. Lalau and Race reported removal of 1105 mg, 694 mg, and 688 mg by HD in 3 patients,3 and Barrueto et al. reported 3.5 g removal by continuous venovenous hemodialysis in 10.5 hours.25 Our data show that high-efficiency dialysis provides enhanced metformin clearance and substantial metformin removal. Interestingly, as shown in other reports, a rebound of metformin concentrations into plasma may be associated with an increase in lactate and worsening clinical condition. Termination of dialysis should only be confirmed once lactate and pH have been normalized; close monitoring of these parameters is required following treatment to evaluate the pertinence of repeat HD sessions.
Conclusion
Based on this case, we report that HD is effective at enhancing elimination of metformin and may quickly reverse life-threatening toxicity.
Disclosure
All the authors declared no competing interests.
References
- 1.Vecchio S., Giampreti A., Petrolini V.M. Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol (Phila) 2014;52:129–135. doi: 10.3109/15563650.2013.860985. [DOI] [PubMed] [Google Scholar]
- 2.Calello D.P., Liu K.D., Wiegand T.J. Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med. 2015;43:1716–1730. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 3.Lalau J.D., Race J.M. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20:377–384. doi: 10.2165/00002018-199920040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sirtori C.R., Franceschini G., Galli-Kienle M. Disposition of metformin (N,N-dimethylbiguanide) in man. Clin Pharmacol Ther. 1978;24:683–693. doi: 10.1002/cpt1978246683. [DOI] [PubMed] [Google Scholar]
- 5.Scheen A.J. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 6.Graham G.G., Punt J., Arora M. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Kajbaf F., Lalau J.D. Mortality rate in so-called “metformin-associated lactic acidosis”: a review of the data since the 1960s. Pharmacoepidemiol Drug Saf. 2014;23:1123–1127. doi: 10.1002/pds.3689. [DOI] [PubMed] [Google Scholar]
- 8.Peters N., Jay N., Barraud D. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12:R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biradar V., Moran J.L., Peake S.L., Peter J.V. Metformin-associated lactic acidosis (MALA): clinical profile and outcomes in patients admitted to the intensive care unit. Crit Care Resusc. 2010;12:191–195. [PubMed] [Google Scholar]
- 10.Nguyen H.L., Concepcion L. Metformin intoxication requiring dialysis. Hemodial Int. 2011;15(suppl 1):S68–S71. doi: 10.1111/j.1542-4758.2011.00605.x. [DOI] [PubMed] [Google Scholar]
- 11.Kajbaf F., Lalau J.D. The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol. 2013;14:22. doi: 10.1186/2050-6511-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calello D.P., Henretig F.M. Pediatric toxicology: specialized approach to the poisoned child. Emerg Med Clin North Am. 2014;32:29–52. doi: 10.1016/j.emc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Roberts D., Duong J., Ray J., Williams K., Furlong T. Therapeutic drug monitoring of metformin in a patient with end stage renal failure on haemodialysis. Nephrology. 2010;15:87–88. [Google Scholar]
- 14.Walsh S., Abesamis M., Cannon R. Severe metformin-associated lactic acidosis from acute ingestion without renal failure. Clin Toxicol. 2010;48:614. [Google Scholar]
- 15.Lalau J.D., Andrejak M., Moriniere P. Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination. Int J Clin Pharmacol Ther Toxicol. 1989;27:285–288. [PubMed] [Google Scholar]
- 16.Kruse J.A. Metformin-associated lactic acidosis. J Emerg Med. 2001;20:267–272. doi: 10.1016/s0736-4679(00)00320-6. [DOI] [PubMed] [Google Scholar]
- 17.Gudmundsdottir H., Aksnes H., Heldal K. Metformin and antihypertensive therapy with drugs blocking the renin angiotensin system, a cause of concern? Clin Nephrol. 2006;66:380–385. doi: 10.5414/cnp66380. [DOI] [PubMed] [Google Scholar]
- 18.Pearlman B.L., Fenves A.Z., Emmett M. Metformin-associated lactic acidosis. Am J Med. 1996;101:109–110. doi: 10.1016/s0002-9343(97)89422-3. [DOI] [PubMed] [Google Scholar]
- 19.Acquistapace G., Rossi M., Garbi M. Acute metformin intoxication: 2012 experience of Emergency Departement of Lodi. Italy. Clin Chem Lab Med. 2014;52:1489–1497. doi: 10.1515/cclm-2014-0208. [DOI] [PubMed] [Google Scholar]
- 20.Doorenbos C.J., Bosma R.J., Lamberts P.J.N. Use of urea containing dialysate to avoid disequilibrium syndrome, enabling intensive dialysis treatment of a diabetic patient with renal failure and severe metformin induced lactic acidosis. Nephrol Dial Transplant. 2001;16:1303–1304. doi: 10.1093/ndt/16.6.1303. [DOI] [PubMed] [Google Scholar]
- 21.Scalzo A.J., Andreone T.A., Wood E.G., Weber J.A. Metformin overdose in an adolescent with severe metabolic acidosis & hyperlacticacidemia treated with bicarbonate-buffer hemodialysis. Clin Toxicol (Phila) 2008;46:605. [Google Scholar]
- 22.Mustafa E., Lai L., Lien Y.H.H. Rapid recovery from acute kidney injury in a patient with metformin-associated lactic acidosis and hypothermia. Am J Med. 2012;125:e1–e2. doi: 10.1016/j.amjmed.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huberlant V., Laterre P.F., Hantson P. Nearly fatal metabolic acidosis: septic or toxic? Eur J Emerg Med. 2010;17:243–244. doi: 10.1097/MEJ.0b013e328331a580. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo A.M., Walroth T.A., Mowry J.B., Kao L.W. The MALAdy of metformin poisoning: Is CVVH the cure? Am J Ther. 2010;17:96–100. doi: 10.1097/MJT.0b013e318197eab6. [DOI] [PubMed] [Google Scholar]
- 25.Barrueto F., Meggs W.J., Barchman M.J. Clearance of metformin by hemofiltration in overdose. J Toxicol Clin Toxicol. 2002;40:177–180. doi: 10.1081/clt-120004407. [DOI] [PubMed] [Google Scholar]
- 26.Mujtaba M., Geara A.S., Madhrira M. Toxicokinetics of metformin-associated lactic acidosis with continuous renal replacement therapy. Eur J Drug Metab Pharmacokinet. 2012;37:249–253. doi: 10.1007/s13318-012-0104-y. [DOI] [PubMed] [Google Scholar]
- 27.Pikwer A., Vernersson E., Frid A., Sterner G. Extreme lactic acidosis type B associated with metformin treatment. NDT Plus. 2011;4:399–401. doi: 10.1093/ndtplus/sfr110. [DOI] [PMC free article] [PubMed] [Google Scholar]