Abstract
Background:
There are challenging reports in the public health sphere regarding associations between oral contraceptive (OC) use and cancer risk.
Methods:
To evaluate possible effects of OCs on cancer susceptibility, we quantified of global 5-methyl cytosine (5-mC) levels and assessed methylation patterns of CpG islands of two key tumor suppressor genes, APC1 and ESR1, in serum of users by enzyme-linked immunosorbent assay and methylation specific PCR methods, respectively.
Results:
Our results indicated that OCs significantly decrease the level of global DNA methylation in users relative to control non-users. However, our data revealed no significant differences between CpG island methylation patterns for ESR1 and APC1 in healthy control and OC-treated women. However, we did find a trend for hypermethylation of both tumor suppressor genes in OC users.
Conclusion:
Our data suggest that the level of 5-mC but not individual CpG island patterns is significantly influenced by OCs in our cross-section of adult users.
Keywords: Oral contraceptives, global methylation, CpG islands, APC1, ESR1
Introduction
Oral contraceptives (OCs) are exogenous synthetic sex hormones that used primarily to inhibit pregnancy through disrupt endogenous endocrine function. The association between OCs use and cancer risk is a key challenge in public health and has been controversial (Bethea et al., 2015; Burkman et al., 2004; Gierisch et al., 2013a; Marchbanks et al., 2012). Some studies indicated that the use of OCs is related to decreases in colorectal and endometrial cancers, whereas, other studies claimed that widespread use of OCs appears to be increased risk of certain cancers including breast, cervical and liver cancer (Beaber et al., 2014; Burkman et al., 2004; Cogliano et al., 2005; Gierisch et al., 2013a; Hunter et al., 2010; Rosenberg et al., 2009; Urban et al., 2012). The precise mechanism between OC use and cancer risk have not been cleared, but, it has been found that OCs contain estrogens and progestins which could cause progression of cancer through applicable mechanisms such as increase circulating levels of estradiol, estrogen and progesterone, and promote angiogenesis (Bethea et al., 2015; Gupta et al., 2007; Isaksson et al., 2001; Iversen et al., 2011; Merki-Feld et al., 2012). Other studies represent epigenetic properties of OCs and suggested another potential mechanism that oral contraceptives alter cancer susceptibility through epigenetic mechanisms such as alteration in DNA methylation (Bredfeldt et al., 2010; Campesi et al., 2012b; Starlard-Davenport et al., 2010; Tao and Freudenheim, 2010). DNA methylation is generated by a family of three active DNA methyltransferases (DNMTs) such as DNMT1, DNMT3A and DNMT3B (Sarabi and Naghibalhossaini, 2015). Many studies have indicated that DNMTs are under the regulation of estrogen and progesterone, therefore, DNA methylation may be influenced by oral contraceptives (Yamagata et al., 2009). Furthermore, other new data showed that exposure to synthetic estrogens induce epigenetic modification in the mammary gland and germ cells (Hilakivi-Clarke et al., 2013). These studies also reported that in utero estrogenic exposures modify the epigenome through alteration in DNA methylation (de Assis et al., 2012; Sato et al., 2006, 2009). Alteration in DNA methylation of tumor suppressor genes is a well-established epigenetic mechanism that plays an integral role in cancer etiology and drive tumorgenesis (Baylin, 2005; Belinsky et al., 1998; Esteller et al., 2001; Esteller et al., 2000b; Prosperi and Goss, 2010; Wu et al., 2012). It has been found that in comparison with normal cells, several classical tumor suppressor genes such as adenomatous polyposis coli (APC), and estrogen receptor α (ESR1) to be hyper methylated in cancer (Esteller et al., 2001; Jin et al., 2001; Lehmann et al., 2002; Tao and Freudenheim, 2010). For example, APC promoter hypermethylation has been linked to approximately 70% and 7% of inflammatory and metaplastic human breast carcinomas, respectively (Hayes et al., 2008; Van der Auwera et al., 2008). Furthermore, it has been observed that APC hypermethylation significantly correlate with cancer survival and its epigenetic silencing, contributes significantly to ER+ and HER2+ breast cancer progression (Prasad et al., 2008; Swift-Scanlan et al., 2011). ESR1 is a key molecular marker to prognosis and prediction of response to endocrine therapy in cancer patients (Martinez-Galan et al., 2014). It is proposed that OC may trigger DNA methylation of the ER genes resulting in impaired estrogen signaling and cancer (Strifert, 2015). Many studies reported a significant association of OC use with increased risk of estrogen receptor positive (ER+) and estrogen receptor negative (ER–) subtypes of breast cancer (Dolle et al., 2009; Ritte et al., 2013; Rosenberg et al., 2010; Rosenberg et al., 2009), while some studies did not observe significant associations of OCs use with either subtype and overall breast cancer risk (Bao et al., 2011; Cotterchio et al., 2003; Hunter et al., 2010; Ma et al., 2010). In breast cancer ER- cases and endometrial cancer tissues, hypermethylation of ESR1 is observed in up to 50% and 94% of cases, respectively, and this cases exhibit a develop resistance to endocrine treatment and will favor to metastasis and death (Johnston et al., 1992; Martinez-Galan et al., 2014; Platet et al., 2004; Sasaki et al., 2001). Therefore, DNA methylation of specific genes is a potential biomarker for many applications and DNA methylation patterns can be used as a surrogate marker for the detection of hidden carcinoma, cancer screening and can also precede tumor formation. In addition to specific gene locus DNA hypermethylation, global genomic DNA hypomethylation was associated with different types of cancer risk through different molecular mechanisms such as microsatellite instability, increased chromosome breakage, loss of imprinting and activation of oncogenes (Choi et al., 2009; Dumitrescu, 2012; Sarabi and Naghibalhossaini, 2015; Szyf et al., 2004; Wu et al., 2012). For this reason and in given the role of OCs on DNA methylation and following previous our studies on OC side effects (Torkzahrani et al., 2014; Zal et al., 2012) in the current study, we have investigated the influence of OCs on APC and ESR1 gene specific and global genomic DNA methylation in serum of OCs exposed mothers.
Materials and Methods
subjects
60 healthy adult women were enrolled and stratified in to two groups: thirty women who did not use oral contraceptives (control) and thirty women who were regular OC users as prevention of pregnancy. The average duration of OC use was 2 to 5 years. All the OC users were taking a contraceptive pill containing 0.03 mg ethinylestradiol and 0.15 mg levonorgestrel 21 days on and 7 days off. The study was approved and performed under the guidelines of the Ethics Committee of Shiraz University of Medical Sciences, and informed consent was and informed consent was obtained from each of the subjects before blood sampling. Baseline characteristics of the subjectsare listed in Table 1.
Table 1.
Baseline Characteristics of Healthy Participant Women
| Variable | Control | OC users |
|---|---|---|
| age | 31.21 (5.3) | 30.36 (6.1) |
| Body mass index (Kg/m2) | 21.6 (2.8) | 22.54(3.4) |
| Blood pressure | ||
| Systolic (mm/Hg) | 114 (12) | 118 (13) |
| Diastolic (mm/Hg) | 63 (6) | 62 (6) |
Values are means (SD)
Genomic DNA preparation
Genomic DNA was extracted from serum of control and OCs users by the standard method of proteinase K digestion, phenol-chloroform extraction and ethanol precipitation.
Methylation specific polymerase chain reaction (MS-PCR)
We determined the status of promoter methylation of APC and ESR1 tumor-related genes in serum by sodium bisulfite chemical treatment of genomic DNA followed by MS-PCR as described (Herman et al., 1996). Briefly, in this technique all un-methylated, but not methylated, cytosines convert to uracil by bisulfite modification. The modified DNA samples were amplified using MS-PCR with specific primers for either the methylated (M) or unmethylated (U) DNA. The primers and PCR conditions for MS-PCR analysis are listed in Table 2. Water without DNA template were included as a control for contamination for each PCR set. In all sodium bisulfite conversion reactions, the universal human methylated DNA standards from Zymo Research (ZYMO Research, Freiburg, Germany) was used as positive methylated controls and DNA from normal lymphocytes was used as negative control for methylated alleles of APC and ESR1. The PCR products were analyzed by electrophoresis on a 1·5% of agarose gel, stained with GelRed (Biotium, Belgium) and visualized under ultraviolet illumination.
Table 2.
Primers’sequence and the Annealing Temperature Used for Methylation-Specific PCR
| Gene | Primer sequence (5′ → 3′) | Annealing T (°C) | Product size (bp) |
|---|---|---|---|
| APC1 | UF: 5′-GTGTTTTATTGTGGAGTGTGGGTT-3′ | 63 | U: 112 M: 97 |
| UR: 5′-CCAATCAACAAACTCCCAACAA-3′ | |||
| MF: 5′-TATTGCGGAGTGCGGGTC-3′ | |||
| MR: 5′-TCGACGAACTCCCGACGA-3′ | |||
| ESR1 | UF: 5’-ATGAGTTGGAGTTTTTGAATTGTTT-3’ | 58 | U: 151 M: 158 |
| UR: 5’-ATAAACCTACACATTAACAACAACCA-3’ | |||
| MF: 5’-CGAGTTGGAGTTTTTGAATCGTTC-3’ | |||
| MR: 5’-CTACGCGTTAACGACGACCG-3 |
Analysis of global DNA methylation
We measured global methylation in DNA isolated from serum of healthy control and OCs users using 5-mC DNA enzyme-linked immunosorbent assay (ELISA) kit (Zymo Research, Germany), as per the manufacturer’s instructions. Briefly, 100ng of genomic DNA extracted from serum of OCs users and controls provided by the kit were denatured and used to coat the wells of the plate with 5-mC coating buffer. After incubation at 37 °C for 1h, the wells were washed with 5-mC ELISA buffer and then an antibody mix consisting of anti-5-mC and the horseradish peroxidase (HRP)-conjugated secondary antibody was added to each well. The plate was incubated at 37°C for 1h in the dark. After washing the antibody mix from the wells with the 5-mC ELISA buffer, an HRP developer was added to each well and incubated at room temperature for 1h. The amount of methylated DNA was proportional to the optical density (OD) intensity measured in an ELISA plate reader at 450 nm. The standard curve was constructed with negative and positive controls supplied with the kit, and the 5-mC percentage was calculated using the following equation: 5-mC%= [(OD sample - OD negative control/100ng) / (OD positive control - OD negative control) ×2/5ng]×100; 2 is a factor for normalization of 5-mC in the positive control to 100%, 100ng is the amount of input genomic DNA, and 5ng is the amount of positive control DNA, which contains 50% 5-mC. Three replicates of each sample were measured in duplicate.
Statistical analysis
SPSS 18 analytic software (SPSS, Inc., Chicago) and GraphPad Prism (Version 6·01) were used for data analysis. Depending upon the sample size, associations between clinical, biological and genotypic features were evaluated using either the Chi square test or the Fisher’s exact test. The correlation between OCs user and global DNA methylation was analyzed by Pearson correlation test. Differences with p value ≤0·05 were set as the level of significance.
Results
Analysis of global DNA methylation
In order to measure the levels of global methylation, we quantified the amount of 5-mC in genomic DNA from serum of healthy control and oral contraceptive treated women using ELISA method. Our data showed that the levels of methylated cytosine in healthy control women DNA were significantly higher (1.007) as compared with oral contraceptive treated women (0.979) (p<0·01) (Figure 1). On the other hand, our results indicated that oral contraceptive users had a significantly lower level of genomic global DNA methylation.
Figure 1.
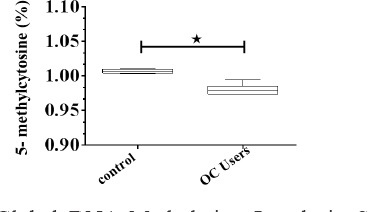
Global DNA Methylation Levels in Serum of Healthy and Oral Contraceptive Treated Women. Percentage of 5-mC was evaluated using ELISA assay. Mean values ±SE of three experiments are given. The asterisk represents significantly different between samples as verified by Mann Whitney t test honestly significant difference comparison test (p<0·05)
CpG islands hypermethylation status of APC1 and ESR1
In addition to the measurement of the global DNA methylation status, CpG island promoter hypermethylation of APC1 and ESR1 genes was analyzed by MS-PCR as described in the section on Materials and Methods. Illustrative examples are shown in Figure 2. Our results showed that 3 of 30 (10.0%) of healthy adult women (control) had APC1 hypermethylation while 4 of 30 (13.33%) of women treated with oral contraceptive had APC1 promoter hypermethylation. Also, 9 of 30 (30.0%) of the healthy adult women (control) had ESR1 hypermethylation and 11 of 30 (36.7%) of oral contraceptive treated women had methylation of ESR1. Although there were no significant differences (p>0.05) between methylation pattern in the healthy control and oral contraceptive treated women for both genes, but ESR1 and APC1 were found to be more methylated (36.7% and 13.33%, respectively) in oral contraceptive treated women compared to control women (30.0% and 10.0% unmethylated, respectively).
Figure 2.

Representative Examples of MSP Products for Ppromoter Methylation Analysis of APC1 and ESR1 Genes in Serum of Healthy Adult and Oral Contraceptive Treated Womens. Universal methylated DNA and un-methylated lymphocytes DNA were used as positive and negative controls, respectively. U, un-methylated genes; M, methylated genes; M, 50 bp DNA size marker
Discussion
Among different contraceptive options, oral contraceptives (OCs) are the most commonly used reversible method of con¬traception in the USA due to effectiveness, convenience and tolerability (Daniels et al., 2013). The relationship between OCs and cancer is a key challenge in public health due to the high prevalence of use of OCs and the serious consequences of cancer as a leading cause of death worldwide. Aberrant methylation, including gene-specific DNA hypermethylation and global genomic hypomethylation can lead to genomic instability, alter gene transcription, and increase mutation rates (Chen et al., 1998) which may impact normal cell growth and increase the likelihood of tumorigenesis (Terry et al., 2011). However, these epigenetic alterations are accepted as playing a fundamental role in cancer, we are still in the early stages of understanding the timing of epigenetic alterations. While an increasing number of studies have reported the presence of whole genome or gene-specific aberrant methylated DNA in matched samples from tumor tissue and plasma in patients with different types of cancer but not in normal control tissues (Goessl et al., 2000; Sanchez-Cespedes et al., 2000; Silva et al., 1999; Silva et al., 2002), Palmisano et al have found that in lung cancer patients aberrant DNA methylation is detectable as early as 3 years prior to diagnosis in the sputum of subjects exposed to carcinogens (uranium miners and smokers) (Palmisano et al., 2000). Furthermore, in a study of hepatocellular carcinoma, Santella et al. detected changes in serum methylation patterns of RASSF1A, p16, and p15 as much as 9 years prior to diagnosis (Zhang et al., 2007). Therefore, detection of aberrant methylation in serum/plasma DNA of cancerous patients could be a marker of disease (an early neoplastic effect) and in healthy subjects may reflect chronic exposure to carcinogenic factors (Hoque et al., 2006) and these results can support the use of methylation status as a screening biomarker for detection of high risk cases or a diagnostic biomarker for early tumor detection. Among the studies on the global methylation status, Fraga et al demonstrated that in the mouse skin cancer progression model the earliest decrease in global methylation status occurred during the early stages of benign tumor growth and normal mouse skin showed the highest level of methylation (Fraga et al., 2004). In contrast to studies that reported the absence of this epigenetic alteration in normal control samples, our results showed that global DNA methylation was significantly decreased under the effect of OCs even in normal adult users. In close agreement with our results Campesi et al. observed that genome-wide methylation was significantly lower in women treated with OCs for at least 3 months (Campesi et al., 2012a). A possible explanation for genome-wide methylation alterations seen in normal cases is that there may be a threshold for methylation to affect gene expression and lead to a growth advantage. Tumor-suppressor genes are known to be frequently methylated in different malignancies and among these are two important ones APC and ESR1 (Esteller et al., 2000a; Issa et al., 1996; Li et al., 2000; Müller et al., 2003; Xu et al., 2011). Our results showed that there were no significant methylation changes in APC and ESR1 genes between the normal adult OCs users and the control non users. Although we found no significant differences between methylation pattern in the healthy control and oral contraceptive users for both genes, but there were a trend for promoter methylation of ESR1 and APC1 tumor suppressor genes in oral contraceptive users relative to control non users. Since our cases were normal women, our results are in line with previous studies, but several studies have reported the presence of significant methylation in several genes in a small proportion of control subjects (Hoque et al., 2006) or in nonmalignant tissues and serum DNA of smokers (Palmisano et al., 2000). One reason for these conflicting results may be due to the differences between the sensitivity of detection techniques because detection of methylation in circulating DNA depends on the ability of the assay to detect methylated DNA in a background of wild type DNA. Another possible reason for this contradictory may be related to difficulties in assessing the risk of cancer associated with the use of OCs including the long latency period of cancer disease, variations in OCs formulations available on the market, as well as duration and patterns of OCs use over a woman’s lifetime. Furthermore, the use of OCs may be influenced by gravidity, parity, breastfeeding and these factors also affect cancer risks (Gierisch et al., 2013b; Moorman et al., 2008). To our best knowledge; this is the first experimental study to examine the association between OCs exposure and methylation status of serum DNA and the most remarkable result to emerge from our data is that OCs usage can affect genome-wide status of methylation. Therefore, based on the fundamental role of epigenetic alterations in cancer, this result may point to the possible involvement of OCs exposure in tumorigenesis. More extensive research with more sensitive DNA methylation detection techniques will require exploring the possible gene-specific methylation changes under the effect of oral contraceptives.
Conflict of interest
The authors have declared that there is no conflict of interest.
Acknowledgements
This work was supported by Grant Number 92-6278 from Vice-chancellor for Research Affairs of Shiraz University of Medical Sciences.
References
- Bao PP, Shu XO, Gao YT, et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol. 2011;174:661–71. doi: 10.1093/aje/kwr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2:4–11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- Beaber EF, Malone KE, Tang MT, et al. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol Biomarkers Prev. 2014;23:755–64. doi: 10.1158/1055-9965.EPI-13-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, Nikula KJ, Palmisano WA, et al. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci U S A. 1998;95:11891–6. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea TN, Rosenberg L, Hong CC, et al. A case-control analysis of oral contraceptive use and breast cancer subtypes in the African American breast cancer epidemiology and risk consortium. Breast Cancer Res. 2015;17:22. doi: 10.1186/s13058-015-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, et al. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190:5–22. doi: 10.1016/j.ajog.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Campesi I, Sanna M, Zinellu A, et al. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012a;3:1. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesi I, Sanna M, Zinellu A, et al. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012b;3:4. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- Choi JY, James SR, Link PA, et al. Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 2009;30:1889–97. doi: 10.1093/carcin/bgp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CoglianoV Grosse Y, Baan R, et al. Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment. Lancet Oncol. 2005;6:552–3. doi: 10.1016/s1470-2045(05)70273-4. [DOI] [PubMed] [Google Scholar]
- Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S. Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev. 2003;12:1053–60. [PubMed] [Google Scholar]
- Daniels K, Mosher WD, Jones J. Contraceptive methods women have ever used:United States 1982–2010. Natl Health Stat Report. 2013;62:2013. [PubMed] [Google Scholar]
- de Assis S, Warri A, Cruz MI, et al. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle JM, Daling JR, White E. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu RG. DNA methylation and histone modifications in breast cancer. Methods Mol Biol. 2012;863:35–45. doi: 10.1007/978-1-61779-612-8_3. [DOI] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–9. [PubMed] [Google Scholar]
- Esteller M, Sparks A, Toyota M, et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000a;60:4366–71. [PubMed] [Google Scholar]
- Esteller M, Sparks A, Toyota M, et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000b;60:4366–71. [PubMed] [Google Scholar]
- Fraga MF, Herranz M, Espada J, et al. A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer Res. 2004;64:5527–34. doi: 10.1158/0008-5472.CAN-03-4061. [DOI] [PubMed] [Google Scholar]
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers:a systematic review. Cancer Epidemiol Biomarkers Prev. 2013a;22:1931–43. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers:A systematic review. Cancer Epidemiol Biomarkers Prev. 2013b;22:1931. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- Goessl C, Krause H, Müller M, et al. Fluorescent Methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 2000;60:5941. [PubMed] [Google Scholar]
- Gupta PB, Proia D, Cingoz O, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–71. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Thomas D, Emmons A, Giordano TJ, Kleer CG. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin Cancer Res. 2008;14:4038–44. doi: 10.1158/1078-0432.CCR-07-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR:a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S, Warri A. Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J Mammary Gland Biol Neoplasia. 2013;18:25–42. doi: 10.1007/s10911-013-9274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque MO, Feng Q, Toure P, et al. Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. Clin Oncol. 2006;24:4262–9. doi: 10.1200/JCO.2005.01.3516. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer:a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19:2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson E, von Schoultz E, Odlind V, et al. Effects of oral contraceptives on breast epithelial proliferation. Breast Cancer Res Treat. 2001;65:163–9. doi: 10.1023/a:1006482418082. [DOI] [PubMed] [Google Scholar]
- Issa J-PJ, Baylin SB, Belinsky SA. Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res. 1996;56:3655–8. [PubMed] [Google Scholar]
- Iversen A, Thune I, McTiernan A, et al. Ovarian hormones and reproductive risk factors for breast cancer in premenopausal women:the Norwegian EBBA-I study. Hum Reprod. 2011;26:1519–29. doi: 10.1093/humrep/der081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Tamura G, Tsuchiya T, et al. Adenomatous polyposis coli (APC) gene promoter hypermethylation in primary breast cancers. Br J Cancer. 2001;85:69–73. doi: 10.1054/bjoc.2001.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SR, Dowsett M, Smith IE. Towards a molecular basis for tamoxifen resistance in breast cancer. Ann Oncol. 1992;3:503–11. doi: 10.1093/oxfordjournals.annonc.a058251. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Langer F, Feist H, et al. Quantitative assessment of promoter hypermethylation during breast cancer development. Am J Pathol. 2002;160:605–12. doi: 10.1016/S0002-9440(10)64880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.-C, Chui R, Nakajima K, et al. Frequent methylation of estrogen receptor in prostate cancer:correlation with tumor progression. Cancer Res. 2000;60:702–6. [PubMed] [Google Scholar]
- Ma H, Wang Y, Sullivan-Halley J, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women's contraceptive and reproductive experiences study. Cancer Res. 2010;70:575–87. doi: 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342–50. doi: 10.1016/j.contraception.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Galan J, Torres-Torres B, Nunez MI, et al. ESR1 gene promoter region methylation in free circulating DNA and its correlation with estrogen receptor protein expression in tumor tissue in breast cancer patients. BMC Cancer. 2014;14:59. doi: 10.1186/1471-2407-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki-Feld GS, Seeger H, Mueck AO. Proliferative effects of estradiol- or ethinylestradiol-progestogen combinations on human breast cancer cells in an intermitted and a long-term regimen. Horm Metab Res. 2012;44:415–21. doi: 10.1055/s-0032-1308999. [DOI] [PubMed] [Google Scholar]
- Moorman PG, Calingaert B, Palmieri RT, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167:1059–69. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HM, Widschwendter A, Fiegl H, et al. DNA methylation in serum of breast cancer patients an independent prognostic marker. Cancer research. 2003;63:7641–7645. [PubMed] [Google Scholar]
- Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–8. [PubMed] [Google Scholar]
- Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression:a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Prasad CP, Mirza S, Sharma G, et al. Epigenetic alterations of CDH1 and APC genes:relationship with activation of Wnt/beta-catenin pathway in invasive ductal carcinoma of breast. Life Sci. 2008;83:318–25. doi: 10.1016/j.lfs.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Prosperi JR, Goss KH. A Wnt-ow of opportunity:targeting the Wnt/beta-catenin pathway in breast cancer. Curr Drug Targets. 2010;11:1074–88. doi: 10.2174/138945010792006780. [DOI] [PubMed] [Google Scholar]
- Ritte R, Tikk K, Lukanova A, et al. Reproductive factors and risk of hormone receptor positive and negative breast cancer:a cohort study. BMC Cancer. 2013;13:584. doi: 10.1186/1471-2407-13-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Boggs DA, Wise LA, Adams-Campbell LL, Palmer JR. Oral contraceptive use and estrogen/progesterone receptor-negative breast cancer among African American women. Cancer Epidemiol Biomarkers Prev. 2010;19:2073–9. doi: 10.1158/1055-9965.EPI-10-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Zhang Y, Coogan PF, Strom BL, Palmer JR. A case-control study of oral contraceptive use and incident breast cancer. Am J Epidemiol. 2009;169:473–9. doi: 10.1093/aje/kwn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Esteller M, Wu L, et al. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 2000;60:892. [PubMed] [Google Scholar]
- Sarabi MM, Naghibalhossaini F. Association of DNA methyltransferases expression with global and gene-specific DNA methylation in colorectal cancer cells. Cell Biochem Funct. 2015;33:427–33. doi: 10.1002/cbf.3126. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kotcherguina L, Dharia A, Fujimoto S, Dahiya R. Cytosine-phosphoguanine methylation of estrogen receptors in endometrial cancer. Cancer Res. 2001;61:3262–6. [PubMed] [Google Scholar]
- Sato K, Fukata H, Kogo Y, et al. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocr J. 2006;53:331–7. doi: 10.1507/endocrj.k06-009. [DOI] [PubMed] [Google Scholar]
- Sato K, Fukata H, Kogo Y, et al. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J. 2009;56:131–9. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- Silva JM, Dominguez G, Garcia JM, et al. Presence of tumor DNA in plasma of breast cancer patients. Cancer Res. 1999;59:3251. [PubMed] [Google Scholar]
- Silva JM, Garcia JM, Dominguez G, et al. Persistence of tumor DNA in plasma of breast cancer patients after mastectomy. Ann Surg Oncol. 2002;9:71–76. doi: 10.1245/aso.2002.9.1.71. [DOI] [PubMed] [Google Scholar]
- Starlard-Davenpor A, Tryndyak VP, James SR, et al. Mechanisms of epigenetic silencing of the Rassf1a gene during estrogen-induced breast carcinogenesis in ACI rats. Carcinogenesis. 2010;31:376–81. doi: 10.1093/carcin/bgp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strifert K. An epigenetic basis for autism spectrum disorder risk and oral contraceptive use. Med Hypotheses. 2015;85:1006–11. doi: 10.1016/j.mehy.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Swift-Scanlan T, Vang R, Blackford A, Fackler MJ, Sukumar S. Methylated genes in breast cancer:associations with clinical and histopathological features in a familial breast cancer cohort. Cancer Biol Ther. 2011;11:853–65. doi: 10.4161/cbt.11.10.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Pakneshan P, Rabbani SA. DNA methylation and breast cancer. Biochem Pharmacol. 2004;68:1187–97. doi: 10.1016/j.bcp.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Tao MH, Freudenheim JL. DNA methylation in endometrial cancer. Epigenetics. 2010;5:491–8. doi: 10.4161/epi.5.6.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells:association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–37. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkzahrani S, Heidari A, Mostafavi-pour Z, Ahmadi M, Zal F. Amelioration of lipid abnormalities by vitamin therapy in women using oral contraceptives. Clin Exp Reprod Med. 2014;41:15–20. doi: 10.5653/cerm.2014.41.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M, Banks E, Egger S, et al. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women:case-control study. PLoS Med. 2012;9:1001182. doi: 10.1371/journal.pmed.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Van Laere SJ, Van den Bosch SM, et al. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter is associated with the inflammatory breast cancer phenotype. Br J Cancer. 2008;99:1735–42. doi: 10.1038/sj.bjc.6604705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Delgado-Cruzata L, Flom JD, et al. Repetitive element DNA methylation levels in white blood cell DNA from sisters discordant for breast cancer from the New York site of the breast cancer family registry. Carcinogenesis. 2012;33:1946–52. doi: 10.1093/carcin/bgs201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gammon MD, Jefferson E, et al. The influence of one-carbon metabolism on gene promoter methylation in a population-based breast cancer study. Epigenetics. 2011;6:1276–83. doi: 10.4161/epi.6.11.17744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Asada H, Tamura I, et al. DNA methyltransferase expression in the human endometrium:down-regulation by progesterone and estrogen. Hum Reprod. 2009;24:1126–32. doi: 10.1093/humrep/dep015. [DOI] [PubMed] [Google Scholar]
- Zal F, Mostafavi-Pour Z, Amini F, Heidari A. Effect of vitamin E and C supplements on lipid peroxidation and GSH-dependent antioxidant enzyme status in the blood of women consuming oral contraceptives. Contraceptio. 2012;86:62–6. doi: 10.1016/j.contraception.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y-J, Wu H-C, Shen J, et al. Predicting hepatocellular carcinoma by detection of aberrant promoter methylation in serum DNA. Clin Cancer Res. 2007;13:2378–4. doi: 10.1158/1078-0432.CCR-06-1900. [DOI] [PubMed] [Google Scholar]


