Abstract
Introduction:
The flavonoids comprise a diverse group of polyphenolic compounds with antioxidant activity that is present in edible plants like soybeans and soy products. In vivo studies have concentrated on the effects of flavonoids on cancer and genistein (GE), a soy-derived isoflavone, has been reported to reduce prostate, colon, hepatic and breast adenocarcinoma risk. Tamoxifen (TAM) is an important drug for cancer treatment worldwide, which can induce apoptosis in various cancers, including examples in the liver, breast and ovaries. The aim of the present study was to evaluate the effects of GE and TAM, alone and in combination, on proliferation and apoptosis in the human hepatocellular carcinoma (HCC) HepG2 cell line.
Materials and Methods:
HepG 2 cells were treated with GE, TAM and GE/TAM and then MTT and flow cytometry assays were conducted to determine effects on viability and apoptosis, respectively.
Results:
GE and TAM inhibited cell proliferation and induced apoptosis in the HepG 2 cell lines.
Discussion:
Our findings clearly indicated that GE and TAM may exert inhibitory and apoptotic effects in liver cancer cells.
Conclusion:
GE and TAM can significantly inhibit growth of HCC cells and play a significant role in apoptosis.
Keywords: Genistein, Tamoxifen, proliferation, apoptosis, hepatocellular carcinoma
Introduction
The flavonoids, natural antioxidants, comprise a diverse group of polyphenolic compounds with antioxidant activity, including more than 4,000 phenylbenzopyrones that present in many edible plants (Nagendran et al., 2006; Harborne et al., 2000). This group contains distinct classes such as flavans and proanthocyanidins, flavonols, anthocyanidins, flavones, flavanones, neoflavonoids and isoflavones. These natural antioxidant compounds present in the legumes, cereals, fruits, nuts, seeds, vegetables, spices, herbs, stems and flowers, tea and cocoa (Harborne et al., 2000; Tim, 2005; Kutaiba et al., 2012; Crozier et al., 2009; Wang et al., 2009; Pier-Giorgio, 2000). In vivo studies have concentrated on the effects of flavonoids on cancer and have reported a variety of actions, including cell cycle arrest, carcinogen inactivation, induction of apoptosis and differentiation, antiproliferation and inhibition of angiogenesis (Chithan et al., 2005). Similar in vivo works have demonstrated that flavonoids inhibit tumor cell growth and induce cell differentiation (Middleton et al., 2005; Haitao et al., 2008; Robert et al., 2001; Monasterio et al., 2004; Hyon et al., 2005; Koen et al., 2005; Wen-Xin et al., 2005). Epidemiological studies have demonstrated that high dietary intake of flavonoids can decrease colon cancer prevalence in humans (Evropi et al., 2007; Uwe et al., 2000). Experimental investigations have showed that flavonoids induce cell cycle arrest in colon cancer HT-29 and Caco-2 cell lines (Sanaz et al., 2016). Genistein (GE), a soy-derived isoflavone, has been reported to reduce prostate (Wang et al., 2002), colon cancer (Hakkak et al., 2001; Raju et al., 2009), prostate adenocarcinoma (Joanne et al., 2009) and MDA-MB-231 breast cancer cells (Lijie et al., 2003). Previously, we reported that GE can induce apoptosis and inhibit proliferation in hepatocellular carcinoma PLC/PRF5 (Dastjerdi et al., 2015) and HepG 2 Cell lines (Sanaei et al., 2016). Tamoxifen (TAM) is an important drug for cancer treatment in the world. Chemoprevention trials have indicated that TAM can reduce the incidence of breast cancer (Fabian et al., 2001). Tamoxifen can induce apoptosis in human hepatocellular carcinoma (HepG2) cell line (Sebastian et al., 2004; Lee et al, 2000). Several clinical trials have reported that TAM is an active anticancer drug in ovarian cancer (Seiji et al., 2004; Hakan et al., 2007; Lee et al., 2012). Previously, we indicated that TAM can inhibit proliferation and induce apoptosis in HepG 2 cell line (Sanaei et al., 2016). It has been reported that TAM and GE combination synergistically inhibit proliferation and induce apoptosis in HepG 2 cell line breast carcinoma MDA-MB-435 cell line (Fei et al., 1999) and BT-474 human breast cancer cells (Zhiming et al., 2007). To establish that GE in combination to TAM can induce apoptosis and inhibit proliferation in human HepG 2 cell line, we investigated whether apoptosis and proliferation are altered by these compounds.
Materials and Methods
Materials
The human HCC cell line, HepG 2, was obtained from the National Cell Bank of Iran-Pasteur Institute. The cells were maintained in DMEM (Dulbecco minimal essential medium) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) and cultured and incubated in 5% CO2, 95% humidified air at 37°C as reported. DMEM, GE, TAM and MTT (3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide) were purchased from Sigma (Sigma, St. Louis, MO). All other chemicals were obtained from the best available sources.
In vitro assays of HepG 2 cell growth and viability
To determine the effect of GE and TAM, the cells were seeded in triplicate in 24-well plates at the density of 1×105 cells with 1mL of medium/well and were treated with various concentrations of GE (1, 5,10, 20 and 40 μM/lit) and TAM (1, 5, 10, 20 and 40 μM/lit), which were dissolved in dimethyl sulfoxide (DMSO); DMSO was present at 0.01–0.3%. After 24, 48 and 72 h of treatment, cell viability was assayed using methyl thiazol tetrazolium (MTT) method based on the conversion of tetrazolium dye (MTT) to a blue formazan product. The absorbance of the cell lysates in DMSO solution was read at 570 nm by a microplate reader (Bio-Rad Hercules, CA). Each assay was performed in triplicate.
Cell cycle analysis
To determine the effect of GE and TAM, the cells were seeded in triplicate in 24-well plates at the density of 5×105/well. After 24 h of seeding, cells were treated with GE (20 µM) and TAM (5 µM) alone and combined except control groups received DMSO, DMSO was present at 0.01–0.3%. After 24, 48 and 72 h of drugs exposure, cells were harvested by trypsinization and then centrifuged, washed with cold phosphate-buffered saline (PBS) and resuspended in Binding buffer (1x). Finally, AnnexinV-FITC and propidium iodide (PI, Becton-Dickinson, San Diego, CA) were added and analysis was carried out according to the manufacturer’s protocol (BMS500F1/100CE AnnexinV-FITC, eBiscience, USA). All experiments were processed independently in triplicate.
Results
In vitro effects of GE and TAM on HepG 2 cell growth
Antiproliferative effects of GE and TAM (combined and alone) were evaluated by MTT assay, which indicated that these compounds can inhibit proliferation of HepG 2 cell than cells in control groups significantly. The percentage of cell proliferation of GE (1, 5, 10, 20 and 40 µM) and TAM (1, 5, 10, 20 and 40 µM) were indicated in Figure 1 and 2. IC50s value for GE and TAM were observed with concentration of 20 µM after 24 h and 5 µM of TAM after 48 h respectively. The percentage of cell proliferation of GE (20µM) were 54, 40 and 36 % and of TAM (5µM) were 73, 54 and 42 % after various time periods (24, 48 and 72 h) respectively (P < 0.001). As shown in the Figure 3, the combined GE (20 µM) with TAM (5 µM) decreased cell proliferation more significant than each compound alone, which decreased cell proliferation to 36, 30 and 28 after different time periods (24, 48 and 72 h) respectively (P < 0.001).
Figure 1.
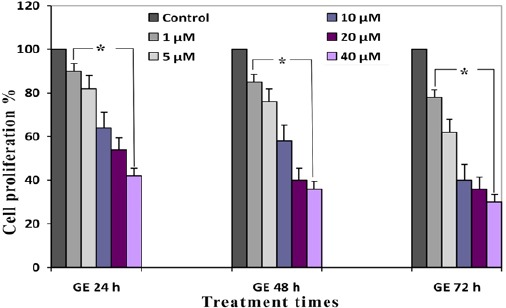
In Vitro Effects of GE on HepG 2 Cell Proliferation Tested by MTT Assay. Values are means of three experiments in triplicate. Standard errors were less than 5 %. Asterisks (*) indicate significant differences between GE treated and the control groups. *P < 0.001 as compared to the control group.
Figure 2.
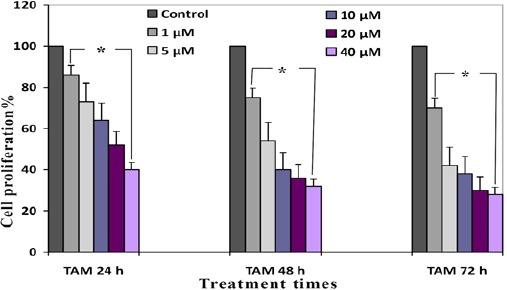
Antiproliferative Effect of TAM on HepG 2 Cells Tested by MTT Assay. Data are means of three experiments in triplicate. Standard errors were less than 5 %. Asterisks (*) indicate significant differences between TAM treated and the control groups. *P < 0.001 as compared to the control group.
Figure 3.
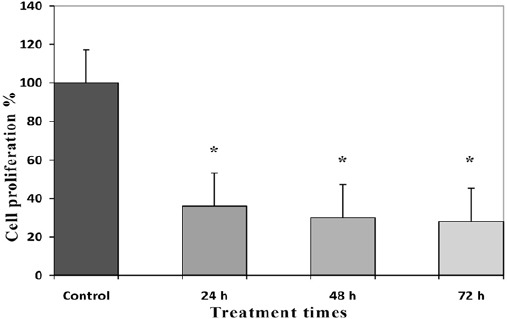
Antiproliferative Effects of Combined GE/TAM on HepG 2 Cells. Data are means of three experiments in triplicate. Standard errors were less than 5 %. Asterisks (*) indicate significant differences between combined treated and the control groups. *P < 0.001 as compared to the control group.
In vitro effects of GE and TAM on cell cycle progression
The results of flow cytometry assay indicated that GE with concentration of 20 µM and TAM with concentration of 5 µM (alone and combined) induced apoptosis in HepG 2 cell than cell in the control groups significantly. The percentage of apoptotic cells in GE-treated groups were 22, 34 and 48 % and of that TAM were 24, 26 and 30 % at different time periods (24, 48 and 72 h) respectively (P < 0.001). The most apoptotic cells were obtained after 72 h in GE, TAM and combined treated (GE/TAM) groups. It should be noted that GE/TAM synergistically induced apoptosis (Figure 4). The percentage of apoptotic cells in combined-treated groups were more than GE and TAM treated groups (Figure 5).
Figure 4.
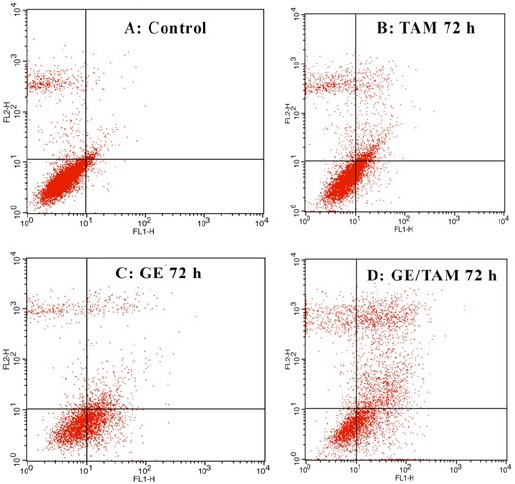
GE, TAM and GE/TAM-Induced Apoptosis in HepG 2 Cells After 24, 48 and 72 h of Treatment Tested by Flow Cytometric Analysis.
Figure 5.
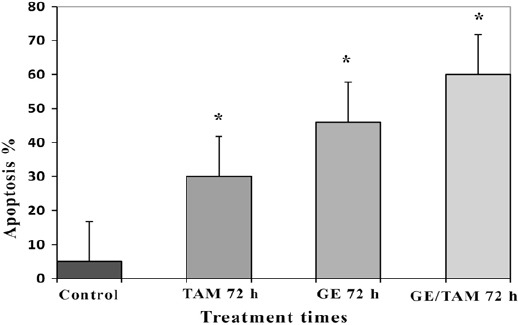
Comprative Analysis between Different Groups Treated with GE, TAM and GE/TAM after 72 h. Bars, mean ± SD, *P<0.01, Significantly Different from Control.
Discussion
GE is a phytoestrogen that presents in many edible plant soybeans and soy products. Epidemological studies support strung evidences between dietary soy consumption and the risk of breast (Jia-Yi et al., 2011; Seiichiro et al., 2003) prostate cancer (Marion et al., 2003) and endometrial cancer (Wang et al., 2004). Besides, the consumption of dietary GE inhibits tumor progression and decreases the incidence of mammary cancer in rat (Xiao et al., 2001). Our previous study indicated that GE can induce apoptosis in human hepatocellular carcinoma PLC/PRF5 cell line (Kavoosi et al., 2016). TAM is widely used in the cancers treatment such as breast cancer (Anthony et al., 2005; Bernard et al., 2005., Radmacher et al., 2000). Clinical and experimental studies have reported that TAM can reduce the risk of breast cancer (Crew et al., 2017; Olver., 2016) and induce cell-cycle arrest and apoptosis of breast cancer cells (Yan et al., 2008). Recently, we reported that TAM can inhibit the growth of HepG2 cells and induced apoptosis significantly with a time- and dose-dependent manner (Sanaei et al., 2016).
In the present report, we announce that GE, TAM and combined GE/TAM inhibit proliferation and induce apoptosis in HCC HepG 2 cell line. Our results are in agreement with those from other researches, showing that GE induces apoptosis and inhibits viability in Bel 7402 HCC cells (Yan et al., 2005) and also plays an important role in the prevention and inhibition of cancers such as prostate cancer, colon cancer, breast cancer, leukemia, melanoma, etc (Herman e al, 2002). Effect of GE and TAM (alone and combined) on human breast carcinoma MDA-MB-435 cells has been reported by other researchers (33) which is in line with our result. GE can induce apoptosis in HCC by different mechanism such as activation of several ER stress-relevant regulators, including caspase-12, m-calpain, GRP78 and GADD153 (Ting-Chun et al., 2007). Besides, activation of caspase-3 in GE treated HCC by which induces apoptosis and inhibits cell proliferation has been reported (Mumtaz et al., 2007). It has been reported that GE inhibits the activation of NF-κB and Akt signaling pathways in the breast and prostate cancers, both of which play a significant role to maintain a balance between cell viabilty and apoptosis (Sanjeev et al., 2008). Previously, we indicated that GE can act by esterogen receptor alpha in HCC PLC/PRF5 (Kavoosi et al., 2016). Primary mechanism of GE is through estrogen receptor inhibition and also non-ER-mediated pathway including modulation of signaling proteins such as calmodulin, protein kinase C (PKC), protooncogene c-myc and transforming growth factor-β (TGFβ). Furthermore, the role of c-Jun N-terminal kinase (JNK) and p38 in TAM-induced apoptotic pathway is significant (Mandlekar et al., 2001). We did not evaluate the mechanism of GE and TAM in this study. It should be evaluated the mechanism of these compounds in HepG 2 cell line and other HCC cell lines.
In conclusion, our research may provide a novel approach for the prevention and treatment of hepatocellular carcinoma and further in vivo studies to verify the efficacy of GE and TAM combination on the growth of other hepatic cell lines and also clinical evaluation of these compounds.
Acknowledgements
This article was supported by adjutancy of research of Jahrom medical University-Iran. This article has been extracted from Ms Soheila Haghighat’s thesis.
References
- Abukhdeir AM, Vitolo MI, Argani P, et al. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci U S A. 2008;105:288–93. doi: 10.1073/pnas.0710887105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JS. Tamoxifen treatment for breast cancer and risk of endometrial cancer:A case–control study. J Natl Cancer Inst. 2005;96:375–84. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- Bernard Fisher JP, Costantino D, Lawrence Wickerham RS, et al. Tamoxifen for the prevention of breast cancer:Current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Chithan K, Lee LT, et al. The ant activities of Fla. In Vivo. 2005;19:895–910. [Google Scholar]
- Crew KD, Albain KS, Hershman DL, et al. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention? J Breast Cancer. 2017;3:20. doi: 10.1038/s41523-017-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A, Burns J, Aziz AA, et al. Antioxidant flavonols from fruits, vegetables and beverages. Measurements and bioavailabilty. Biol Res. 2009;33:79–88. doi: 10.4067/s0716-97602000000200007. [DOI] [PubMed] [Google Scholar]
- Dastjerdi NM, Kavoosi F, Valiani A, et al. Inhibitory Effect of Genistein on PLC/PRF5 Hepatocellular Carcinoma Cell Line. Int J Prev Med. 2015;176:242–51. doi: 10.4103/2008-7802.158914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evropi T, Janet K, Roseanne C, et al. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684–93. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF. Beyond tamoxifen new endpoints for breast cancer chemoprevention, new drugs for breast cancer prevention. Ann NY Acad Sci. 2001;952:44–59. doi: 10.1111/j.1749-6632.2001.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Fei S, Xinjian X, George W. Tamoxifen and genistein synergistically down-regulate signal transduction and proliferation in estrogen receptor-negative human breast carcinoma MDA-MB-435 Cells. Anticancer Res. 1999;19:1657–62. [PubMed] [Google Scholar]
- Haitao L, Bing-Hua J, Sarah M. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–9. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- Hakan K, Pinar S, Kazim U, et al. The efficacy of tamoxifen in patients with advanced epithelial ovarian cancer. Med Oncol. 2007;24:39–43. doi: 10.1007/BF02685901. [DOI] [PubMed] [Google Scholar]
- Hakkak R, Korourian S, Ronis MJ, et al. Soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats. Cancer Lett. 2001;166:27–32. doi: 10.1016/s0304-3835(01)00441-4. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Herman A. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–73. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- Hyon S, Sung H, Young O, et al. Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta Discipline. 2005;1726:309–16. doi: 10.1016/j.bbagen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jia-Yi D, Li-Qiang Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence:a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315–23. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- Joanne ND, Brahmchetna S, Mahbubur B, et al. Genistein-induced upregulation of p21 WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 2009;32:123–31. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- Kavoosi F, Dastjerdi NM, Valiani A, et al. Genistein potentiates the effect of 17-beta estradiol on human hepatocellular carcinoma cell line. Adv Biomed Res. 2016;5:241–7. doi: 10.4103/2277-9175.187395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen B, Ruth V, Guido V, et al. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–45. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- Kutaiba IA, Mohamed AM. Flavonoids:chemistry, biochemistry and antioxidant activity. J Pharm Res. 2012;5:4013–20. [Google Scholar]
- Lee JY, Shin JY, Kim HS, et al. Effect of combined treatment with progesterone and tamoxifen on the growth and apoptosis of human ovarian cancer cells. Oncol Rep. 2012;27:87–93. doi: 10.3892/or.2011.1460. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kang YS, Lee SH, et al. Role of NAD (PH) oxidase in the tamoxifen-induced generation of reactive oxygen species and apoptosis in HepG2 human hepatoblastoma cells. Cell Death Differ. 2000;7:925–32. doi: 10.1038/sj.cdd.4400717. [DOI] [PubMed] [Google Scholar]
- Lijie G, Yiwei L, Ana NK, et al. Inactivation of NF-jB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–9. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–77. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- Marion ML, Scarlett LG, Jeffrey SC, et al. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–8. [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharidis TC. The impact of plant flavonoids on mammalian biology:implications for inflammations, heart disease and cancer. Pharmacol Rev. 2005;52:673–751. [PubMed] [Google Scholar]
- Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- Mumtaz Banu S, Padmavathi R, Sakthisekaran D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol Biol Cell. 2007;297:73–9. doi: 10.1007/s11010-006-9324-2. [DOI] [PubMed] [Google Scholar]
- Nagendran B, Kalyana S, Samir S. Phenolic compounds in plants and agri-industrial by-products:Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;1:191–203. [Google Scholar]
- Olver IN. Prevention of breast cancer. Med J Aust. 2016;205:475–9. doi: 10.5694/mja16.01007. [DOI] [PubMed] [Google Scholar]
- Pier-Giorgio P. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Radmacher MD, Simon R. Estimation of tamoxifens's efficiency for preventing the formation and growth of breast tumors. J Natl Cancer Inst. 2000;92:48–53. doi: 10.1093/jnci/92.1.48. [DOI] [PubMed] [Google Scholar]
- Raju J, Bielecki A, Caldwell D, et al. Soy isoflavones modulate azoxymethane-induced rat colon carcinogenesis exposed pre- and postnatally and inhibit growth of DLD-1 human colon adenocarcinoma cells by increasing the expression of estrogen receptor-beta. J Nutr. 2009;139:474–81. doi: 10.3945/jn.108.099200. [DOI] [PubMed] [Google Scholar]
- Robert JT, Els VN, Danny EC, et al. Flavonoids:a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Arezoo M. Apoptotic effect of genistein on hepatocellular carcinoma HepG 2 cell line. Glob J Med Res. 2016;3:1–8. [Google Scholar]
- Sanaei M, Kavoosi F, Rakhshandehroo S. Apoptotic Effect of Tamoxifen on Hepatocellular Carcinoma HepG 2 Cell Line. Glob J Med Res. 2016;3:9–14. [Google Scholar]
- Sanaz K, Mohammed AA, Chung YL, et al. An association map on the effect of flavonoids on the signaling pathways in colorectal cancer. Int J Med Sci. 2016;13:374–85. doi: 10.7150/ijms.14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjeev B, Yiwei L, Zhiwei W, et al. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;8:226–42. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian B, Hartmut H, Klaus-Dieter S, et al. Tamoxifen induces suppression of cell viability and apoptosis in the human hepatoblastoma cell line HepG2 via down-regulation of telomerase activity. Liver Int. 2004;24:46–54. doi: 10.1111/j.1478-3231.2004.00887.x. [DOI] [PubMed] [Google Scholar]
- Seiichiro Y, Tomotaka S, Minatsu K, et al. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–13. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- Seiji M, Masahide O, Akiko K, et al. Tamoxifen inhibits cell proliferation via mitogen activated protein kinase cascades in human ovarian cancer cell lines in a Manner Not Dependent on the Expression of Estrogen Receptor or the sensitivity to cisplatin. Endocrinology. 2004;145:1302–13. doi: 10.1210/en.2003-0709. [DOI] [PubMed] [Google Scholar]
- Tim C. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-Chun Y, Po-Cheng C, Tsai-Kun L, et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782–92. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Uwe W, Sabine K, Mathias DB, et al. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000;60:3823–31. [PubMed] [Google Scholar]
- Wang HX, Wei Z, Yong BX, et al. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai:population based case-control study. BMJ. 2004;328:1285. doi: 10.1136/bmj.38093.646215.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett. 2002;186:8–11. doi: 10.1016/s0304-3835(01)00811-4. [DOI] [PubMed] [Google Scholar]
- Wang U, Lee IM, Shumin MZ, et al. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009;89:905–12. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen-Xin L, Cheng-Bin C, Bing C, et al. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J Asian Nat Prod Res. 2005;7:615–26. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- Xiao OS, Fan J, Qi D, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8. [PubMed] [Google Scholar]
- Yan G, Chen-Fang Z, Hitoshi I, et al. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. Word J Gastroenterol. 2005;11:6512–17. doi: 10.3748/wjg.v11.i41.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiming M, George LB, Jin-Rong Z. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007;46:534–42. doi: 10.1002/mc.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]


