Abstract
Background:
Colorectal cancer (CRC) is one of the most diffuse malignancy in the world. In Southern Europe, the incidence and prevalence are lower than in most Western countries, although some hot spots of increased risk are emerging. In Sardinia, the cancer rate has risen steeply in the last years. Among risk factors for CRC, genomic homozygosity has been postulated. Glucose-6-phosphate dehydrogenase (G6PD) deficiency has been hypothesized to decrease CRC risk. In Sardinians, this disorder has a frequency of 12-24% due to selection by past malaria. In this study the relationship between mortality for CRC, homozygosity and G6PD deficiency was analysed using spatial analysis.
Methods:
The spatial association between CRC mortality and G6PD deficiency and homozygosity was assessed in the 377 municipalities of the island using ordinary least squares regression and geographically weighted regression.
Results:
A consanguinity index, available across all municipalities, was used as a proxy for homozygosity. A significant inverse correlation was found between CRC mortality and G6PD deficiency (ρ = -0.216; p = 0.002) whereas no association was found for consanguinity (ρ = -0.077; p = 0.498). The geographical map of CRC mortality showed a significant clustering in mountain areas compared to the population living in lowlands, whereas hot spot areas of G6PD deficiency were observed on the south-western side of Sardinia.
Conclusions:
These results indicate that G6PD deficiency might contribute to reduce colon carcinogenesis, and is in line with in vitro and in vivo studies.
Keywords: Colorectal cancer, glucose-6-phosphate dehydrogenase deficiency, consanguinity, spatial analysis
Introduction
Colorectal cancer (CRC) is the second most frequent cancer in the world after lung cancer, and is still characterized by a poor prognosis. Incidence, prevalence and mortality of CRC may vary substantially across different countries according to the exposure to risk factors and the population genetic background (Jensen et al., 1990). Among these factors, age, a family history of sporadic CRC, inflammatory bowel disease, and abdominal irradiation influence recommendations for screening in the individual subject (Levi et al., 2014). In addition, consanguinity has also been associated to higher rates of cancer in several studies, suggesting that homozygosity may lead to carcinogenesis in inbred populations via recessive CRC-predisposing loci (Bacolod et al., 2009).
Observational studies identified also sex, black race, obesity, diabetes, acromegaly, sedentariness, excessive consumption of alcohol and processed/red meat, and smoking habits as factors raising the risk of developing CRC (Haggar and Boushey, 2009).
On the other hand, there is robust evidence supporting a protective effect of nonsteroidal anti–inflammatory drugs including aspirin and their role for chemoprevention in CRC (Hamoya et al., 2016). Other factors suggested as protective are regular exercise and a diet rich in fruit, vegetables and poor in meat and animal fats (Haggar and Boushey, 2009).
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, one of the most common inherited enzyme defects, has been associated with a lower cancer risk (Beaconsfield et al., 1965). The disorder is widespread in the Mediterranean area due to the selection of malaria in the past (Tognotti, 2009). In the island of Sardinia, the overall prevalence of G6PD deficiency ranges between 12% and 24%, depending on the area, and in the majority of cases is due to the G6PD Mediterranean variant (a C→T transition at nucleotide 563) (Fiorelli et al., 1990). An inverse relationship between geographic distribution of G6PD deficiency and CRC was reported by Beaconsfield et al., (1965). In addition, Sulis et al. in Sardinia observed a cancer prevalence lower than in the rest of Europe, where G6PD deficiency is uncommon (Sulis, 1972). More recently, in a retrospective case-control study of 3901 Sardinian patients with known G6PD status a 43.2% reduction of CRC risk was reported among G6PD-deficient compared with subjects with normal G6PD enzyme activity (OR 0.57, 95%CI 0.37-0.87, p=0.010) (Dore et al., 2016). In this report. although age, gender, family history of CRC, type 1 diabetes and ischemic heart disease were significantly associated with CRC rate, the protective effect of G6PD deficiency remained significant at logistic regression analysis after adjusting for all covariates (Dore et al., 2016).
Spatial analysis may help analysing geographically distributed data by complementing conventional statistics (Chun and Griffth, 2012). It includes descriptive spatial statistics, namely formal techniques to analyze the distribution of data in a given geographic area, as well as the identification of spatial patterns of data, and the relationship between two or more spatial variables (spatial regression).
In this ecological study, spatial analysis was applied to test the relationship between CRC mortality rate, G6PD deficiency and consanguinity in Sardinia.
Materials and Methods
Study design and setting
The Mediterranean island of Sardinia, Italy, has a surface of 24,100 square kilometres and a total population of 1,651,793 residents (ISTAT, 2014). In this study we analysed data from all Sardinian municipalities, corresponding to 377 territorial units. Raw data for CRC cancer between 1981 and 1988 were available from the “Atlante di Mortalità in Sardegna” (Sardinia Mortality Registry) (2005) which provided the number of observed and expected deaths for each municipality and each disease category. The prevalence of G6PD deficiency was extracted from the data reported by Sanna et al., (1997). In that study, the G6PD status was assessed in 11,052 military conscripts from all Sardinian municipalities using the method of Beutler and Mitchell (1968) modified by Dow et al., (1974). In addition, the consanguinity coefficient α, estimated through the average inbreeding coefficients of the marriages which occurred in each Sardinian municipality, was collected from the study of Lisa et al., (2015) and categorized into quartiles as reported by the authors.
Spatial analysis
The standardized mortality ratio (SMR) for the cancer categories 153-154 (cancer of colon and rectum) according to the International Classification of Diseases 9th revision (ICD-9) was calculated from the observed and expected deaths. A ratio greater than 1.0 indicates that more than expected deaths have occurred. Since G6PD data were limited to male gender, only data for men were selected. The calculated SMR for CRC in each municipality was entered as dependent variable in all statistical models. As SMR has an asymmetrical distribution based on the results of a linearity tests, it was log-transformed before analysis.
The association between the variables was assessed (i) by using the Spearman correlation coefficient ρ, taking each Sardinian municipality as the smallest statistical unit; (ii) by performing an Ordinary Least Squares (OLS) regression with SPSS version 16.0 software (Chicago, IL) to compute parameter estimates, entering SMR for CRC as dependent variable, whereas consanguinity index (coded into quartiles) and G6PD deficiency prevalence (coded as a continuous variable) were entered as independent variables. Adjusted R² values and F-tests were used to assess the model fitting. To complement the OLS regression analysis, a Geographically Weighted Regression (GWR) modelling was also fitted to the data using the same covariates, using the GWR 4.0 software able to generate parameter estimates for each unit by borrowing information from the surrounding units (Nakaya et al., 2009). The regression coefficients for consanguinity and G6PD deficiency obtained from the GWR model were finally mapped to display spatial variation of parameters.
Results
The summary of geographic and demographic data on Sardinia are reported in Table 1. The distribution of CRC mortality between 1981 and 1988 and the prevalence of G6PD deficiency across the 377 municipalities of Sardinia is illustrated in Figure 1. Overall, CRC mortality was scattered all over the island with a tendency for clustering in the central area. In contrast G6PD deficiency appears concentrated in villages of the North-Eastern and Southern Western flatlands, corresponding to the ancient wetlands previously infested by malaria since pre-Roman times. The correlation between SMR for CRC and G6PD deficiency was negative (Spearman ρ = -0.216; p=0.002), underlying a moderate inverse relationship (Figure 2). In contrast, the correlation coefficient for consanguinity was not statistically significant (-0.077, p=0.498). The model summary for OLS and GWR analysis is reported in Table 2. When both explanatory variables were entered in the OLS model, they showed β coefficients of -0.071 and -0.172 for consanguinity and G6PD deficiency, respectively. However, only the coefficient for the latter was statistically significant (p=0.006), confirming the result of the correlation analysis. The global model fit gave an adjusted R² of 0.026, suggesting that a large proportion of variability in CRC mortality was not explained by the variables selected. The finding of a mild clustering of CRC mortality in the central area of the island suggested to proceed to GWR.
Table 1.
Geographic and Demographic Data on Sardinia
| Geographic coordinates | Latitude: 40° 7’ 15.151” N |
|---|---|
| Longitude: 9° 0’ 46.413” E | |
| Surface, square kilometers | 24100 |
| Population density, inh./square kilometers | 68.54 |
| Total no. of municipalities | 377 |
| Total population in the study period | 1,651,793 |
| Average population in the smallest municipality | 186 |
| Average population in the largest municipality | 431,819 |
| Total male population | 809,289 |
| Total female population | 842,505 |
Figure 1.
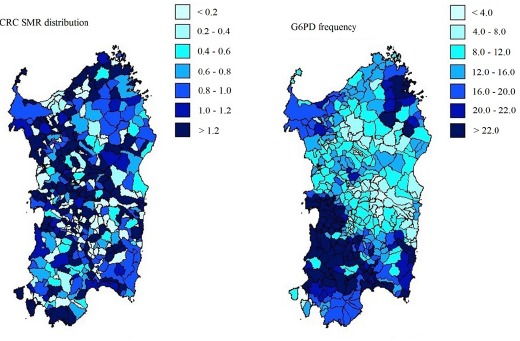
A. Standardized mortality ratio for cancer of colon and rectum (153-154-159, according to ICD-9 Cause-of-Death Lists of Multiple Risk Factor Intervention Trial (MRFIT) as reported in Atlante della Mortalità Sarda 1981-1988. B. Distribution of Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency in Sardinia (Sanna et al., 1997). ISTAT, Roma, Italy.
Figure 2.
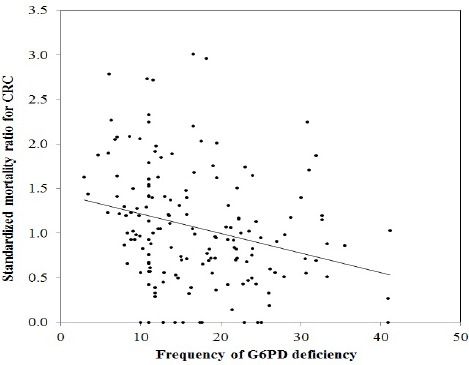
Linear Regression of Standardized Mortality Ratio (SMR) for Colorectal Cancer in Sardinia and Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency.
Table 2.
Parameters of OLS and GWR Regression Models
| OLS | GWR | |||||
|---|---|---|---|---|---|---|
| ß coefficient | SE | Standardized ß | p -value | Mean | STD | |
| Intercept | 0.431 | 0.128 | – | – | – | – |
| Consanguinity | –0.038 | 0.033 | –0.071 | 0.252 | 8.292 | 1.371 |
| G6PD deficiency | –0.014 | 0.005 | –0.172 | 0.006 | –2.932 | 0.643 |
Adjusted R2, 0.026; G6PD, glucose-6-phosphate dehydrogenase; SE, Standard Error; OLS, Ordinary Least Squares; GWR, Geographically; Weighted Regression
The spatial distribution of the local coefficients in the geographically weighted regression model is illustrated in Figure 3. They varied between -0.20 and 0.0 and indicate that the model has a better explanatory capacity in the eastern and southern coastal areas of the island where G6PD deficiency is prevalent. The adjustment decreased in the North-Western zone, where the magnitude of both dependent and independent variables is lower. As for consanguinity, the GWR model did not show better explanatory capacity than the OLS model, and did not support a significant association of this variable with CRC mortality.
Figure 3.
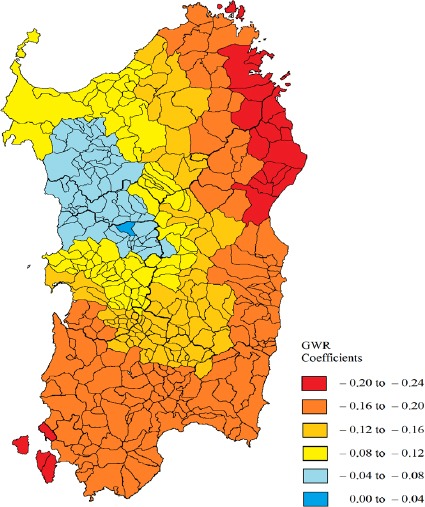
Spatial Distribution of the Local Coefficients for G6PD Deficiency in the Geographically Weighted Regression Model
Discussion
Colorectal cancer is a malignancy common worldwide, characterized by high morbidity and mortality. Incidence and prevalence of CRC varies up to 15 folds across and within different countries (Welch and Robertson, 2016). The majority of CRCs follow the sequence adenoma–carcinoma through dysplastic changes resulting from inherited and acquired genetic alterations. Several tests and procedures are available for CRC screening including non–invasive tests such as stool– or plasma–based tests and Tissue Resonance Interaction Method prob (TRIMprob) (Dore et al., 2015); or invasive tests such as endoscopic or radiologic tests. However, screening recommendation about when and how to test needs to be tailored on the individual patient and preferences. Risk factors such as age and a family history are the most important for screening recommendations although genetic factors also play a role in colorectal carcinogenesis.
Among genetic factors, Sulis et al. almost half a century ago hypothesised a protective role of G6PD deficiency against cancer (Sulis, 1972). Further research carried out by Cocco et al. in Sardinia added evidence supporting this hypothesis (Cocco et al., 1987; Cocco et al., 1989). More specifically, the analysis of a spatial correlation between G6PD deficiency and cancer in 24 Sardinian towns showed a negative correlation coefficient (r = -0.418) (Cocco et al., 1987). However, the large variability of mortality data and the lack of randomization for samples selection did not allow to draw definite conclusions. To the best of our knowledge a systematic spatial analysis between CRC and G6PD based on aggregate data was never been attempted in Sardinia where the frequency of this hereditary disorder is one of the highest in the world (Fiorelli et al., 1990; Cocco et al., 1998).
More recently, a retrospective case-control study performed in 3901 patients from Sardinia who underwent a colonoscopy, the proportion of pre- and malignant colorectal lesions was compared in cases (G6PD deficient) and controls (G6PD normal) (Dore et al., 2016). Subjects with G6PD-deficiency showed a CRC risk reduction of 43.2% compared with normal G6PD subjects. The protective effect of G6PD deficiency remained significant across all age groups by logistic regression analysis after adjusting for all covariates (Dore et al., 2016).
According to these findings and the observations reported in the literature (Sulis, 1972), the present ecological study revealed an inverse spatial distribution between CRC mortality and G6PD deficiency at aggregated level.
Among the hypotheses proposed to explain the observed protective effect of G6PD deficiency against cancer are: reduction of free radical such as superoxide anion which increases the DNA mutation rate and the synthesis of pro-inflammatory cytokines (Pascale et al., 1987). The enzyme catalyses the first step of the pentose phosphate pathway (PPP) which synthesises coenzyme nicotinamide-adenine dinucleotide phosphate (NADPH) (Luzzato and Notaro, 2001). Experiments in vitro showed that colon cancer cell need NADPH to proliferate (Vizàn et al., 2009; Bravard et al., 1994). Thus, NADPH deficiency due to the block of PPP may reduce nitric oxide production and delay carcinogenesis. Recently it has been demonstrated that siRNAs against G6PD decrease cancer cell proliferation (De Preter et al., 2016). Evidence has also shown that among genetic factors, consanguinity may promote carcinogenesis (Bacolod et al., 2009). The human genome, through the contribution of parental germ-lines, contains long homozygous chromosomal segments. Explanation for the presence of these stretches in the individual genome may be due to shared ancestry between parents. In some geographical areas, the number of marriages among relatives is relatively high (Kirin et al., 2010). Among these, Sardinian population experienced a longstanding history of inbreeding and consanguinity, making the island the ideal scenario to test the association between homozygosity and CRC mortality. Although a mild clustering of CRC deaths was observed in the central area of the island, we were unable to find a significant correlation between consanguinity and CRC mortality. Our findings are consistent with recent data reported by other authors who did not confirm the role of homozygosity in CRC risk (Spain et al., 2009). However, in our analysis homozygosity was estimated using consanguinity rate, which may have affected the results.
As in most ecological studies, our investigation displays several methodological limitations that should be taken into consideration. First, other risk factors for CRC not considered in this study such as diet, smoke and environmental pollution, might have affected the CRC mortality. As a matter of fact, the number of variables available in all Sardinian municipalities for the period considered was limited, and we cannot rule out the existence of other potential confounders. Second, ecological studies analyse only aggregate data, and therefore their results cannot be extended to individual subjects. Another limitation is that G6PD data were available only for males, therefore only SMR among males was considered. More importantly, our ecological approach cannot be used to support directly a cause-effect relationship and should be considered as a mere hypothesis-generating study.
In conclusion, the negative spatial association found between CRC mortality and G6PD deficiency is an interesting finding that seems to support previous epidemiological observations. However, further analyses are necessary to understand why CRC mortality in Sardinia show a heterogeneous spatial distribution and especially a trend toward higher rates in the central area of the island.
Conflict of interest disclosure
All authors declare that they have no conflict of interest.
References
- Atlante della MortalitàSarda 1981-1988. Rome, Italy: ISTAT; 2005. [Google Scholar]
- Bacolod MD, Schemmann GS, Giardina SF, et al. Emerging paradigms in cancer genetics:some important findings from high-density single nucleotide polymorphism array studies. Cancer Res. 2009;69:723–7. doi: 10.1158/0008-5472.CAN-08-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaconsfield P, Rainsbury R, Kalton G. Glucose‒6‒phosphate dehydrogenase deficiency and the incidence of cancer. Oncologia. 1965;19:11–19. doi: 10.1159/000224280. [DOI] [PubMed] [Google Scholar]
- Beutler E, Mitchell M. Special modifications of the fluorescent screening method for glucose‒6‒phosphate dehydrogenase deficiency. Blood. 1968;32:816–8. [PubMed] [Google Scholar]
- Bravard A, Beaumatin J, Dussaulx E, et al. Modifications of the anti-oxidant metabolism during proliferation and differentiation of colon tumor cell lines. Int J Cancer. 1994;59:843–7. doi: 10.1002/ijc.2910590622. [DOI] [PubMed] [Google Scholar]
- Chun Y, Griffith D. Spatial statistics and geostatistics:Theory and applications for geographic information science and technology. 2455 Teller Road, Thousand Oaks, California 91320, USA: SAGE publication; 2012. [Google Scholar]
- Cocco P, Todde P, Fornera S, et al. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood. 1998;91:706–9. [PubMed] [Google Scholar]
- Cocco PL, Dessì S, Avataneo G, Picchiri GF, Heineman EF. Glucose‒6‒phosphate dehydrogenase deficiency and cancer in a Sardinian male population:A case‒control study. Carcinogenesis. 1989;10:813–6. doi: 10.1093/carcin/10.5.813. [DOI] [PubMed] [Google Scholar]
- Cocco PL, Manca P, Dessì S. Preliminary results of a geographic correlation study on G6PD deficiency and cancer. Toxicol Pathol. 1987;15:106–8. doi: 10.1177/019262338701500116. [DOI] [PubMed] [Google Scholar]
- De Preter G, Neveu MA, Danhier P, et al. Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. Oncotarget. 2016;7:2910–20. doi: 10.18632/oncotarget.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore MP, Davoli A, Longo N, Marras G, Pes GM. Glucose-6-phosphate dehydrogenase deficiency and risk of colorectal cancer in Northern Sardinia:A retrospective observational study. Medicine (Baltimore) 2016;95:5254. doi: 10.1097/MD.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore MP, Tufano MO, Pes GM, et al. Tissue resonance interaction accurately detects colon lesions:A double-blind pilot study. World J Gastroenterol. 2015;21:7851–9. doi: 10.3748/wjg.v21.i25.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow PA, Petteway MB, Alperin JB. Simplified method for G‒6‒PD screening using blood collected on filter paper. Am J Clin Pathol. 1974;61:333–6. doi: 10.1093/ajcp/61.3.333. [DOI] [PubMed] [Google Scholar]
- Fiorelli G, Meloni T, Palomba V, et al. Gene frequency of glucose-6-phosphate dehydrogenase (G6PD) polymorphic variants in Sardinia. Gene Geogr. 1990;4:139–42. [PubMed] [Google Scholar]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology:incidence, mortality, survival, and risk Factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoya T, Fujii G, Miyamoto S, et al. Effects of NSAIDs on the risk factors of colorectal cancer:a mini review. Genes Environ. 2016;38:6. doi: 10.1186/s41021-016-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istituto Nazionale di Statistica. Atlante della MortalitàSarda 1981-1988. Roma, Italy: ISTAT; 2014. [Google Scholar]
- Jensen OM, Estève J, Møller H, Renard H. Cancer in the European Community and its member states. Eur J Cancer. 1990;26:1167–256. doi: 10.1016/0277-5379(90)90278-2. [DOI] [PubMed] [Google Scholar]
- Kirin M, McQuillan R, Franklin CS, et al. Genomic runs of homozygosity record population history and consanguinity. PLoS One. 2010;5:13996. doi: 10.1371/journal.pone.0013996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Randimbison L, Blanc-Moya R, La Vecchia C. Age-specific incidence of all neoplasms after colorectal cancer. Ann Epidemiol. 2014;24:785–8. doi: 10.1016/j.annepidem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Lisa A, Astolfi P, Zei G, Tentoni S. Consanguinity and late fertility:spatial analysis reveals positive association patterns. Ann Hum Genet. 2015;79:37–45. doi: 10.1111/ahg.12092. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, Notaro R Malaria. Protecting against bad air. Science. 2001;293:442–3. doi: 10.1126/science.1063292. [DOI] [PubMed] [Google Scholar]
- Nakaya T, Fotheringham A, Charlton M, Brunsdon C. Semiparametric geographically weighted generalised linear modelling in GWR 4.0. In: Lees B.G, Laffan S.W, editors. 10th International Conference on GeoComputation, November-December, 2009. Sidney: UNSW; 2009. [Google Scholar]
- Pascale R, Garcea R, Ruggiu ME, et al. Decreased stimulation by 12-O-tetradecanoylphorbol-13-acetate of superoxide radical production by polymorphonuclear leukocytes carrying the Mediterranean variant of glucose-6-phosphate dehydrogenase. Carcinogenesis. 1987;8:1567–70. doi: 10.1093/carcin/8.10.1567. [DOI] [PubMed] [Google Scholar]
- Sanna E, Cosseddu GG, Floris G, Liguori A, Silvetti M. Micromapping the distribution of G6PD deficiency in Sardinia with data collected from the 1950s to the 1980s, in adaptation to Malaria. In: L. S Greene, M. E Danubio., editors. The interaction of biology and culture. New York, NY, USA: 1997. pp. 293–322. [Google Scholar]
- Spain SL, Cazier JB, et al. CORGI Consortium. Colorectal cancer risk is not associated with increased levels of homozygosity in a population from the United Kingdom. Cancer Res. 2009;69:7422–9. doi: 10.1158/0008-5472.CAN-09-0659. [DOI] [PubMed] [Google Scholar]
- Sulis E. G6PD deficiency and cancer. Lancet. 1972;1185 doi: 10.1016/s0140-6736(72)91416-x. [DOI] [PubMed] [Google Scholar]
- Tognotti E. Program to eradicate malaria in Sardinia 1946-1950. Emerg Infect Dis. 2009;15:1460–66. doi: 10.3201/eid1509.081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizán P, Alcarraz-Vizán G, Díaz-Moralli S, et al. Modulation of pentose phosphate pathway during cell cycle progression in human colon adenocarcinoma cell line HT29. Int J Cancer. 2009;124:2789–96. doi: 10.1002/ijc.24262. [DOI] [PubMed] [Google Scholar]
- Welch HG, Robertson DJ. Colorectal cancer on the Decline‒Why Screening Can't Explain It All. N Engl J Med. 2016;374:1605–7. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]


