Abstract
Purpose:
Recurrence is one of the most important factors influencing survival of colorectal cancer patients.
Subjects and Methods:
In this cohort study, clinical and demographic characteristics of 561 patients with colorectal cancer were collected from 2010 to 2015. Medical records and telephone interviews were used to define the patient’s clinical status including the date of any recurrence during the study period. The multivariate Cox model was used as the main strategy for analyzing data.
Results:
Some 239 (42.6%) patients experienced cancer recurrence during the 5-year follow-up period. Those with an older age at diagnosis had a higher risk of cancer recurrence than their younger counterparts [Hazard Ratio (HR) >70 y /<50 y= 1.65, P=0.01]. Rectal cancer had a greater risk of disease recurrence compared with other tumor sites [HR colon/ rectum=1.53, P=0.02]. Stage 3 cancer had a higher risk than stage 1 cancer [HR stage 3/ stage 1=4.30, P<0.001], and positive lympho-vascular invasion was also a risk factor [HR yes/ no=2.03, P<0.001]. Finally, tumor size, number of dissected lymph nodes, proportion of positive lymph nodes, perineural invasion and type of treatment did not significantly predict recurrence.
Conclusion:
Access to enhanced medical services including cancer diagnosis at an early stage and optimal treatment is needed to improve the survival and quality of life of CRC patients.
Keywords: Colorectal cancer, recurrence, stage, risk factors, Iran
Introduction
Colorectal cancer (CRC) is the third most common type of cancer in the world and the fourth in Iran with an incidence rate of 5000 new cases every year consisting about % 8.12 of all cases of cancer in the country (Dianatinasab et al., 2016b; Zare-Bandamiri et al., 2016). In recent years, studies suggested a worldwide increase in the incidence of CRC among young people (Ferlay et al., 2010; Jemal et al., 2011; Torre et al., 2015; Dianatinasab et al., 2016b). In Iran, the rates of incidence and mortality of CRC are rising constantly resulting in an increase in the burden of disease in the country (Malekzadeh et al., 2009; Kolahdoozan et al., 2010; Fararouei et al., 2015).
Several factors seem to influence survival of CRC patients, among which, recurrence is possibly the most important (Moghimi-Dehkordi et al., 2008; Yuan et al., 2013). Gan et al reported that 30% to 50% of patients with treated CRC relapse and die due to the disease (Gan et al., 2007). Despite the availability of different types of treatment‚ the chance of recurrence and metastasis of CRC among patients is high. This means that our knowledge about factors influencing the chance of recurrence is either limited or not applicable. Identification of these factors along with better treatment strategies may help in reducing the probability of recurrence and raising the life expectancy of the patients. The aim of this study was to identify factors which are influencing recurrence of CRC in a large sample of Iranian CRC patients.
Materials and Methods
Patients
In this historical cohort study, we obtained demographic and clinical data of 561 CRC patients who referred to oncology department of Shiraz Namazi hospital from 2010 to 2015 (in total 1783.5 person-years). The hospital is located in Shiraz (the capital of Fars province) and is considered as a referral diagnosis and treatment center for all types of cancer in southern of Iran and provides medical services to patients from southern part of the country (Dianatinasab et al., 2017).
Data collection
From 607 CRC patients, 46 were excluded due to incomplete data, leading to a final sample size of 561 patients. Data was collected using the patient’s medical records. In addition, a telephone interview was conducted by a trained female nurse to confirm patients’ current clinical status and if applicable the date of recurrence. A verbal consent was obtained from participants at the beginning of the phone interview. Ethical approval was obtained from Shiraz University of Medical Sciences ethical committee.
Data on gender, age at diagnosis, number of dissected lymph nodes and positive lymph nodes (N-stage), tumor site, tumor stage, T-stage (based on the American Joint Committee on Cancer classification), tumor differentiation level, tumor size, type of treatment (adjuvant vs. neoadjuvant therapy), lympho-vascular and perineural invasion was obtained from the patient’s medical file. For recurrent cases‚ site of recurrence (local‚ distant or local and distant recurrence) was also defined.
Sampling and statistical analysis
All patients newly registered with the department of oncology during 2010 to 2015 were included in the analysis. Survival analysis (considering the recurrence as event) was performed using multivariable Cox’s modelling. Survival probability was estimated by the Kaplan-Meier curves and life table. Log-rank test was used to compare survival between different groups of categorical variables. The proportional hazard assumption (a key assumption of Cox proportional hazards models) was tested using diagnostic graph with -Ln [-Ln (St)] on the y-axis and time on the x-axis. To conduct multivariate analysis, variables with a p-value less than 0.2 were included in the analysis. Since tumor stage consists N-stage and T-stage, and given the collinearity between these two variables, these variables were included in two multivariate Cox models separately and the one with smaller p-value were selected to be included in the final model. All statistical approaches were applied assuming a two-sided test based on a 5% level of type I error. P<0.05 was considered to be statistically significant. STATA (version 12) was used to conduct the analysis.
Results
Selected characteristic of participant
Clinical and demographic characteristics of 561 patients with CRC are shown in table 1. More than half of the subjects were male (57.6%). The mean [±SD] age of diagnosis was 55.7 [±13.6] years. Nearly half of the patients (45.5%) were 50 to 70 years old at the time of interview and the rectum was the primary cancer site in half of the patients. Approximately, 81% of the patients underwent surgery before chemotherapy and radiotherapy. In addition, most patients diagnosed at stage II (44%) and stage 3 (36.7). Majority of patients were reported to have well differentiation cells (66.3%).
Table 1.
Demographic and Clinical Characteristics of Colorectal Cancer Cases
| Variables | Recurrence | No recurrence | Total |
|---|---|---|---|
| Age (Mean ± SD) | 57.2 ±14.1 | 54.65 ±13.3 | 55.7 ± 13.7 |
| Sex (Number (%)) | |||
| Male | 135 (56.5%) | 188 (58.3%) | 323 (57.6%) |
| Female | 104 (43.5%) | 134 (41.7%) | 238 (42.4%) |
| Number of dissected lymph nodes | 8.4 ±6.6 | 9.4 ±8.6 | 9 ± 6.8 |
| Number of positive lymph nodes | 2.1 ±3.9 | 0.9 ±3.9 | 3.2 ± 1.5 |
| Tumor Size | 4.7 ±1.8 | 4.89 ±1.9 | 4.8 ± 1.9 |
| Site of tumor (number (percent)) | |||
| Rectum | 131 (54.8%) | 152 (47.0%) | 283(50.4%) |
| Right and Transverse colon | 41 (17.2%) | 68 (21.2%) | 109 (19.4%) |
| Left colon | 17 (7.1%) | 35 (10.9%) | 52(9.3%) |
| Sigmoid | 50 (20.9%) | 67 (20.9%) | 117 (20.9%) |
| T-stage | |||
| T1 | 1 (0.4%) | 6 (1.9%) | 7 (1.2%) |
| T2 | 18 (7.5%) | 93 (29%) | 111 (19.8%) |
| T3 | 206 (86.2%) | 218 (67.6%) | 424 (75.6%) |
| T4 | 14 (5.9%) | 5 (1.5%) | 19 (3.4%) |
| N-stage | |||
| N0 | 119 (49.7%) | 227 (70.8%) | 346 (61%) |
| N1 | 62 (25.9%) | 71 (22.0%) | 133 (23%) |
| N2 | 58 (24.4%) | 24 (7.2%) | 82 (16%) |
| Stage | |||
| I | 14 (5.8%) | 90 (27.9%) | 104 (19.3%) |
| II | 109 (45.6%) | 142 (44.2%) | 251 (44%) |
| III | 116 (48.6%) | 90 (27.9%) | 206 (36.7%) |
| Grade | |||
| Well differentiated | 140 (58.6%) | 232 (72%) | 372 (66.3%) |
| Moderately differentiated | 78 (32.7%) | 77 (23.7%) | 155 (27.6%) |
| Poorly differentiated | 20 (8.7%) | 14 (4.3%) | 34 (6.1%) |
| Lymphovascular invasion | |||
| Yes | 127 (53.2%) | 76 (23.6%) | 203 (36%) |
| No | 112 (46.8%) | 246 (76.4%) | 358(64%) |
| Perineural invasion | |||
| Yes | 96 (40.1%) | 66 (20.4%) | 162 (28.8%) |
| No | 143 (59.9%) | 256 (79.6%) | 399 (71.2%) |
| Treatment method | |||
| Adjuvant therapy | 179 (74.9%) | 275 (85.4%) | 454 (80.9%) |
| Neoadjuvant therapy | 60 (25.1%) | 47 (14.6%) | 107 (19.1%) |
| Site of recurrence | |||
| Local | 64(11.4%) | - | 64(11.4%) |
| Distant | 50(8.9%) | - | 50(8.9%) |
| Locodistant | 125(22.3%) | - | 125(22.3%) |
At the end of study, from 561 patients, 239 had recurrence (42.6%) and most recurrent cases were loco-distant. The mean [±SD] survival time was 62.14 [±1.66] months and the five-year survival rate was 56.8%.
Age of patients with CRC recurrence 57.21 [±14.03] was significantly higher than those without recurrence 54.65 [±13.32] (P=0.035). Women had less chance of recurrence (43.5%) compared to men (56.5%), (P=0.041). Lympho-vascular invasion was reported less frequent in patients without recurrence (23.6%), compared to (53.2%) in patients who experienced recurrence (P<0.001).
Univariable analysis
Univariate analysis suggested that variables such as age of diagnosis, stage and primary tumor site, T-stage‚ N-stage, tumor differentiation level, proportion of involved lymph nodes, type of treatment, lympho-vascular and perineural invasion were significantly associated with CRC recurrence.
Figure 1.
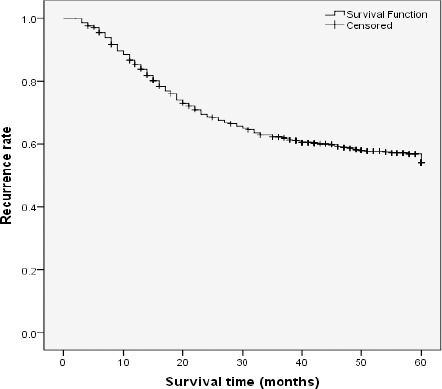
Overall Recurrence Rate in Colorectal Cancer Patients During Study Period (60 Month)
Multivariable analysis
As shown in table 2, after controlling for the effect of other study variables, multiple Cox model analysis suggested that; older patients had a higher risk of cancer recurrence than those who were diagnosed at younger age [HR>70/<50 years= 1.65, CI95%:1.09 to 2.49, P=0.012]. In addition, those with rectum site of cancer had more risk of recurrence compare to patients with other sites of CRC [HR colon/rectum= 1.53, CI95%: 1.05 to 2.24, P=0.024]. Patients with stage III of cancer had a higher risk of recurrence compared to those with stage 1 cancer [HR stageIII/stageI= 4.30, CI95%: 2.25 to 8.20, P<0.001]. Those patients who experienced lympho-vascular invasion of tumor were also at a higher risk of recurrence compare to those with no LV invasion of tumor [HR lympho-vascular invasion / no LV invasion = 2.03, CI95%: 1.52 to 3.72, P<0.001]. However, no significant association was found between the risk of recurrence and size of tumor, number of dissected lymph node, grade, proportion of positive lymph nodes, perineural invasion and type of treatment [P<0.05 for all].
Table 2.
Univariable and Multivariable Analysis of Prognostic rRecurrence Factors in Patients with Colorectal Cancer
| Variables | Univariable HR (95% CI) | P-Value | Multivariable HR (95%CI) | P-Value |
|---|---|---|---|---|
| Age | ||||
| <50 year (reference) | 1* | 1* | ||
| 50-70 year | 1.17 (0.84_1.62) | 0.211 | 1.15 (0.84_1.58) | 0.331 |
| >70 year | 1.811 (1.280_2.561) | <0.001 | 1.65 (1.09_2.49) | 0.012 |
| Sex | ||||
| Female (reference) | 1 | NI | ||
| Male | 1.01 (0.78_1.30) | 0.946 | - | |
| Site of tumor | ||||
| Right and transverse colon (reference) | 1 | 1 | ||
| Left colon | 0.81 (0.46_1.44) | 0.482 | 0.76 (0.40_1.48) | 0.437 |
| Sigmoid | 1.18 (0.78_1.79) | 0.417 | 1.17 (0.75_1.81) | 0.470 |
| Rectum | 1.35 (0.95_1.92) | 0.092 | 1.53 (1.05_2.24) | 0.024 |
| Size of tumor | ||||
| ≤5 cm (reference) | 1 | 1 | ||
| >5cm | 0.78 (0.58_1.03) | 0.098 | 0.86 (0.62_1.19) | 0.360 |
| T-stage | ||||
| T1 and T2 (reference) | 1 | NI | ||
| T3 | 3.89 (2.43_6.23) | <0.001 | - | |
| T4 | 9.37 (4.69_18.74) | <0.001 | - | |
| N-stage | ||||
| N0 (reference) | 1 | NI | ||
| N1 | 1.60 (1.16_2.19) | 0.003 | - | |
| N2 | 2.61 (1.88_3.62) | <0.001 | - | |
| Stage | ||||
| I (reference) | 1 | 1 | ||
| II | 3.61 (2.07_6.31) | <0.001 | 3.11 (1.65_5.86) | <0.001 |
| III | 5.38 (2.88_9.29) | <0.001 | 4.30 (2.25_8.20) | <0.001 |
| Grade | ||||
| Well differentiated | 1 | 1 | ||
| Moderately differentiated | 1.45 (1.10_1.91) | 0.008 | 1.263 (0.932_1.712) 0.133 | 0.133 |
| Poorely differentiated | 1.71 (1.07_2.73) | 0.029 | 1.39 (0.81_2.37) | 0.226 |
| Dissected Lymph node | ||||
| >12 (reference) | 1 | 1 | ||
| ≤12 | 0.80 (0.58_1.11) | 0.195 | 0.85 (0.60_1.22) | 0.391 |
| Proportion of positive Lymph nodes | ||||
| ≤0.16 | 1 | 1 | ||
| >0.16 | 2.02 (1.54_2.64) | <0.001 | 1.41 (0.85_2.33) | 0.170 |
| Lympho-vascular invasion | ||||
| No (reference) | 1 | 1 | ||
| Yes | 2.44 (1.89_3.16) | <0.001 | 2.03 (1.52_3.72) | <0.001 |
| Perineural invasion | ||||
| No (reference) | 1 | 1 | ||
| Yes | 1.81 (1.40_2.36) | <0.001 | 1.08 (0.75_1.56) | 0.656 |
| Treatment (colon) | ||||
| Adjuvant therapy | 1 | 1 | ||
| Neoadjuvant therapy | 2.04 (1.28_3.24) | 0.002 | 1.4 (0.77_2.35) | 0.269 |
| Treatment (rectum) | ||||
| Adjuvant therapy | 1 | 1 | ||
| Neoadjuvant therapy | 1.54 (1.05_2.26) | 0.028 | 1.28(0.80_2.07) | 0.291 |
Reference category; NI, Not included
Discussion
This study examined factors that influence the recurrence of CRC among study participants referring to the department of radiation oncology at Namazi hospital, Shiraz, Iran.
In this study the overall five-year recurrence rate was 56.8%, which seems higher from what is reported by previous studies in Iran (35%), US (46%) and Korea (28.5%) (DeMatteo et al., 2000; Huh et al., 2009; Aghili et al., 2010). Findings of similar studies in China suggested that 5-year survival rate of CRC is 67.9% (Chen et al., 2016b). Also Merkel et al., (2017) reported that 5year disease free survival rate for rectum cancer was 72% One explanation for this finding is younger age of CRC diagnosis, which results in worse prognosis of patients (O’Connell et al., 2004; Dianatinasab et al., 2016). As shown in the result section, the mean age of the study patients was lower than what reported before (O’Connell et al., 2004). According to a study in the United States CRC incidence rate is becoming more prevalent among younger individuals. In the present study, 36.7% of all patients with CRC were diagnosed at stage III. As reported by a study in Iran, access to enhanced medical services including cancer diagnosis and treatment is poor. As the result, most patients receive the required medical services when they are severely ill at advanced stage (Dianatinasab et al., 2016a).
According to the results from multiple Cox regression analysis, older age at diagnosis is directly associated with the recurrence of CRC. This finding is consistent with previous studies suggesting higher risk of recurrence in older patients (Aghili et al., 2010; Westberg et al., 2015). However, this finding is not supported by a study in which suggested a by Dina et al., (2015). Similar to Fatemi et al., (2015) the present study found no significant association between gender and risk of CRC recurrence.
In this study, one of the most important factors influencing the recurrence of disease was tumor site. According to the results, patient with rectal site of tumor had significantly higher risk of recurrence (Sloothaak et al., 2014). A previous study reported the same result supporting the association between rectum cancer and a higher risk of recurrence (Leşe et al., 2013). In addition, this study showed that tumor stage is associated with the risk of recurrence. In accordance to this finding, results of a study also suggested that patients with colorectal cancer at stage III have worse prognosis and more chance for recurrence compared to patients with stage I of the tumor (Sloothaak et al., 2014). Also Dianatinasab et al., (2016c) reported that higher tumor stage is significantly associated with worse prognosis of CRC. Westberg et al., (2015) report that early local recurrence in patients with a stage III primary tumor were more common compared with the late local recurrence.
Figure 2.
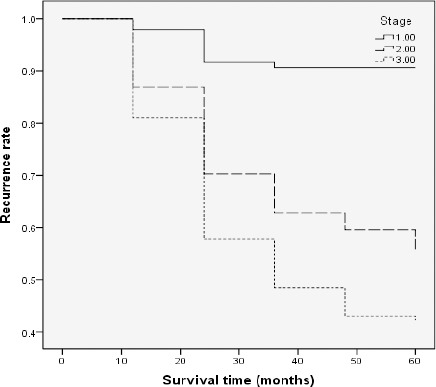
Cumulative Survival (Relapse) Curve for Colorectal Cancer According to Stage (P<0.001)
According to univariate analysis, grade of tumor was significantly associated with recurrence of disease. However, after controlling for other potential confounders, no statistically significant association between CRC grade and recurrence was found. This finding is in accordance to the results of other studies suggesting no significant association between grade and recurrence of CRC (Aghili et al., 2010; Sloothaak et al., 2014). As mentioned in the results section, no significant association was found between tumor size and recurrence. This finding is supported by other study by (Dina, 2015).
Surgery was the main treatment strategy for CRC, with the exception of relatively small number of patients with metastatic cancer. Adjuvant therapy such as chemotherapy and radiation therapy, are often used before (neoadjuvant therapy) and after surgery to reduce the risk of recurrence and metastasis (Huh et al., 2009; Van Cutsem and Oliveira, 2009; Barbosa et al., 2016; Chen et al., 2016a). Although in univariable analysis neoadjuvant therapy showed a significant association with cancer recurrence, but after controlling the effect of other variables this association was not statistically significant. Previous studies suggested that adjuvant chemotherapy or radiation therapy after surgery for colon cancer is more effective for patients aged 70 and older compared to younger patients (Popescu et al., 1999). In the present study, many participants were relatively younger which may justify the contradicting results. Another study also showed that applying both radiotherapy and chemotherapy had no significant effect on preventing recurrence (Huh et al., 2011).
Finally, the lymph node ratio (the relationship between the number of total lymph nodes examined) among patients who experienced cancer recurrence and patients without cancer recurrence was not significantly different. This finding is in contrast with what was reported by Bogdan (Dina, 2015) who reported that more lymph node ratio has worse prognosis in colorectal cancer treatment. Also in Ooki and et al study they found that higher LNR value was significantly associated with recurrence (Ooki et al., 2017).
Strengths and Limitations
The present study used all patients who visited the biggest referral medical center in the southern part of the country. This advantage makes the results generalizable to the population of the country. However, some prognostic factors such as education level and socioeconomic status may be related with prognosis of CRC (Khazaei et al., 2015) which have not been fully investigated in the present study.
In conclusion, this cohort study revealed that several factors can affect patient’s recurrence rate. As the stage of disease is associated with survival of CRC, it seems beneficial to encourage people to do screening. Access to enhanced medical services for early diagnosis of cancer diagnosis and treatment is needed to improve the survival and quality of life of CRC patients.
Declarations
List of abbreviations
Abbreviations: HR= Hazard ratio, CRC= Colorectal cancer, LV= Lympho-vascular invasion invasion, PN= Perineural invasion, 95%CI= 95% confidence interval.
Source of funding
The present study was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (No: 95-01-04-12557).
Authors’ contributions
MZ contributed in the conception of the work, conducting the study and preparing the draft. MD contributed in the conception of the work, drafting and revising the draft, MF contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. ShZ contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MM and ND contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. All authors approve the final version that is submitted to the APJCP. The corresponding author affirms the information mentioned here on behalf of research team and has been given the authority to modify the manuscript if requested by the journal’s editorial team.
Conflict of interest
All members of the study declare that they have no conflicts of interest.
References
- Aghili M, Izadi S, Madani H, et al. Clinical and pathological evaluation of patients with early and late recurrence of colorectal cancer. Asian Pac J Clin Onco. 2010;l(6):35–41. doi: 10.1111/j.1743-7563.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- Barbosa N, Barbosa E, Taveira-Gomes T, et al. Laparoscopy and laparotomy for colorectal cancer:a comparative single-center study. Colorectal Cancer. 2016;5:135–45. [Google Scholar]
- Chen C-H, Wei P-L, Hsieh M-C, et al. The outcomes of therapeutic decision in lower 3rd rectal cancer patients. Medicine. 2016a;95:4638. doi: 10.1097/MD.0000000000004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Wei P-L, Hsieh M-C, et al. The outcomes of therapeutic decision in lower 3rd rectal cancer patients. Medicine. 2016b;95:4638. doi: 10.1097/MD.0000000000004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors:recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianatinasab M, Ghaem H, Rezaianzadeh A, Hosseini SV, Khazraei H. Colorectal cancer mortality in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:4103–7. [PubMed] [Google Scholar]
- Dianatinasab M, Fararouei M, Mohammadianpanah M, et al. Impact of social and clinical factors on diagnostic delay of breast cancer:A Cross-sectional study. Medicine. 2016a;95:4704. doi: 10.1097/MD.0000000000004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianatinasab M, Fararouei M, Mohammadianpanah M, et al. Hair coloring, stress, and smoking increase the risk of breast cancer:A case-control study. Clin Breast Cancer. 2017 doi: 10.1016/j.clbc.2017.04.012. doi.org/10.1016/j.clbc.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Dianatinasab M, Ghaem H, Rezaianzadeh A, et al. Colorectal cancer mortality in Shiraz, Iran. Asian Pac J Cancer Prev. 2016b;17:4103–7. [PubMed] [Google Scholar]
- Dianatinasab M, Ghaem H, Rezaianzadeh A, et al. Colorectal cancer mortality in Shiraz, Iran. Asian Pac J Cancer Prev. 2016c;17:4101–5. [PubMed] [Google Scholar]
- Dina L. Assessment of clinical and pathological prognostic factors for colorectal cancer recurrence after surgery. Human Veterinary Med. 2015;7:47–54. [Google Scholar]
- Fararouei M, Parisai Z, Farahmand M, et al. Cancer incidence appears to be rising in a small province in Islamic Republic of Iran:a population-based cohort study. East Mediterr Health J. 2015;21:319. doi: 10.26719/2015.21.5.319. [DOI] [PubMed] [Google Scholar]
- Fatemi SR, Pourhoseingholi MA, Asadi F, et al. Recurrence and five-year survival in colorectal cancer patients after surgery. Iran J Cancer Prev. 2015;8:3439. doi: 10.17795/ijcp.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gan S, Wilson K, Hollington P. Surveillance of patients following surgery with curative intent for colorectal cancer. World J Gastroenterol. 2007;13:3816–23. doi: 10.3748/wjg.v13.i28.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Lim SW, Kim HR, et al. Effects of postoperative adjuvant radiotherapy on recurrence and survival in stage III rectal cancer. J Gastrointest Surg. 2011;15:963–70. doi: 10.1007/s11605-011-1497-7. [DOI] [PubMed] [Google Scholar]
- Huh JW, Park YA, Lee KY, et al. Recurrences after local excision for early rectal adenocarcinoma. Yonsei Med J. 2009;50:704–8. doi: 10.3349/ymj.2009.50.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Khazaei S, Rezaeian S, Mansori K, et al. Effects of human development index and its components on colorectal cancer incidence and mortality:a global ecological study. Asian Pac J Cancer Prev. 2015;17:253–6. doi: 10.7314/apjcp.2016.17.s3.253. [DOI] [PubMed] [Google Scholar]
- Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- Leşe M, Petric M, Mare C. Epidemiologic factors of colorectal cancer in a county hospital in Romania. J Vet Med. 2013;5:19–23. [Google Scholar]
- Malekzadeh R, Bishehsari F, Mahdavinia M, et al. Epidemiology and molecular genetics of colorectal cancer in iran:a review. Arch Iran Med. 2009;12:161–9. [PubMed] [Google Scholar]
- Merkel S, Weber K, Göhl J, et al. Survival analysis in rectal carcinoma after neoadjuvant chemoradiation:various methods with different results. Int J Colorectal Dis. 2017;32:1295–1301. doi: 10.1007/s00384-017-2861-1. [DOI] [PubMed] [Google Scholar]
- Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Di. 2008;23:683–8. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- O'Connell JB, Maggard MA, Livingston EH, et al. Colorectal cancer in the young. Am J Surg. 2004;187:343–8. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Ooki A, Akagi K, Yatsuoka T, et al. Lymph node ratio as a risk factor for recurrence after adjuvant chemotherapy in stage III colorectal cancer. J Gastrointest Surg. 2017;21:867–78. doi: 10.1007/s11605-017-3382-5. [DOI] [PubMed] [Google Scholar]
- Popescu R, Norman A, Ross P, et al. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- Sloothaak D, Sahami S, van der Zaag-Loonen HJ, et al. The prognostic value of micrometastases and isolated tumour cells in histologically negative lymph nodes of patients with colorectal cancer:a systematic review and meta-analysis. Eur J Surg Oncol. 2014;40:263–9. doi: 10.1016/j.ejso.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Oliveira J. Primary colon cancer:ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2009;20:49–50. doi: 10.1093/annonc/mdp126. [DOI] [PubMed] [Google Scholar]
- Westberg K, Palmer G, Johansson H, et al. Time to local recurrence as a prognostic factor in patients with rectal cancer. Eur J Surg Oncol. 2015;41:659–66. doi: 10.1016/j.ejso.2015.01.035. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Li M-D, Hu H-G, et al. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World J Gastroenterol. 2013;19:2650–9. doi: 10.3748/wjg.v19.i17.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare-Bandamiri M1, Khanjani N, Jahani Y, Mohammadianpanah M. Factors affecting survival in patients with colorectal cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159–63. doi: 10.7314/apjcp.2016.17.1.159. [DOI] [PubMed] [Google Scholar]


