Abstract
Purpose:
To determine expression levels of CD44 and ALDH1/2, known cancer stem cell (CSC) markers, in stomach adenocarcinomas and assess relationships with clinicopathologic parameters and prognosis.
Methods:
Eighty patients diagnosed with gastric cancer between the years 2011-2015 were included in this study of clinicopathologic characteristics, postoperative prognostic indexes and stem cell marker CD44 and ALDH1/2 expression in paraffin-embedded tumour sections analyzed immunohistochemically. Clinicopathologic parameters were evaluated using the chi-square test and t-test. Survival analyses were conducted using Kaplan-Meier statistics.
Results:
We observed positive CD44 and ALDH1/2 staining in 45.0 % and 67.5% of tumour tissues, respectively, but not in normal gastric mucosa. Recurrence-free survival (RFS) was found to be shorter in cases with high levels of CD44 expression (p=0.004). Similarly, short RFS was observed in patients with high levels of CD44 and ALDH1/2 co-expression (p=0.004). Furthermore, tumour invasion depth was found to correlate with high CD44 and ALDH1/2 co-expression (p=0.028).
Conclusion:
The cancer stem cell markers CD44 and ALDH1/2 may indicate poor patient prognosis and play a role in tumour development and invasion.
Keywords: ALDH1/2, cancer stem cell, CD44, gastric cancer, prognosis
Introduction
Cancer stem cells (CSCs) are cells that have the ability to initiate tumour development and that are responsible for tumour self-renewal. CSCs have differentiation abilities and most of the characteristics of embryonic cells. In studies performed on mice, it was shown that within the tumour, only a specific group of cells contributed to tumour growth, whereas other cells did not (Singh et al., 2004). Thus, CSCs have two main functions: self-renewal and differentiation. CSCs are simultaneously responsible for not only initiating tumour development, metastasis, and recurrence but also resistance to cancer treatments (Soltanian et al., 2011). In recent years, CSCs were successfully identified and isolated in various cancers. Established CSC markers include CD44, CD24, CD166, and CD133, stage-specific antigen-1 (SSEA), SSEA4 and aldehyde dehydrogenase (ALDH) (Takaishi et al., 2009). CD44 is a transmembrane glycoprotein that was first identified on lymphocytes and that has an important role in the adhesion and migration of the cells. CD44 is found in the extracellular matrix and exerts its adhesion characteristics by interacting with hyaluronic acid. This glycoprotein plays an important role in the invasiveness of tumour cells and can change the adhesive characteristics of these cells. CD44 may affect tumour cell migration towards the veins or cause embolus formation through the accumulation of cells (Chen et al., 2005). CD44 participates in many cellular processes such as cell growth and differentiation (Vigetti et al., 2008). Previous studies have emphasized the importance of CD44 expression in tumour advancement, metastasis, and prognosis in gastric, urothelial, pancreatic, colorectal, liver and cervical tumours (Liu et al., 2005; Visca et al., 2002). ALDH1 is a new promising marker for CSCs. ALDH enzymes are members of an intracellular enzyme family that includes ALDH1, ALDH1A1, ALDH1A2 and ALDH1A3, as well as the ALDH2 subfamily. These enzymes are responsible for the oxidation of intracellular aldehydes to carboxylic acids and are responsible for drug resistance through the oxidation of cellular aldehydes (Moreb et al., 1996). ALDH enzymes are present in normal tissues and CSCs. ALDH1 is specifically used in identifying CSCs (Marchitti et al., 2008).
It has been recently reported that ALDH1 is related to poor prognosis in breast cancer, lung cancer, ovarian cancer and esophageal cancer (Ginestier et al., 2007).
Although a few studies evaluated these markers together in gastric cancer, these studies were performed on cultured cells. This study is the first to investigate the combined expression of CD44 and ALDH1/2, which are CSC markers, in paraffin-embedded tissues from patients with GC. This study aimed to determine the relationship of these biomarkers with patient survival and other clinicopathologic features.
Material and Methods
Study population
In present study, total of 80 primary gastric carcinoma cases were selected from archives of Kayseri Training and Research Hospital Pathology Department. All the operations were performed between March 2011 and November 2015. In this retrospective study paraffin embedded blocks of patients were used.
Tumour samples that had been removed during surgery and then embedded in paraffin blocks were immunohistochemically stained.
Tumour stages were classified according to the American Joint Committee on Cancer (AJCC) TNM staging system (Edge et al., 2010).
Immunohistochemical assay and evaluation
When the samples were selected, the tumour tissues that had neighboring normal stomach mucosal tissue were preferred. For the immunohistochemical examination, two paired sections treated with lysine were selected from the stored tumour samples obtained from each patient. Paraffin-embedded tissues fixed with formalin were cut to a 4-mm thickness and were incubated for 15 minutes in a drying oven. Antibodies against CD44 (CD44 Std. /HCAM Ab-4, Thermo Scientific, USA, dilution: 1:100) and ALDH1/2 (ALDH1/2 (H-8): sc-166362, Santa Cruz Biotechnology, Inc., Europe, dilution: 1:200) were applied using a fully automated Benchmark Ultra, Ventana (Roche Group) immunohistochemistry machine. The selected sections were submerged in alcohol for 10 minutes and then in xylene for 5 minutes, after which the system was turned off. The results were evaluated by two pathologists. According to the descriptions of positive staining in previous reports, if there was staining in at least 10% of the tumour cells, the result was considered positive. If less than 10% of the tumour cells were stained, the result was considered negative (Jiang et al., 2009). The tumour samples were classified according to the percentage of positive tumor cells as follows: very low, 0 (<5% positive cells); low, 1(5-10% positive cells); moderate, 2(11-50% positive cells); and high, 3(>50% positive cells). Tumours were categorized based on the following scores: ≤ 1, negative; > 1, positive. The patients were evaluated in terms of age, gender, histological type, invasion depth, lymph node metastasis, tumour location, lymphatic invasion, perineural invasion, differentiation degree, tumour stage, overall survival (OS), and recurrence-free survival (RFS).
Statistical analysis
Data were analyzed using SPSS Statistics for Windows, Version 19.0 (IBM Corporation; Chicago, IL, USA).
The significance of the relationship between CD44 and ALDH1/2 and gender, histological type, invasion depth, lymph node metastasis, tumour location, lymphatic invasion, perineural invasion, differentiation degree, and tumour stage was evaluated statistically using the chi-square test(X2). The survival analyses were conducted using the Kaplan-Meier test. The significance of the relationship with age was evaluated with the t-test and was accepted to be statistically significant if p<0.05.
Results
Eighty patients with gastric cancer were analyzed in this study. According to the results of immunohistochemistry, we correlated ALDH1/2 and CD44 status gastric cancer specimens with clinic pathologic parameters (Table 1). The tumour invasion depth was found to correlate with high CD44 and ALDH1/2 co-expression (p=0.028). There was no statistically significant relationship between the expression of these two cancer stem cell markers and the patient age, patient gender, lymphatic invasion, perineural invasion, lymph node metastasis, tumour localization, histological tumour type, tumour differentiation degree or tumour stage (p>0.05) (Table 1).
Table 1.
Relationship of CD44 and ALDH1/2 Expression with Clinicopathologic Parameters
| CD44 expression36/80 (45.0%) | ALDH1/2 expression54/80 (67.5%) | Co-expression 25/80 (31.25%) | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total number | Positivite rate | P-Value | Positivite rate | P-Value | Positivite rat | P-Value |
| Sex | |||||||
| *Male | 48 | 24/48(66.7%) | 0.36 | 34/48 (63.0%) | 0.47 | 41/48 (63.1%) | 0.381 |
| *Female | 32 | 12/32(33.3%) | 20/32 (37.0%) | 24/32 (36.9%) | |||
| Histology | |||||||
| *intestinal | 58 | 28/58(77.8%) | 0.115 | 40/58 (74.1%) | 0.93 | 48/58 (73.8%) | 0.635 |
| *diffuse | 14 | 3/14 (8.3%) | 9/14 (16.7%) | 10/14 (15.4%) | |||
| *mixed | 8 | 5/8 (13.9%) | 5/8 (9.3%) | 7/8 (10.8%) | |||
| Differentiation | |||||||
| *well | 9 | 3/9 (8.3%) | 0.335 | 6/9 (11.1%) | 1.000 | 6/9 (9.2%) | 1.190 |
| *moderate | 37 | 20/37(55.6%) | 25/37 (46.3%) | 33/37 (50.8%) | |||
| *poorly | 34 | 13/34(36.1%) | 23/34 (42.6%) | 26/34 (40.0%) | |||
| Lymphatic invasion | |||||||
| *Yes | 66 | 31/66(86.1%) | 0.559 | 45/66 (83.3%) | 1.000 | 55/66 (84.6%) | 0.450 |
| Perineural invasion | |||||||
| *Yes | 56 | 24/56(66.7%) | 0.628 | 38/56 (70.4%) | 1.000 | 46/56 (70.8%) | 1.000 |
| Lymph node metastasis | |||||||
| *Yes | 66 | 31/66(86.1%) | 0.559 | 45/66 (83.3%) | 1.000 | 55/66 (84.6%) | 0.450 |
| Tumor location | |||||||
| *proximal | 28 | 12/28(33.7%) | 0.817 | 17/28 (31.5%) | 0.4 | 21/28 (32.3%) | 0.370 |
| *distally | 52 | 24/52(66.7%) | 37/52 (68.5%) | 44/52 (67.7%) | |||
| Depth of invasion | |||||||
| *mucosa | 4 | 1/4 (2.8%) | 0.795 | 1/4 (1.9%) | 0.22 | 1/4 (1.5%) | |
| *muscularis propria | 8 | 4/8 (11.1%) | 6/8 (11.1%) | 7/8 (10.8%) | |||
| *serosa | 68 | 31/68(86.1%) | 47/68 (87.0%) | 57/68 (87.7%) | 0.028* | ||
| TNM Stage | |||||||
| *I | 6 | 2/6 (33.3%) | 0.507 | 2/6 (33.3%) | 0.19 | 1/6 (16.6%) | 0.714 |
| *II | 15 | 5/15 (33.3%) | 10/15 (66.7%) | 4/15 (26.6%) | |||
| *III | 59 | 29/59(49.2%) | 42/59 (71.2%) | 20/59 (33.9%) | |||
X2 test, p<0.05 was considered significant; Tumor stage was classified according to the American Joint Committee on Cancer (AJCC) TNM staging system; ALDH1/2: aldehyde dehydrogenase ½.
Relationship of positive ALDH 1/2 staining with clinicopathologic parameters
ALDH1/2 was stained in the cytoplasm of tumour cells in both the intestinal type and diffuse type (i.e., signet-ring cell type) (Figure 1a, b). The positivity rate of ALDH1/2 was 67.5% in gastric cancer samples. According to the results of immunohistochemistry, we correlated ALDH1/2 status in 80 gastric cancer specimens with clinicopathologic parameters (Table 1). There was no statistically significant relationship between the expression of ALDH1/2 and clinicopathologic parameters.
Figure 1.
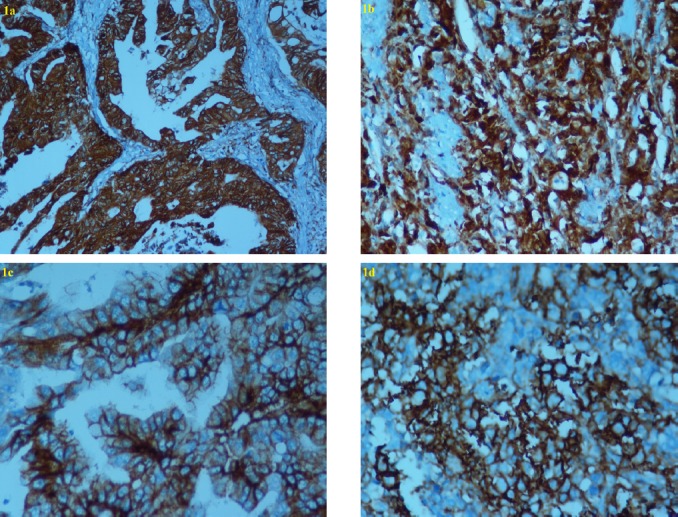
Immunohistochemical Analysis of ALDH1/2 And CD44 in Gastric Cancer Tissue. Figure 1 Subtitles, Cytoplasmic staining of aldehyde dehydrogenase ½ (ALDH ½) was observed in intestinal-type (a) and diffuse type (b) GC (original magnification: X400). Membranous staining of CD44 was observed in intestinal type (c) and diffuse type (d) GC (Original magnification: X400).
Relationship of positive CD44 staining with clinicopathologic parameters
Membrane staining with CD44 was observed in the intestinal and diffuse types of tumour cells (Figure 1c, d). CD44 was stained in the lymphocytes, stromal cells and sometimes the intestinal metaplasia (2 cases); however, staining was not observed in the epithelial cells. The positivity rate of CD44 was 45.0 in gastric cancer samples. According to the results of immunohistochemistry, we correlated CD44 status in 80 gastric cancer specimens with clinicopathologic parameters (Table 1). There was no statistically significant relationship between the expression of CD44 and clinicopathologic parameters.
The relationship of ALDH 1/2 and CD44 co-expression with clinicopathologic parameters
The positivity rate of ALDH1/2 and CD44 co-expression was 31.25% in gastric cancer samples. In patients with serosal invasion, a statically significant relationship between ALDH1/2 and CD44 co-expression was determined (p=0.028, X2test), (Table 1). Patients demonstrating ALDH1/2 and CD44 expression were more inclined towards serosal invasion.
Relationship of CD44 and ALDH positivity with tumour stage and patient survival
Short RFS was observed in patients who demonstrated high CD44 expression compared to those who did not (13 months vs. NR, respectively; p=0.004) (Figure 2). Similarly, short RFS was observed in patients who demonstrated ALDH1/2 and CD44 co-expression compared to those who did not (10 months vs. NR, respectively; p=0.004) (Figure 3). There was no statistically significant relationship between the expression of ALDH1/2 and RFS.
Figure 2.
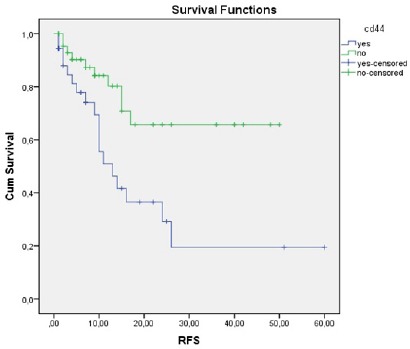
The Relationship between CD44 Expression and RFS
Figure 3.
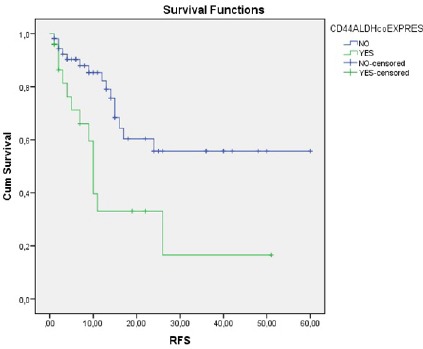
The Relationship between CD44 and ALDH1/2 co-Expression and RFS
We evaluated the OS of patients with or without high CD44 expression (12 months vs. 13 months, p=0.69), patients with or without high expression of ALDH1/2 (11 months vs. 18 months, p=0.65), and patients with or without high co-expression of CD44 and ALDH1/2 (11 months vs. 15 months, p=0.2). Those patients who demonstrated elevated cancer stem cell marker expression levels were inclined to have short OS, but this result was not significant.
Of the enrolled patients, 6 were stage I, 15 were stage II, and 59 were stage III. Of the patients with high CD44 expression, 33.3% were stage I, 33.3% were stage II, and 49.2 were stage III. Of the patients with high ALDH1/2 expression, 33.3% were stage I, 66.7% were stage II, and 71.2% were stage III. A significant relationship between CD44 and ALDH1/2 expression and the stage was not determined (p>0.05, X2test).
Discussion
Gastric cancer is one of the most prevalent causes of death worldwide (Compare et al., 2010). Despite advanced treatments, the prognosis of patients with gastric cancer is poor due to tumour recurrence and metastasis. The cancer stem cell model explains the high relapse rate and treatment resistance (Vermeulen et al., 2012). According to the stem cell hypothesis, CSCs are responsible for cancer initiation and development. CSCs were identified in some solid cancers, and these markers might serve as targets to prevent tumour spread and recurrence (Brungs et al., 2016). In this study, the distribution and expression of the CSC markers CD44 and ALDH1/2 in stomach tissue samples from GC patients were examined. The staining pattern of these CSC markers in the tumour area and non-tumour stomach tissue was observed, and the relationship between the staining pattern and the clinicopathologic characteristics and prognosis of the patients was investigated. In our study, strong and intense staining for these two CSC markers was observed in some cancer cells. In normal stomach mucosa, staining was observed in the intestinal metaplasia area in some cases, consistent with previous reports (Wakamatsu et al., 2012). While staining was not observed in normal stomach tissue in our study, because CD44 and ALDH1/2 expression was observed in tumour cells, these markers were considered related to malignant transformation.
CD44 is a marker that plays an important role in the invasiveness of tumour cells. CD44 expression has been investigated in breast, cervical, colon, lung, esophageal, liver and gastric cancers; this protein was determined to be important in tumour advancement and metastasis (Castella et al., 1998). In previous GC studies, an increase in the percentage of CD44-positive cells was observed as the invasion depth increased (Chen et al., 2005). In our study, a statistically significant relationship between CD44 and ALDH1/2 positivity was observed (p=0.028) in patients who had serosal invasion. Thus, CD44 and ALDH1/2 may have important roles in tumour invasion. However, conflicting results have been obtained in different studies. For instance, Liu et al. reported that CD44 is more highly expressed in gastric cancer and is related to tumour formation, metastasis and clinically aggressive behaviours (Liu et al., 2005). In contrast, Arıcı et al. observed CD44 expression in tumour tissues but did not find a significant relationship between CD44 expression and the tumour invasion depth, lymph node metastasis, or venous invasion (Arıcı et al., 2006). In our study, focal CD44 expression was observed in the normal stomach mucosa in the intestinal metaplasia area, and ALDH1/2 expression was observed in the intestinal metaplasia area. This finding was also reported in previous publications (Arıcı et al., 2006). As reported in studies of Harn et al., ALDH1/2 staining was observed in basal cells within the intestinal metaplasia area (5 case) and in the cytoplasm of parietal cells (Harn et al.,1995). While many studies have examined CD44, studies of ALDH are limited. Wakamatsu et al. found that overexpression of ALDH was related to the tumour invasion depth. In gastric cancer, the state of ALDH expression is responsible for tumour aggressiveness, and ALDH can be a target for cancer treatment (Wakamatsu et al., 2012). In this study, no statistically significant relationship was found between ALDH1/2 and CD44 expression and the patient age, patient gender, tumour type, tumour differentiation degree, lymphatic invasion, perineural invasion, lymph node metastasis, or tumour location (p >0.05). Xiao-shan Li et al. reported that patients expressing ALDH were more inclined towards tumour recurrence (Xiao-shan et al., 2014). In our study, ALDH1/2 and CD44 expression were evaluated together, and the patients expressing high levels of CD44 had shorter RFS and were more likely at an advanced stage. Additionally, a relationship between elevated CD44 and ALDH1/2 expression and overall survival could not be shown. We showed the staining characteristics of two CSC markers in normal, metaplastic and cancerous stomach tissue. Various parameters were evaluated, and it was concluded that these two CSC markers were related to the tumour invasion depth and poor patient prognosis.
The present study revealed that high expression of CD44 and ALDH1/2 correlated with increased tumour invasion depth and short RFS. Similarly, OS tended to be shorter in patients who expressed these markers at high levels. Thus, CD44 and ALDH1/2 expression may help in identifying high-risk GC patients and in choosing more aggressive treatments. Further prospective studies are needed to determine the importance of CSC markers in gastric cancers.
References
- ArıcıS KıvançF, Özer H, et al. CD44 and Ki 67 expression in gastric adenocarcinomas (correlation with clinicopathological parameters) Turk J Gastroenterol. 2006;28:289–97. [Google Scholar]
- Brungs D, Aghmesheh M, Vine KL, et al. Gastric cancer stem cells:evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51:313–26. doi: 10.1007/s00535-015-1125-5. [DOI] [PubMed] [Google Scholar]
- Castella EM, Ariza A, Pellicer I, et al. Differential expression of CD44V6 in metastases of intestinal and diffuse types of gastric carcinoma. J Clin Pathol. 1998;51:134–7. doi: 10.1136/jcp.51.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Wang ZC, Li H, et al. Nuclear translocations of beta-catenin and TCF4 in gastric cancers correlate with lymph node metastasis but probably not with CD44 expression. Hum Pathol. 2005;36:1294–1301. doi: 10.1016/j.humpath.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–8. [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American joint committee on cancer:the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn HJ, Ho LI, Chang JY, et al. Differential expression of the human metastasis adhesion molecule CD44V in normal and carcinomatous stomach mucosa of Chinese subjects. Cancer. 1995;75:1065–71. doi: 10.1002/1097-0142(19950301)75:5<1065::aid-cncr2820750503>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell- associated marker in lung cancer. Mol Cancer Res. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44s, CD44v6, and mRNA inhuman cancer. World J Gastroenterol. 2005;11:6601–6. doi: 10.3748/wjg.v11.i42.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes:the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreb j, Schweder M, Suresh A, Zucali JR. Overexpression of the human aldehyde dehydrogenase class I results in increased resistance to 4- hydroperoxycyclophosphamide. Cancer Gene Ther. 1996;3:24–30. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumourinitiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soltanian S, Matin MM. Cancer stem cells and cancer therapy. Tumour Biol. 2011;32:425–40. doi: 10.1007/s13277-011-0155-8. [DOI] [PubMed] [Google Scholar]
- Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells Int. 2009;27:1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, de Sousae Melo F, Richel DJ, Medema JP. The developing cancerstem-cell model:clinical challenges and opportunities. Lancet Oncol. 2012;13:83–9. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Viola M, Karousou E, et al. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem. 2008;283:4448–58. doi: 10.1074/jbc.M709051200. [DOI] [PubMed] [Google Scholar]
- Visca P, Del Nonno F, Botti C, et al. Role and prognostic significance of CD44s expression in colorectal cancer. Anticancer Res. 2002;22:2671–6. [PubMed] [Google Scholar]
- Wakamatsu Y, Sakamoto N, Oo HZ, et al. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62:112–19. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- Xiao-shan Li, Qing Xu, Xiang-yang Fu, Wei-sheng Luo. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer. 2014;14:705. doi: 10.1186/1471-2407-14-705. [DOI] [PMC free article] [PubMed] [Google Scholar]


