Abstract
Osteopontin (OPN) is an extracellular structural protein that is secreted by osteoblasts and hematopoietic cells. It suppresses the proliferation of hematopoietic stem and also plays an important role in promoting survival and drug resistance in leukemic stem cells (LSCs). Since the role of OPN isoforms in AML angiogenesis are remaining controversial, in the present study, we aimed to evaluate whether curcumin (CUR), as a known natural component with anti-angiogenesis effects, in a combination of AML conventional regiment has the potency to preclude induced anti-angiogenesis effects of OPN isoforms or not? Leukemia cells were treated with different concentration of CUR and AML conventional drugs alone and/or in combination with together to find effective doses and IC50 values. Percentages of apoptotic cells were evaluated by Annexin/PI staining and mRNA levels of OPN isoforms and AKT/ VEGF-A and VEGF-C/ STAT3/ β-catenin/ CXCR4/ IL-6/ KDR gene expression were investigated by Real Time-PCR method. Moreover, to confirm OPN gene expression data, we investigated the effect of simvastatin and OPN siRNA as an OPN inhibitor on the cell proliferation and induction of apoptosis in the indicated cell lines. Our data display that Ara-c (2μM and 1μM in KG-1 and U937 cell lines respectively), CUR (40μM in both cell lines), and also their combination significantly increased the percentage of apoptotic cells. Moreover, the mRNA level of OPN isoforms were down regulated in the KG-1and U937 cell lines treated with Ara-c while, upregulated in KG-1and U937 cell lines treated with CUR and its combination. Our results suggest that despite anti-angiogenesis effects of CUR, AML cells probably evade from anti-angiogenesis effects of CUR via induction of OPN b and c isoform and related molecular pathways.
Keywords: Osteopontin, anti-angiogenesis, chemoresistance, acute myeloid leukemia
Introduction
Acute Myeloid Leukemia (AML), is one of the most common hematologic disorders that, described by the prevented homeostatic mechanisms of normal hematopoietic stem cells (Shahrabi et al., 2016; Zahedpanah et al., 2016). Treatment for AML has comprised a combination of Cytarabine (Ara-c), an anthracycline (often daunorubicin) or anthracycline mitoxantrone (Bishop, 1997). However, 40 to 50% of AML patients achieve complete remission after intensive chemotherapy; there is a widespread variation in the incidence and recurrence of the disease (Kavianpour et al., 2016). Curcumin (CUR) is the major extracted component of Curry family (Huang et al., 1994; Bailly et al., 1997; Rao et al., 2011; Mohammadi et al., 2017c). In vitro studies have demonstrated that CUR specifically hinders the development of tumor cells as well as induction of cell apoptosis in a dose-dependent manner (Menon et al., 1995; Jiang et al., 1996; Wu et al., 2000). It is recommended that CUR has an exceptionally developing prospect in antitumor activities. In spite of the fact that CUR instigates apoptosis in the flexibility of AML cell lines, cytotoxic impacts of CUR in AMLs remain indistinct (Mohammadi et al., 2016b; Mohammadi et al., 2017a).
Osteopontin (OPN) is a glycoprotein and overexpressed in many cancers (Vejda et al., 2005; Rangel et al., 2008). The association of OPN, with different cancers and distinct stages of disease progression, suggests that it is a viable target for therapeutic interposition (Mi et al., 2009; Dai et al., 2010; Mohammadi et al., 2017c). In spite of the knowledge and understanding of OPN in soft tissue tumors, there is little information in connection with OPN in leukemia (Zahedpanah et al., 2016). Recent studies have shown that the oncogenic roles of OPN, including excitation of cell proliferation, invasion and migration might be regulated through different OPN isoforms such as OPN-a, OPN-b and OPN-c (Liu et al., 2004; Flamant et al., 2005; Nilsson et al., 2005; Mirza et al., 2008; Powell et al., 2009; Zduniak et al., 2015). Although many studies have been conducted on the effect of OPN in solid tumors, but not addressed, the effect of different isoforms of OPN in the hematologic malignancies (Philip et al., 2001; Philip and Kundu, 2003; Rangel et al., 2008; Shevde and Samant, 2014). Our previous study revealed that upregulation of OPN-b and c in AML cells were concurrently associated with the upregulation of AKT/VEGF/CXCR4/STAT3/ IL-6 genes expression as a part of molecular loop involved in angiogenesis (Mirzaei et al., 2017). Based on the critical role of CUR in the suppression of angiogenesis in cancer cells (Ding et al., 2014; Huang et al., 2015), it seems reasonable to hypothesize that combination of CUR with conventional AML regiment results in inhibition of OPN b and c isoforms as LSCs molecular surrogate. Therefore, we analyzed the expression of OPN isoforms in both resistants (KG-1) as an LSCs model (Zhang et al., 2010) and sensitive (U937) AML cell lines upon treatment with IDR, DNR, Ara-C as a conventional regiment in AML chemotherapy in a combination of CUR. Our results declare that OPN b and c isoforms probably veto anti-angiogenesis effects of CUR in combination with conventional AML regiment through induction of angiogenesis molecular loop.
Materials and Methods
Reagents
Annexin V-FITC apoptosis detection kit, dimethylsulfoxide (DMSO), DEPC treated water, Daunorubicin (DNR), Cytarabine (Ara-C), Idarubicin (IDR) and CUR were obtained from the Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). RPMI 1640 medium and fetal bovine serum (FBS) were purchased from (Gibco; Invitrogen, USA). The cDNA synthesis kit and SYBR Premix Ex Taq were obtained from Takara Bio Inc. (Takara Bio Inc., Otsu, Japan). Tripure isolation Reagent was purchased from Roche Applied Science (Roche Applied Science, Peuzberg, Germany).
Cell lines and Cell culture
The human leukemia cell lines KG-1 and U937 were obtained from the National Cell Bank of Pasteur Institute (Tehran, Iran). These cell lines were cultured in RPMI-1640 medium supplemented with 10% and 20% heat inactivated FBS for U937 and KG-1 cell line respectively, 2mM L-glutamine, and 100 units/mL penicillin and 100 µg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Dose Determination MTT assay and Annexin/PI Assay
Cell lines were treated with different doses of DNR (0-2μM), Ara-C (0-10μM), IDR (0-4 μM) and CUR (0-100 μM) at different times 24, 48 and 72 hours are compared with the control group. The experiments were performed in triplicate. Results were expressed as a proliferation rate, with 100% representing control cells treated with 0.1% DMSO alone. For simvastatin treatment, a relevant amount of working solution of simvastatin was added to the culture medium to achieve the concentrations of 2, 4, 6, 8, 10 µM.
For confirmation of MTT assay Annexin/PI staining was performed. Both AML cell lines were seeded at 5×105/ml per well in 6-well plates treated for 48 hours in absence and presence of various concentrations of CUR, Ara-c, IDR, DNR and their combinations. Discrimination of cells was performed as apoptosis (Annexin+ /PI- [early apoptosis] and Annexin+ /PI+ [late apoptosis]).
DNA Cell Cycle Analysis
Both AML cell lines KG-1 and U937 (5×105cells/well), permeabilized and treated with demonstrated concentrations of CUR and Ara-c for 48h, afterwards, fixed in 70% in cold ethanol and dye DNA quantitatively with 500 µL propidium iodide (PI) (50µg/mL in 0.1% Triton X-100/0.1% sodium citrate). Cells were distinguished by BD flow cytometer instrument analyzed with the flowjo program. According to PI staining, the cells with sub-G0/G1 (before DNA synthesis) pattern were considered as apoptotic cells.
Inhibition of OPN by Simvastatin and siRNA transfection
The effect of OPN mRNA expression and also the efficacy of cited drugs in the induction of apoptosis were tested by use of siRNA and also simvastatin (Sigma-Aldrich, St.Louis, MO, USA) in both cell lines. siRNA transfection was performed based described method (Mohammadi et al., 2016a). Alongside siRNA, Simvastatin was used as a natural component (made from Aspergillus terreus) for OPN inhibition.(Table-1)
Table 1.
OPN Specific siRNA Sequence
| Size (bp) | Sequence | Name |
|---|---|---|
| 21 | GGAAUAUUACUGUGGGAAAdTdT | Sense (5’-3’) |
| UUUCCCACAGUAAUAUUCCdTdT | Antisense (5’-3’) |
RNA isolation, cDNA synthesis, and Real Time PCR
Total RNA was isolated from the cells by using Tripure Isolation Reagent (Roche Applied Science, Peuzberg, Germany) according to manufacturer’s instructions. The quantity RNA samples were assessed spectrophotometrically using Nano drop ND-1000 (Nano-drop Technologies, Wilmington, DE). cDNA synthesis kit (Takara Bio Inc., Otsu, Japan) used for making cDNA from RNA. Real-Time PCR was performed with Step One Plus™ ABI instrument (Apply Bio systems, USA) using 2 μl of a 2-fold diluted cDNA, 0.5 μl of each forward and reverse primers (10 pMol) and SYBR Premix Ex Taq technology (Takara Bio Inc, Otsu, Japan) in a final volume of 20 μl. HPRT mRNA expression levels were used to estimate the relative expression levels. Thermal cycling conditions included a predenaturation step for the 30s at 950C followed by 45 cycles including a denaturation step for 5s at 950C and a combined annealing/extension step for 20s at 600C. The specificity of PCR reactions was confirmed by melting curve analysis. HPRT1 mRNA expression levels were used to estimate the relative expression levels, and the relative expression was calculated based on the 2−ΔΔCT method Table 2 Nucleotide sequences of the primers used for Real Time PCR and siRNA sequence.
Table 2.
Nucleotide Sequences of the OPN Isoforms and the Primers Used for Real Time PCR
| Gene | Accession number | Forward primer (5′-3′) | Reverse primer (5′-3′) | Size (bp) |
|---|---|---|---|---|
| OPN-a | NM_001040058.1 | ATCTCCTAGCCCCACAGAAT | CATCAGACTGGTGAGAATCATC | 208 |
| OPN-b | NM_000582.2 | ATCTCCTAGCCCCAGAGAC | AAAATCAGTGACCAGTTCATCAG | 209 |
| OPN-c | NM_001040060.1 | TGAGGAAAAGCAGAATGCTG | GTCAATGGAGTCCTGGCTGT | 155 |
| IL6 | NM_000600.4 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG | 132 |
| CXCR4 | NM_001008540.1 | TACACCGAGGAAATGGGCTCA | AGATGATGGAGTAGATGGTGGG | 127 |
| STAT3 | NM_003150.3 | GAGCGGGCCATCCTAAGCACA | TTGGTCTTCAGGTACGGGGCAGC | 176 |
| AKT | NM_005163 | AGCGACGTGGCTATTGTGAAG | GTACTCCCCTCGTTTGTGCAG | 51 |
| B-Catenin | NM_001098209 | TACCTCCCAAGTCCTGTATGAG | TGAGCAGCATCAAACTGTGTAG | 180 |
| VEGF-A | NM_001316955.1 | CTCACCAAGGCCAGCACATAGG | ATCTGGTTCCGAAAACCCTGAG | 159 |
| VEGF-C | NM_005429.4 | GTCTGTGTCCAGTGTAGATG | AGGTAGCTCGTGCTGGTGTT | 360 |
| VEGFR-2 (KDR) | NM_002253.2 | GTGACCAACATGGAGTCGTG | CCAGAGATTCCATGCCACTT | 660 |
| HPRT | NM_000194 | TGGACAGGACTGAACGTCTTG | CCAGCAGGTCAGCAAAGAATTTA | 111 |
Statistical Analysis
All data were presented as means ± SE of triplicate determinants. Data were analyzed using an unpaired two-tailed t-test or χ2 test. Statistical significances were defined at *P<0. 05, **P<0. 01, and ***P<0. 001 compared to the corresponding controls.
Results
Synergic Effects of CUR and Ara-C
The cytotoxic effects of CUR and three drugs such as Ara-c, DNR and IDR alone as well as in combination with CUR was evaluated in two distinct cell types of AML. After treatment with different concentrations of drugs for 24–72 h, growth suppressive effects were assessed by an MTT assay. Cells were cultured with different concentration of CUR (20μM, 40μM, 60μM, 80μM and 100 μM) (Figure 1), Ara-c (1-10 μM), IDA and DNR (1-2 μM) (Figure 2) for 24h, 48h and 72h to examine whether CUR has the potential to sensitize primary leukemic cells to the conventional AML regimen. We did not comprehend a significant difference between 48h and 72h. Our result demonstrated that Ara-C, DNR, and IDA inhibited cell proliferation with IC50 values of 1, 0.5, 0.2 μM for U937 cells and 2, 0.5, 0.4 μM for KG-1 cells respectively. Our results indicate a substantial increase in Ara-c sensitivity with the addition of CUR- 40μM at 24h (p<0.05) in both cell lines. This result suggests that combination of CUR and Ara-c was more effective than either treatment like a combination of CUR with DNR and IDR, clearly indicating that Ara-c and CUR treatment has synergistic effects (Figure 3) and (Figure 4).
Figure 1.
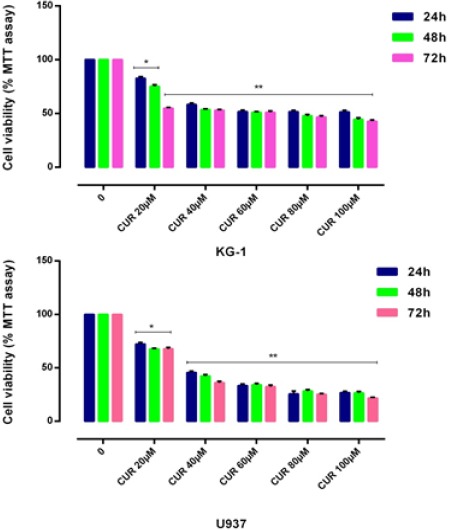
Effects of CUR with Different Concentration (0-100μm) on Cell Proliferation. Antiproliferative effect of CUR was measured by MTT assay following 24, 48 and 72h in KG-1 and U937 cell lines. There was no significant difference between 48 and 72 hours treatment. We detected of suitable dose for CUR (40μM for both cell lines). Data are mean ± SE of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Figure 2.
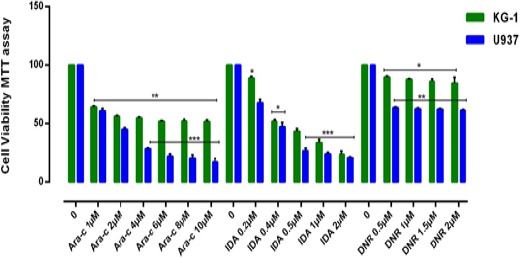
Effects of Ara-C, IDA and DNR with Different Concentration (0-10μM) on Viability of KG-1 and U937 Cell Lines. The anti-growth effect of each three drugs was measured by MTT assay following 24-48h exposed to cell lines. IC50 pharmaceutical doses for KG-1 cells, 0.5µM, 0.4µM and 2µM and for U937 cells 0.5µM, 0.2µM and 1µM were determined respectively for DNR, IDA, and Ara-c. Data indicated that the antiproliferative effects of these drugs leads to a reduction of viability and number of cells in a dose and time dependent manner. Data are mean ± SE of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Figure 3.
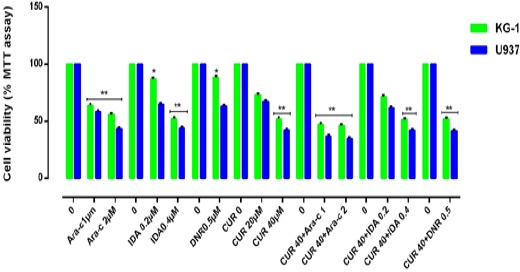
Effects of Combination of CUR with Ara-c, IDA, and DNR on Cell Viability. The anti-growth effect of CUR and combination with three drugs were measured by MTT assay following 24-48h in KG-1 and U937 cell lines. Data indicated that the antiproliferative effect of this drugs leads to a reduction of viability and number of cells in a dose and time dependent manner. The combination of CUR and three drugs were highly effective in inhibiting cell growth and promoting massive apoptosis in both cell lines. The combination of CUR and Ara-c is better than two other combinations. Combination effect of CUR and Ara-c compared to the control or even single compound could significantly decrease cell proliferation in both U937 and KG-1 cell lines. Data are mean ± SE of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Figure 4.
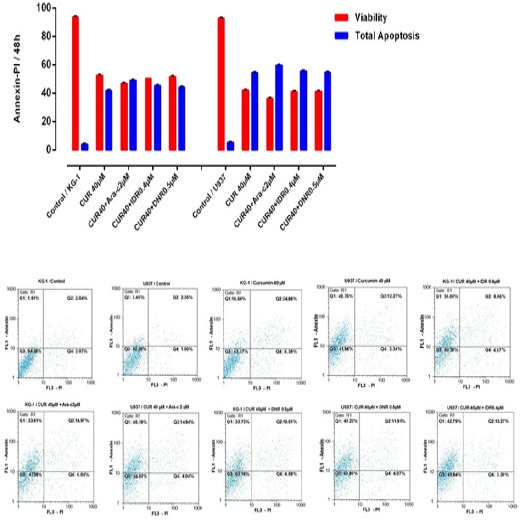
Effects of CUR and Ara-c on Apoptosis of U937 and KG1 Cell Lines were Evaluated by Annexin/PI Staining. The cells were treated with 40μM CUR and 1-2 μM Ara-c in U937 and KG1 respectively and harvested every 24 h. Apoptosis assay in KG-1 and U937 cell lines after treated with CUR in combination with three drugs in 48h. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Cell cycle assay
DNA content of KG-1 and U937 cells evaluated during cell cycle to get information about cell cycle progression. In present study after treatment of U937, the cell population in G0/G1 phase increased in all doses especially when cells treated with a combination of CUR and Ara-c for 48h. Moreover, our data exhibited that combination of CUR and Ara-C increased the Hypodiploid sub G0/G1 DNA fraction in dose depended manner 22.5% for KG-1 cell and 37.35% for U937 cell respectively)indicating apoptotic population) (Figure 5).
Figure 5.
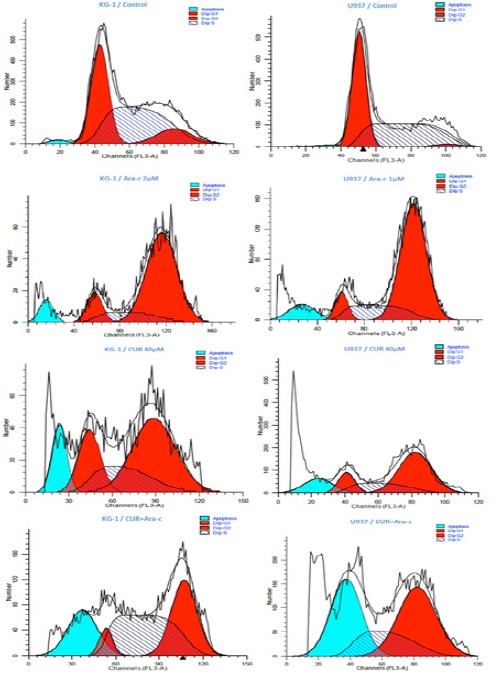
Effects of CUR and Ara-c on Cell Cycle of U937 and KG1 Cell Lines. The cells were treated with 40μM CUR and 1-2 μM Ara-c in U937 and KG1 respectively and harvested every 24 h. there upon Cell-cycle distribution was analyzed using flow cytometry.
Effect of CUR on OPN isoforms expression level in treated AML cells
KG-1 and U937 cells were treated with 3 drugs for 48h and then examined for expression of OPN isoforms by Real Time PCR. As shown in (Figure 6), in contrast of Ara-C, the expression level of OPN isoforms gene expression was strongly increased in CUR, IDA and DNR in comparison to the untreated cells. Ara-C decreases each three OPN isoforms, while, combination CUR and Ara-c result in an increase in each three OPN isoforms in KG-1 as well as a decrease in two isoforms a and b in U937 cell line.
Figure 6.
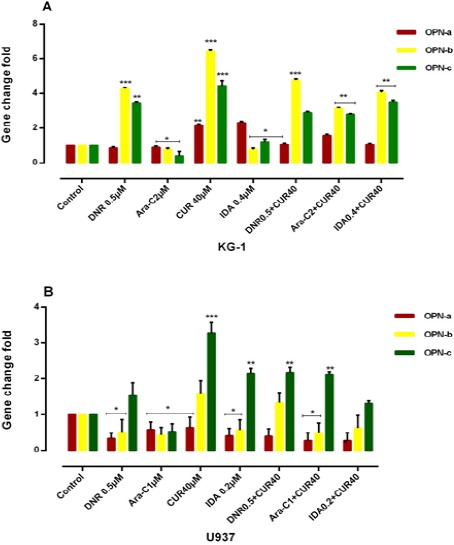
Combination of CUR with 3 Drugs (DNR, ARA-c, IDA, and CUR) on the mRNA Level of OPN Isoforms in KG-1(A) and U937(B) Cells. The effect of 3 drugs on the expression level of OPN was determined by Real Time-PCR analysis. Values were normalized using the expression of the housekeeping HPRT. The expression level of OPN isoforms gene expression was markedly increased in DNR, CUR and IDA-treated KG-1 cells compared to the untreated cells and cells that treated with drugs separately. OPN isoforms b and c considered as the predominant isoforms which showed an increased expression trend in treatment-resistant KG-1 cells as a result of a response to treatment. Values are given as mean ± S.E of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
Simvastatin and OPN specific siRNA reduces OPN gene expression in AML cell lines
In order to prove the effectiveness of drugs on gene expression of OPN and also to determine whether suppression of this gene has an effect on the response to the above-mentioned drugs in our cell lines or not, we used two OPN inhibitors. In the present study, we used simvastatin (3-hydroxy-3-methyl glutaryl coenzyme A reductase inhibitor) as a natural OPN inhibitor (Matsuura et al., 2010) and OPN specific siRNA for OPN gene expression inhibition (Figure 7). KG-1 and U937 cell lines were treated with simvastatin and in combination with 3 drugs and CUR for 24-48h to determine the effect of simvastatin on OPN expression in vitro. As our results show, simvastatin inhibited cell proliferation with IC50 values of 4μM and 8μM in U937 and KG-1 cells respectively. The best result was seen in the combination of simvastatin and Ara-c. Total RNA was then isolated from the cells, and OPN isoforms mRNA expression of AML cell lines determined by Real time PCR.
Figure 7.
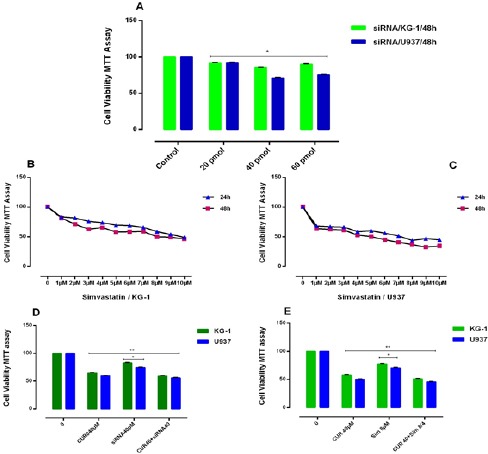
(A): Effects of siRNA against OPN with different concentration (0-60μM) on cell viability. siRNA inhibited cell proliferation with IC50 values of 40μM for each both cells lines. The anti-growth effect of simvastatin (0-10 μM) was measured by MTT assay following 24-48h exposed to KG-1(B) and U937 (C) cell lines. Simvastatin inhibited cell proliferation with IC50 values of 4μM and 8μM for U937 and KG-1 cells respectively. Data indicated that the antiproliferative effect of this drugs leads to a reduction of viability and number of cells in a dose dependent manner. (D): The effect of the combination of CUR40μM with siRNA OPN on cell viability in KG-1 and U937 cells. The suppressive effect of siRNA OPN (40 pM) for KG-1 and U937 on viability was assessed by MTT assay after 48h chemotrophic treatments in both cell lines. Combined treatments have resulted in a decrease in cell count and viability of cells, more than either drug treatment alone. The suppression of OPN gene by siRNA increased the susceptibility of two cell lines to apoptosis. (E): Synergistic effect of the combination of CUR 40μM with simvastatin ((8μM) for KG-1 and (4μM) for U937 and conventional AML regimen drugs on KG-1 and U937 cells. KG-1 and U937 leukemic cells were treated with drugs alone or in combination for 48h, and then cell count and the metabolic activity were assessed by MTT assays. Combined treatments resulted in significant decrease in cell count and viability of cells, more than either compound alone in comparison to control group. Data are mean ± SE of three independent experiments. Statistical significance was defined at *p<0.05 compared to corresponding control.
Our result notifies that OPN gene isoforms expression was significantly decreased in simvastatin treated groups in KG-1 and U937 cell lines. OPN gene isoforms expression had a slight decrease in a combination of CUR and simvastatin in KG-1 cell lines. When cells treated with CUR alone, all three isoforms of OPN increased in KG-1 but OPN-a gene isoform expression was decreased in U937 when cells treated with CUR alone. OPN-a gene isoform expression was significantly decreased in a combination of CUR and simvastatin in U937 cell lines, (*P<0.05). Moreover, we used the specific siRNA against OPN. The suitable dose for siRNA was tested using MTT test, which finally does of 40μM within 24 hours selected for transfection(Mohammadi et al., 2016a). Our data showed that the results of siRNA OPN effects alone and/or combination with CUR on both cell lines were similar to simvastatin effects on both cell lines. On the other hand, our results offer that CUR by enhancement of OPN isoforms gene at mRNA level could be nullified siRNA and drug -induced apoptosis in AML cell lines (Figure 7) and (Figure 8).
Figure 8.
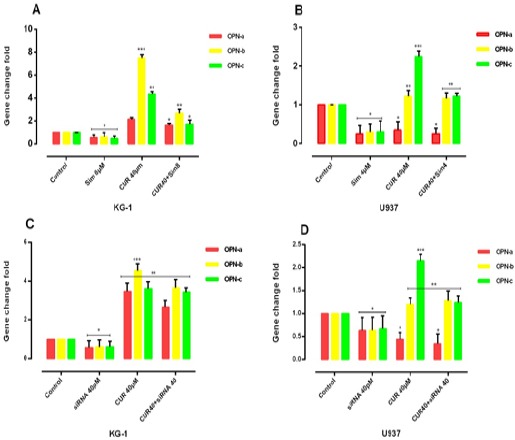
The effect of the combination of CUR with simvastatin on the expression level of OPN isoforms was determined by qRT-PCR analysis. Simvastatin can reduce the OPN isoforms in two cell lines treated with CUR and CUR by enhancement of OPN isoforms gene at mRNA level could be nullified drug -induced apoptosis in both AML cell lines, KG-1(A) and U937(B). Simvastatin with reduction of the expression of OPN might have a significant contribution to the effectiveness of the drugs. Values were normalized using the expression of the housekeeping HPRT. The effect of siRNA OPN on the expression level of OPN isoforms was determined by qRT-PCR analysis. The effect of the combination of CUR with OPN specific siRNA on the expression level of OPN isoforms was determined by qRT-PCR analysis. OPN specific siRNA can reduce the OPN isoforms in two cell lines KG-1(C) and U937(D) treated with CUR. Values were normalized using the expression of the housekeeping HPRT. Values are given as mean ± S.E. of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
OPN isoforms likely prevent CUR-induced Anti-angiogenesis through AKT/ VEGF-A and C/ STAT3/ β-catenin / CXCR4/ IL-6/ KDR
As shown in Figure-9, significantly the mRNA levels of OPN isoforms/VEGF-C/STAT3/CXCR4/IL-6/β-catenin/KDR were decreased in the Ara-c plus or without simvastatin-treated U937cells (P≤0. 05) After 48 h. The mRNA levels of OPN isoforms /STAT3 and β-catenin gene expression level were decreased in KG-1 cell lines after treatment with Ara-c. Likewise, KG-1 cells in treatment with CUR showed a decrease in STAT3 and in U937 cells in treatment with CUR shown a decrease in OPNa/VEGF-C/ β-catenin and KDR. Also, cells in treatment with simvastatin showed a decrease in VEGF-A, β-catenin, and AKT and three isoforms of OPN gene expression level in KG-1 and U937 cells.
Figure 9.
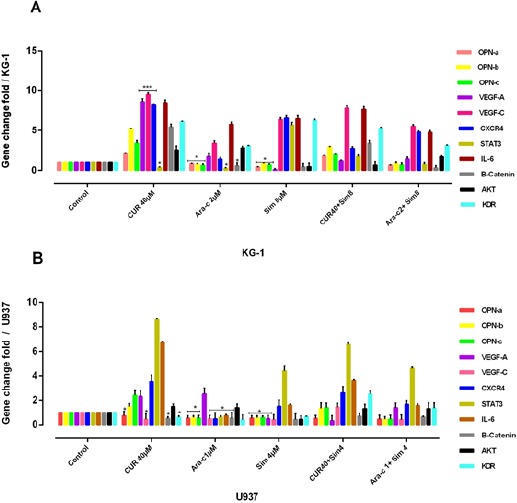
The Effect of OPN Isoforms Inhibitor on VEGF-A, VEGF-C, KDR, CXCR4, STAT3, IL-6 and β-Catenin Gene Expression in KG-1 and U937 Cells. The suppressive effect of OPN inhibitor alone or in combination with Ara-c and CUR treatment on KG-1(A) and U937 (B) and their combination on VEGF-A/VEGF-C/KDR/CXCR4/STAT3/IL-6/β-Catenin gene expression was assessed after 24h by qRT-PCR analysis in both KG-1 and U937 cell lines. Values were normalized using the expression of the housekeeping HPRT. The mRNA levels of OPN isoforms/VEGF-C/STAT3/CXCR4/IL-6/B-catenin/KDR were decreased significantly in the Ara-c plus or without simvastatin-treated U937cells (P≤0. 05) After 48 h. while, The mRNA levels of OPN isoforms /STAT3 and β-catenin gene expression level were decreased in KG-1 cell lines after treatment with Ara-c, on the other hand, KG-1 cells in treatment with CUR shown a decrease in STAT3 and in U937 cells in treatment with CUR shown a decrease in OPN-a/VEGF-C/B-catenin and KDR. Also cells in treatment with simvastatin shown a decrease in VEGF-A, β-catenin, and AKT and three isoforms of OPN gene expression level in KG-1 and U937 cells. Values were normalized using the expression of the housekeeping HPRT. Values are given as mean ± S.E. of three independent experiments. Statistical significance was defined at *p<0.05, **p<0.01 and ***p<0.001 compared to corresponding control.
In contrast, β-catenin/AKT/IL-6/CXCR4/VEGF-A/VEGF-C and VEGFR2 (KDR) gene expression level were increased in KG-1 cell lines after treatment with CUR in line with OPN isoforms. VEGF-C/STAT3/CXCR4/IL-6/β-catenin/KDR gene expression level was increased after combination CUR with simvastatin in parallel with OPN isoforms in both cell lines. VEGF-A and AKT gene expression level were increased in U937 cell lines after treatment with Ara-c. Hence, it sensible to promulgate a connection between angiogenesis and OPN isoforms.
Discussion
It has been well-documented that OPN is a secreted protein and may act an autocrine/paracrine manner in AML cell lines (Mohammadi et al., 2017a). It may affect on numerous survival signaling pathways and angiogenesis (Mohammadi et al., 2017b). The recent study declares that OPN deficiency affects angiogenesis in the bone dysfunctions and OPN is required for effective angiogenesis (Asou et al., 2001). Furthermore, OPN and VEGF overexpression are linked with angiogenesis in different cancers such as gastric carcinoma, myeloid leukemia, breast, and prostate cancer (Ramchandani and Weber, 2015). Our previous study revealed that OPNb and c upregulation in AML cell lines were concurrently associated with the angiogenesis molecular loop upregulation (Mirzaei et al., 2017). Regarding anti-angiogenesis potency of CUR in cancer cells (Ding et al., 2014; Huang et al., 2015), we assume that combination of CUR with conventional AML regimens likely lead to inhibition of OPNb and c isoforms as LSCs molecular surrogate. For the first time, our results propose that despite the fact that CUR has angiogenesis effects, AML cells probably evade from anti-angiogenesis effects of CUR in a combination of AML conventional regiment through induction of OPN b and c isoform and consequently AKT/VEGF/STAT3/CXCR4/IL-6 anti-angiogenesis molecular pathway. It seems that overexpression of both isoforms OPN-b and OPN-c cells secrete factors that are able to activate angiogenic processes.
Bhandarkar and Arbiser (2007) showed that CUR is an angiogenesis inhibitor and also down regulates numerous proangiogenic proteins via the expression of proangiogenic factors, cell adhesion molecules, and signal transduction pathways. Also, in some study has been shown that the CUR has anticarcinogenic activities and mediated in ovarian carcinoma by targeting of NF-κB pathway and STATs (Kurzrock and Li, 2005; Yoysungnoen et al., 2005; Yoysungnoen et al., 2006; Lin et al., 2007; Kunnumakkara et al., 2008; Yoysungnoen et al., 2008).
Robertson and Chellaiah (2010) and Zhao et al., (2016) showed that cells treated with Ara-c leads to a decrease in expression OPN splicing isoforms, STAT3, β-Catenin in the KG-1 cell and OPN splicing isoforms, β-catenin, VEGF-C, CXCR4, STAT3, IL-6 and KDR genes expression in U937 cells
Many studies have shown that IL-6 is accomplished of prompting VEGF-C via the JAK-STAT3 signal pathway. CXCR4 overexpression is related to AML, colorectal cancer, and kidney cell carcinoma and renal, and is relevant to invasion, cell proliferation chemotaxis, and angiogenesis (Huang et al., 2016; Zhao et al., 2016). STAT3 is considered as an important pathway that can adjust metastasis in cancer cells. STAT3 has an important role in numerous pro-oncogenic mechanisms to stimulate resistance to apoptosis, cell proliferation and survival, angiogenesis, invasion, and immune suppression (Hirano et al., 2000; Gopinathan et al., 2015).
In parallel of our research, Haghi et al., (2017) suggested that The VEGF pathway may have a potential effect on AML cell lines progression. Moreover, some studies showed that OPN overexpression launched angiogenesis of cells via PI3K/AKT signaling pathway (Wang et al., 2011; Shevde and Samant, 2014). Dai and et al., (2009) and Xu et al., (2015) showed that OPN enhancements angiogenesis directly via PI3K/AKT pathway and extracellular-signal-regulated kinase (ERK)-mediated pathways with VEGF acting as a positive feedback signal. Here, we reported that Ara-c inhibited each three isoforms of OPN, STAT3, B-Catenin, IL-6, CXCR4, VEGF-C and KDR in AML cell lines and CUR decrease OPNa in U937 cell line that they are necessary for angiogenesis. As Tilli et al., (2014) has proved, OPN-c stimulated different steps of angiogenesis. Ovcar-3 and overexpressing of OPNc PC-3 cells secrete specific proteins that induction of angiogenesis and OPN has been approximately characterized as an inducer of tumor angiogenesis, with an association with VEGF expression .
Syed and Mukhtar (2011) showed that Curcumin prevented phosphorylation of STAT3 and proto-oncogene tyrosine-protein kinase Src through down regulation of phosphatase of regenerating liver 3 (PRL-3) and inhibited melanoma cells from attacking the lymph nodes that are in harmony with our study. In line with our findings, Tilli et al, discovered that OPNc induces higher levels of VEGF in prostate cancer cells between all type of OPN isoforms that followed by OPNb, while this direct effect is not seen by OPNa (Tilli et al., 2012). Also, Kumar et al., (2012) displayed that meaningfully CUR hampers adhesion, motility, and invasion of breast cancer cells by inhibiting STAT3 activity.
In conclusion, taken all together, it seems that overexpression of both isoforms OPN-b and OPN-c cells secrete factors that are able to activate angiogenic processes. Indeed, our results demonstrated that OPN b and c isoforms probably preclude anti-angiogenesis effects of CUR in combination with the conventional AML regiment. Regarding the effect of CUR and Ara-c, this might give better combination treatment strategy and reduce the toxicity profile and therefore may be useful for the patients.
Conflicts of inters
The authors declare no conflicts of interest.
Acknowledgments
This study was performed in Hematology, Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences.
References
- Asou Y, Rittling SR, Yoshitake H, et al. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology. 2001;142:1325–32. doi: 10.1210/endo.142.3.8006. [DOI] [PubMed] [Google Scholar]
- Bailly J, Skladanowski A, Bettaieb A, et al. Natural resistance of acute myeloid leukemia cell lines to mitoxantrone is associated with lack of apoptosis. Leukemia. 1997;11:1523–32. doi: 10.1038/sj.leu.2400762. [DOI] [PubMed] [Google Scholar]
- Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. Adv Exp Med Biol. 2007;595:185–95. doi: 10.1007/978-0-387-46401-5_7. [DOI] [PubMed] [Google Scholar]
- Bishop JF. The treatment of adult acute myeloid leukemia. Semin Oncol. 1997;24:57–69. [PubMed] [Google Scholar]
- Dai J, Li B, Shi J, et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59:355–66. doi: 10.1007/s00262-009-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- Ding Q, Niu T, Yang Y, et al. Preparation of curcumin-loaded poly(ester amine) nanoparticles for the treatment of anti-angiogenesis. J Biomed Nanotechnol. 2014;10:632–41. doi: 10.1166/jbn.2014.1829. [DOI] [PubMed] [Google Scholar]
- Flamant S, Kortulewski T, Dugray A, et al. Osteopontin is upregulated by BCR-ABL. Biochem Biophys Res Commun. 2005;333:1378–84. doi: 10.1016/j.bbrc.2005.05.203. [DOI] [PubMed] [Google Scholar]
- Gopinathan G, Milagre C, Pearce OM, et al. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015;75:3098–107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghi A, Mohammadi S, Heshmati M, et al. Anti-vascular endothelial growth factor effects of sorafenib and arsenic trioxide in acute myeloid leukemia cell lines. Asian Pac J Cancer Prev. 2017;18:1655–61. doi: 10.22034/APJCP.2017.18.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Huang M-T, Lou Y-R, Ma W, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–7. [PubMed] [Google Scholar]
- Huang Y-H, Yang H-Y, Huang S-W, et al. Interleukin-6 induces vascular endothelial growth factor-C expression via Src-FAK-STAT3 signaling in lymphatic endothelial cells. PLoS One. 2016;11:0158839. doi: 10.1371/journal.pone.0158839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YF, Zhu XX, Ding ZS, et al. Study on anti-angiogenesis effect of three curcumin pigments and expression of their relevant factors. Zhongguo Zhong Yao Za Zhi. 2015;40:324–9. [PubMed] [Google Scholar]
- Jiang MC, Yang-Yen HF, Yen JJ, Lin JK. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer. 1996;26:111–20. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- Kavianpour M, Ahmadzadeh A, Shahrabi S, et al. Significance of oncogenes and tumor suppressor genes in AML prognosis. Tumour Biol. 2016;37:10041–52. doi: 10.1007/s13277-016-5067-1. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kumar M, Saravanan C, et al. Curcumin:a potential candidate for matrix metalloproteinase inhibitors. Expert Opin Ther Targets. 2012;16:959–72. doi: 10.1517/14728222.2012.710603. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Kurzrock R, Li L. Liposome-encapsulated curcumin:in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. J Clin Oncol. 2005;23:4091. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- Liu Y-N, Kang B-B, Chen J. Transcriptional regulation of human osteopontin promoter by C/EBPαand AML-1 in metastatic cancer cells. Oncogene. 2004;23:278–88. doi: 10.1038/sj.onc.1207022. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Suzuki T, Saito T. Osteopontin is a new target molecule for ovarian clear cell carcinoma therapy. Cancer Sci. 2010;101:1828–33. doi: 10.1111/j.1349-7006.2010.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon LG, Kuttan R, Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–5. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- Mi Z, Guo H, Russell MB, et al. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17:153–61. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Shaughnessy E, Hurley JK, et al. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122:889–97. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- Mirzaei A, Mohammadi S, Ghaffari SH, et al. Osteopontin b and c isoforms:Molecular candidates associated with leukemic stem cell chemoresistance in acute myeloid leukemia. Asian Pac J Cancer Prev. 2017;18:1707–15. doi: 10.22034/APJCP.2017.18.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Ghaffari SH, Shaiegan M, et al. Acquired expression of osteopontin selectively promotes enrichment of leukemia stem cells through AKT/mTOR/PTEN/β-catenin pathways in AML cells. Life Sci. 2016a;152:190–8. doi: 10.1016/j.lfs.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Ghaffari SH, Shaiegan M, et al. Curcumin veto the effects of osteopontin (OPN) specific inhibitor on leukemic stem cell colony forming potential via promotion of OPN overexpression. Int J Hematol Oncol Stem Cell Res. 2016b;10:120. [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Nikbakht M, Sajjadi SM, et al. Reciprocal interactions of leukemic cells with bone marrow stromal cells promote enrichment of leukemic stem cell compartments in response to curcumin and daunorubicin. Asian Pac J Cancer Prev. 2017a;18:831–40. doi: 10.22034/APJCP.2017.18.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Nikbakht M, Sajjadi SM, et al. Reciprocal interactions of leukemic cells with bone marrow stromal cells promote enrichment of leukemic stell cell compartments in response to curcumin and daunorubicin. Asian Pac J Cancer Prev. 2017b;18:831. doi: 10.22034/APJCP.2017.18.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Zahedpanah M, Nikbakht M, et al. Parthenolide reduces gene transcription of prosurvival mediators in U937 cells. Exp Oncol. 2017c;39:30–5. [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-κB-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926–35. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- Philip S, Kundu GC. Osteopontin induces nuclear factor κB-mediated promatrix metalloproteinase-2 activation through IκBα/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. 2003;278:14487–97. doi: 10.1074/jbc.M207309200. [DOI] [PubMed] [Google Scholar]
- Powell JA, Thomas D, Barry EF, et al. Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood. 2009;114:4859–70. doi: 10.1182/blood-2009-02-204818. [DOI] [PubMed] [Google Scholar]
- Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor:Implications for cancer. Biochim Biophys Acta. 2015;1855:202–22. doi: 10.1016/j.bbcan.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–50. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- Rao J, Xu D-R, Zheng F-M, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+acute myeloid leukemia cell lines and primary sorted CD34+acute myeloid leukemia cells. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BW, Chellaiah MA. Osteopontin induces β-catenin signaling through activation of Akt in prostate cancer cells. Exp Cell Res. 2010;316:1–11. doi: 10.1016/j.yexcr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrabi S, Rezaeeyan H, Ahmadzadeh A, et al. Bone marrow blood vessels:Normal and neoplastic niche. Oncol Rev. 2016;10:306. doi: 10.4081/oncol.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–41. doi: 10.1016/j.matbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed DN, Mukhtar H. Botanicals for the prevention and treatment of cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24:688–702. doi: 10.1111/j.1755-148X.2011.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilli TM, Bellahcène A, Castronovo V, et al. Changes in the transcriptional profile in response to overexpression of the osteopontin-c splice isoform in ovarian (OvCar-3) and prostate (PC-3) cancer cell lines. BMC Cancer. 2014;14:433. doi: 10.1186/1471-2407-14-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilli TM, Mello KD, Ferreira LB, et al. Both osteopontin-c and osteopontin-b splicing isoforms exert pro-tumorigenic roles in prostate cancer cells. The Prostate. 2012;72:1688–99. doi: 10.1002/pros.22523. [DOI] [PubMed] [Google Scholar]
- Vejda S, Piwocka K, McKenna SL, et al. Autocrine secretion of osteopontin results in degradation of IκB in Bcr-Abl-expressing cells. Br J Haematol. 2005;128:711–21. doi: 10.1111/j.1365-2141.2004.05355.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yan W, Lu X, et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol. 2011;90:642–8. doi: 10.1016/j.ejcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Wu Y-d, Chen Y, Chen W-j, et al. Effects of curcumin on proliferation and apoptosis in acute myeloid leukemia cells HL-60. Chin J Cancer Res. 2000;12:96–8. [Google Scholar]
- Xu J, Yi Y, Li L, et al. Osteopontin induces vascular endothelial growth factor expression in articular cartilage through PI3K/AKT and ERK1/2 signaling. Mol Med Rep. 2015;12:4708–12. doi: 10.3892/mmr.2015.3975. [DOI] [PubMed] [Google Scholar]
- Yoysungnoen P, Wirachwong P, Bhattarakosol P, et al. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin Hemorheol Microcirc. 2005;33:127–35. [PubMed] [Google Scholar]
- Yoysungnoen P, Wirachwong P, Bhattarakosol P, et al. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109–15. [PubMed] [Google Scholar]
- Yoysungnoen P, Wirachwong P, Changtam C, et al. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14:2003. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedpanah M, Shaiegan M, Ghaffari SH, et al. Parthenolide induces apoptosis in committed progenitor AML cell line U937 via reduction in osteopontin. Rep Biochem Mol Biol. 2016;4:82–8. [PMC free article] [PubMed] [Google Scholar]
- Zduniak K, Ziolkowski P, Ahlin C, et al. Nuclear osteopontin-c is a prognostic breast cancer marker. Br J Cancer. 2015;112:729–38. doi: 10.1038/bjc.2014.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang S, He YJ, et al. Fluorouracil selectively enriches stem-like leukemic cells in a leukemic cell line. Int J Biol Sci. 2010;6:419–27. doi: 10.7150/ijbs.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Zhu G, Huang Y, et al. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–95. doi: 10.3892/or.2016.4544. [DOI] [PubMed] [Google Scholar]


