Abstract
Timely recognition of patients at risk or with possible acute kidney injury (AKI) is essential for early intervention to minimize further damage and improve outcome. Initial management of patients with suspected and persistent AKI should include thorough clinical assessment of all patients with AKI to identify reversible factors, including fluid volume status, potential nephrotoxins, and an assessment of the underlying health of the kidney. Based on these assessments, early interventions to provide appropriate and adequate fluid resuscitation while avoiding fluid overload, removal of nephrotoxins, and adjustment of drug doses according to the level of kidney function derangement are important. The judicious use of diuretics for fluid overload and/or in cardiac decompensated patients and introduction of early enteral nutritional support need to be considered to improve outcomes in AKI. Although these basic principles are well recognized, their application in clinical practice in low resource settings is often limited due to lack of education, availability of resources, and lack of trained personnel, which limits access to care. We report the consensus recommendations of the 18th Acute Dialysis Quality Initiative meeting in Hyderabad, India, on strategies to evaluate patients with suspected AKI and initiate measures for prevention and management to improve outcomes, particularly in low resource settings. These recomendations provide a framework for caregivers, who are often primary care physicians, nurses, and other allied healthcare personnel, to manage patients with AKI in resource poor countries.
Keywords: acute kidney injury, confirmed, developing countries, management, prevention, resources, suspected, transient
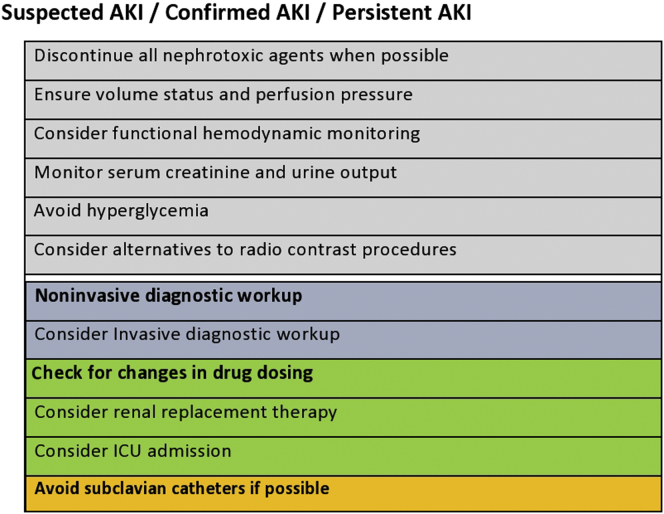
Acute kidney injury (AKI) is associated with significant morbidity and mortality. Timely recognition of patients at risk for AKI, or with possible AKI, is essential to allow early intervention to minimize further renal injury, and may likely result in better outcomes than treating established AKI.1 Protective measures to avoid worsening should be started immediately, with special attention to ensure adequate hydration, maintain hemodynamic stability and oxygenation, and prevent nephrotoxicity of drugs (Figure 1).2 Treatment goals in patients with AKI include: preservation and optimization of renal function; correction and maintenance of electrolyte, acid-base, and mineral homeostasis; minimize secondary organ damage from the consequences of AKI; and manage effects of decreased renal function. The spectrum of AKI includes rapid reversal of AKI, persistent AKI, and acute kidney disease (AKD) as defined previously3,4 (Table 1).
Figure 1.
Management strategies in acute kidney injury (AKI). ICU, intensive care unit.
Table 1.
Definitions
| Fluid bolus: a rapid infusion to correct hypotensive shock. It typically includes the infusion of at least 500 ml over a maximum of 15 min |
| Fluid challenge: 100–200 ml over 5–10 min with reassessment to optimize tissue perfusion |
| Fluid infusion: continuous delivery of i.v. fluids to maintain homeostasis, replace losses, or prevent organ injury (e.g., prehydration before operation to prevent intraoperative hypotension or for contrast nephropathy) |
| Maintenance: fluid administration for the provision of fluids for patients who cannot meet their needs by oral route. This should be titrated to patient need and context, and should include replacement of ongoing losses. In a patient without ongoing losses, this should probably be no more than 1–2 m/kg per hour |
| Daily fluid balance: daily sum of all intakes and outputs |
| Cumulative fluid balance: sum total of fluid accumulation over a set period of time |
| Fluid overload: cumulative fluid balance expressed as a proportion of baseline body weight. A value of 10% is associated with adverse outcomes |
| Response: Achieving hemodynamic goal and/or improvement of UOP: >0.5 ml/kg per hour |
| Persistent AKI is characterized by the continuance of AKI by creatinine or urine output criteria (defined by KDIGO criteria) beyond 48 hours from onset. |
| Complete reversal of AKI by KDIGO criteria within 48 hours of the onset characterizes rapid reversal of AKI |
| AKD is defined as a condition wherein AKI Stage Ia or greater criteria is present 7 days (or more) after an exposure.a AKD that persists beyond 90 days is then considered CKD |
AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; KDIGO, Kidney Disease Improving Global Outcomes; UOP, urinary output.
ADQI 16 workgroup report.4
The spectrum of AKI, its presentation, and the site of development is quite different in poor resource countries compared with the resource rich countries (Table 2). AKI occurs frequently in the community (community-acquired AKI [CAKI]) in rural areas where access to care is limited, and patients may not present to a hospital. In contrast, hospital-acquired AKI (HAKI) is less frequent than that in the developed world,5 and is potentially related to poor recognition due to limited resources and lack of knowledge and training. In most countries in the developing world, the number of nephrologists is insufficient; it is primary care physicians, nurses, and allied health personnel who manage AKI patients. They need to be trained to raise awareness, promote prevention, and provide practical management of AKI. Although the Kidney Disease Improving Global Outcomes (KDIGO) guidelines for management of AKI, published in 2012, are available, their application and use for individual patient care in the developing world are often limited secondary to resource limitation, economic disparities, and lack of trained personnel. Management of AKI in these settings needs to consider these limitations. To address these issues, the steering committee of the 18th Acute Dialysis Quality Initiative (ADQI) conference dedicated a work group with the task of identifying elements that might affect the evaluation and management of AKI based on the availability of resources. Using a modified Delphi process, this group reached consensus regarding strategies to manage AKI risk in low resource settings. In this article, we provide consensus recommendations that address the following 4 questions:
-
Q1.
What should be the initial management of patients with suspected AKI and/or persistent AKI?
-
Q2.
How should patients with suspected AKI and/or persistent AKI be monitored for drug selection and dosages?
-
Q3.
Should diuretics be used in patients with suspected AKI and/or persistent AKI? Or are diuretics useful in patients with suspected AKI and/or persistent AKI?
-
Q4.
What are the nutritional requirements in patients with suspected AKI and/or persistent AKI?
Table 2.
Typical characteristics of acute kidney injury (AKI) in high- and low-income countries
| Characteristics | AKI in high-income coutries | AKI in low-income and middle-income countries |
|---|---|---|
| Pattern of occurrence | Occurs predominantly in intensive care units | Occurs in health centers and hospitals in rural areas and large hospitals and intensive care units in large cities |
| Disease patterns | Associated with multiple organ failure | Often caused by a single disease; multiple organ failure less common |
| Associations | Associated with sepsis and complex surgery (major trauma, cardiovascular surgery) | Frequently associated with specific disease (e.g., diarrhea) and specific infection (e.g., malaria) |
| Mortality | High mortality | Same or lower mortality than in high-income countries |
| Populations affected | A disease of older adult populations | A disease of young, otherwise healthy people |
| Prevalence | Could be increasingly prevalent | Could be increasingly prevalent |
| Sufficiency of reporting | Accurately reported | Severely under-reported |
| Preventable status | Difficult to prevent | Preventable |
| Expense | Very expensive to treat | Very inexpensive to treat at early stages, too costly for most |
Methods
This consensus meeting followed the established ADQI process, as previously described.6 The broad objective of ADQI is to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment, as well as identify evidence care gaps to establish research priorities. The 18th ADQI Consensus Conference focused on “Management of AKI in the Developing World,” convening a diverse panel for a 2-1/2 day meeting in Hyderabad, India from September 27 to 30, 2016. The consensus-building process was informed by preconference, conference, and postconference activities. Before the conference, the work group searched PubMed for English language articles on prevention and management for AKI. This search included the following terms: acute kidney injury in developing world, acute kidney failure, systematic review/meta-analysis in acute kidney injury, prevention, treatment, fluid resuscitation in AKI/critically ill patients, diuretics in AKI, nutritional support in acute kidney injury, and drug selection and/or dosing in AKI.
A preconference series of emails that involved work group members was used to identify the current state of knowledge and enable the formulation of key questions. A formal systematic review was not conducted. At the in-person meeting, the work group developed consensus statements through a series of alternating breakout and plenary sessions. In each breakout session, the work group refined the key questions, identified the supporting evidence, and generated consensus statements. Work group members presented the results for feedback to all ADQI participants during the plenary sessions, and then revised the drafts based upon the plenary comments until a final version was accepted. We developed recommendations and consensus of expert opinion with evidence, when possible, to distill the current literature. To address important unanswered questions, we articulated a research agenda.
Following the conference, this summary report was generated, revised, and approved by all members of the work group.
Q1: What Should Be the Initial Management of Patients With Suspected AKI?
Consensus Statements
-
1.1.
Every patient with suspected and/or persistent AKI should be assessed for volume status.
-
1.2.
The volume status should be assessed by history, physical examination, laboratory testing, and imaging, depending on clinical severity and the setting.
Context
Every patient with suspected AKI, confirmed AKI, and/or persistent AKI should be assessed for volume status as a part of hemodynamic optimization. Volume depletion is one of the major risk factors for AKI. In contrast, some suspected, confirmed, and/or persistent AKI can present with volume overload and worsening renal function, such as congestive heart failure, or cardiorenal syndrome. Fluid overload was defined as the difference between cumulative fluid intake and cumulative fluid output, divided by initial body weight.7 There is no specific study that used these parameters to show the benefit for AKI outcomes. A combination of history taking, including medications, physical examination, laboratory testing, and hemodynamic parameters (both static and dynamic) should be performed to obtain the best information for fluid assessment. Clinical variables used for fluid assessment include baseline body weight, history of recent fluid loss, cumulative fluid balance, vital signs, urine output, capillary refill, and skin turgor, whereas laboratory variables should include blood lactate, lactate clearance, mixed venous oxygen saturation, and urinary indexes (fractional excretion of sodium, lithium, urea). Several trials have shown the limitation of static hemodynamic parameters such as central venous pressure and pulmonary capillary wedge pressure in guiding fluid responsiveness.8 Dynamic hemodynamic variables (stroke volume or pulse pressure variation, change in vena cava diameter, and passive leg raising test) have been used as part of clinical decision-making during fluid assessment and have shown superior results over static hemodynamic variables.9, 10 However, no study has shown superiority of a particular method in determining clinical outcomes. Therefore, it is advised to combine these variables to make a decision on fluid administration.
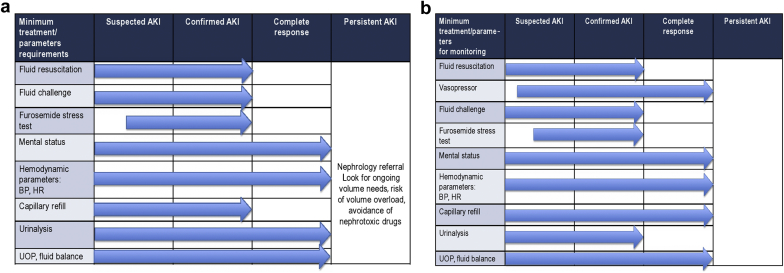
Clinical reassessment is the key concept of fluid administration. It is becoming apparent that the concept “one size fits all” cannot be applied to fluid therapy in patients with suspected, confirmed, and/or persistent AKI. The amount of fluid should be based on requirements of the individual. Careful fluid assessment can be performed many ways, depending on sites of care and stage of disease. We have proposed the minimum parameters for fluid assessment in Figures 2a (community setting) and Figure 2b (hospital setting).
Figure 2.
(a) Minimum treatment parameter requirements in the community setting. (b) Minimum treatment and parameter requirements in the hospital setting. AKI, acute kidney injury; BP, blood pressure; HR, heart rate; UOP, urinary output.
Consensus Statement
-
1.3.
We recommend use of crystalloid over colloid for initial fluid resuscitation as initial treatment for suspected, confirmed, and/or persistent AKI.
Context
Many trials have been conducted to compare fluid types for fluid resuscitation using survival as the primary outcome and using AKI outcome as the secondary outcome. No study has used AKI as a primary outcome.
Crystalloid Versus Colloid. Colloids have been widely used for fluid therapy in the critical care setting during the past few years.11 The amount of colloids used for fluid resuscitation were expected to be less than the amount of crystalloid by approximately 3 times.12, 13 Since the publication of 2 large randomized controlled trial (RCTs), 6S and the CHEST trials 4 years ago, use of hydroxyethyl starch (HES) has been restricted by regulatory authorities with a warning for the potential of worsening kidney function.14, 15 The 6S trial studied fluid optimization in patients with severe sepsis and/or septic shock. The third-generation HES (6% HES 130/0.4) increased the mortality or dialysis dependent rate higher than Ringer’s acetate (51% vs. 43%; P = 0.03). Moreover, the HES group had a higher incidence rate of renal replacement therapy (RRT) than the Ringer’s acetate group (22% vs. 16%; P = 0.04). The CHEST trial studied fluid optimization in patients in the intensive care unit (ICU). There was no difference in the mortality rate between the HES and saline groups, but the HES group had a higher incidence rate of RRT than the saline group (7% vs. 5.8%; P = 0.04). In theory, human albumin is the main protein for maintaining plasma colloid oncotic pressure. It also works as a carrier for several endogenous and exogenous compounds with antioxidant and anti-inflammatory properties. Moreover, albumin can act as a buffer molecule for controlling acid-base homeostasis.16, 17, 18, 19, 20 The result from large RCTs, the SAFE study in the ICU setting and the latest ALBIOS study in the setting of severe sepsis and/or septic shock, did not show the benefit of human albumin over crystalloids. In addition, there was no difference in renal outcomes between human albumin and crystalloid in both studies.21, 22 However, albumin is safe for the kidney when used in the high-risk setting. With the high cost and no obvious advantage over crystalloids, human albumin should not be used as the firstline therapy. In tropical infections, Wills et al. conducted a double-blind RCT of 3 fluids (Ringer’s lactate, 6% dextran 70, or 6% HES) for initial resuscitation in Vietnamese children with dengue shock syndrome. The primary outcome was rescue colloid at any time after administration of the study fluid. There was no difference of requirement of rescue colloid among children with moderate shock.23
Balanced Crystalloid Solution Versus Nonbalanced Crystalloid Solution. There were several studies that addressed the adverse effect of nonbalanced crystalloid solution (isotonic saline) on the kidney.24, 25, 26 Isotonic saline contains 154 mmol/L chloride, and administration of a large volume can result in hyperchloremic metabolic acidosis. This condition can lead to renal vasoconstriction, decrease in renal artery flow velocity and in renal blood flow, afferent arteriolar vasoconstriction, and a decreased glomerular filtration rate (GFR).27 Current evidence from 3 large observational studies also suggested that the high chloride content of isotonic saline might cause harm, especially to the kidney. The first study, by Shaw et al.,28 in 30,994 adult patients who underwent major abdominal surgery, showed that patients who received isotonic saline had significantly greater blood transfusion requirements, more infectious complications, and more renal support requirements than those who received balanced crystalloids. However, there was no difference in the mortality rate between the 2 groups.28 The second study, by Yunos et al.,29 was an open-label prospective sequential study that compared traditional chloride-rich solutions (isotonic sodium chloride, 4% succinylated gelatin solution, or 4% albumin solution) and chloride-restricted fluids (Hartmann’s solution, Plasma-Lyte 148, or chloride-poor 20% albumin). After adjusting for confounding variables, the chloride-restricted group had decreased incidence of AKI (odds ratio [OR]: 0.52; P = 0.001) and reduced use of RRT (OR: 0.52; P = 0.004). Again, there were no differences in hospital mortality and hospital or ICU length of stay.29 A third study by McCluskey et al.30 on postoperative patients showed that the incidence of acute postoperative hyperchloremia was 22%. Patients with hyperchloremia were found to be at increased risk of 30-day postoperative mortality (3.0% vs 1.9%; OR: = 1.58), had a longer hospital length of stay, and were more likely to have postoperative renal dysfunction. These large observational studies suggest that it might be time to consider the use of balanced crystalloid solution as the fluid of choice, especially with metabolic acidosis. However, the SPLIT trial, the largest RCT that aimed to compare the effect of balanced crystalloid and nonbalanced crystalloid on kidney injury, did not show a difference of AKI incidence within 90 days between Plasma-Lyte 148 solution and isotonic saline (9.6% vs. 0.2%; P = 0.77). Moreover, there was no difference in the RRT incidence rate and hospital mortality rate between the 2 groups. The incidence of AKI in this study was quite low, and the adverse effects of isotonic saline on AKI outcome might not have been evident.31 Therefore, based on this study, it would be too early to conclude that there is no harmful effect of isotonic saline on kidney function.
With the limited resource setting in developing countries and considering the number one cause of out-of-hospital AKI (volume depletion), the work group concluded that isotonic saline could still be the crystalloid of choice for fluid resuscitation. We need more large RCTs to study the beneficial effect of balanced crystalloid solution on kidney function, especially in the high-risk setting of kidney injury.
Consensus Statement
-
1.4a.
The amount of fluid to be given should be individualized based on the initial assessment of volume status and clinical background and/or associated comorbidities.
-
1.4b.
Oral fluid administration should be considered in the community setting.
-
1.4c.
Fluid overload should be avoided.
Context
Since the publication of the Early Goal-Directed Therapy (EGDT) study by River et al.32 15 years ago, the concept of a protocolized strategy that consisted of fluids, vasopressors, and blood transfusion that targeted hemodynamic parameters has been widely adopted.33 The average fluid administration during the first 72 hours in this single center study was 13 L. However, during the past few years, there were many studies that showed the adverse effects of fluid overload on patient outcome.7 Supporting the concept of restricted fluid therapy by 3 large RCTs, the PROCESS,34 ARISE,35 and PROMISE36 studies, which compared the mortality between protocolized care and the usual care in patients with sepsis, showed that only 3 to 4 L of fluid intake was adequate in the first 72 hours. All of these 3 major RCTs also revealed that protocolized therapy and the usual care provided comparable outcomes. This emphasized the concept that the amount of fluid to be given should be individualized based on the initial assessment of volume status and clinical background and/or associated comorbidities.
In patients with tropical infections that cause AKI, some specific infections show unique features of hemodynamic alterations and require a different pattern of fluid administration compared with patients with severe sepsis and/or septic shock. Patients with dengue shock syndrome versus septic shock syndrome presented with narrower pulse pressures (25 ± 8 mm Hg vs. 43 ± 8 mm Hg; P < 0.01), less presence of systemic inflammatory response syndrome (9/16 vs. 15/16; P < 0.05), and a lower requirement of fluid administration (28.5 ml/kg vs. 57.5 ml/kg; P = 0.03).37 Concern regarding fluid bolus in tropical infections causing AKI was raised following the Fluid Expansion As Supportive Therapy (FEAST) study by Maitland et al.38 African children who had severe sepsis (mainly malaria) were randomized to receive no fluid boluses or to receive fluid boluses with either isotonic saline or albumin. At 48 hours, patients who received fluid boluses had higher mortality compared with control patients (relative risk: 1.45; P =0.003). This trial was conducted in a limited resources setting with no access to ventilation to optimize the management of sepsis.
Many observational studies of pediatric AKI reported that volume overload status at the beginning of RRT affected survival, although it is known that fluid proportion is greater in a child than in an adult. As a parameter of volume status, percent fluid overload (%FO) is use for pediatric care: %FO = (fluid in – fluid out)/body weight on pediatric ICU admission × 100 (%). Goldstein et al.39 reported a prospective multicenter study of pediatric AKI with continuous renal replacement therapy (CRRT), namely the pediatric patients CRRT study. In this study, 116 children started CRRT due to multi-organ failure with AKI and examined predictive factors capable of distinguishing survivors from nonsurvivors. After adjusting for disease severity confounding factors, PRISM (pediatric risk of mortality) score, % FO of survivors was significantly low compared with nonsurvivors (14 ± 15.9 vs. 25.4 ± 32.9; P < 0.05). The mortality rate was significant in subjects with % FO >20%. Lower % FO at CRRT start was reportedly crucial for better survival in multi-organ failure40, 41, 42, 43 and AKI under extra-corporeal membrane oxygenation after cardiac surgery.44 However, another observational study showed opposite results.45 Future prospective multicenter studies are necessary for fluid management of pediatric AKI.
Oral fluid administration should be considered in the community setting. This strategy with thorough clinical assessment could decrease the rate of hospitalization. In mild dengue infection, ingestion of fluid in 24 hours before being seen by a clinician was found to be protective against hospitalization after adjusting for the distance from a health facility, date of symptom onset, and thrombocytopenia (OR: 0.74 per each additional glass consumed; P < 0.01). The most common liquid ingested was water (70%), followed by fruit juice (42%), lemonade (27%), milk (25%), coffee (14%), oral dehydration serum (6%), and tea (2%).46
Consensus Statement
-
1.5.
Vasopressors should be considered as soon as possible if volume repletion has not achieved the hemodynamic goal.
-
1.6.
We recommend a target of mean arterial pressure of 65 to 85 mm Hg, depending on clinical condition.
Context
The vasopressor requirement is the essential component of treatment to achieve the hemodynamic goal after intravascular volume restoration. Persistent hypotension after initial fluid administration place patients at risk for AKI. There was no clinical study to show which vasopressor agents (norepinephrine, dopamine, and vasopressin and/or terlipressin) were the most effective for prevention or treatment of patients with AKI. A study that compared the efficacy between norepinephrine and dopamine did not show any difference in mortality and AKI incidence between the groups.46 However, the use of dopamine was associated with more adverse events, such as cardiac arrhythmia in the subgroup of patients with cardiogenic shock. Vasopressin, another potent vasopressor agent, which acts as a vasopressin receptor of smooth muscle cells, is used in the treatment of shock refractory to norepinephrine.47 Compared with norepinephrine, it increases blood pressure, enhances diuresis, and may lower rate of AKI progression, but it has not as yet been proven to enhance survival nor to reduce the need for RRT.48, 49
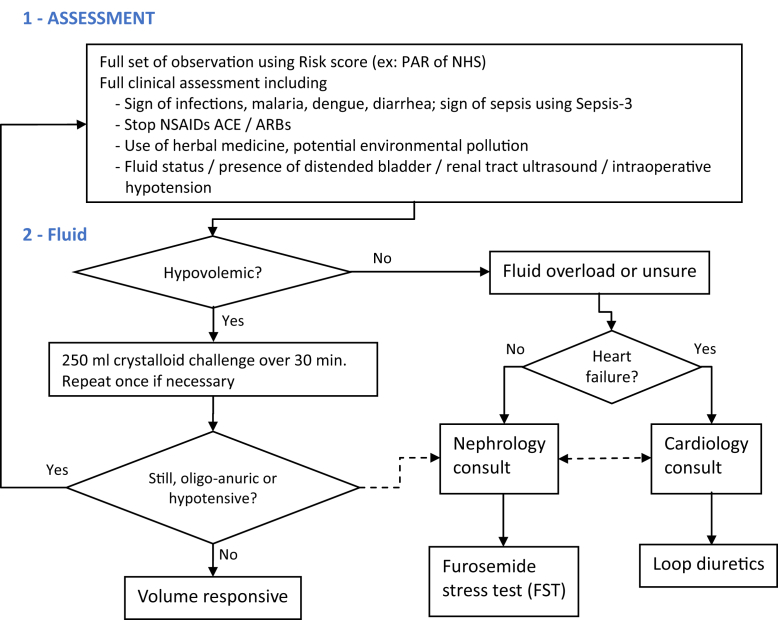
The guideline of the Surviving Sepsis Campaign recommended initial resuscitation with vasopressors to reverse hypotension, with a mean arterial pressure target of at least 65 mm Hg.50 This recommendation was based on previous studies, which showed no significant differences in lactate levels or regional blood flow when the mean arterial pressure was elevated to >65 mm Hg in patients with septic shock.51 The kidney is one of the organs prone to compromising blood supply when mean arterial pressure is decreased. A recent large, retrospective study showed that a mean arterial pressure of >75 mm Hg might be required to maintain kidney function.52 Results from the SEPSISPAM investigators group, a multicenter, open-label RCT in patients with septic shock who underwent resuscitation, showed a mean arterial pressure target of either 80 to 85 mm Hg or 65 to 70 mm Hg. There were differences in the mortality rate, but among patients with chronic hypertension, those in the high-target group required less RRT and less doubling of serum creatinine than in those in the low-target group.53 Although AKI in the developing world is more often CAKI, HAKI is not uncommon because of the increasing affordability and availability of tertiary health care facilities. Emerging new evidence suggests a strong link between intraoperative hypotension and postoperative AKI.54, 55, 56 Preventing intraoperative hypotension is included in the initial care bundle for AKI (Figure 3).
Figure 3.
Acute kidney injury initial care bundle.
Research Recommendations
-
•
Study the process of care (hemodynamic optimization) and triage patients from primary care to tertiary care to improve AKI outcome in limited resource settings.
-
•
Randomized trials to study the effect of balanced and nonbalanced crystalloid to kidney injury in the high-risk AKI setting.
-
•
Randomized trials to study the role of oral fluid intake in preventing AKI in the community setting.
-
•
Randomized trials are needed that compare different types of vasopressors for prevention and treatment of AKI.
Q2: How Should Patients With Suspected AKI and/or Persistent AKI Be Monitored for Drug Selection and Dosages?
Consensus Statement
-
2.1.1.
We recommend daily monitoring of renal function in AKI.
Context
AKI is a dynamic condition with frequent alteration in GFR. At times, alterations occur in a few hours with a rapidly deteriorating GFR. In view of these rapid changes, daily monitoring of renal functions by estimating serum creatinine and monitoring urine output is recommended. Patients need to be monitored with measurements of AKI criteria (serum creatinine and urine output), and the management of blood pressure and cardiac output require careful titration of fluids and vasoactive medication. Minimum treatment and parameters for monitoring are adapted to the nature of the site of care with the timeline (Figures 2a and 2b). An early nephrology referral and intervention is likely to result in better outcomes. Delayed or absent nephrology referral has been associated with higher mortality, dialysis dependence, and a longer length of hospital stay.57, 58, 59, 60 When the patient with confirmed AKI presents with a significant risk of increased progression of AKI and does not reach the initial treatment goals within the timeframe of 6 hours (Figure 3), escalating care to the secondary level or tertiary level, and probably nephrology referral, may be warranted. Nephrology consultation should be also considered if the etiology is not clear or subspecialist care is needed.3
Consensus Statement
-
2.2.
We recommend to withdraw or stop all nephrotoxic drugs, unless needed for life-saving conditions.
Context
Drugs contribute to 20% of CAKI episodes that result in hospitalization.61, 62 Mortality or morbidity of drug-associated AKI is similar to AKI due to other causes. Early reversal of AKI occurs with withdrawal of the offending drug. AKI, unlike chronic kidney disease (CKD), is a dynamic condition with changes in GFR that occur rapidly. Drug dosing in AKI requires the treating physician to be aware of markedly altered and constantly changing physiology in the context of many diseases that cause AKI. Multiorgan dysfunction syndrome or multisystem organ failure are common in critically ill patients.63, 64, 65, 66 The metabolism and effects of drugs in patients with AKI depends on a number of factors, such as changes in drug clearance (glomerular and tubular functions, nonrenal metabolism) and altered pharmacokinetic parameters due to decreased kidney functions (volume overload, metabolic demands). In the developing world, there is easy access to multiple drugs without much regulation; polypharmacy and indiscriminate use of various drugs, as well as use of indigenous drugs, is rampant, and their contribution to development of AKI is an important cause.
In patients at risk of developing AKI or suspected AKI and/or persistent AKI, AKD, AKI, or CKD, it is advisable to withdraw or not to add potential drugs that might be nephrotoxic, unless lifesaving; no suitable alternative is available (ADQI 16).3, 67, 68, 69 The following considerations should be made before deciding to discontinue, introduce, and/or reintroduce medications in patients with persistent AKI (ADQI 16)3:
-
•
Individualize the prescription;
-
•
Renal versus nonrenal excretion;
-
•
Potential for nephrotoxicity;
-
•
Effect of AKI on metabolites and/or the effect of AKI on the nonrenal metabolism of drugs;
-
•
Strength of indications and/or urgency for use of the drug; and
-
•
Availability of suitable alternative.
The preceding considerations may have varying relevance for a particular drug and stage of AKI and transition from one AKI stage to another (ADQI 16).3
In persistent AKI and acute CKD, in the early stages when GFR starts declining, avoidance of nephrotoxic drugs and drugs with renovascular effects is recommended3, 70 (e.g., avoiding nonsteroidal anti-inflammatory drugs [NSAIDs], aminoglycosides, and withdrawing angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers [ACEIs/ARBs]). Certain antibiotics, especially aminoglycosides, have certain favorable characteristics; they are highly potent bacterial antibiotics with predictable pharmacokinetics, low incidence of immunologically-mediated side effects, and lack hematotoxic and hepatotoxic effects. Aminoglycosides, because of their potent bactericidal activity, can help reverse sepsis and sepsis-related hemodynamic instability, and thus, the risk for AKI and/or worsening of AKI. They are also inexpensive.71, 72, 73, 74, 75 Restricting use of aminoglycoside in all patients would have significant affect on treatment costs and antimicrobial stewardship, more so in resource-limited countries.76, 77 Individualizing the treatment with proper judgment and weighing the risk-to-benefit ratio before starting and/or adding or withdrawing a nephrotoxic drug is needed. In all phases of AKI, avoidance and/or withdrawal of nephrotoxic drugs or selection of less nephrotoxic drugs (e.g., netilmicin over amikacin) should be the goal. It is advisable to use sick day rules for patients at risk of AKI. The important aspect of these rules is informing patients at risk of AKI to temporarily stop and/or withhold medications, including ACEIs/ARBs, diuretics, and NSAIDs during acute illness.
Implementing such a policy would help prevent and/or reduce incidence of AKI, especially in developing countries where accessibility to health care may be limited or delayed.78
Consensus Statement
-
2.3.
We recommend adjusting drug dosage following the nomogram using an appropriate estimated GFR (eGFR) formula. with serum creatinine adjusted for fluid balance.
Context
Estimates of kidney function are used to modify drug dosing in patients with AKI. Assessment of kidney function in patients with AKI or multisystem organ failure and/or multiorgan dysfunction syndrome is challenging.79, 80, 81 Gold standard measures of GFR (such as inulin clearance) cannot be used because they are expensive, not readily available, and are impractical in the ICU. The eGFR has been widely adopted for drug dosages, although its role remains unclear in the AKI and ICU settings because these equations were designed for CKD. The Cockcroft-Gault equation and modification of diet in renal disease formula are known to overestimate kidney function in AKI.79 Use of the Jelliffe and modified Jelliffe equation (serum creatinine adjusted for fluid balance) could help avoid this pitfall in the setting of AKI.79, 80 Jelliffe and modified Jelliffe equations can be easily integrated into a computer program to facilitate the dosage requirement of drugs with a narrow therapeutic index. If other equations to estimate eGFR (e.g., Cockcroft-Gault equation equations, modification of diet in renal disease, and so on) are used, it is advisable to use the adjusted serum creatinine according to the cumulative daily fluid balance using the following equation82:
It is recommended that once eGFR is calculated considering all the preceding factors, drug dosages should be calculated using the product label that contains the drug dosage recommendations according to eGFR, taking into consideration loading and maintenance doses. Volume of distribution of many drugs, especially hydrophilic antibiotics, is significantly increased in AKI, and administration of a loading dose (25%−50%) greater than normal is recommended.66 Consideration should be given to dialysis clearance of drugs, drug adsorption to dialyzer membrane, and altered nonrenal clearance due to RRT in patients with AKI on RRT (hemodialysis, peritoneal dialysis, sustained low-efficiency dialysis, or CRRT). Antibiotic concentration in patients with AKI who receive CRRT may vary considerably.83 Vancomycin clearance is markedly increased during hemodialysis using polysulfone and high-flux dialysis. Conventional once-a-week dosing of vancomycin can result in subtherapeutic serum levels.84, 85
Consensus Statement
-
2.4.
We recommend therapeutic drug-level monitoring if available.
Context
When available, therapeutic drug monitoring should be done, especially for those drugs with a narrow therapeutic range. In a resource-constrained setting, if rapid specific analytical methods for serum drug concentration monitoring are not available, close monitoring of excessive pharmacological effects or physical signs of toxicity may indicate the need to modify drug dosage.
Research Recommendations
-
•
Formulation and validation of reliable formulas for estimating kidney function (eGFR) in AKI are needed.
-
•
Rapid and specific assays for drugs (especially drugs with a narrow therapeutic window) should be developed and made available easily and at an affordable cost.
-
•
Study of pharmacodynamics and pharmacokinetics of commonly used drugs in the AKI setting to establish a proper loading dose and a maintenance dose in various forms of RRTs.
Q3: Should Diuretics Be Used in Patients With Suspected AKI and/or Persistent AKI?
Consensus Statement
-
3.1.
We do not recommend diuretics for prevention and/or treatment of AKI.
Context
The consideration to prescribe diuretics to patients with potential AKI varies depending on the clinical context, which may be different in terms of timing, location, and occasion. The use of diuretics will be justified for the patients with volume overload in AKI. Initial prescriptions might be diuretics in patients with heart failure and pulmonary edema with potential AKI. Therefore, the current recommendation does not preclude the prescription of diuretics in the appropriate clinical setting.
There were 3 RCTs for AKI prevention that compared placebo and/or standard medication versus loop diuretics.86, 87, 88 A meta-analysis by Ho and Power89 reported that the use of furosemide did not improve the hospital mortality rate and the rate of RRT to a significant level. It is arguable that the definition of AKI varies in different studies, but there is no report that favors the effectiveness of furosemide to reduce the occurrence of AKI. Moreover, 1 RCT by Lassnigg et al. showed deterioration of AKI in the furosemide group.87 In summary, we do not recommend diuretics for the purpose of prevention of AKI.
There are 7 RCTs90, 91, 92, 93, 94, 95, 96 for AKI treatment that compared placebo and/or standard medication versus loop diuretics. A meta-analysis by Ho and Sheridan97 aggregated these and reported that the use of furosemide did not improve hospital mortality rate or the rate of RRT. Some studies looked at AKI recovery, but there were no reports that showed efficacy of furosemide for recovery of AKI, although the definitions of AKI recovery varied. The use of furosemide in AKI with RRT was examined in 2 RCTs,95, 96 but furosemide did not reduce the treatment period of RRT nor did it hasten the recovery from AKI. Therefore, the current recommendation is not to use diuretics to specifically treat AKI.
Consensus Statement
-
3.2.
Diuretics may be used in the setting of fluid overload.
Context
It is certainly necessary under clinical circumstances to use diuretics in patients with potential AKI who have reduced urine output, with volume overload and/or hyperkalemia. However, there is no epidemiological report on this particular clinical scenario. The other factor why diuretics have not provided beneficial effects might be related to drug interaction with preprescribed medications, such as NSAIDs and ACEi/ARBs, as recently reported by Lapi et al.98
Consensus Statement
-
3.3.
We recommend a furosemide stress test (FST) after adequate fluid repletion under monitored conditions.
Context
The use of i.v. furosemide has been recommended in suspected and persistent AKI. Intravenous administration of high-dose furosemide (1.0 or 1.5 mg/kg depending on previous furosemide exposure), the so-called FST, may predict the severity of AKI.99 Seventy-seven critically ill patients with AKI stage 1 or 2 of acute kidney injury network, some of whom had already received standard dose of furosemide were given a single dose i.v. of furosemide 1.5 mg/kg (in cases with previous diuretic exposure) or 1.0 mg/kg (for loop diuretic−naïve cases). To minimize the risk of hypovolemia in the 1.5 mg/kg cohort, urine output was replaced milliliter for milliliters each hour with either Ringer’s lactate or normal saline for6 hours after the FST. The treating team could elect not to replace the volume if net volume loss was considered clinically desirable. Urine output was measured hourly for 6 hours and in total for 24 hours. They found that subjects with progressive AKI had significantly lower urine output following FST in each of the first 6 hours (P < 0.001). The area under the receiver-operator characteristic curves for the total urine output over the first 2 hours following FST to predict progression to acute kidney injury network stage III was 0.87 (P = 0.001). The ideal cutoff for predicting AKI progression during the first 2 hours following FST was a urine volume of <200 ml (100 ml/hours), with a sensitivity of 87.1% and specificity 84.1%. The FST is a dynamic test and should be performed in the appropriate clinical setting, where urine output, heart rate, and blood pressure can be monitored frequently. In the tropical zone, the physician should evaluate the feasibility of doing the FST keeping in mind the basal conditions and the gastrointestinal-related infectious disease prevalent in the region. The study, which combined FST with a AKI biomarker, further proved outstanding prediction capability of AKI severity as seen in the area under the curve for progression to stage 3, which improved to 0.90 ± 0.06, and the area under the curve for patients on RRT, which improved to 0.91±0.08.100 The current protocol of The FST is not handy for the general physician or community hospital settings. The combination of an efficient AKI biomarker may facilitate reducing the challenge dose of FST to develop a concise version of FST with a reasonable AKI severity prediction level. Urine output response after an increase in FST expects that furosemide might potentially convert oliguric AKI to nonoliguric AKI, and therefore, would be therapeutically useful. However, this opinion is not supported by previously mentioned studies in this consensus statement. This is because inappropriate use of furosemide will often have a negative impact on renal microcirculation and further results in worse outcome. It remains still unclear if FST reveals the severity of AKI (the structure damage) or the loss of tubular functional capacity (functional injury).
Research Recommendations
-
•
The clinical impact of diuretics in subjects with potential AKI who have reduced urine output with volume overload and/or hyperkalemia need to be evaluated.
-
•
The reduction of the challenge dose of diuretics in FST needs to be evaluated to ascertain if it is user-friendly with the combination of AKI biomarkers.
-
•
The severity of AKI and/or tubular functional injury should be evaluated in conjunction with FST.
Q4: What Are the Nutritional Requirements in Patients With Suspected AKI and/or Confirmed AKI and/or Persistent AKI?
Consensus Statements
-
4.1.
We recommend enteral nutrition support be started as early as possible to prevent malnutrition.
-
4.2.
We recommend prescribing 25 to 30 kcal/kg per day intake of calories.
-
4.3.
We recommend prescribing 0.8 to 1.0 g/kg per day of protein intake for AKI without RRT and/or in the noncatabolic state.
-
4.4.
We recommend prescribing 1.5 to 2.0 g/kg per day of protein intake for AKI with RRT and/or in the hypercatabolic state.
Context
The importance of nutritional status evaluation and management in AKI and the need to use an integrated and practical terminology have been recognized in a recent consensus statement by the International Society of Renal Nutrition and Metabolism.101 Protein energy wasting (PEW) is common in patients with AKI. Up to 40% of patient with AKI in the ICU have severe PEW, which is an independent risk factor for in-hospital mortality. Preexisting malnutrition, especially in resource poor countries, together along with anorexia, oxidative stress, the hypercatabolic state, impaired protein transport and metabolism, metabolic acidosis, and nutrient losses during the dialysis procedure are important contributing factors for PEW in AKI.102 The currently available nutritional parameters for evaluation of nutritional status in AKI have low sensitivity and specificity103 (Table 3).
Table 3.
Nutritional markers and their limitations in patient with acute kidney injury (AKI)
| Parameter | Comment |
|---|---|
| Anthropometry (triceps skin fold, arm circumference, etc.) | Influenced by edema |
| Changes in body weight | Total body water is increased in AKI. Hypervolemia can mask changes in muscle mass. |
| Albumin, prealbumin, and cholesterol | May be reduced regardless of PEW (negative inflammation markers) |
| Leukocyte count | Low specificity |
| Protein catabolic rate of protein equivalent to nitrogen emergence | Measurement based on urea kinetics during RRT + collection of the dialysate |
| Energy spending | Prediction formulas are not always accurate in critically ill patients (they are usually based on body weight) |
| Nutritional score (SGA and its modifications) | Most data from patients with chronic kidney disease |
PEW, protein-energy wasting; RRT, renal replacement therapy; SGA, subjective global assessment.
The main aim of nutritional support in AKI is to provide adequate intake of energy, proteins, macro and micronutrients, and vitamins with the aim of modulating increased catabolism in AKI while maintaining lean body mass.104 Because PEW has been associated with poor renal and survival outcomes in AKI in ICU settings, it is believed that feeding should improve kidney injury and survival.105 There are many meta-analyses of enteral versus parenteral nutrition, and parenteral nutrition has been associated with poor outcomes.106, 107, 108, 109 A large randomized trial of early parenteral nutrition versus enteral nutrition showed that early parenteral nutrition was associated with prolonged ICU and hospital stay, increased infections, prolonged ventilatory support, and prolonged AKI recovery.110 In the absence of adequately designed trials, most nutritional guidelines for AKI are based on expert opinions.111, 112, 113 Enteral feeding helps to maintain gut integrity, decreases gut atrophy, and bacterial translocation. AKI may be associated with impairment of gastrointestinal mortality, and therefore, decreased absorption. However, these become aggravated with parenteral nutrition, and enteral nutrition reverses many of these impairments faster in patients with AKI.114, 115, 116, 117, 118 If oral feeding is not possible, enteral feeding should be initiated as soon as possible. Meta-analyses of critically ill patients, including AKI, showed significant decreases in mortality rates, infectious complications, and hospital stay when subjects received enteral nutrition within 2 hours of admission.111, 119, 120, 121 Parenteral nutrition should be given only if enteral nutrition is not possible or is inadequate to provide nutrition.
The optimal energy-to-nitrogen ratio during AKI has not been studied well.122 There are alterations in carbohydrate and lipid metabolism in AKI. Peripheral insulin resistance leads to hyperglycemia. Amino acids released during accelerated protein catabolism accelerate hepatic gluconeogenesis. Alterations in lipolysis in AKI lead to hypertriglyceridemia. Impaired clearance of exogenous lipids in AKI has also been reported. Because fatty acid oxidation is preserved in AKI, up to 35% of total nonprotein energy can be given as lipids.123, 124 The insulin basal-bolus method is reportedly not recommended in critically ill patients. It will be reasonable to start insulin in patients with blood sugars >180 mg/dl with target of reaching 140 to 180 mg/dl.2, 125, 126 An energy intake of 25 kcal/kg per day in a retrospective study of patients who underwent continuous veno-venous hemofiltration resulted in only a weak positive nitrogen balance.127 In another randomized trial, even higher 30 to 40 kcal/kg per day of energy nutrition did not result in greater positive nitrogen balance, but it was associated with negative impacts, such as hyperglycemia, hypertriglyceridemia, and increased fluid balance.128 The Harris-Benedict formula may overestimate the target energy in subjects with metabolic syndrome.
Optimal amount of protein supplementation in AKI is unknown. Adequate protein intake is essential to prevent aggravation of malnutrition, which is highly prevalent in patients with AKI, and is an important risk factor for increased mortality. Nutritional protein intake should not be restricted in patients with AKI due to fear of increased urea generation and earlier need for RRT. Malnutrition is a far greater risk for mortality. In patients on CRRT, nitrogen balance was achieved with protein intake of 1.4 to 1.8 g/kg per day.129, 130 Negative nitrogen balance will get worse on RRT if protein intake is <1 g/kg per day. High amounts of protein intake in cancer patients with AKI in the hypercatabolic state and on sustained low-efficiency dialysis had improved nitrogen balance and had a lower risk of mortality.131
All forms of RRT lead to loss of protein and/or amino acids in the dialysis procedure; 5 to 10 g of protein or its equivalent may be lost in various RRT procedures. It has been estimated that in CRRT, 10 to 15 g/d of amino acids are lost. Therefore, patients with AKI on RRT need to be compensated with a higher protein intake (1–1.5 g/kg per day), and those with hypercatabolism require additional protein supplementation (1.5–2 gm/kg per day) to provide for negative nitrogen balance related to the hypercatabolic state.
After starting CRRT, electrolyte disorders such as hypopotassemia and hypophosphatemia often happen, and therefore, their appropriate correction is necessary to prevent arrhythmic events due to hypopotassemia and ventilator dependency due to hypophosphatemia; the target level of potassium correction will be 3.5 to 4.0 mEq/L and that of phosphate will be 2.5 mg/dl.
Research Recommendations
-
•
Determine the nutritional requirements of patients with AKI to optimize recovery.
-
•
Establish the best options for measuring nutritional needs in patients with AKI and those who require RRT.
-
•
Determine the best formulations of nutritional supplementations in patients with AKI and RRT to reduce time to recovery.
Summary
In conclusion, early recognition of AKI and/or AKI risk factors and thorough clinical assessment of fluid and volume status for adequate and prompt resuscitation with normal saline, removing nephrotoxins, and providing early and adequate enteral nutritional support remain the cornerstone for preventing and managing AKI in the developing and the developed world.
Disclosure
VK has received research funding from Novartis India, Sanofi Aventis India, and Astellas India and has received honoraria from Novartis India, Roche India, Astellas India, Torrent India, and Reddy’s India; is a Scientific Advisor for Roche India, Novartis India, Torrent, Sanofi Aventis, Reddy’s India, Biocon India, and Medtronics; and is a member of the Speakers’ Bureau for Novartis India, Roche India, Panacea India, Sanofi Aventis India, Intas India, Biocon India, Pfizer, Medtronics, and wipro GE, India. All the other authors declared no competing interests.
Author Contributions
VK, NS, EM, MB, MS, RC, and RM all participated in the consensus-building process and drafting of this paper. LY, RM, RC, and AB provided a critical review of this paper.
Acknowledgment
Supported through the UAB-UCSD O’Brien Center NIH-NIDDK Grant DK079337.
References
- 1.KDIGO Clinical Practice Guideline for Acute Kidney Injury Section 2: AKI Definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDIGO Clinical Practice Guideline for Acute Kidney Injury Section 3: Prevention and Treatment of AKI. Kidney Int Suppl. 2012;2:37–68. doi: 10.1038/kisup.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: guideline report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Clin J Am Soc Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 4.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Worgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 5.Mehta R.L., Burdmann E.A., Cerda J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet (London, England) 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 6.Kellum J.A., Bellomo R., Ronco C. Acute Dialysis Quality Initiative (ADQI): methodology. Int J Artif Organs. 2008;31:90–93. doi: 10.1177/039139880803100202. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard J., Soroko S.B., Chertow G.M. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 8.Osman D., Ridel C., Ray P. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 9.Feissel M., Teboul J.L., Merlani P. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med. 2007;33:993–999. doi: 10.1007/s00134-007-0602-6. [DOI] [PubMed] [Google Scholar]
- 10.Gruenewald M., Meybohm P., Koerner S. Dynamic and volumetric variables of fluid responsiveness fail during immediate postresuscitation period. Crit Care Med. 2011;39:1953–1959. doi: 10.1097/CCM.0b013e31821cb751. [DOI] [PubMed] [Google Scholar]
- 11.Finfer S., Vincent J.L. Critical care–an all-encompassing specialty. N Engl J Med. 2013;369:669–670. doi: 10.1056/NEJMe1304035. [DOI] [PubMed] [Google Scholar]
- 12.Starling E.H. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadhan K.K., Lobo D.N. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69:488–498. doi: 10.1017/S0029665110001734. [DOI] [PubMed] [Google Scholar]
- 14.Perner A., Haase N., Guttormsen A.B. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 15.Myburgh J.A., Finfer S., Bellomo R. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 16.Quinlan G.J., Martin G.S., Evans T.W. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 17.Weil M.H., Henning R.J., Puri V.K. Colloid oncotic pressure: clinical significance. Crit Care Med. 1979;7:113–116. doi: 10.1097/00003246-197903000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Sudlow G., Birkett D.J., Wade D.N. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–832. [PubMed] [Google Scholar]
- 19.King T.P. On the sulfhydryl group of human plasma albumin. J Biol Chem. 1961;236:PC5. [PubMed] [Google Scholar]
- 20.Quinlan G.J., Margarson M.P., Mumby S. Administration of albumin to patients with sepsis syndrome: a possible beneficial role in plasma thiol repletion. Clin Sci (Lond) 1998;95:459–465. [PubMed] [Google Scholar]
- 21.Finfer S., Bellomo R., Boyce N. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 22.Caironi P., Tognoni G., Masson S. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 23.Wills B.A., Nguyen M.D., Ha T.L. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- 24.Hadimioglu N., Saadawy I., Saglam T. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264–269. doi: 10.1213/ane.0b013e3181732d64. [DOI] [PubMed] [Google Scholar]
- 25.Hasman H., Cinar O., Uzun A. A randomized clinical trial comparing the effect of rapidly infused crystalloids on acid-base status in dehydrated patients in the emergency department. Int J Med Sci. 2012;9:59–64. doi: 10.7150/ijms.9.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khajavi M.R., Etezadi F., Moharari R.S. Effects of normal saline vs. lactated ringer's during renal transplantation. Ren Fail. 2008;30:535–539. doi: 10.1080/08860220802064770. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox C.S. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw A.D., Bagshaw S.M., Goldstein S.L. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 29.Yunos N.M., Bellomo R., Hegarty C. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 30.McCluskey S.A., Karkouti K., Wijeysundera D. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 31.Young P., Bailey M., Beasley R. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 32.Rivers E., Nguyen B., Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger R.P., Levy M.M., Carlet J.M. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 34.Pro C.I., Yealy D.M., Kellum J.A. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ARISE Investigators; ANZICS Clinical Trials Group. Peake S.L. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 36.Mouncey P.R., Osborn T.M., Power G.S. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 37.Ranjit S., Kissoon N., Gandhi D. Early differentiation between dengue and septic shock by comparison of admission hemodynamic, clinical, and laboratory variables: a pilot study. Pediatr Emerg Care. 2007;23:368–375. doi: 10.1097/01.pec.0000278403.22450.a2. [DOI] [PubMed] [Google Scholar]
- 38.Maitland K., Kiguli S., Opoka R.O. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein S.L., Somers M.J., Baum M.A. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 40.Foland J.A., Fortenberry J.D., Warshaw B.L. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 41.Gillespie R.S., Seidel K., Symons J.M. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 42.Hayes L.W., Oster R.A., Tofil N.M. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24:394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Michael M., Kuehnle I., Goldstein S.L. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19:91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 44.Selewski D.T., Cornell T.T., Blatt N.B. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40:2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Galasso L., Emma F., Picca S. Continuous renal replacement therapy in children: fluid overload does not always predict mortality. Pediatr Nephrol. 2016;31:651–659. doi: 10.1007/s00467-015-3248-6. [DOI] [PubMed] [Google Scholar]
- 46.De Backer D., Biston P., Devriendt J. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 47.Delmas A., Leone M., Rousseau S. Clinical review: vasopressin and terlipressin in septic shock patients. Crit Care. 2005;9:212–222. doi: 10.1186/cc2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell J.A., Walley K.R., Singer J. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 49.Gordon A.C., Russell J.A., Walley K.R. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36:83–91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- 50.Dellinger R.P., Levy M.M., Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 51.LeDoux D., Astiz M.E., Carpati C.M. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Dunser M.W., Takala J., Ulmer H. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35:1225–1233. doi: 10.1007/s00134-009-1427-2. [DOI] [PubMed] [Google Scholar]
- 53.Asfar P., Meziani F., Hamel J.F. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 54.Salmasi V., Maheshwari K., Yang Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 55.Sun L.Y., Wijeysundera D.N., Tait G.A., Beattie W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 56.Onuigbo M.A.C., Agbasi N. Intraoperative hypotension - a negalected causative factor in hospital-acquired acute kidney injury;a Mayo Clinic Health System experience revisited. J Renal Inj Prev. 2015;4:61–67. doi: 10.12861/jrip.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta R.L., McDonald B., Gabbai F. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113:456–461. doi: 10.1016/s0002-9343(02)01230-5. [DOI] [PubMed] [Google Scholar]
- 58.Costa e Silva V.T., Liano F., Muriel A. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLoS One. 2013;8:e70482. doi: 10.1371/journal.pone.0070482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponce D., Zorzenon Cde P., dos Santos N.Y. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant. 2011;26:3202–3206. doi: 10.1093/ndt/gfr359. [DOI] [PubMed] [Google Scholar]
- 60.Meier P., Bonfils R.M., Vogt B. Referral patterns and outcomes in noncritically ill patients with hospital-acquired acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2215–2225. doi: 10.2215/CJN.01880211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaufman J., Dhakal M., Patel B. Community-acquired acute renal failure. Am J Kidney Dis. 1991;17:191–198. doi: 10.1016/s0272-6386(12)81128-0. [DOI] [PubMed] [Google Scholar]
- 62.Wu T.Y., Jen M.H., Bottle A. Ten-year trends in hospital admissions for adverse drug reactions in England 1999-2009. J R Soc Med. 2010;103:239–250. doi: 10.1258/jrsm.2010.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lameire N., Van Biesen W., Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 64.Bagshaw S.M., George C., Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizock B.A. The multiple organ dysfunction syndrome. Dis Mon. 2009;55:476–526. doi: 10.1016/j.disamonth.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Proulx F., Joyal J.S., Mariscalco M.M. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 67.Lewis S.J., Mueller B.A. Antibiotic dosing in patients with acute kidney injury: “enough but not too much”. J Intensive Care Med. 2016;31:164–176. doi: 10.1177/0885066614555490. [DOI] [PubMed] [Google Scholar]
- 68.Eyler R.F., Mueller B.A. Medscape. Antibiotic dosing in critically ill patients with acute kidney injury. Nat Rev Nephrol. 2011;7:226–235. doi: 10.1038/nrneph.2011.12. [DOI] [PubMed] [Google Scholar]
- 69.Mehta R.L., Pascual M.T., Soroko S. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 70.KDIGO Clinical Practice Guideline for Acute Kidney Injury Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2:69–88. doi: 10.1038/kisup.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cosgrove S.E., Vigliani G.A., Fowler V.G., Jr. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48:713–721. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- 72.Falagas M.E., Matthaiou D.K., Bliziotis I.A. The role of aminoglycosides in combination with a beta-lactam for the treatment of bacterial endocarditis: a meta-analysis of comparative trials. J Antimicrob Chemother. 2006;57:639–647. doi: 10.1093/jac/dkl044. [DOI] [PubMed] [Google Scholar]
- 73.Falagas M.E., Matthaiou D.K., Karveli E.A. Meta-analysis: randomized controlled trials of clindamycin/aminoglycoside vs. beta-lactam monotherapy for the treatment of intra-abdominal infections. Aliment Pharmacol Ther. 2007;25:537–556. doi: 10.1111/j.1365-2036.2006.03240.x. [DOI] [PubMed] [Google Scholar]
- 74.Glasmacher A., von Lilienfeld-Toal M., Schulte S. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clin Microbiol Infect. 2005;11 Suppl 5:17–23. doi: 10.1111/j.1469-0691.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 75.Paul M., Benuri-Silbiger I., Soares-Weiser K. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.James M., Bouchard J., Ho J. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:673–685. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 77.Ad-hoc Working Group of ERBP. Fliser D., Laville M. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–4272. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blakeman T., Harding S., O'Donoghue D. Acute kidney injury in the community: why primary care has an important role. Br J Gen Pract. 2013;63:173–174. doi: 10.3399/bjgp13X664207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouchard J., Macedo E., Soroko S. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25:102–107. doi: 10.1093/ndt/gfp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol. 2002;22:320–324. doi: 10.1159/000065221. [DOI] [PubMed] [Google Scholar]
- 81.Brater D.C. ADIS Health Science Press; Balgowlah, Australia: 1983. Drug Use in Renal Disease. [Google Scholar]
- 82.Macedo E., Bouchard J., Soroko S.H. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts D.M., Roberts J.A., Roberts M.S. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–1528. doi: 10.1097/CCM.0b013e318241e553. [DOI] [PubMed] [Google Scholar]
- 84.Lanese D.M., Alfrey P.S., Molitoris B.A. Markedly increased clearance of vancomycin during hemodialysis using polysulfone dialyzers. Kidney Int. 1989;35:1409–1412. doi: 10.1038/ki.1989.141. [DOI] [PubMed] [Google Scholar]
- 85.Barth R.H., DeVincenzo N. Use of vancomycin in high-flux hemodialysis: experience with 130 courses of therapy. Kidney Int. 1996;50:929–936. doi: 10.1038/ki.1996.393. [DOI] [PubMed] [Google Scholar]
- 86.Hager B., Betschart M., Krapf R. Effect of postoperative intravenous loop diuretic on renal function after major surgery. Schweiz Med Wochenschr. 1996;126:666–673. [PubMed] [Google Scholar]
- 87.Lassnigg A., Donner E., Grubhofer G. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97–104. doi: 10.1681/ASN.V11197. [DOI] [PubMed] [Google Scholar]
- 88.Mahesh B., Yim B., Robson D. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial. Eur J Cardiothorac Surg. 2008;33:370–376. doi: 10.1016/j.ejcts.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 89.Ho K.M., Power B.M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65:283–293. doi: 10.1111/j.1365-2044.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 90.Cantarovich F., Fernandez J.C., Locatelli A. Frusemide in high doses in the treatment of acute renal failure. Postgrad Med J. 1971;47 Suppl:13–17. [PubMed] [Google Scholar]
- 91.Letter: high-dose frusemide in renal failure. BMJ. 1974;2:278–279. doi: 10.1136/bmj.2.5913.278-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kleinknecht D., Ganeval D., Gonzalez-Duque L.A. Furosemide in acute oliguric renal failure. A controlled trial. Nephron. 1976;17:51–58. doi: 10.1159/000180710. [DOI] [PubMed] [Google Scholar]
- 93.Brown C.B., Ogg C.S., Cameron J.S. High dose frusemide in acute renal failure: a controlled trial. Clin Nephrol. 1981;15:90–96. [PubMed] [Google Scholar]
- 94.Shilliday I.R., Quinn K.J., Allison M.E. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997;12:2592–2596. doi: 10.1093/ndt/12.12.2592. [DOI] [PubMed] [Google Scholar]
- 95.Cantarovich F., Rangoonwala B., Lorenz H. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44:402–409. [PubMed] [Google Scholar]
- 96.van der Voort P.H., Boerma E.C., Koopmans M. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med. 2009;37:533–538. doi: 10.1097/CCM.0b013e318195424d. [DOI] [PubMed] [Google Scholar]
- 97.Ho K.M., Sheridan D.J. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lapi F., Azoulay L., Yin H. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. doi: 10.1136/bmj.e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chawla L.S., Davison D.L., Brasha-Mitchell E. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koyner J.L., Davison D.L., Brasha-Mitchell E. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol. 2015;26:2023–2031. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fouque D., Kalantar-Zadeh K., Kopple J. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 102.Fiaccadori E., Lombardi M., Leonardi S. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10:581–593. doi: 10.1681/ASN.V103581. [DOI] [PubMed] [Google Scholar]
- 103.Fiaccadori E., Cremaschi E., Regolisti G. Nutritional assessment and delivery in renal replacement therapy patients. Semin Dial. 2011;24:169–175. doi: 10.1111/j.1525-139X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 104.Kritmetapak K., Peerapornratana S., Srisawat N. The impact of macro−and micronutrients on predicting outcomes of critically ill patients requiring continuous renal replacement therapy. PLoS One. 2016;11:e0156634. doi: 10.1371/journal.pone.0156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fiaccadori E., Maggiore U., Cabassi A. Nutritional evaluation and management of AKI patients. J Ren Nutr. 2013;23:255–258. doi: 10.1053/j.jrn.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Braunschweig C.L., Levy P., Sheean P.M. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 107.Gramlich L., Kichian K., Pinilla J. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Peter J.V., Moran J.L., Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med. 2005;33:213–220. doi: 10.1097/01.ccm.0000150960.36228.c0. discussion 260–211. [DOI] [PubMed] [Google Scholar]
- 109.Simpson F., Doig G.S. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med. 2005;31:12–23. doi: 10.1007/s00134-004-2511-2. [DOI] [PubMed] [Google Scholar]
- 110.Casaer M.P., Mesotten D., Hermans G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 111.Heyland D.K., Dhaliwal R., Drover J.W. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 112.Singer P., Berger M.M., Van den Berghe G. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 113.Martindale R.G., McClave S.A., Vanek V.W. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care. Med. 2009;37:1757–1761. doi: 10.1097/CCM.0b013e3181a40116. [DOI] [PubMed] [Google Scholar]
- 114.Scheinkestel C.D., Kar L., Marshall K. Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition. 2003;19:909–916. doi: 10.1016/s0899-9007(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 115.Fiaccadori E., Maggiore U., Clima B. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int. 2001;59:1510–1519. doi: 10.1046/j.1523-1755.2001.0590041510.x. [DOI] [PubMed] [Google Scholar]
- 116.Metnitz P.G., Krenn C.G., Steltzer H. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 117.Fiaccadori E., Maggiore U., Giacosa R. Enteral nutrition in patients with acute renal failure. Kidney Int. 2004;65:999–1008. doi: 10.1111/j.1523-1755.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- 118.Barnert J., Dumitrascu D., Neeser G. Gastric emptying of a liquid meal in intensive care unit patients. Gastroenterology. 1998;114:A865. [Google Scholar]
- 119.Marik P.E., Zaloga G.P. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 120.Doig G.S., Heighes P.T., Simpson F. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: a meta-analysis of randomised controlled trials. Injury. 2011;42:50–56. doi: 10.1016/j.injury.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 121.Bost R.B., Tjan D.H., van Zanten A.R. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Ann Intensive Care. 2014;4:31. doi: 10.1186/s13613-014-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kopple J.D. McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr. 1997;65:1544–1557. doi: 10.1093/ajcn/65.5.1544. [DOI] [PubMed] [Google Scholar]
- 123.Druml W., Mitch W.E. Metabolic abnormalities in acute renal failure. Semin Dial. 1996;9:484–490. [Google Scholar]
- 124.Schneeweiss B., Graninger W., Stockenhuber F. Energy metabolism in acute and chronic renal failure. Am J Clin Nutr. 1990;52:596–601. doi: 10.1093/ajcn/52.4.596. [DOI] [PubMed] [Google Scholar]
- 125.Investigators N.-S.S., Finfer S., Liu B. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 126.Fiaccadori E., Sabatino A., Morabito S. Hyper/hypoglycemia and acute kidney injury in critically ill patients. Clin Nutr. 2016;35:317–321. doi: 10.1016/j.clnu.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Macias W.L., Alaka K.J., Murphy M.H. Impact of the nutritional regimen on protein catabolism and nitrogen balance in patients with acute renal failure. JPEN J Parenter Enteral. Nutr. 1996;20:56–62. doi: 10.1177/014860719602000156. [DOI] [PubMed] [Google Scholar]
- 128.Fiaccadori E., Maggiore U., Rotelli C. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005;20:1976–1980. doi: 10.1093/ndt/gfh956. [DOI] [PubMed] [Google Scholar]
- 129.Chima C.S., Meyer L., Hummell A.C. Protein catabolic rate in patients with acute renal failure on continuous arteriovenous hemofiltration and total parenteral nutrition. J Am Soc Nephrol. 1993;3:1516–1521. doi: 10.1681/ASN.V381516. [DOI] [PubMed] [Google Scholar]
- 130.Leblanc M., Garred L.J., Cardinal J. Catabolism in critical illness: estimation from urea nitrogen appearance and creatinine production during continuous renal replacement therapy. Am J Kidney Dis. 1998;32:444–453. doi: 10.1053/ajkd.1998.v32.pm9740161. [DOI] [PubMed] [Google Scholar]
- 131.Salahudeen A.K., Kumar V., Madan N. Sustained low efficiency dialysis in the continuous mode (C-SLED): dialysis efficacy, clinical outcomes, and survival predictors in critically ill cancer patients. Clin J Am Soc Nephrol. 2009;4:1338–1346. doi: 10.2215/CJN.02130309. [DOI] [PMC free article] [PubMed] [Google Scholar]