Abstract
Cardiovascular disease (CVD) accounts for more deaths globally than any other single disease. There are on average 1.5 million episodes of myocardial infarction (heart attack) each year in the United States alone with roughly one third resulting in death. There is therefore a major need for developing new and effective strategies to promote cardiac repair. Intramyocardial transplantation of mesenchymal stem cells (MSCs) has emerged as a leading contender in the pursuit of clinical intervention and therapy. MSCs are potent mediators of cardiac repair and are therefore an attractive tool in the development of pre-clinical and clinical trials. MSCs are capable of secreting a large array of soluble factors, which have had demonstrated effects on pathogenic cardiac remolding, fibrosis, immune activation and cardiac stem cell proliferation within the damaged heart. MSCs are also capable of differentiation into cardiomyocytes, endothelial cells and vascular smooth muscle cells, although the relative contribution of trilineage differentiation and paracrine effectors on cardiac repair remains the subject of active investigation.
Introduction
While our knowledge of the developmental origin of MSCs is still relatively limited, it is widely believed that MSCs are derived from mesoderm, one of the three germ layers that form at gastrulation during the development of the mammalian embryo[1]. It is from this mesodermal layer that cells destined to form the myocardium of the heart are also derived[2]. During heart tube formation, promyocardial cells migrate from the lateral plate mesoderm to populate the primordium of the left ventricle and sinus venosus. The outflow tract and right ventricle are then simultaneously populated with cells migrating from a second cardiogenic area located posterior to the dorsal wall of the pericardial cavity[3].
Within 3 weeks of gestation the human heart demonstrates the first signs of peristaltic contraction, while mesoderm-derived cells continue to migrate into the heart as it grows. This hyperplastic growth continues until birth at which point the organ has received a full complement of cardiac cells[2, 3]. During the first few days following birth, cardiomyocytes undergo a final round of karyokinesis in the absence of cytokinesis resulting in binucleation and exit from the cell cycle[4, 5]. Postpartum organ growth to adulthood is then primarily the result of cardiomyocyte hypertrophy rather than cellular proliferation. For decades it was believed that this program made the heart a post mitotic organ, unable to replenish lost cells once depleted[6]. However, during the 1990s several researchers began describing an ability of mature cardiomyocytes to re-enter into the cell cycle in vitro, although this process resulted in rapid apoptosis of the cells[7]. More recently some key studies have expanded on this observation, demonstrating sustained cytokinesis of postpartum mammalian cardiomyocytes in vivo. The first of these studies identified a transient ability of the murine neonatal heart to repair in response to partial resection of the left ventricular apex[8]. Within a finite developmental window, mature cardiomyocytes can undergo sarcomeric disassembly and re-enter mitosis with the resulting progeny contributing directly to recellularization of the injury site. The second study demonstrated an ability of human cardiac tissue to replace between 0.45–1% of cells per year throughout the human lifespan[9], based on abrupt changes in the cellular incorporation of the radioisotope 14C of humans exposed following the Limited Nuclear Test Ban Treaty of 1963. Together these observations have lead to a revolution in the perception of cardiovascular disease, dispelling concerns that repair of the mammalian heart was not feasible and giving hope to interventional strategies geared toward promoting endogenous repair mechanisms.
Cardiovascular disease
Cardiovascular disease (CVD) continues to account for more deaths globally than any other single disease. The most recent data shows that Americans suffer an average of 1.5 million episodes of acute myocardial infarction (AMI) each year with roughly one third resulting in death[10]. This rate translates to about one heart attack every 30 seconds and one cardiac-related death every 1.5 minute within the United States alone. Morbidity associated with post-infarction cardiomyopathy is also a significant problem and accounts for approximately 6 million hospital visits per year, thereby contributing significantly to annual healthcare costs.
AMI is the result of blockage to one or more of the main coronary arteries. Upon occlusion, a region of permanent injury containing dead and dying cells, known as an infarct, develops. Blood supply is interrupted within the developing infarct and the area rapidly becomes hypoxic[11]. Cardiomyocytes are comparatively resistant to chronic hypoxia at neutral pH. However, when the extracellular pH drops below 6.5 cardiomyocytes undergo extensive hypoxia-induced death. Hypoxia in cardiomyocytes causes a switch from oxidative to glycolytic energy generation, resulting in increased glucose consumption, lactic acid production and lower intracellular pH. Increased plasma lactate levels reflect this metabolic shift and are diagnostic of infarction in ischemic heart disease[12]. Chronic hypoxia in the presence of high glucose leads to progressive acidosis of cardiac myocytes. The resulting hypoxia-acidosis leads to apoptosis of cardiac cells within the infarct zone followed by vascular collapse and extensive tissue necrosis[12, 13]. A typical human infarct can result in the loss of over 1 billion cardiomyocytes[14], the tissue being replaced by the formation of a permanent, avascular collagenous scar, that averts an otherwise inevitable ventricular rupture. This process of cardiac remolding, while rapid in onset, can take several weeks to complete. When completed, these changes lead to significant reduction in cardiac function and ultimately to heart failure and death. While examples exist in nature that demonstrate an innate regenerative ability of cardiovascular tissue following injury[15, 16], this capability has been largely lost in mammalians, possibly as a consequence of increased body mass and greater systemic blood pressure.
Studying large and small animal models of AMI have led to the development of strategies to improve the reparative response of mammalian cardiac tissue. In this regard recent focus has been directed at engaging endogenous repair mechanisms through various interventions including the use of exogenous stem cell transplantation. The rationale for this approach is that the proliferative and multilineage differentiation capacity of stem cells conveys latent potential for organ regeneration through the formation of new tissue and also through the initiation of neovascularization. Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) were early candidates for regenerative therapy as they retain the most potential for multilineage differentiation and proliferation. However, as a therapeutic cell for cardiac regeneration they are fraught with biological (and in the case of ESCs, ethical) issues, including risks of teratoma formation[17] and have therefore proved to be of little direct use.
The limited differentiation capacity of adult stem cells suggested a more refined approach and in 2003 the first phase I clinical trial using adult skeletal myoblast stem cells was carried out[18]. After initial optimism triggered by cell engraftment and enhancements in left ventricular function, the effects were found not only to be unsustainable, but also lead to arrhythmias due to a lack of electrical coupling with host cardiomyocytes[19]. However, with the discovery of cardiac stem cells (CSCs)[20] came an exciting opportunity to improve engraftment and enhance functional integration. The recent SCIPIO trial[21] established CSCs as an effective therapeutic cell demonstrating cardiac engraftment, enhancement of coronary vasculature and a contemporaneous reduction in scar volume. These initial results are promising, and efforts aimed at increasing cell numbers obtained from tissue biopsies are currently moving forward. In the interim a variety of other adult cell types have been examined in pre-clinical and clinical trials. Some of the most consistently successful results for the induction of cardiac repair have been seen with the use of MSCs, which have been isolated from bone marrow and adipose tissue, expanded in culture and used successfully in pre-clinical and clinical trials (see Table 1 for list of clinical trails).
Table 1.
Clinical Trials with mesenchymal stem cells (MSC);(http://clinicaltrials.gov)
| Study | Year/ Country | Study ID | Phase | Cell type | Delivery method |
No. Treated |
Primary Outcome |
|---|---|---|---|---|---|---|---|
| Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) | 2007 – 2011/ USA | NCT00587990 | 1–2 | Autologous | Intraoperative Intramyocardial | 45 | Safety/ Efficacy Study |
| Intracoronary Autologous Mesenchymal Stem Cells Implantation in Patients With Ischemic Dilated Cardiomyopathy | 2012 – Pres./ Malasya | NCT01720888 | 2 | Autologous | Intracoronary | 80 | Efficacy Study |
| Left Ventricular Assist Device Combined With Allogeneic Mesenchymal Stem Cells Implantation in Patients With End-stage Heart Failure | 2012 – Pres. / Greece | NCT01759212 | 2–3 | Allogeneic | Intraoperative Intramyocardial | 5 | Efficacy Study |
| Allogeneic Stem Cells Implantation Combined With Coronary Bypass Grafting in Patients With Ischemic Cardiomyopathy | 2012 – Pres. / Greece | NCT01753440 | 2–3 | Allogeneic | Intraoperative Intramyocardial | 30 | Efficacy Study |
| A Phase IIa, Single-blind, Placebo-controlled, Crossover, Multi-center, Randomized Study to Assess the Safety, Tolerability, and Preliminary Efficacy of a Single Intravenous Dose of Ischemia-tolerant Allogeneic Mesenchymal Bone Marrow Cells to Subjects With Heart Failure of Non-ischemic Etiology | 2012 – Pres. / USA | NCT02123706 | 2a | Allogeneic | Intravenous | 20 | Safety / Efficacy Study |
| The Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis Pilot Study (The POSEIDON-Pilot Study) | 2010 – 2013 / USA | NCT01087996 | 1–2 | Allogeneic/ Autologous | Transendocardial Injection | 30 | Safety / Efficacy Study |
| A Phase 1 Randomized, Double-blind, Placebo-controlled, Dose Escalation, Multicenter Study to Determine the Safety of Intravenous Ex-vivo Cultured Adult Human Mesenchymal Stem Cells (Provacel) Following Acute Myocardial Infarction | 2005 – 2009 / USA | NCT00114452 | 1 | Allogeneic | Intravenous | 53 | Safety Study |
| Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy (RIMECARD) / Dilated Cardiomyopathy | 2012 – Pres. / Chile | NCT01739777 | 1 | Allogeneic | Intravenous | 30 | Safety/ Efficacy Study |
| / Safety and Efficacy of Intracoronary Adult Human Mesenchymal Stem Cells After Acute Myocardial Infarction (SEED-MSC) | 2007–2010 / Korea | NCT01392105 | 2 | Autologous | Intracoronary | 80 | Safety/ Efficacy Study |
| "ESTIMATION Study" for Endocardial Mesenchymal Stem cells Implantation in Patients After Acute Myocardial Infarction | 2011-Pres. / Russia | NCT01394432 | 3 | Autologous | Transendocardial Injection | 50 | Efficacy Study |
| Umbilical Cord Derived Mesenchymal stem cells Therapy in Ischemic Cardiomyopathy | 2013–2014 / China | NCT01946048 | 1 | Allogeneic | Intraoperative Intramyocardial | 10 | Safety/ Efficacy Study |
| Mesenchymal stem cells and Myocardial Ischemia (MESAMI) | 2010–2014 / France | NCT01076920 | 1, 2 | Autologous | Transendocardial Injection | 10 | Safety / Efficacy Study |
| Transendocardial Autologous Cells (hMSC or hBMC) in Ischemic Heart Failure Trial (TAC-HFT) | 2008–2012 / USA | NCT00768066 | 1,2 | Autologous | Transendocardial Injection | 67 | Safety/ Efficacy Study |
| TRansendocardial Stem Cell Injection Delivery Effects on Neomyogenesis STudy (The TRIDENT Study) | 2013-Pres. / USA | NCT02013674 | 2 | Allogeneic | Transendocardial Injection | 45 | Safety/ Efficacy Study |
| PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis in Dilated CardioMyopathy (The POSEIDON-DCM Study) | 2011- Present. / USA | NCT01392625 | 1, 2 | Autologous/ Allogeneic | Transendocardial Injection | 36 | Safety/ Efficacy Study |
| Mesenchymal Stem Cells to Treat Ischemic Cardiomyopathy | 2010–2013 / Brazil | NCT01913886 | 1, 2 | Autologous | Intracoronary | 10 | Safety/ Efficacy Study |
| Combined CABG and Stem-Cell Transplantation for Heart Failure | 2006–2010 / Finland | NCT00418418 | 2 | Autologous | Intraoperative Intramyocardial | 60 | Efficacy Study |
| A II, Multi-center, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of PROCHYMAL® | 2009–2016 / USA | NCT00877903 | 2 | Allogeneic | Intravenous | 220 | Safety/ Efficacy Study |
| Clinical Trial of Autologous Adipose Tissue Derived Stromal Cell Therapy for Ischemic Heart Failure | 2012–2014 / Japan | NCT01709279 | 1 | Autologous | Intracoronary | 6 | Safety/ Efficacy Study |
| Stem Cell Therapy for Vasculogenesis in Patients With Severe Myocardial Ischemia | 2005–2009 / Denmark | NCT00260338 | 1, 2 | Autologous | Intraoperative Intramyocardial | 31 | Safety/ Efficacy Study |
| Phase II Combination Stem Cell Therapy for the Treatment of Severe Coronary Ischemia (CI) MESENDO | 2008–2014 / USA | NCT00790764 | 2 | Autologous | Intracoronary | 60 | Safety/ Efficacy Study |
| Autologous Mesenchymal Stromal Cell Therapy in Heart Failure | 2008–2014 / Denmark | NCT00644410 | 1, 2 | Autologous | Transendocardial Injection (NOGA) | 59 | Safety/ Efficacy Study |
| RELIEF (A Randomized,Open labEled, muLticenter Trial for Safety and Efficacy of Intracoronary Adult Human Mesenchymal stEm Cells Acute Myocardial inFarction) | 2013-Pres. / Korea, Republic of | NCT01652209 | 3 | Autologous | Intracoronary | 135 | Safety/ Efficacy Study |
| Mesenchymal Stem Cells for Idiopathic Dilated Cardiomyopathy | 2013–2016 / Spain | NCT01957826 | 1, 2 | Autologous | Transendocardial Injection | 70 | Safety/ Efficacy Study |
| Stem Cell Injection to Treat Heart Damage During Open Heart Surgery | 2012–2014 / USA | NCT01557543 | 1 | Autologous | Intraoperative Intramyocardial | 24 | Safety Study |
| Intracoronary Human Wharton's Jelly- Derived Mesenchymal Stem Cells (WJ-MSCs) Transfer in Patients With Acute Myocardial Infarction (AMI) | 2011–2012 / China | NCT01291329 | 2 | Allogeneic | Intracoronary | 160 | Safety/ Efficacy Study |
| Ex Vivo Cultured Bone Marrow Derived Allogeneic MSCs in AMI | 2009–2012 / India | NCT00883727 | Allogeneic | Intravenous | 20 | Safety/ Efficacy Study | |
| Intracoronary Infusion of Autologous Bone Marrow Cells for Treatment of Idiopathic Dilated Cardiomyopathy | 2008–2010 / Spain | NCT00629096 | 2 | Autologous | Intracoronary | 27 | Efficacy Study |
| A Study of Allogeneic Mesenchymal Bone Marrow Cells in Subjects With ST Segment Elevation Myocardial Infarction (STEMI) | 2013–2017 / USA | NCT01770613 | 2 | Allogeneic | intravenously | 50 | Safety/ Efficacy Study |
| Effect of Intramyocardial Injection of Mesenchymal Precursor Cells on Heart Function in People Receiving an LVAD | 2009–2011 / USA | NCT00927784 | 2 | Allogeneic | Intraoperative Intramyocardial | 10 | Safety/ Efficacy Study |
| The Purpose of This Study is to Evaluate the Efficacy and Safety of a Allogeneic Mesenchymal Precursor Cells (CEP-41750) for the Treatment of Chronic Heart Failure | 2014–2018 / USA | NCT02032004 | 3 | Allogeneic | Transendocardial Injection | 1730 | Safety/ Efficacy Study |
| Safety Study of Allogeneic Mesenchymal Precursor Cell Infusion in MyoCardial Infarction | 2012–2014 / New Zealand; Denmark; Belgium; Australia | NCT01781390 | 2 | Allogeneic | Intracoronary | 225 | Safety/ Efficacy Study |
Mesenchymal Stem Cells (MSCs)
The term “Mesenchymal Stem Cell” was coined by Arnold Caplan in the early 1990s[22, 23], although the cells were first described in the 1970s by Alexander Friedenstein as a population of bone marrow stromal cells capable of mesodermal differentiation and trophic support of hematopoiesis[24, 25]. Since their discovery, there have been over 20,000 publications on the subject of MSC biology and their clinical applications. However, despite this attention a consensus has yet to be reached regarding the exact identity of these cells. In an attempt to standardize the field, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) set minimal criteria in 2001 for defining MSCs. The primary feature set forth by the committee, as definitively representative of MSCs, is an adherence to plastic substrate together with a capacity for multilineage differentiation towards osteo-, adipo- and chondrocytic lineages. The committee also recommended that MSCs should express cluster of differentiation (CD) markers: CD90, CD105, CD44, CD106, CD166, CD73 and CD29 while not expressing CD45, CD34, CD14, CD11b, CD19, CD31 and HLA-DR[26]. While broadly accepted, labs have not universally adopted these criteria. Indeed, the designation Mesenchymal Stem Cell and Multipotent Stromal Cell in reference to MSCs is routinely used interchangeably. This lack of consensus has made navigating the MSC literature and following the development MSC biology more of a challenge.
MSCs have been isolated from several different species and from nearly every tissue type, suggesting that MSCs likely reside in all postnatal organs[22, 27, 28]. The cells reside within organs in a perivascular distribution where they contribute to niche maintenance and tissue homeostasis[29–31]. MSCs typically do not mobilize to the peripheral blood and constitute only around 1 in 108 of the total peripheral mononuclear cell population[32, 33]. Therefore, to obtain sufficient numbers for transplantation it is necessary to isolate MSCs from a tissue or organ and expand them in vitro, using specific protocols [34–39]. The fact that tissue isolation and culturing techniques appear to contribute to differences in cell characteristics, combined with their apparent ubiquitous distribution, highlights the potential heterogeneity within various MSC isolates. Consequently, several subpopulations of MSCs have been described, some of which are described below:
Recycling Stem (RS) cells represent the smallest population, and most rapidly dividing in culture. These bone marrow-derived cells are considered the most primitive and exhibit a greater potential to differentiate into osteoblasts, adipocytes and chondrocytes under standard conditions[40, 41].
Multipotent Adult Progenitor Cells (MAPCs) are the only MSCs to display immortality in culture, and demonstrate a capacity to differentiate into cells of all three germ layers (endoderm, mesoderm and ectoderm)[42, 43].
Human Bone Marrow-derived Multipotent Stem Cells (hBMSCs)[44] also have the capacity to differentiate into cells of all three germ layers.
Human Marrow-Isolated Adult Multilineage Inducible (MIAMI) cells, which in addition to being multi-potent, express numerous markers found among embryonic stem cells and pancreatic islet cells[45].
Cardiac Stromal Cells (CStCs) are a novel MSC subpopulation arising from cardiac tissue[46]. Compared to BM-derived MSCs, these cells demonstrate an enhanced ability to express cardiovascular markers and differentiate into cardiomyocytes[46], while exhibiting a reduced ability to differentiate along the osteogenic and adipogenic lineages.
Subpopulations of c-kit (CD117) positive MSCs. While not a defining characteristic, there is some evidence to suggest that these cells may constitute a more homogeneous group of primitive MSCs exhibiting greater capacity for endodermal differentiation and enhanced multilineage differentiation efficiency[40, 47, 48].
In summary, MSCs comprise a heterogeneous population of multipotent adult mesodermal progenitors. They possess several biological properties, such as a broad differentiation potential, low immunogenicity, an ability to modulate host immune responses and to produce and secrete an array of factors that promote tissue remodeling. These features make them attractive candidates for cell-based therapies, and will be explored in detail below as they pertain to myocardial infarction as the primary cause of cardiovascular disease in humans.
Cardiac immunobiology
Perhaps one the most striking features of MSCs is their capacity for immune obscurity[49–51]. This property is (in part) due to the absence of Major Histocompatibility Complex (MHC) class II, CD40 ligand and CD80/86 (B7 costimulatory molecules) expression [52–54], all of which are involved in allogeneic tissue rejection. MSCs also lack, or express at very low levels, MHC class I, which is typically used by natural killer cells (NK) and cytotoxic T cells (CTLs) to differentiate healthy “self” from unhealthy cells or “non-self”. This lack of MHC-I shields MSCs from detection by allogeneic CTLs, but makes them conspicuous to activated NK cells, which exhibit cytolytic activity against both autologous and allogeneic MSCs[55, 56]. Despite the potency of immune modulation exerted by MSCs (or perhaps because of it) a mechanism seems to have evolved that systematically removes autologous MSCs over time whether infection or malignant transformation has occurred or not. Elimination is comparatively enhanced for allogeneic MSCs, the rate of which seems to be dictated by a balance between their relative expression of immunogenic and immunosuppressive factors[57, 58]. While some concerns regarding the relative immunogenicity of allogeneic MSCs might be obviated by using autologous MSCs, there are compelling reasons for developing an “off the shelf” modality for allogeneic MSCs. The low numbers of autologous MSCs that can be isolated from an individual necessitates ex-vivo expansion of cells leading to unavoidable delays between isolation and re-infusion. Moreover, the quantity and quality of MSCs dramatically diminish with age[59], and several genetic diseases preclude the use of autologous MSCs[60]. Furthermore, the effect ex vivo culture has upon the immunogenicity of autologous MSCs has yet to be determined experimentally.
One of the challenges facing the use of MSCs for cell therapies is that their immune evasion is hampered upon differentiation, when the cells upregulate MHC expression, thus compromising their covert status and making them visible to the immune system[61]. Clearly this detectability has implications for their use in the treatment of AMI, where MSC differentiation to cardiomyocytes may play a therapeutic role. In a swine model of chronic ischemic cardiomyopathy, our group and others have reported the capacity of allogeneic MSCs to engraft and undergo multilineage differentiation[62, 63]. However, long-term success of MSC engraftment into post-infarct myocardium has yet to be demonstrated satisfactorily, possibly due to the fact that the cells are being attacked by the immune system upon differentiation. The future success of MSC-based cellular therapies for the treatment of CVD will therefore benefit from studies aimed at exploiting and enhancing the immune evasive properties of MSCs.
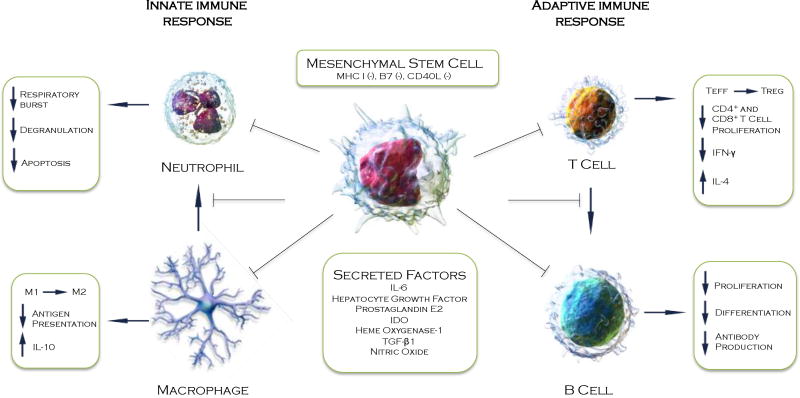
The immunomodulatory properties of MSCs have been extensively described throughout the literature and represent arguably one of the principal mechanisms of action against adverse cardiac remodeling following AMI. Immune modulation is not, however, a default function of MSCs and specific activation is required by inflammatory mediators to stimulate the immunomodulatory activity of MSCs[64]. Following AMI, pro-inflammatory chemokines are rapidly released by tissue-resident macrophages activated by damage-associated molecular patterns (DAMPs)[65]. These chemokines bind to glycosaminoglycans on endothelial cell surfaces and in the extracellular matrix, causing a respiratory burst in neutrophils and their subsequent degranulation[66]. The release of reactive oxygen species, proteases, arachidonic metabolites and other proinflammatory mediators leads to robust immune activation and severe damage to the vascular endothelium and myocardium[67]. MSCs used in models of lung injury[68], diabetes[69] and sepsis[70] appear to be motivated by this stimulus to adopt a modulatory role, diminishing the apoptosis and degranulation of neutrophils, therefore tempering the magnitude of the innate response.
Once “primed” by pro-immunogenic stimuli, MSCs exhibit modulatory activity on several aspects of the immune system, most notably a major effect on resident macrophages. Macrophages can be broadly separated into two distinct categories. Classically activated (M1) macrophages represent the pro-inflammatory arm, whereas alternatively activated (M2) macrophages represent an anti-inflammatory, reparative branch[71, 72]. Through the secretion of PGE2, MSCs promote the M2 phonotype even in the presence of heavy pro-inflammatory stimuli that would normally lead to M1 phenotypes[70, 73, 74]. A shift from an M1 to an M2 phenotype results in decreased production of INF-γ and TNF-α, potent proinflammatory cytokines, and promotes the production of the anti-inflammatory cytokine IL-10. The effect is a further tempering of neutrophil recruitment and activation. Cardiac fibroblasts, responsible for the production and deposition of collagen leading to the establishment of a permanent scar, respond to an array of proinflammatory cytokines (e.g., tumor necrosis factor-α, interleukin (IL)-1, IL-6, and transforming growth factor-beta (TGF-β) all of which are produced by M1 macrophages in the myocardium post-MI[73, 75]. The healing process requires a precise balance between removal of debris and regulation of scar formation. Depletion of macrophages in infarcted hearts impairs collagen deposition, however it also inhibits necrotic cell clearance, angiogenesis and predisposes the heart to rupture[76]. The molecular pathways that control the balance between the pro-inflammatory (M1) and reparative (M2) functions of macrophages therefore represent one potential target for MSC-mediated modulation of pathogenic cardiac remodeling and enhancement of repair[77].
Dendritic cells (DC) are phagocytic antigen presenting cells, which link the innate immune system to the adaptive immune system. MSCs modulate the function and maturation of DCs in co-culture experiments[78, 79], and hamper migration to lymph nodes in vivo and mitigate their T cell allostimulatory capacity[80]. Activated MSCs also act directly upon the adaptive immune response by suppressing T-cell proliferation, as demonstrated in mixed lymphocyte reactions, though the release of soluble factors including indoleamine 2,3-dioxygenase (IDO)[81], PGE2[82], nitric oxide (NO)[83], heme oxygenase-1 (HO-1)[84], hepatocyte growth factor (HGF) and transforming growth factor-β (TGF-β)[54, 85]. In addition to inhibiting T cell proliferation, MSCs can also influence T cell lineage commitment. In a rat allograft model combined intrathymic (i.t.) and intravenous (i.v.) injection of MSCs prolonged survival of the transplanted heart. The allograft survival was associated with a shift in the Th1/Th2 balance, and an up-regulation of CD4+, CD25+, and Foxp3+ T regulatory (Treg) cell differentiation. A concordant decrease in the level of pro-inflammatory cytokines interleukin 2 (IL-2) and interferon-gamma (IFN-γ) and an increase in the levels of anti-inflammatory IL-4 and IL-10 were also reported. The B cell, which produces antibodies, is highly dependent on T cells and MSC inhibition of T cell activity and proliferation likely contribute a major role in MSC-dependent B cell modulation[86]. However, human MSCs have been observed to directly inhibit the proliferation, differentiation, and chemotactic behavior of mature B cells when pre-activated with exogenous IFN-γ[87]. In an allogeneic co-culture experiment, B-cells arrested in the G0/G1 phase of the cell cycle, IgM, IgG, and IgA production was significantly impaired and expression of chemotactic receptors CXCR4, CXCR5, and CCR7 were significantly downregulated[88].
Thus, this evidence makes it clear that MSCs have a broad and significant impact on the immune system (Figure 1). Their capacity for immune modulation and homing to the sites of injury makes them a compelling delivery system for secreted soluble factors that potentially protect the heart from acute injury. However, MSCs are also capable of producing anti-fibrotic factors and matrix metalloproteinases (MMPs) that are able to remodel extracellular matrix. It is thought, therefore, that MSCs have a potential role in chronic cardiac disease through reverse remolding of the scar resulting in sufficient reperfusion and rigidity to promote cardiomyocyte regeneration[89, 90].
Figure 1. Paracrine immunomodulation in the infarcted myocardium.
MSCs are immune-evasive and modulate the response of both the adaptive and innate immune system during acute inflammation of the ischemic myocardium. Illustration of cells used with permission from Wikimedia commons.
Cardiomyogenesis and neoangiogenesis
As previously discussed, MSCs possess several key characteristics that set them apart from other cell types, making them an attractive therapy for cardiomyoplasty. In addition to their immunoregulatory and homing properties, their genetic stability and ability to be expanded in culture means that vast numbers of cells capable of multilineage differentiation can be generated.
Human MSCs retain sufficient plasticity to adopt cardiac specific characteristics in culture and can be induced to express several cardiomyocyte-specific makers (α-actinin, myosin heavy chain, and troponin-T) and transcription factors (GATA-4 and Nkx2–5)[91] [92, 93] when driven by exogenous stimuli. Mechanical strain[94], electrostimulation[95, 96], DNA demethylation using 5-azacytidine[97], culture with pro-cardiogenic factors such as BMP-2[98], HGF[99], TGF-β[100] and Jagged1[101], or co-culture with mature xenogeneic cardiomyocytes[92, 102] or cardiomyocyte lysate[103] have each proven efficacious in driving myocyte differentiation in vitro. However, whether the physiological conditions within the heart are sufficient to promote such efficient differentiation of transplanted MSCs toward a myocyte lineage is a topic of discussion. Our group and others have demonstrated cardiac differentiation in vivo, but this has not been observed by others[14, 62, 104–109] possibly due to species differences or distinct handing of the cells, which could affect viability or differentiation frequency towards cardiomyogenesis. Nevertheless, a direct role for transplanted MSCs in neomyogenesis may be of little significant importance from a physiological standpoint. Evidence from our laboratory and others suggests that MSCs may stimulate the proliferation and maturation of resident cardiac stem cells through paracrine signaling, thereby indirectly supporting innate neomyogenesis[110]. Indeed, such a supporting role would be in concordance with the theory that MSCs, as perivascular cells, function as supportive cells in the maintenance of stem cells within the vascular niche of the organ.
An ideal cell type for use in ischemic AMI would be one that not only promotes cardiomyogenesis, but also supports neoangiogenesis. There appears to be clear evidence that MSCs retain sufficient ability to differentiate into endothelial and vascular smooth muscle cells both in vitro and in vivo[62, 109, 111–114]. Moreover, MSCs have potent proangiogenic activity both in vitro and in vivo through the secretion of VEGF, FGFs, angiopoeitins and extracellular matrix components[89, 115–120]. The significance of the angiogenic action of MSCs in post-ischemic myocardial protection was highlighted specifically by Markel and colleagues using small-interfering RNA (si)RNA directed against VEGF. In these studies knock down of VEGF in transplanted MSCs resulted in a marked negative effect on recovery of myocardial function in a rat model of AMI[121]. This effect was further demonstrated in suicide gene-based studies carried out by Yoon et al.[122]. In this system targeted elimination of cells acquiring a vascular lineage dramatically reduced functional benefits, whereas depletion of cardiomyogenic cells showed no significant effect[122]. Our group recently demonstrated that this effect of VEGF on MSC differentiation towards a vascular phenotype was dependent on the activation of PDGFR via nitric oxide synthase[123]. Thus, accumulating evidence suggests a crucial role for MSCs in neoangiogenesis and vascular homeostasis that might parallel their endogenous role as perivascular cells[124].
While MSCs produce a battery of factors that have a demonstrable biochemical effect on cardiac disease[125], the measurable effects on tissue regeneration have been less than expected. Low engrafting efficiency, low viability and hostile environmental conditions within the injury site have been implicated as potential explanations[126, 127]. If the medicinal benefit of MSCs is conveyed though recapitulation of their pericytic function, inasmuch as their stimulation of resident cardiac stem cells and induction of angiogenesis, then engraftment of MSCs with host vasculature would be a necessity. However, the ischemic scar tissue is, by its nature, avascular and therefore may not provide the ideal substrate or niche environment for robust engraftment and MSC activation. Recent efforts have therefore been geared towards improving the therapeutic efficiency, engraftment, survival and immune evasion of MSCs, the results of which are several preconditioning approaches and genetic modifications aimed at amplifying existing characteristics and imparting novel capabilities onto the cells.
MSC Modifications
In order to utilize MSCs for cardiac repair, it is necessary to expand the cells in vitro and then prepare them as a suspension for injection. However, MSCs are normally grown attached to a substrate, and this adhesion to structural glycoproteins of the extracellular matrix (ECM) is necessary for their survival[128, 129]. Removal of this matrix support, as occurs when the cells are prepared for injection, will therefore lead to increased anoikis i.e., apoptosis induced by loss of matrix attachments. This lack of matrix, coupled with the fact that infusion of MSCs into the circulation or directly into the infarcted region of the heart exposes them to harsh environmental conditions, means that the number of culture-expanded MSCs engrafting at sites of injury rapidly declines following initial infusion[130]. A number of studies have therefore focused on improving the engraftment efficiency of MSCs following their injection into the damaged heart. As MSCs are capable of long-term transgene expression[131], it has been possible to develop strategies based on genetic engineering to enhance homing efficiency and improve cell survival.
Mangi et al. were the first to demonstrate that genetic modification of MSCs to over-express the anti-apoptotic transcription factor Akt, resulted in a greater resistance to apoptosis both in vitro and in vivo and lead to an increase in cardiac function in a rodent model of MI[132, 133]. Transgenic over-expression of another pro-survival gene, Bcl-2 by rat MSCs decreased apoptosis by 32%, enhanced secretion of VEGF by 60% under hypoxic conditions and improved capillary density in the infarct border zone[134]. Similarly, transfection of MSCs with basic-fibroblast growth factor (bFGF) enhanced cytoprotection under hypoxic conditions and caused greater neovascularization compared to untransfected MSCs[135]. MSCs overexpressing VEGF demonstrated an improvement in myocardial perfusion and in restoration of heart function compared to control groups[136] and MSCs transduced with hemoxygenase, an enzyme preventing oxidative damage, showed both better engraftment and enhanced cell survival following intra-myocardial delivery relative to non-transduced MSCs[137].
Culture expanded MSCs demonstrate reduced expression of receptors for chemokines and adhesion molecules such as CXCR4 and CCR1, which significantly compromises homing capacity. Studies in which MSCs were modified to overexpress CXCR4 and CCR1 demonstrated enhanced engraftment and cardiac performance[138, 139]. Over-expression of tissue transglutaminase (tTG), which crosslinks proteins, leads to enhanced adhesion of MSCs and survival of implanted cells via an integrin-dependent mechanism[140]. Indeed, integrin signaling is a critical component of MSC engraftment and the integrin-linked kinase (ILK) is crucial for hypoxic MSCs to establish cell adhesion with ischemic myocardium[127]. ILK enhances phosphorylation of PKB/Akt, which plays a major role in the regulation of adhesion-mediated cell survival signals. Hypoxic conditions suppress expression of ILK, however forced expression though transfection of the ILK gene results in enhanced MSC survival, decreases in infarct size and a greater improvement of left ventricular function[127].
Although the engraftment efficiency of MSCs might be enhanced by such genetic modification, these approaches are currently restricted to basic and translational research due to limited clinical experience with gene therapy and genetically modified cell products. However, these ongoing studies will further elucidate the biology of MSCs and will contribute to our understanding of the mechanisms of their reparative action.
MSC preconditioning
Modifications to MSCs with translational pragmatism include various methods of priming or preconditioning [141]. In this regard, improvements in engraftment of transplanted MSCs have been demonstrated in the absence of direct genetic manipulation, by the use of combinatorial pre-treatment with several exogenous growth factors. In a rat model of MI, pre-treatment with insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (bFGF) and bone morphogenetic protein-2 (BMP-2) enhanced connexing-43 (Cx43) gap junction formation and imparted cytoprotective effects on cardiomyocytes[98]. Behfar et al. pretreated human MSCs with a recombinant cocktail of transforming growth factor-beta(1), BMP-4, activin-A, retinoic acid, IGF-1, bFGF, alpha-thrombin, and interleukin-6, which directed differentiation of MSCs into cardiopoiesis. These cells were subsequently injected into the myocardium of infarcted murine hearts, which led to functional and structural benefits[142].
Pharmacologic pretreatment of MSCs with steroids such as estrogen[143], which influences myocardial remodeling though stimulation of growth hormone production, or statins such as atorvastatin[144, 145], which enhance cell survival and differentiation into cardiomyocytes, have also received attention, as has tadalafil, a phosphodiesterase inhibitor used on adipose-derived MSCs in rat models of cardiomyopathy[146]. Non-biochemical and non-pharmacologic treatments such as hypoxic and anoxic preconditioning have also demonstrated significant improvements on MSC survival. Hypoxic preconditioning activates the Akt signaling pathway leading to the expression of several prosurvival and proangiogenic factors such as Bcl-2, Bcl-xL, Hif-1, VEGF, Ang-1 and erythropoietin[147]. Hypoxia-preconditioning enhanced MSCs survival together with improved angiogenesis of the infarct border zone and as a consequence improved cardioprotection following MI[148]. Similarly anoxic-preconditioned MSCs demonstrated reduced apoptosis, which is thought to be mediated through upregulation of the Bcl-2/Bax ratio and by inhibition of caspase-3 activation in the myocardium[108].
Clinical Trials with MSCs for Heart Disease
Thirty-two clinical trials using MSCs to treat different heart conditions including AMI, severe coronary ischemia, ischemic cardiomyopathy, dilated cardiomyopathy, and heart failure are registered on https://clinicaltrials.gov (a web-based service by the US National Institute of Health; see Table 1). Most have used or are using adult bone marrow-derived MSCs; while several trials chose cell-based treatment with MSCs derived from adipose tissue or umbilical cord. Many of these studies used allogeneic sources of MSCs from healthy donors, which, as discussed above, is possible due to the immune evasive and immunomodulatory properties exhibited by MSCs. Allogeneic MSCs are an attractive and convenient cell type, the major advantage being their immediate availability. In contrast, autologous cells require expansion for 4–6 weeks prior to treatment, a delay that may reduce the efficacy of stem cell therapy. In addition, their therapeutic ability may be compromised by the health of the donor/patient.
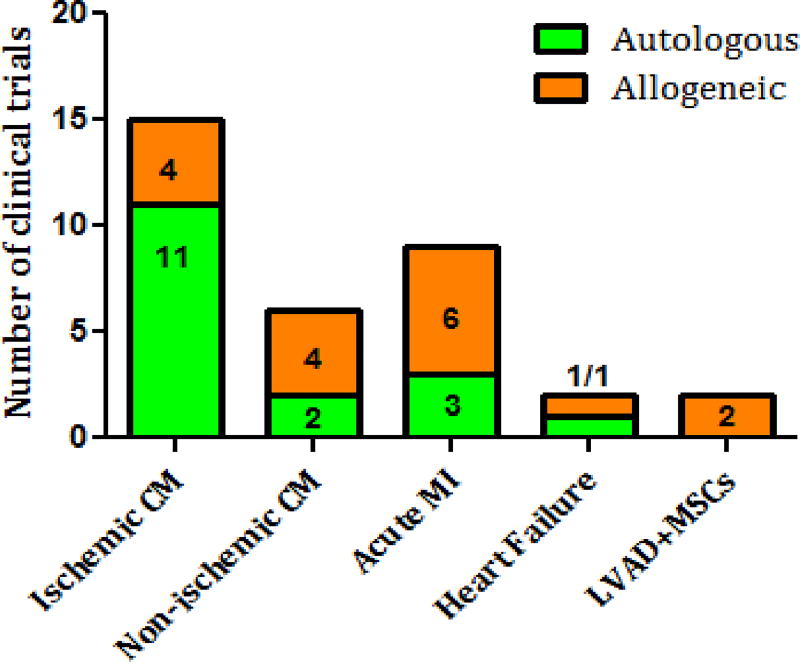
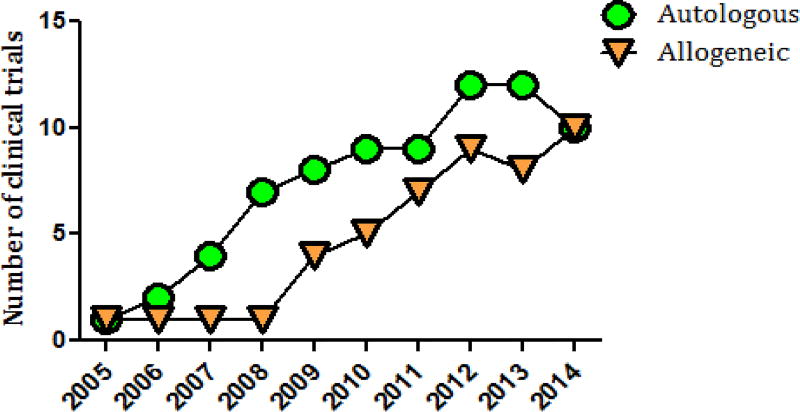
The initial MSC studies for acute and chronic MI in 2004–2008 used intracoronary administration of autologous cells[149, 150]. However, in 2009, the first double-blinded trial on 53 patients with AMI “Osiris”[151] showed that allogeneic intravenous cell infusion was well tolerated and had a significantly greater effect on left ventricular ejection fraction and a lower incidence of arrhythmia and chest pain (Figure 2). This study began the era of allogeneic MSC use for cardiac pathology, suggesting that allogeneic cell-based therapy is safe and effective. Although autologous MSC treatment was prevalent in ischemic cardiomyopathy (11/15 clinical trials), non-ischemic cardiomyopathy and AMI trials adopted allogeneic MSC treatment early on (Figure 3). By 2014, an equal number of clinical trials used allogeneic and autologous MSCs (Figure 4).
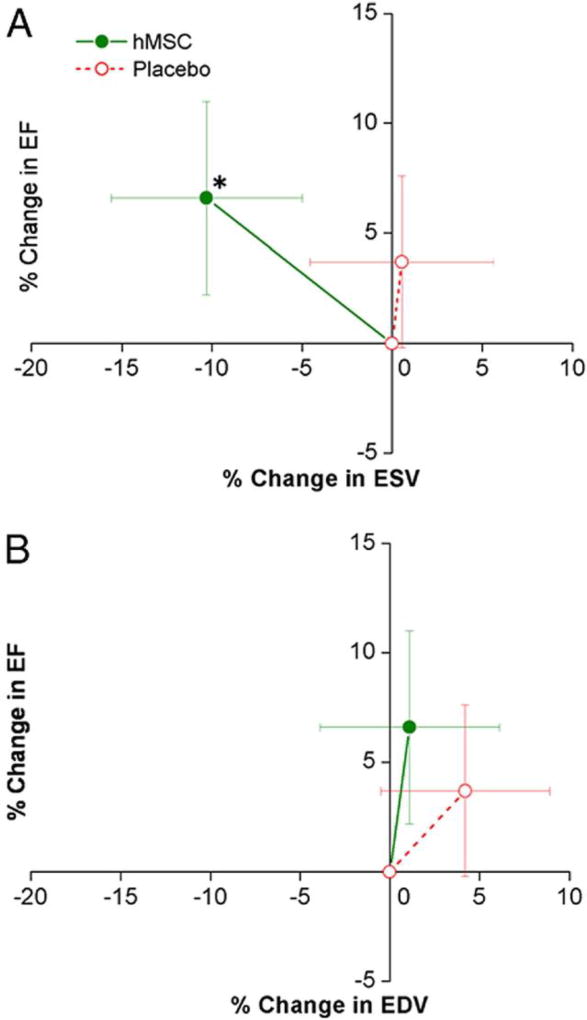
Figure 2. Impact of hMSC Treatment on LV Remodeling.
Changes in left ventricular (LV) ejection fraction (EF) are plotted against the changes in LV end-systolic volume (ESV) (A) and end-diastolic volume (EDV) (B) during follow-up. Human mesenchymal stem cell (hMSC) patients (n = 18 at 12 months) exhibit evidence of reverse remodeling with no increase in LV EDV and a decline in LV ESV, whereas placebo patients (n = 11 at 12 months) demonstrate evidence of LV chamber enlargement. *p = 0.005 versus baseline. [151]
Figure 3.
Autologous and allogeneic mesenchymal stem cell-based treatment according to heart pathology.
Figure 4.
Progressive growth of allogeneic and autologous mesenchymal stem cell-based clinical trials.
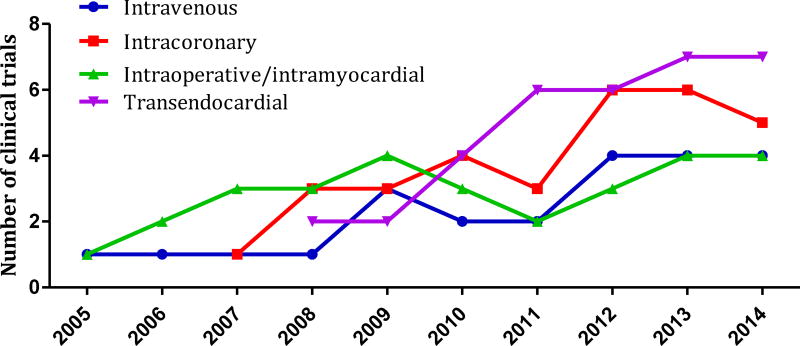
Consistent with the registered data from https://clinicaltrials.gov, there are ongoing phase 1, 2, and 3 clinical trials evaluating MSC safety and efficacy for cardiac regeneration using a variety of delivery systems. The method of stem cell delivery can influence cell-therapy outcome, which is why intracoronary, intravenous, intraoperative/intramyocardial injections and catheter-based transendocardial injections are currently in use in order to establish the most efficient stem cell transplantation technique in compliance with heart pathology (Figure 5).
Figure 5.
MSC delivery methods used in clinical trials from 2005 to 2014.
The first clinical trials for acute and chronic MI[150] used catheter-based intracoronary autologous MSC delivery during percutaneous coronary intervention (PCI). These studies showed no serious adverse events, significantly better regional and global left-ventricular function, up to 10% increased left ventricular ejection fraction (LVEF), increased exercise capacity, and improvement in New York Heart Association (NYHA) heart failure class. Using the same method of cell delivery, Katritsis et al.[149] combined autologous MSCs with endothelial progenitor cells for AMI treatment and showed better left ventricular function and myocardial perfusion with evidence of regeneration of the previously nonviable infarct scar. A pilot study conducted by Mohyeddin-Bonab et al. in 2007 tested whether intracoronary delivery during PCI or intraoperative/intramyocardial treatment with autologous MSCs is safe and efficient for old myocardial infarction[152]. Their results demonstrated increased LVEF, change in NYHA class, and significant improvement in viable myocardium in the MSC-treated group. However, there was no data presented (probably because of small sample size) regarding which delivery route was more beneficial. The PROMETHEUS study showed that intraoperative/ intramyocardial autologous MSC injections together with Coronary Artery Bypass Grafting (GABG) procedure into akinetic, yet non-revascularized segments, was safe and produced comprehensive regional functional restitution, which in turn drives improvement in global left ventricular function (Figure 6)[153]. The TAC-HFT trial compared transendocardial injections of autologous MSCs, bone marrow mononuclear cells (BMCs), and placebo in chronic ischemic heart failure[154, 155]. This study again showed safety of the delivery method, and MSCs and BMCs significantly improved regional contractility[156] and increased 6-minute walk distance, but only the MSCs reduced scar size. There were no changes in LVEF or heart chamber volumes[154].
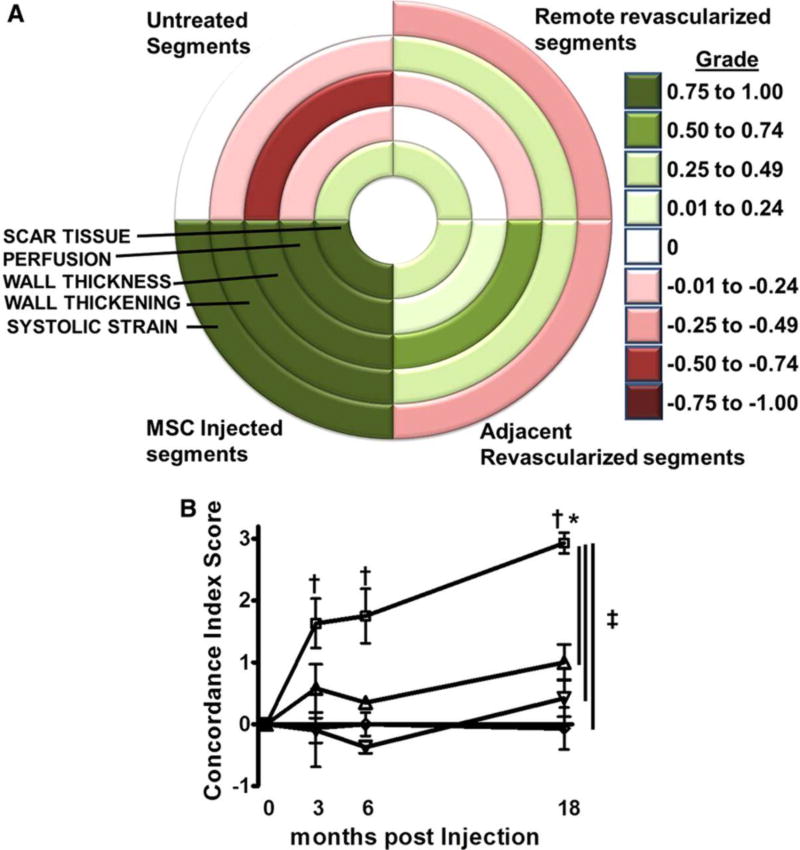
FIGURE 6. Concordance index score is an indicator of simultaneous and comprehensive improvement. (PROMETHEUS Trial).
A, Bull’s-eye map depicting the concordance of change for each variable used for the concordance index score based on the average grade for each group at 18 months post-treatment. A grade closer to 1 signifies a concordant improvement in the variable and a grade closer to −1 represents deterioration. The concordance index score is then derived by adding the grades for changes in scar tissue size, perfusion and the average of the grades for changes in wall thickness, wall thickening and systolic strain. The highest value (3) signifies simultaneous improvement in all 5 CMR indices and the lowest (−3) a simultaneous deterioration, respectively. B, In the MSCs plus CABG group, the injected nonrevascularized segments improved comprehensively and thus had a higher concordance index score compared with all other groups. The effect of the MSCs dissipated by a function of distance from the actual injection site (P=0.03 adjacent vs. remote revascularized and nontreated segments). □, MSC injected; ▲, adjacent revascularized; ▼, remote revascularized; and ◊, untreated. *P<0.05 1-way ANOVA repeated measures; †P<0.05 vs. baseline, Bonferroni post-tests; ‡P<0.05 2-way ANOVA. [153]
Anastasiadis et al.[157] combined left ventricular mechanical support device (LVAD) implantation with allogeneic MSC injections in a case-report study and showed improved LVEF when the device was turned off. This study from 2012 formed the basis for a recent study with LVAD + allogeneic MSC treatment for patients with end-stage heart failure. In 2013 our group compared allogeneic vs. autologous bone marrow-derived MSCs delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized dose-escalation trial (20, 100, 200 million cells). This study showed that allogeneic and autologous MSCs are safe and reduced mean early enhancement defect (scar size) by 33.21%. Allogeneic MSCs reduced left ventricular end-diastolic volumes and interestingly, the lowest concentration of MSCs (20 million cells) produced the greatest reductions in left ventricular volume and increased ejection fraction. Notably, allogeneic MSCs did not stimulate significant donor-specific alloimmune reactions[51]. The segmental ejection fraction analysis from the POSEIDON study showed that injected and non-injected segments improved regional contractile performance with the greater scar reduction in injected sites (Figure 8)[158]. In 2014, Lee et al.[159] published results of a multicenter trial examining the safety and efficacy of intracoronary administration of autologous bone marrow-derived MSCs in patients with AMI and showed improved global LVEF in MSC-treated patients.
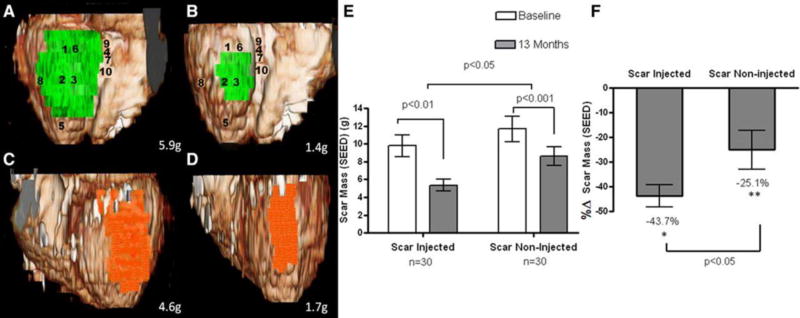
Figure 8. Volume rendered 3-dimensional (3D) reformats of left ventricle with color encoding of scar tissue. (POSEIDON Trial).
A and B, Scar mass (green) of inferior segments treated by transendocardial stem cell injection (TESI) at baseline and 13 months after TESI, respectively (numbers represent sites of injection). C and D, Scar mass (orange) of lateral segments not treated by TESI at baseline and at 13-month follow-up, respectively. Actual scar mass (grams) is depicted in the lower right corner of each panel. Three-dimensional reconstructions in this figure correspond to segmental early enhancement defect (SEED) measurements of the same patient as in Figure 2 and Online Movie I. E and F, Absolute values and percentage changes of scar mass obtained by segmental imaging analysis approach. When considering the autologous and allogeneic groups combined, there is greater scar size reduction in the scar-injected segments (−43.7±4.4%, from 9.8±1.2 to 5.4±0.7 g; n=30; *P<0.01) when compared with the scar-noninjected segments (−25.1±7.8%, from 11.7±1.4 to 8.6±1.0 g; n=30; **P<0.001; between-group comparison scar-injected vs. scar-noninjected P<0.05)[158].
Twenty ongoing clinical trials are testing the regenerative potential of MSCs for cardiac disease, in particular ischemic cardiomyopathy and AMI. However, there is still a need for larger clinical trials comparing autologous and allogeneic MSCs in order to answer questions about the most effective dose and frequency for each cardiac pathology, the best cell delivery system, and the use of single vs. multiple cell types.
Conclusion
Collectively the studies using MSCs to treat CVD are numerous and consensus favors MSCs as potent mediators of cardiac repair. MSCs clearly retain significant plasticity and are capable of formidable immunomodulation and neo-angiogenesis. However, a feature common in MSC transplantation is a conspicuous lack of proliferation, differentiation or engraftment. Several explanations have been presented to explain this and many modifications and pre-treatments are being attempted to address it. However, there are other considerations, perhaps more difficult to address and have not been described as thoroughly. Stem cell division is driven by contextual signals from the 3D structure of the niche. MSCs being pericytic or adventitial cells require adhesion to a substrate to maintain viability, which in vivo constitutes perivascular adhesion to sinusoids or large vessels, respectively[29, 160]. In the absence of the niche microenvironment, stem cells can irreversibly lose their inherent stemness[161]. Although they initially survive and retain many stem cell markers, their efficacy can begin to wane very rapidly following transplantation[161]. When MSCs are injected into the myocardium or circulation no such orientation or structure exists and the transplanted cells therefore may not receive the mitogenic signals necessary to initiate cell cycle or drive differentiation. Moreover, a lack of substantive adhesion may cause a loss of cellular identity, anoikis and depletion by NK cells, leading to an irreversible decline in viable cell numbers. It is clear that while we have made significant progress, much work remains as we learn to fully exploit the power of MSCs in cardiac repair.
Moving forward, an exciting prospect aimed at improving stem cell potency, viability and engraftment involves cell combination therapy. An example includes the use of ex-vivo stromal cell aggregates known as cardiospheres[162]. These floating clusters are comprised of a central core of primitive c-kit+ cardiac stem cells surrounded by layers of early-stage committed differentiating cells, and an outside cell layer of MSCs[163]. Culture of these cells as a 3D structure is thought to potentially recapitulate cardiac niche biology in the in vitro environment[164]. Preclinical models using autologous as well as allogeneic cardiosphere-derived cells (CDCs) have demonstrated a reduction in scar size and improvements in cardiac function after MI, although the mechanism of action is not clear[165–168]. Results from a phase I clinical trial using intracoronary infusion of cardiosphere-derived cells (CADUCEUS) further supports the notion that CDCs are capable of regenerating heart tissue after AMI in humans[169].
Our group recently reported preclinical findings from studies combining MSCs with c-kit+ cardiac stem cells (CSC)[170]. Co-culture with MSCs enhanced CSC proliferation and lineage commitment toward a cardiac phenotype, suggesting that important biological interactions exist between these cells (Figure 9)[110]. These interactions are dependent on gap junction formation mediated through expression of Cx43[110, 171]. In a porcine model of AMI, co-injection of human MSCs and human c-kit+ CSCs resulted in a 7-fold greater engraftment of stem cells than with either cell type alone, a significant reduction in scar size and significant restoration of diastolic and systolic function[170]. These observations, together with the success of safety trials of MSCs (POSEIDON)[51] and c-kit+ stem cells (SCIPIO)[21] have created an opportunity for exploring therapeutic enhancement of this combination therapy in humans. As a result, a new phase I clinical trial has recently received regulatory approval from the FDA and is currently in the process of recruiting patients.
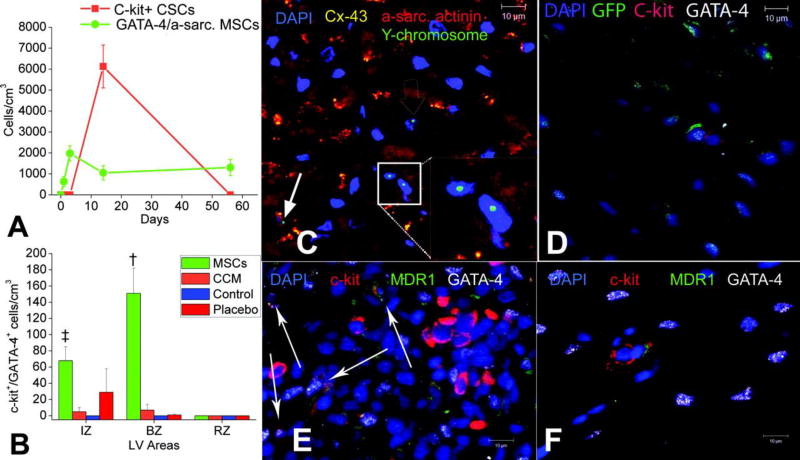
Figure 9. MSCs stimulate endogenous CSCs.
A, The contribution of cardiomyocyte precursors following exogenous administration of MSCs (green line) and endogenous CSCs (orange line) during cardiac repair after MI. MSC differentiation occurs rapidly after delivery. At 2 weeks, MSCs activate endogenous expansion of c-kit+ CSCs (orange line). B, Two weeks following TEI, the number of C-kit+ cells coexpressing GATA-4 is greater in MSCs versus non-MSCs treated hearts. The cardiac precursors are preferentially located in the IZ and BZ of the MI, indicating an active process of endogenous regeneration (‡P=0.019 and †P<0.0001). C and D, The 2-week-old chimeric myocardium contains mature cardiomyocytes (open arrow), immature MSCs (inset), and cardiac precursors of MSCs origin (arrow), coupled to host myocardium by connexin-43 gap junctions. Interestingly, endogenous c-kit+ CSCs are found in close proximity to MSCs (D). E, Cluster of c-kit+ CSCs in an MSC-treated heart; numerous CSCs are committed to cardiac lineage documented by GATA-4 and MDR-1 coexpression (arrows). F, Few isolated c-kit+ cells were found in non-MSC–treated animals[110].
The debate enveloping stem cell clinical trials for use against cardiac disease has intensified recently with the publication of two high profile reports[172, 173]. These critical reports clearly have implications for future clinical trials involving bone marrow cells and CSCs and the tide of criticism would seem to also threaten clinical trials utilizing MSCs. However, this criticism will likely not affect MSCs, which stand alone in their unprecedented ability to perform a number of beneficial functions, as discussed above. The data coming from pre-clinical and clinical trails clearly supports a continued effort to exploit the medicinal potential of MSCs. The biochemical activity of MSCs has been demonstrated beyond doubt with observable results. Our understanding of MSC biology has evolved significantly over the past 2 decades and significant advancements have been made, yet much work still remains. Therefore, the focus moving forward will be directed at expanding the current successes through discovery of the critical factors mediating MSC repair and optimization of the system.
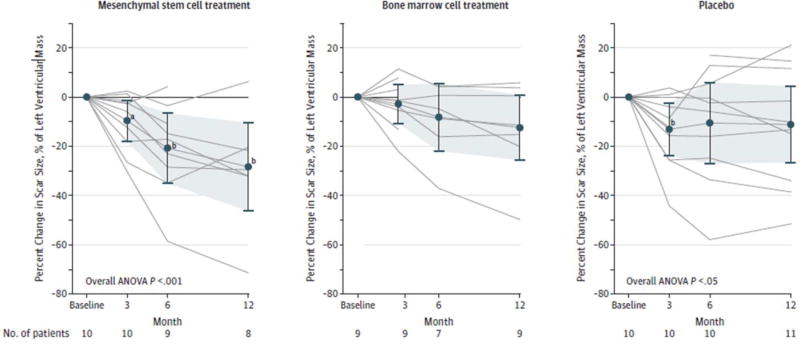
FIGURE 7. Impact of Transendocardial Stem Cell Injection of Mesenchymal Stem Cells, Bone Marrow Cells, or Placebo on the Scar Size. (TAC-HFT Randomized Trial).
Significant reduction in scar size as the percentage of left ventricular mass for patients treated with mesenchymal stem cells (MSCs) and those in the placebo group who underwent serial magnetic resonance imaging. Repeated measures of analysis of variance model P values: treatment group, P=.99; time, P=.007; treatment group × time, P=.22. Data markers represent means; error bars, 95% CIs. Analysis of variance (ANOVA) was conducted with repeated measures. aWithin group, P<.05 vs. baseline. bWithin group, P<.01 vs. baseline.[154]
Literature cited
- 1.Vodyanik MA, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7(6):718–29. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 3.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258(1):1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 4.Montserrat N, Jopling C, Izpisua Belmonte JC. Understanding the molecular basis for cardiomyocyte cell cycle regulation: new insights in cardiac regeneration after injury? Expert Rev Cardiovasc Ther. 2010;8(8):1043–5. doi: 10.1586/erc.10.91. [DOI] [PubMed] [Google Scholar]
- 5.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90(10):1044–54. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 6.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 7.Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ Res. 2000;86(11):1107–13. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 8.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go AS, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings RB, Steenbergen C, Jr, Reimer KA. Myocardial ischemia and reperfusion. Monogr Pathol. 1995;37:47–80. [PubMed] [Google Scholar]
- 12.Graham RM, et al. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J Exp Biol. 2004;207(Pt 18):3189–200. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 13.Kubasiak LA, et al. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99(20):12825–30. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23(7):845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 15.Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248(5444):145–7. doi: 10.1038/248145a0. [DOI] [PubMed] [Google Scholar]
- 16.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 17.Hatzistergos KE, et al. What is the oncologic risk of stem cell treatment for heart disease? Circ Res. 2011;108(11):1300–3. doi: 10.1161/CIRCRESAHA.111.246611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menasche P, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41(7):1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 19.Menasche P. Myoblast-based cell transplantation. Heart Fail Rev. 2003;8(3):221–7. doi: 10.1023/a:1024705214292. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 21.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 23.Caplan AI. Molecular and cellular differentiation of muscle, cartilage, and bone in the developing limb. Prog Clin Biol Res. 1986;217B:307–18. [PubMed] [Google Scholar]
- 24.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedenstein AJ, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 28.Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012;2012:461718. doi: 10.1155/2012/461718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roufosse CA, et al. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36(4):585–97. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 33.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25(1):69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 34.Caterson EJ, et al. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20(3):245–56. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y, Hsieh PH, Chao CC. The efficiency of Percoll and Ficoll density gradient media in the isolation of marrow derived human mesenchymal stem cells with osteogenic potential. Chang Gung Med J. 2009;32(3):264–75. [PubMed] [Google Scholar]
- 36.Harichandan A, Sivasubramaniyan K, Buhring HJ. Prospective isolation and characterization of human bone marrow-derived MSCs. Adv Biochem Eng Biotechnol. 2013;129:1–17. doi: 10.1007/10_2012_147. [DOI] [PubMed] [Google Scholar]
- 37.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34(11):1604–5. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Majumdar MK, et al. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185(1):98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe M, et al. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs) Methods Mol Biol. 2008;449:3–25. doi: 10.1007/978-1-60327-169-1_1. [DOI] [PubMed] [Google Scholar]
- 40.Colter DC, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, et al. Comparative study on various subpopulations in mesenchymal stem cells of adult bone marrow. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(1):54–8. [PubMed] [Google Scholar]
- 42.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30(8):896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 44.Yoon YS, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115(2):326–38. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Ippolito G, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–81. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 46.Rossini A, et al. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res. 2011;89(3):650–60. doi: 10.1093/cvr/cvq290. [DOI] [PubMed] [Google Scholar]
- 47.Blazquez-Martinez A, et al. c-Kit identifies a subpopulation of mesenchymal stem cells in adipose tissue with higher telomerase expression and differentiation potential. Differentiation. 2014 doi: 10.1016/j.diff.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Varma MJ, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16(1):91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 49.Abarbanell AM, et al. Proinflammatory cytokine effects on mesenchymal stem cell therapy for the ischemic heart. Ann Thorac Surg. 2009;88(3):1036–43. doi: 10.1016/j.athoracsur.2009.02.093. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 51.Hare JM, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Blanc K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 53.Majumdar MK, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10(2):228–41. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 54.Tse WT, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 55.Rasmusson I, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 56.Spaggiari GM, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 57.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zangi L, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–74. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 59.Fan M, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13(4):429–38. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 60.O'Marcaigh AS, Cowan MJ. Bone marrow transplantation for inherited diseases. Curr Opin Oncol. 1997;9(2):126–30. [PubMed] [Google Scholar]
- 61.Huang XP, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122(23):2419–29. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 62.Quevedo HC, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makkar RR, et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10(4):225–33. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 64.Shi Y, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–8. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 65.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 66.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43(4):860–78. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz LA, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volarevic V, et al. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 2010;43(4):255–63. doi: 10.3109/08916930903305641. [DOI] [PubMed] [Google Scholar]
- 70.Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang QZ, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez MA, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136(3):978–89. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 75.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 76.van Amerongen MJ, et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170(3):818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho DI, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jung YJ, et al. MSC-DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy. 2007;9(5):451–8. doi: 10.1080/14653240701452057. [DOI] [PubMed] [Google Scholar]
- 79.Spaggiari GM, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–83. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 80.Chiesa S, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108(42):17384–9. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DelaRosa O, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15(10):2795–806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 82.Najar M, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 2010;264(2):171–9. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Ren G, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 84.Chabannes D, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 85.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 86.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 87.Krampera M, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 88.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 89.Merfeld-Clauss S, et al. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16(9):2953–66. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traktuev DO, et al. Adipose tissue stromal cells -- multipotent cells with therapeutic potential for stimulation of angiogenesis in tissue ischemia. Kardiologiia. 2006;46(6):53–63. [PubMed] [Google Scholar]
- 91.Makino S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42(2):295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 93.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 94.Pijnappels DA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103(2):167–76. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 95.Genovese JA, et al. Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Front Biosci (Landmark Ed) 2009;14:2996–3002. doi: 10.2741/3429. [DOI] [PubMed] [Google Scholar]
- 96.Wen L, et al. Mild electrical pulse current stimulation upregulates S100A4 and promotes cardiogenesis in MSC and cardiac myocytes coculture monolayer. Cell Biochem Biophys. 2013;65(1):43–55. doi: 10.1007/s12013-012-9402-x. [DOI] [PubMed] [Google Scholar]
- 97.Xu W, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229(7):623–31. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 98.Hahn JY, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51(9):933–43. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 99.Forte G, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24(1):23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 100.Mohanty S, et al. TGFbeta1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2013;163(1):93–9. doi: 10.1016/j.ijcard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Li H, et al. Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;341(2):320–5. doi: 10.1016/j.bbrc.2005.12.182. [DOI] [PubMed] [Google Scholar]
- 102.Xu M, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110(17):2658–65. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 103.Xie XJ, et al. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27(9):1153–8. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 104.Toma C, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 105.Rota M, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104(45):17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–48. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 107.Wei F, et al. Mesenchymal stem cells neither fully acquire the electrophysiological properties of mature cardiomyocytes nor promote ventricular arrhythmias in infarcted rats. Basic Res Cardiol. 2012;107(4):274. doi: 10.1007/s00395-012-0274-4. [DOI] [PubMed] [Google Scholar]
- 108.Rose RA, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 109.Silva GV, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 110.Hatzistergos KE, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang T, et al. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 112.Jazayeri M, et al. Molecular and ultrastructural characterization of endothelial cells differentiated from human bone marrow mesenchymal stem cells. Cell Biol Int. 2008;32(10):1183–92. doi: 10.1016/j.cellbi.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 113.Li Q, et al. Investigation of canine mesenchymal stem cells differentiation to vascular endothelial cell in vitro. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007;24(6):1348–51. [PubMed] [Google Scholar]
- 114.Oswald J, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 115.Martens TP, et al. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S18–22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- 116.Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 117.Oskowitz A, et al. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res. 2011;6(3):215–25. doi: 10.1016/j.scr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang YL, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 119.Rubina K, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15(8):2039–50. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]
- 120.Tang J, et al. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–61. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 121.Markel TA, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295(6):H2308–14. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoon CH, et al. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121(18):2001–11. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- 123.Gomes SA, et al. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110(8):2834–9. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Traktuev DO, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 125.Ranganath SH, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58(11):1095–104. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 127.Song H, et al. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. 2010;10(3):309–19. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 128.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4(9):953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Assis AC, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19(2):219–30. doi: 10.3727/096368909X479677. [DOI] [PubMed] [Google Scholar]
- 131.Zhang XY, et al. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol Ther. 2002;5(5 Pt 1):555–65. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 132.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 133.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 134.Li W, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 135.Song H, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19(3):402–7. [PubMed] [Google Scholar]
- 136.Yang J, et al. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007;107(1):17–29. doi: 10.1159/000093609. [DOI] [PubMed] [Google Scholar]
- 137.Tang YL, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46(7):1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 138.Cheng Z, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16(3):571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 139.Huang J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106(11):1753–62. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song H, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25(6):1431–8. doi: 10.1634/stemcells.2006-0467. [DOI] [PubMed] [Google Scholar]
- 141.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51(4):619–25. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Behfar A, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56(9):721–34. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ray R, et al. Sex steroids and stem cell function. Mol Med. 2008;14(7–8):493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang YJ, et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J. 2008;29(12):1578–90. doi: 10.1093/eurheartj/ehn167. [DOI] [PubMed] [Google Scholar]
- 145.Li N, et al. Atorvastatin induces autophagy of mesenchymal stem cells under hypoxia and serum deprivation conditions by activating the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. Chin Med J (Engl) 2014;127(6):1046–51. [PubMed] [Google Scholar]
- 146.Haider H, et al. Phosphodiesterase inhibition with tadalafil provides longer and sustained protection of stem cells. Am J Physiol Heart Circ Physiol. 2010;299(5):H1395–404. doi: 10.1152/ajpheart.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chacko SM, et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299(6):C1562–70. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu X, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 149.Katritsis DG, et al. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9(3):167–71. doi: 10.1093/europace/eul184. [DOI] [PubMed] [Google Scholar]
- 150.Chen SL, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 151.Hare JM, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohyeddin-Bonab M, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10(4):467–73. [PubMed] [Google Scholar]
- 153.Karantalis V, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302–10. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heldman AW, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trachtenberg B, et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161(3):487–93. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 156.Williams AR, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108(7):792–6. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Anastasiadis K, et al. Stem cells transplantation combined with long-term mechanical circulatory support enhances myocardial viability in end-stage ischemic cardiomyopathy. Int J Cardiol. 2012;155(3):e51–3. doi: 10.1016/j.ijcard.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 158.Suncion VY, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292–301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JW, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29(1):23–31. doi: 10.3346/jkms.2014.29.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95(7):671–6. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- 161.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chimenti I, et al. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol. 2012;879:327–38. doi: 10.1007/978-1-61779-815-3_19. [DOI] [PubMed] [Google Scholar]
- 163.Smith RR, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 164.Karantalis V, et al. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303(3):H256–70. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carr CA, et al. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks--an MRI study. PLoS One. 2011;6(10):e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee ST, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57(4):455–65. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 167.Li TS, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–53. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malliaras K, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–12. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Makkar RR, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Williams AR, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127(2):213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mureli S, et al. Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. Am J Physiol Heart Circ Physiol. 2013;304(4):H600–9. doi: 10.1152/ajpheart.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nowbar AN, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Berlo JH, et al. c-kit cells minimally contribute cardiomyocytes to the heart. Nature. 2014 doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]