Abstract
Background
Identifying priority areas for vector control is of considerable public health relevance. Arthropod-borne viruses (arboviruses) spread by Aedes mosquitoes are (re)emerging in many parts of the tropics, partially explained by changes in agricultural land-use. We explored the effects of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes mosquitoes along a gradient of anthropogenic disturbance in oil palm-dominated landscapes in southeastern Côte d’Ivoire.
Methodology
Between January and December 2014, eggs, larvae, pupae, and adults of Aedes mosquitoes were sampled in four types of macrohabitats (rainforest, polyculture, oil palm monoculture, and rural housing areas), using standard procedures (bamboo-ovitraps, metallic-ovitraps, larval surveys, and human-baited double-net traps). Immature stages were reared and adult mosquitoes identified at species level.
Principal findings
A total of 28,276 Aedes specimens belonging to 11 species were collected. No Aedes-positive microhabitat and only four specimens of Ae. aegypti were found in oil palm monoculture. The highest abundance of Aedes mosquitoes (60.9%) was found in polyculture, while the highest species richness (11 species) was observed in rainforest. Ae. aegypti was the predominant Aedes species, and exhibited high anthropophilic behavior inflicting 93.0% of total biting to humans. The biting rate of Aedes mosquitoes was 34.6 and 7.2-fold higher in polyculture and rural housing areas, respectively, compared to rainforest. Three species (Ae. aegypti, Ae. dendrophilus, and Ae. vittatus) bit humans in polyculture and rural housing areas, with respective biting rates of 21.48 and 4.48 females/person/day. Unexpectedly, all three species were also feeding during darkness. Aedes females showed bimodal daily feeding cycles with peaks at around 08:00 a.m. and 05:00 p.m. Host-seeking activities were interrupted between 11:00 a.m. and 02:00 p.m. in rural housing areas, while no such interruption was observed in polyculture. Some rainforest-dwelling Aedes species displayed little preference to feed on humans.
Conclusions
In southeastern Côte d’Ivoire, the agricultural land-use/land-cover changes due to the conversion of rainforest into oil palm monocultures influence the abundance, distribution, and host-seeking behaviors of anthropophagic and non-anthropophagic Aedes vectors. As a result, there is higher risk of humans to arbovirus transmission in polyculture and rural housing areas. There is a need for integrated vector management, including landscape epidemiology and ecotope-based vector control.
Introduction
Aedes mosquito-transmitted arthropod-borne viruses (arboviruses) have (re)emerged from their sylvatic reservoirs of Africa and the Americas under landscape anthropization forces [1]. Indeed, arboviruses are dispersed globally, and they are responsible for various diseases [1]. Several Aedes species act as vectors of arboviral diseases, such as yellow fever, dengue, chikungunya, Rift valley fever, and Zika that are of considerable public health relevance [1]. The resurgence of these mosquito-borne diseases and their geographic expansion has long been associated with human-induced modifications of terrestrial ecosystems [2]. Identifying priority areas for integrated vector management (IVM) is crucial for public health because the ecology (e.g., abundance distribution, and behavior) of Aedes mosquito vectors is likely to alter with human-induced land-use changes, including deforestation, intensification of agriculture, and urbanization [2–4].
The expansion of tropical oil palm (Elaesis guineensis) plantations is a major driver of deforestation and threatens biodiversity, including arthropods [5, 6]. Wild palm trees have a life-span of up to 200 years, and an economic life-span of 25–30 years, after which trees are cut down and replaced with young palm plants. The planting density ranges from 120 to 160 palms/ha. Changes in land-use can result in the losses of Aedes mosquito habitats, hosts, and predators, which, in turn, affect the dynamics, abundance, oviposition, and host-seeking behaviors of vectors searching for alternative habitats and new blood-feeding sources [2]. In contrast, other cultivations such as rubber plantations, and plants with sheathing leaf axils (e.g., banana, bromeliads, and taro), and fruit husks (e.g., coconuts) can be important sources of Aedes mosquito breeding as they retain water for larval breeding [7, 8]. Additionally, containers used to supply water to animals and plants support Aedes mosquito larval growth [9]. Anthropogenic chemicals, such as pesticides (e.g., insecticides, fungicides, herbicides, and rodenticides), are drivers of changes in mosquito populations [10]. While the transformation of native rainforests into human settlements might destroy natural breeding sites of Aedes, it might result in an increase of artificial containers (e.g., tires, discarded containers, and water storage receptacles) that serve as microhabitats for immature Aedes [2]. Moreover, open areas directly exposed to sunlight that are created after the removal of natural vegetation accelerate mosquito development and survivorship [4, 8]. Tropical rainforests are rich in biodiversity, including Aedes that might breed in tree holes that are protected by foliage and contain microbial food sources for mosquito larvae [2, 7]. In addition, the diverse fauna in the rainforest [7] serves as blood sources for host-seeking Aedes females, thereby maintaining the circulation of arboviruses among non-human primates (sylvatic cycle) [11, 12]. Deforestation, forest-degradation, and forest-fragmentation have been associated with arbovirus emergence or re-emergence [11, 12]. The effects of these multiple anthropogenic changes in land-use on mosquito communities and the risk of disease transmission in the tropics may be further amplified by changing patterns of precipitation [2, 13].
In the southeastern part of Côte d’Ivoire, where large parts of rainforest have been converted into oil palm plantations, several outbreaks of yellow fever and dengue have been documented [14]. Yellow fever and dengue viruses have been associated with vectors such as Ae. aegypti, Ae. africanus, Ae. furcifer, Ae. luteocephalus, Ae. opok, and Ae. vittatus [15, 16]. At present, Côte d’Ivoire is the third largest African producer of palm oil with an annual production of about 1.8 million tons. Palm oil production generates 3.1% of the national gross domestic product (GDP) [17]. There are plans to enlarge the national production of palm oil, which might increase human-induced pressures on rainforest [18].
Meanwhile, there is a lack of knowledge on how agricultural land-use changes affect the ecology of Aedes vectors in oil palm-dominated landscapes of Côte d’Ivoire. It is important to deepen the understanding of this relationship to identify priority areas for IVM and to provide a better land-use strategy for the reduction of arboviral disease risks. Hence, our study aimed at assessing the effects of land-use changes on the ecology of Aedes mosquitoes among four major land-cover types (rainforest, polyculture, oil palm monoculture, and rural housing areas) derived from human-driven landscape transformation in large industrial oil palm areas in southeastern Côte d’Ivoire. We hypothesized that the abundance, distribution, oviposition, and host-seeking behaviors of Aedes mosquito species differ depending on the main landscape type.
Methods
Ethics statement
The study protocol was approved by the local health and administrative authorities of PALMCI, which manages the industrial oil palm plantations where our study was conducted. The management of PALMCI provided a field permit for mosquito sampling. Before starting the study, informed oral consent was provided by village leaders. In addition, all entomologic surveys and sample collections carried out on private lands or private residential areas were done with the permission and written informed consent of the residents.
The volunteers participating to the human-baited double-net trapping gave written, informed consent for their participation. They were between 21 and 45 years old, and were given a small remuneration for their participation. Volunteers were vaccinated against yellow fever and protected against malaria with medical prophylaxis. Participants were also offered the opportunity to receive free medical treatment when they showed any symptoms suspected to be caused by mosquito-borne diseases. Moreover, the volunteers were not directly exposed to mosquito females’ biting because they were protected by the inner nets of the double-net trap device. The volunteers who sampled Aedes mosquitoes for this study are among the authors, rather than being subjects of the study. This study did not involve endangered or protected species.
Study area
The study was carried out in the Sud-Comoé region (geographic coordinates 5° 28’ N latitude, 3° 12’ W longitude) located in the southeastern part of Côte d’Ivoire (Fig 1). The estimated human population in the Sud-Comoé region is 642,000 with people mainly living in rural settings. The economic activities are primarily based on subsistence agriculture. Additionally, there is some industrial exploitations of oil palm monocultures (approximately 30,000 ha), managed by PALMCI. Chemical products (i.e., insecticides, fungicides, and herbicides) are intensively used for oil palm plantation and crop protection [19]. The natural vegetation mostly constitutes of rainforest. Several small villages are dispersed across the landscape. The rainforest and traditional agriculture host trees, bamboo, and diverse animal species (primates, and birds).
Fig 1. Location of the study areas in south-eastern Côte d’Ivoire.
The study was carried out in the villages located in oil palm plantation areas belonging to the Sud-Comoé region. The study area covers the villages of Ehania-V1, Cité-cadre and Akakro situated at the interface between the industrial oil palm plantation and traditional agricultural smallholdings. The industrial exploitations are devoted to the monoculture of oil palm plantations (Eleasis guineensis) covering over 30,000 hectares managed by an integrated agro-industrial unit of PALMCI. In the industrial part, a primary rainforest of over 100 ha has been preserved intact and forbidden of any human activities. In the traditional lands, the agricultural exploitation systems are polycultures comprising oil palm trees, rubber trees, banana, taro, bromeliads, and cocoa growing in the same space. Several small villages averaging 20 people are dispersed in these smallholdings.
The climate in the study area is characterized by high temperature and precipitation with two rainy seasons. The seasons are mainly distinguished by rainfall. The main rainy season extends from May to July, while the shorter rainy season lasts from October to November, with distinct dry seasons in between. The average annual precipitation ranges from 1,200 to 2,400 mm. The annual average temperature and relative humidity are around 26.5°C and 80–90%, respectively.
Our study was conducted in the Aboisso department, covering some 625 km2 and an estimated population of 21,300 people, many of whom are employees of PALMCI. The workers leave the villages in the morning to work in the plantations and return in the afternoon.
Study design
The study area was divided into 10 blocks around eight villages of Ehania (Ehania-V1-8), Cité-Cadre, and Akakro. In each block, four types of macrohabitats of roughly equal size were classified as rainforest, polyculture, oil palm monoculture, and rural housing areas based on the land-cover features defined by remote sensing and geospatial analyses (Table 1 and Figures A-D in S1 Fig). The blocks with the villages of Ehania-V1, Cité-Cadre, and Akakro were selected for this study (Fig 1).
Table 1. Classification of Aedes mosquito habitats sampled in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to October 2014.
| Term | Definition | |
|---|---|---|
| I | Macrohabitat1 | Landscape covering specific floristic area and presenting ecological or phyto-geographic aspects that are roughly homogeneous |
| A | Rainforesta | Area covered with dense forest showing natural ecosystems with strong canopy coverage and comprising big trees, creepers, fixed masses of bamboo (Bambusae), and wild vertebrate animals such as primates, birds, and reptiles |
| B | Polyculturea | Area covered with a mosaic of oil palm trees (Eleasis guineensis) mixed with other multiple crops composed of the plants of several industrial crops, such as rubber (Hevea brasiliensis), cocoa (Theobroma cacao), coffee (Coffea spp.), papaya (Carica papaya), coconuts (Cocos spp.), and avocado (Persea Americana), and food-crops such as bananas (Musa spp.), taro (Colocasia spp.), bromeliads (Ananas comosus), yam (Dioscorea spp.), maize (Zea mays), and cassava (Manihot esculenta) growing in the same space. Natural trees, fixed masses of bamboo (Bambusae), and degraded or secondary forest relicts are dispersed in several places in the area |
| Oil palm monoculturea | Area covered uniquely with the monoculture of oil palm trees (Eleasis guineensis) | |
| D | Rural housing areasa | Area covered with human-inhabited space comprising buildings such as houses, markets, hospitals, schools, and other social structures |
| II | Microhabitat1 | Containers that might hold water and serve as breeding sites for Aedes mosquito larvae |
| II.1 | Naturally-occurring microhabitat2 | Containers created without or by indirect intervention of humans |
| E | Natural tree holeb | Rot and pan holes of different shapes and volume located up to 2 m above the ground level |
| F | Bamboo holeb | Cut of fixed masses of bamboo (Bambusae) |
| G | Natural plant leafb | Sheathing leaf axils from plants such as Sanseviera spp. and Xanthosoma spp., and sheets from Thaumatococcus daniellii fallen on the floor |
| H | Other natural microhabitatb | Non-ligneous containers such as snail shells and rock holes |
| II.2 | Agriculturally-occurring microhabitat2 | Containers created by growing crops cultivated by humans |
| I | Crop fruit huskb | Skins of coconuts (Cocos spp.) and cocoa (Theobroma cacao) |
| J | Crop flowerb | Flowers of bananas (Musa spp.) |
| K | Crop leafb | Sheathing leaf axils from plants such as bromeliads (Ananas comosus), taros (Colocasia spp.), and bananas (Musa spp.), and fallen sheets on the floor |
| L | Cultivated plant hole | Growing plant holes of different shapes and volume located up to 2 m above the ground level such as papaya (Carica papaya), coffee (Coffea spp.), avocado (Persea Americana), and cocoa (Theobroma cacao) |
| II.3 | Man-made microhabitat2 | Containers created by direct intervention of humans |
| M | Crop collection containerb | Containers such as ceramic, cemented, glass, plastic, and metallic receptacles used to collect crops such as rubber latex collection cups |
| N | Husbandry watering containerb | Containers such as ceramic, cemented, glass, plastic, and metallic receptacles used to store water for watering plant or animal husbandry |
| O | Discarded containerb | Discarded cans, tires, tarps, broken bottles, buckets, shoes, calabashes, mortars, building tools, and debris of abandoned cars and machines |
| P | Household water containerb | Containers such as ceramic, cemented, glass, plastic, and metallic receptacles used to store potable water or collect rainwater for drinking, cooking, or washing |
1: habitat class,
a: macrohabitat type,
2: microhabitat category,
b:microhabitat sub-category.
Eggs, larvae, pupae, and adults of Aedes mosquitoes were sampled every month during 12 cross-sectional surveys from January to December 2014. There were four defined macrohabitats and we used metallic-ovitraps, bamboo-ovitraps, larvae surveys, and human-baited double-net traps for mosquito collection (Figures A-D in S2 Fig).
Aedes mosquito egg collection
Aedes mosquito eggs were collected monthly using 30 bamboo-ovitraps and 30 metallic-ovitraps during the 12 cross-sectional surveys in each macrohabitat. Bamboo-ovitraps were made of cut bamboo, while metallic-ovitraps were made of a tin can cut to imitate natural and artificial breeding sites of Aedes mosquitoes, respectively. Metallic-ovitraps were painted black, while bamboo-ovitraps were left unpainted. Both ovitrap types had a volume of 400 cm3 and were filled to ¾ with water. The water was a mix of distilled water, rainwater, and a 10% hey infusion with Panicum maximum to increase the attractiveness of the ovitraps [20]. A 5 cm x 7 cm x 0.3 cm paddle made of hardboard served with its rough surface as an oviposition substrate and was plunged into each container and left for one week during each of the 12 surveys.
Microhabitat surveys and Aedes spp. larval sampling
In a preliminary survey, existing larval breeding sites, such as natural and artificial cavities or containers with a potential to contain water, were defined as microhabitats for Aedes larvae. Based on this preliminary survey, microhabitats were classified into three categories and 12 sub-categories depending on their occurring process and use (Table 1 and Figures E-P in S1 Fig). We sampled up to 30 microhabitats of each of the 12 sub-category types among each macrohabitat.
Microhabitats were examined monthly, over a 12-month period (January-December 2014), for the presence of water and immature stages of mosquitoes. Whenever mosquito larvae and/or pupae were present, the content of microhabitat was completely removed using the following equipment: flexible rubber tube connected to a manual suction pump, ladles, and pipettes. Immature forms of Aedes and other non-Aedes mosquitoes such as Anopheles spp., Culex spp., Eretmapodites spp., and Toxorhynchites spp. were sampled and recorded separately. The Aedes mosquito immatures were counted and classified as young larvae (1–2 instar), old larvae (3–4 instar), and pupae. The predacious larvae of mosquitoes, such as Cx. tigripes, Eretmapodites spp., and Toxorhynchites spp., were removed from the samples and preserved separately to avoid predation on the other species. The microhabitats sampled were refilled to their initial volume with the original water, and topped up with distilled water or rainwater according to their flooding mechanism. The presence of shade, predators, and plant leaves in the microhabitats were recorded.
Aedes adult abundance and host-seeking behavior surveillance
Adult mosquitoes were sampled using four human-baited double-net traps in each macrohabitat type for three consecutive days from 04:00 a.m. to 08:00 p.m. during 12 monthly cross-sectional surveys. A double-net trap was a combination of two untreated nets: an inner, smaller net that protected the human bait and an outer, larger net with two openings on each of the four sides which allowed the entry of mosquitoes yet precluded their exit [21, 22]. For each double-net trap, there was a pair of persons: one person was located inside the small net and served as bait to attract mosquitoes. The other person was located outside the double-net device and collected the mosquitoes trapped within the outer net, once every hour. Each trap was monitored by two teams of two persons each that took turns beginning at 12:00 a.m.
Laboratory treatment procedures
All mosquito samples were stored separately in plastic boxes and transferred in a cool-box to a nearby field laboratory. In the laboratory, mosquito larvae were reared until they became adult. In order to minimize mortality, a maximum of 20 larvae were placed in 200 ml plastic cups, filled with 150 ml distilled water and covered with netting. Larvae of Aedes and other mosquitoes were fed each morning between 07:00 and 08:00 a.m. with Tetramin baby fish food. Predacious larvae (e.g., Toxorhynchites spp. and Cx. tigripes) were fed with larvae from additionally sampled mosquitoes from the study area. Emerging adults and collected adult mosquitoes were identified to species level using readily available morphological keys [20, 23]. As larval mortalities were low, the proportion of mosquito species was estimated on the basis of emerging adults. Adult specimens were stored by species and recorded in an entomology collection database.
Statistical analysis
The Aedes-positive index (PI) was calculated as the percentage of bamboo-ovitraps, metallic-ovitraps, microhabitats, or human-baited double-net traps which collected or held at least one egg, larva, pupa, or adult Aedes mosquito (numerator) among the total bamboo-ovitraps, metallic-ovitraps, wet microhabitats, or double-net traps found (denominator), respectively. The Aedes microhabitat positive proportion (PPM) refers to the percentage of each Aedes-positive microhabitat type (numerator) among the total Aedes-positive containers (denominator) in a specific macrohabitat. The Aedes microhabitat positive proportion (PPSA) was calculated as the percentage of each Aedes-positive microhabitat type (numerator) among the total Aedes-positive containers (denominator) in the study area. The proportions of Aedes species were calculated as percentage of specimens among Aedes fauna. We used Fisher’s exact test to determine the relationship between species composition and the macro- and microhabitats. Fisher’s exact test was employed because expected numbers of specimens were equal or less than five. Aedes species richness was expressed as the number of collected species in each study area [24, 25] and compared using a one-way analysis of variance (ANOVA), followed by Bonferroni’s correction. The species diversity, dominance, and community similarity of Aedes mosquitoes in the study area and among the macrohabitats were estimated by Shannon index (H) (1), Simpson index (D) (2), and Sorenson’s coefficient (CC) (3) [24, 25], and analyzed by Kruskal-Wallis test because the log-transformed data exhibited significant deviations from normality. The abundance of Aedes mosquitoes was the number of specimens per species and calculated as the mean numbers of specimens per bamboo-ovitrap, metallic-ovitrap, wet microhabitat and human-baited double-net trap according to sampling methods. The Aedes females’ biting rate was expressed as the mean number of female specimens per person per day. The number of persons was equal to the number of participants used as attractants during human-baited double-net trap sampling. The bamboo-ovitrap, metallic-ovitrap, and human-baited double-net trap data were tested using repeated measures approaches in generalized linear mixed models (GLMM), in order to take into account possible interactions between the variables “macrohabitats” and “month” [26]. We used repeated measures approaches in GLMM framework because the bamboo-ovitrap, metallic-ovitrap, and human-baited double-net trap were repeatedly installed in the same sampling location over time (months). The microhabitat survey data were analyzed using a generalized linear model (GLM) approach. To account for overdispersion due to excessive number of zeroes, the data were log-transformed [log (number of specimens + 1)]. A significance level of 5% was set for statistical testing. All statistical analyses were conducted using Stata version 14.0 (Stata Corporation; College Station, TX, United States of America).
The formulas of the biodiversity indicators were [24, 25]:
| (1) |
| (2) |
| (3) |
where pi is the proportion (n/N) of specimens of one particular species i found (n) divided by the total number of specimens found (N), ln is the natural log, ∑ is the sum of the calculations, s is the number of species, C is the number of species that the two communities have in common, S1 is the total number of species found in community 1, and S2 is the total number of species found in community 2. The Shannon index (H) is an information statistic index which assumes that all species are represented in a sample and are randomly sampled. Note that, the higher the value of H, the higher the species diversity; while the lower the value of H, the lower the species diversity. The Simpson index (D) is a dominance index as it gives more weight to common or dominant species and assumes that a few rare species with only a few representatives will not affect the diversity. The higher the value of D, the higher the species abundance; whereas the lower the value of H, the lower the species abundance. Sorenson’s coefficient (CC) gives information on community similarity and helps to know how much two communities have overlap or similarity. CC ranges from 0 to 1. The closer the value is to 1, the more the communities have species in common; complete community overlap is equal to 1; and complete community dissimilarity is equal to 0.
Results
Mosquito species composition
Table 2 shows the species composition of adult mosquitoes collected as eggs, larvae, pupae, and adults using bamboo-ovitrap, metallic-ovitrap, larval survey, and human-baited double-net trap methods. A total of 30,449 mosquito specimens were collected, comprising different medically important genera, such as Aedes, Anopheles, Culex, Mansonia, and predatory larvae of Eretmapodites and Toxorhynchites. For any sampling method, Aedes mosquitoes dominated the fauna, representing 92.9% of the total fauna with 11 species. The proportions, sex, and the numbers of mosquito species varied substantially between sampling methods.
Table 2. Species composition of mosquitoes sampled in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to December 2014.
| Genus | Species | Bamboo-ovitrap | Metallic-ovitrap | Larval survey | Double-net trap | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | T | % | F | M | T | % | F | M | T | % | F | M | T | % | F | M | T | % | ||
| Aedes | Ae. aegypti | 1,382 | 1,343 | 2,725 | 8.9 | 2,052 | 1,952 | 4,004 | 13.1 | 3,909 | 3,742 | 7,651 | 25.1 | 6,735 | 1,286 | 8,021 | 26.3 | 14,078 | 8,323 | 22,401 | 73.6 |
| Ae. africanus | 163 | 167 | 330 | 1.1 | 199 | 193 | 392 | 1.3 | 120 | 141 | 261 | 0.9 | 59 | 9 | 68 | 0.2 | 541 | 510 | 1,051 | 3.5 | |
| Ae. dendrophilus | 410 | 408 | 818 | 2.7 | 528 | 481 | 1,009 | 3.3 | 405 | 384 | 789 | 2.6 | 302 | 58 | 360 | 1.2 | 1,645 | 1,331 | 2,976 | 9.8 | |
| Ae. fraseri | 16 | 11 | 27 | 0.1 | 27 | 38 | 65 | 0.2 | 16 | 21 | 37 | 0.1 | 0 | 0 | 0 | 0.0 | 59 | 70 | 129 | 0.4 | |
| Ae. furcifer | 41 | 35 | 76 | 0.2 | 62 | 70 | 132 | 0.4 | 145 | 122 | 267 | 0.9 | 23 | 3 | 26 | 0.1 | 271 | 230 | 501 | 1.6 | |
| Ae. lilii | 26 | 16 | 42 | 0.1 | 13 | 15 | 28 | 0.1 | 9 | 5 | 14 | 0.0 | 0 | 0 | 0 | 0.0 | 48 | 36 | 84 | 0.3 | |
| Ae. luteocephalus | 42 | 50 | 92 | 0.3 | 67 | 49 | 116 | 0.4 | 27 | 27 | 54 | 0.2 | 0 | 0 | 0 | 0.0 | 136 | 126 | 262 | 0.9 | |
| Ae. metallicus | 13 | 16 | 29 | 0.1 | 44 | 49 | 93 | 0.3 | 25 | 23 | 48 | 0.2 | 0 | 0 | 0 | 0.0 | 82 | 88 | 170 | 0.6 | |
| Ae. opok | 13 | 30 | 43 | 0.1 | 9 | 1 | 10 | 0.0 | 8 | 7 | 15 | 0.0 | 0 | 0 | 0 | 0.0 | 30 | 38 | 68 | 0.2 | |
| Ae. palpalis | 6 | 6 | 12 | 0.0 | 19 | 13 | 32 | 0.1 | 55 | 62 | 117 | 0.4 | 3 | 1 | 4 | 0.0 | 83 | 82 | 165 | 0.5 | |
| Ae. vittatus | 29 | 13 | 42 | 0.1 | 98 | 80 | 178 | 0.6 | 57 | 38 | 95 | 0.3 | 119 | 35 | 154 | 0.5 | 303 | 166 | 469 | 1.5 | |
| Total | 2,141 | 2,095 | 4,236 | 13.9 | 3,118 | 2,941 | 6,059 | 19.9 | 4,776 | 4,572 | 9,348 | 30.7 | 7,241 | 1,392 | 8,633 | 28.4 | 17,276 | 11,000 | 28,276 | 92.9 | |
| Anopheles | An. pharoensis | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 8 | 2 | 10 | 0.0 | 0 | 0 | 0 | 0.0 | 8 | 2 | 10 | 0.0 |
| An. gambiae | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 39 | 48 | 87 | 0.3 | 19 | 2 | 21 | 0.1 | 58 | 50 | 108 | 0.4 | |
| An. ziemani | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 1 | 0 | 1 | 0.0 | 1 | 0 | 1 | 0.0 | |
| Total | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 47 | 50 | 97 | 0.3 | 20 | 2 | 22 | 0.1 | 67 | 52 | 119 | 0.4 | |
| Culex | Cx. nebulosus | 19 | 27 | 46 | 0.2 | 52 | 43 | 95 | 0.3 | 15 | 19 | 34 | 0.1 | 6 | 0 | 6 | 0.0 | 92 | 89 | 181 | 0.6 |
| Cx. poicilipes | 32 | 36 | 68 | 0.2 | 29 | 41 | 70 | 0.2 | 73 | 54 | 127 | 0.4 | 48 | 5 | 53 | 0.2 | 182 | 136 | 318 | 1.0 | |
| Cx. quinquefasciatus | 74 | 62 | 136 | 0.4 | 89 | 71 | 160 | 0.5 | 218 | 176 | 394 | 1.3 | 56 | 11 | 67 | 0.2 | 437 | 320 | 757 | 2.5 | |
| Cx. tigripes | 3 | 4 | 7 | 0.0 | 13 | 6 | 19 | 0.1 | 79 | 95 | 174 | 0.6 | 3 | 0 | 3 | 0.0 | 98 | 105 | 203 | 0.7 | |
| Total | 128 | 129 | 257 | 0.8 | 183 | 161 | 344 | 1.1 | 385 | 344 | 729 | 2.4 | 113 | 16 | 129 | 0.4 | 809 | 650 | 1,459 | 4.8 | |
| Eretmapodites | Er. chrysogaster | 87 | 69 | 156 | 0.5 | 76 | 76 | 0.2 | 112 | 97 | 209 | 0.7 | 48 | 14 | 62 | 0.2 | 323 | 180 | 503 | 1.7 | |
| Total | 87 | 69 | 156 | 0.5 | 76 | 0 | 76 | 0.2 | 112 | 97 | 209 | 0.7 | 48 | 14 | 62 | 0.2 | 323 | 180 | 503 | 1.7 | |
| Mansonia | Ma. africana | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 6 | 0 | 6 | 0.0 | 6 | 0 | 6 | 0.0 |
| Ma. uniformis | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 2 | 1 | 3 | 0.0 | 2 | 1 | 3 | 0.0 | |
| Total | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 8 | 1 | 9 | 0.0 | 8 | 1 | 9 | 0.0 | |
| Toxorhynchites | Tx. brevipalpis | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 47 | 36 | 83 | 0.3 | 0 | 0 | 0 | 0.0 | 47 | 36 | 83 | 0.3 |
| Total | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 47 | 36 | 83 | 0.3 | 0 | 0 | 0 | 0.0 | 47 | 36 | 83 | 0.3 | |
| Total | Abundance | 2,356 | 2,293 | 4,649 | 15.3 | 3,377 | 3,102 | 6,479 | 21.3 | 5,367 | 5,099 | 10,466 | 34.4 | 7,430 | 1,425 | 8,855 | 29.1 | 18,530 | 11,919 | 30,449 | 100 |
| No. of species | 16 | 16 | 19 | 15 | 22 | ||||||||||||||||
F: female, M: male, T: total, %: percentage.
Distribution of Aedes immature stages across macrohabitats
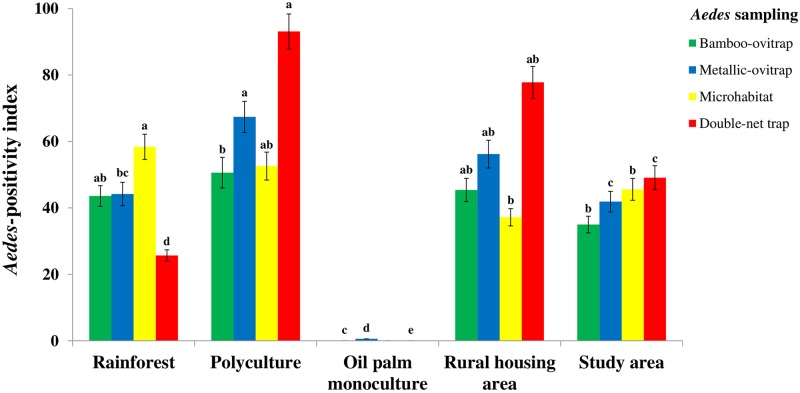
Fig 2 and Table 3 illustrate immature Aedes species occurrence, stratified by macrohabitats. Overall, the study area showed variable Aedes-positivity indices, with PI values of 35.0% (482/1,378) in the bamboo-ovitraps, 41.9% (577/1,377) in metallic-ovitraps, and 45.6% (801/1,756) in the microhabitats. The highest Aedes-positivity indices in the bamboo-ovitraps (177/350; PI = 50.6%) and in the metallic-ovitraps (232/344; PI = 67.4%) were found in the polyculture environment. Conversely, GLMM indicated that Aedes-positivity indices were significantly lower in oil palm monoculture compared to the other macrohabitats (p <0.05) (S1 Table).
Fig 2. Aedes mosquito species occurrence among the macrohabitats in oil in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to December 2014.
Error bars represent the standard error (SE). Letters indicate the results of the GLMM. Groups that do not share the same letter for the same sampling method are significantly different.
Table 3. Aedes mosquito positivity patterns among the macrohabitats, and the study area in southeastern Côte d’Ivoire from January to December 2014.
| Term | Macrohabitat | Study area | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainforest | Polyculture | Oil palm monoculture | Rural housing area | ||||||||||||
| n1 | n2 | PI | n1 | n2 | PI | n1 | n2 | PI | n1 | n2 | PI | n1 | n2 | PI | |
| Bamboo-ovitrap1 | 346 | 151 | 43.6 | 350 | 177 | 50.6 | 343 | 0 | 0.0 | 339 | 154 | 45.4 | 1,378 | 482 | 35.0 |
| Metallic-ovitrap2 | 344 | 152 | 44.2 | 344 | 232 | 67.4 | 349 | 2 | 0.6 | 340 | 191 | 56.2 | 1,377 | 577 | 41.9 |
| Microhabitat3 | 161 | 94 | 58.4 | 737 | 388 | 52.6 | 0 | 0 | NA | 858 | 319 | 37.2 | 1,756 | 801 | 45.6 |
| Naturally-occurring microhabitat3 | 161 | 94 | 58.4 | 148 | 94 | 63.5 | 0 | 0 | NA | 47 | 10 | 21.3 | 356 | 198 | 55.6 |
| Natural tree hole3 | 54 | 45 | 83.3 | 42 | 33 | 78.6 | 0 | 0 | NA | 4 | 1 | 25.0 | 100 | 79 | 79.0 |
| Bamboo hole3 | 51 | 38 | 74.5 | 29 | 21 | 72.4 | 0 | 0 | NA | 13 | 4 | 30.8 | 93 | 63 | 67.7 |
| Natural plant leaf3 | 52 | 9 | 17.3 | 29 | 7 | 24.1 | 0 | 0 | NA | 11 | 0 | 0.0 | 92 | 16 | 17.4 |
| Other natural microhabitat3 | 4 | 2 | 50.0 | 48 | 33 | 68.8 | 0 | 0 | NA | 19 | 5 | 26.3 | 71 | 40 | 56.3 |
| Agriculturally-occurring microhabitat3 | 0 | 0 | NA | 314 | 96 | 30.6 | 0 | 0 | NA | 49 | 6 | 12.2 | 363 | 102 | 28.1 |
| Crop fruit husk3 | 0 | 0 | NA | 91 | 47 | 51.6 | 0 | 0 | NA | 26 | 6 | 23.1 | 117 | 53 | 45.3 |
| Crop flower3 | 0 | 0 | NA | 68 | 3 | 4.4 | 0 | 0 | NA | 16 | 0 | 0.0 | 84 | 3 | 3.6 |
| Crop leaf3 | 0 | 0 | NA | 96 | 11 | 11.5 | 0 | 0 | NA | 0 | 0 | NA | 96 | 11 | 11.5 |
| Cultivated plant hole3 | 0 | 0 | NA | 59 | 35 | 59.3 | 0 | 0 | NA | 7 | 0 | 0.0 | 66 | 35 | 53.0 |
| Man-made microhabitat3 | 0 | 0 | NA | 275 | 198 | 72.0 | 0 | 0 | NA | 762 | 303 | 39.8 | 1,037 | 501 | 48.3 |
| Crop collection container3 | 0 | 0 | NA | 57 | 33 | 57.9 | 0 | 0 | NA | 6 | 2 | 33.3 | 63 | 35 | 55.6 |
| Husbandry watering container3 | 0 | 0 | NA | 51 | 30 | 58.8 | 0 | 0 | NA | 229 | 159 | 69.4 | 280 | 189 | 67.5 |
| Discarded container3 | 0 | 0 | NA | 167 | 135 | 80.8 | 0 | 0 | NA | 167 | 105 | 62.9 | 334 | 240 | 71.9 |
| Household water container3 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 360 | 37 | 10.3 | 360 | 37 | 10.3 |
| Double-net trap4 | 144 | 37 | 25.7 | 144 | 134 | 93.1 | 144 | 0 | 0.0 | 144 | 112 | 77.8 | 576 | 283 | 49.1 |
n1: numbers of bamboo-ovitraps recovered1, metallic-ovitraps recovered2, wet microhabitats3, or double-net traps installed4, n2: numbers of Aedes-positive bamboo-ovitraps1, numbers of Aedes-positive metallic-ovitraps2, Aedes-positive microhabitats3, or Aedes-positive double-net traps4, PI: Aedes-positivity index. PI is expressed as percentage (%).
Microhabitat Aedes-positivity indices widely varied from one macrohabitat to another (Table 3 and S3 Fig). No Aedes-positive microhabitats were found in oil palm monoculture. In contrast, the highest Aedes-microhabitat positivity index was estimated for the rainforest (94/161; PI = 58.4%), followed by the polyculture (388/737; PI = 52.6%), and the rural housing areas (319/858; PI = 37.2%). In the rural housing areas, husbandry watering containers were often infested with Aedes larvae (159/229; PI = 69.4%), and reached a PI of 86.4% (19/22) in December 2014 during the long dry season. In the polyculture site, the highest Aedes-positivity index (135/167; PI = 80.8%) was observed among the discarded containers.
Table 4 shows the proportions of each type of Aedes-positive microhabitats among the whole Aedes-positive microhabitats in each macrohabitat. In the rainforest, all the Aedes-positive breeding sites (94/94; PPM = 100%) were naturally occurring microhabitats, while 95.0% (303/319, PPM = 95.0%) of Aedes-positive microhabitats were man-made containers in the rural housing areas. The polyculture macrohabitat had substantial proportions of all Aedes-positive microhabitat types, with PPM of 24.2% (94/388) of naturally-occurring, 24.8% (96/388) of agriculturally-occurring, and 51.0% (198/388) of man-made microhabitats. In the study area, Aedes-positive breeding sites were dominated by man-made microhabitats (501/801; PPSA = 62.6%), followed by naturally-occurring microhabitats (198/801; PPSA = 24.7%), and agricultural microhabitats (102/801; PPSA = 12.7%) (Table 4 and S4 Fig). Overall, apart from the oil palm monocultures, Aedes microhabitat positivity indices were higher during the dry season (January, February, November, and December), in the other macrohabitats and the study area (S5 Fig). Conversely, the highest proportions of Aedes-positive microhabitats were recorded during the rainy seasons (June, July, and October; see S6 Fig).
Table 4. Proportion (%) of each Aedes-positive microhabitat type among Aedes-positive microhabitats, macrohabitats, and study area in southeastern Côte d’Ivoire from January to December 2014.
| Term | Macrohabitat | Study area | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainforest | Polyculture | Oil palm monoculture | Rural-housing area | |||||||||||
| n | PPM | PPSA | n | PPM | PPSA | n | PPM | PPSA | n | PPM | PPSA | n | PPSA | |
| Naturally-occurring microhabitat | 94 | 100.0 | 11.7 | 94 | 24.2 | 11.7 | 0 | NA | 0.0 | 10 | 3.1 | 1.2 | 198 | 24.7 |
| Natural tree hole | 45 | 47.9 | 5.6 | 33 | 8.5 | 4.1 | 0 | NA | 0.0 | 1 | 0.3 | 0.1 | 79 | 9.9 |
| Bamboo hole | 38 | 40.4 | 4.7 | 21 | 5.4 | 2.6 | 0 | NA | 0.0 | 4 | 1.3 | 0.5 | 63 | 7.9 |
| Natural plant leaf | 9 | 9.6 | 1.1 | 7 | 1.8 | 0.9 | 0 | NA | 0.0 | 0 | 0.0 | 0.0 | 16 | 2.0 |
| Other natural microhabitats | 2 | 2.1 | 0.2 | 33 | 8.5 | 4.1 | 0 | NA | 0.0 | 5 | 1.6 | 0.6 | 40 | 5.0 |
| Agriculturally-occurring microhabitat | 0 | 0.0 | 0.0 | 96 | 24.8 | 12.0 | 0 | NA | 0.0 | 6 | 1.9 | 0.7 | 102 | 12.7 |
| Crop fruit husk | 0 | 0.0 | 0.0 | 47 | 12.1 | 5.9 | 0 | NA | 0.0 | 6 | 1.9 | 0.7 | 53 | 6.6 |
| Crop flower | 0 | 0.0 | 0.0 | 3 | 0.8 | 0.4 | 0 | NA | 0.0 | 0 | 0.0 | 0.0 | 3 | 0.4 |
| Crop leaf | 0 | 0.0 | 0.0 | 11 | 2.8 | 1.4 | 0 | NA | 0.0 | 0 | 0.0 | 0.0 | 11 | 1.4 |
| Cultivated plant hole | 0 | 0.0 | 0.0 | 35 | 9.0 | 4.4 | 0 | NA | 0.0 | 0 | 0.0 | 0.0 | 35 | 4.4 |
| Man-made microhabitat | 0 | 0.0 | 0.0 | 198 | 51.0 | 24.7 | 0 | NA | 0.0 | 303 | 95.0 | 37.8 | 501 | 62.6 |
| Crop collection container | 0 | 0.0 | 0.0 | 33 | 8.5 | 4.1 | 0 | NA | 0.0 | 2 | 0.6 | 0.2 | 35 | 4.4 |
| Husbandry watering container | 0 | 0.0 | 0.0 | 30 | 7.7 | 3.7 | 0 | NA | 0.0 | 159 | 49.8 | 19.9 | 189 | 23.6 |
| Discarded container | 0 | 0.0 | 0.0 | 135 | 34.8 | 16.9 | 0 | NA | 0.0 | 105 | 32.9 | 13.1 | 240 | 30.0 |
| Household water container | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0 | NA | 0.0 | 37 | 11.6 | 4.6 | 37 | 4.6 |
| Total | 94 | 100 | 11.7 | 388 | 100 | 48.5 | 0 | NA | 0.0 | 319 | 100 | 39.8 | 801 | 100 |
n: number of Aedes-positive microhabitats, PPM: proportions of Aedes-positive microhabitat type among the whole Aedes-positive microhabitats in each macrohabitat, PPSA: proportions of Aedes-positive microhabitat type among the whole Aedes-positive microhabitats in the study area. PPM and PPSA are expressed as percentage (%).
The frequency of microhabitats with shade, plant leaves, and predators varied among the macrohabitats. The highest proportions of shaded microhabitats (n = 607; 96.9%), and microhabitats with plant leaves (92.6%) were found in the rainforest. Wet microhabitats containing at least one of the predatory larvae of Toxorhynchites spp., Eretmapodites spp., and Cx. tigripes mosquitoes were also mostly encountered in the rainforest (n = 161; 63.4%). The polyculture area also hosted higher numbers of microhabitats with shade (n = 2,117; 54.5%), plant leaves (n = 2,117; 59.6%), and predators (n = 737; 29.9%), compared to the rural housing areas.
Aedes species distribution, biodiversity, and dynamics
Table 5 presents the geographic distribution and biodiversity of Aedes species among the macrohabitats in the study area. Ae. aegypti was the predominant species in the study area (n = 28,276; 79.2%). Ae. aegypti was also the most abundant species among Aedes mosquitoes collected in the polyculture, rural housing areas, and rainforest macrohabitats, with 49.2% (n = 28,276), 25.7%, and 4.3% of total fauna, respectively. Other Aedes species such as Ae. dendrophilus (10.5%), Ae. africanus (3.7%), Ae. furcifer (1.8%), and Ae. vittatus (1.7%), represented more than 1% of the total Aedes fauna in the study area. However, Ae. africanus (3.4%) showed its highest abundance in the rainforest, whereas the highest proportion of Ae. dendrophilus (7.6%) and Ae. furcifer (1.2%) were found in the polyculture area. The proportion of Ae. dendrophilus was above 1% in the rural housing area.
Table 5. Aedes species distribution and biodiversity among macrohabitats in oil palm-dominated landscapes in southeastern Côte d’Ivoire between January and December 2014.
| Species | Macrohabitat | Study area | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rainforest | Polyculture | Oil palm monoculture | Rural housing area | |||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | |
| Ae. aegypti | 1,213 | 4.3 | 13,903 | 49.2 | 4 | 0.01 | 7,281 | 25.7 | 22,401 | 79.2 |
| Ae. africanus | 948 | 3.4 | 61 | 0.2 | 0 | 0.0 | 42 | 0.1 | 1,051 | 3.7 |
| Ae. dendrophilus | 544 | 1.9 | 2,150 | 7.6 | 0 | 0.0 | 282 | 1 | 2,976 | 10.5 |
| Ae. fraseri | 129 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 129 | 0.5 |
| Ae. furcifer | 24 | 0.1 | 352 | 1.2 | 0 | 0.0 | 125 | 0.4 | 501 | 1.8 |
| Ae. lilii | 53 | 0.2 | 31 | 0.1 | 0 | 0.0 | 0 | 0.0 | 84 | 0.3 |
| Ae. luteocephalus | 96 | 0.3 | 158 | 0.6 | 0 | 0.0 | 8 | 0.0 | 262 | 0.9 |
| Ae. metallicus | 25 | 0.1 | 126 | 0.4 | 0 | 0.0 | 19 | 0.1 | 170 | 0.6 |
| Ae. opok | 24 | 0.1 | 34 | 0.1 | 0 | 0.0 | 10 | 0.0 | 68 | 0.2 |
| Ae. palpalis | 35 | 0.1 | 130 | 0.5 | 0 | 0.0 | 0 | 0.0 | 165 | 0.6 |
| Ae. vittatus | 24 | 0.1 | 289 | 1 | 0 | 0.0 | 156 | 0.6 | 469 | 1.7 |
| Abundance (no. of specimens) | 3,115 | 11.0 | 17,234 | 60.9 | 4 | 0.01 | 7,923 | 28.0 | 28,276 | 100 |
| Species richness (no. of species) | 11 | 10 | 1 | 8 | 11 | |||||
| Species diversity (Shannon index H) | 1.54 | 0.74 | 0.00 | 0.40 | 0.84 | |||||
| Species dominance (Simpson index D) | 0.28 | 0.67 | 1.00 | 0.85 | 0.64 | |||||
| Community similarity (Sorenson’s coefficient CC) | 1.00 | 0.95 | 0.17 | 0.84 | 1.00 | |||||
| 0.95 | 1.00 | 0.18 | 0.89 | 0.95 | ||||||
| 0.17 | 0.18 | 1.00 | 0.22 | 0.17 | ||||||
| 0.84 | 0.89 | 0.22 | 1.00 | 0.84 | ||||||
| 1.00 | 0.95 | 0.17 | 0.84 | 1.00 | ||||||
%: proportion of Aedes specimens calculated as percentages (%). In each row, a macrohabitat with a Sorenson’s coefficient CC of 1 was used as a reference to calculate the Sorenson’s coefficients for the other macrohabitats.
Aedes species number, diversity (F = 17.12; df = 3, p <0.05), and dominance (F = 11.04; df = 3, p <0.05) varied among the study area and the macrohabitats (Table 5). The highest Aedes species richness (n = 11) and the highest species diversity (Shannon index H = 1.54) were observed in the rainforest, while oil palm monoculture exhibited the poorest diversity with one species and null Shannon index. The rural housing areas displayed significantly higher Aedes species dominance (Simpson index D = 0.085) compared with the rainforest (Simpson index D = 0.28), the study area (Simpson index D = 0.64), and the polyculture (Simpson index D = 0.67). The community similarity of Aedes species between the macrohabitats also significantly altered (χ2 = 13.36; df = 3, p <0.05) (Table 5). According to Sorenson’s coefficient (CC = 1), Aedes mosquito community in the study area were similar to those inhabiting the rainforest. Compared with the rainforest, the polyculture showed the highest community similarity with Sorenson’s coefficient of 0.95, followed by the rural-housing areas with a Sorenson’s coefficient of 0.85. In contrast, the Aedes communities in the rainforest and oil palm monoculture showed with 0.17 the lowest value for the Sorenson’s coefficient.
Table 6 indicates Aedes species abundance among the macrohabitats in the study area. No Aedes eggs, larvae, pupae, or adults were collected in the oil palm monoculture using bamboo-ovitrap, larval survey, and double-net trap methods, except four eggs sampled with the metallic-ovitraps. However, higher mean numbers (mean ± standard error) of Aedes specimens with 2.32 ± 0.07 eggs/bamboo-ovitrap/week, 4.18 ± 0.07 eggs/metallic-ovitrap/week, and 26.01 ± 0.12 adults/double-net trap/day were found in the polyculture. The mean number in bamboo-ovitrap deployed in oil palm monoculture was significantly lower than the rainforest (Z = 1.96, p <0.05) and rural housing areas (Z = 2.06, p <0.05) (S2 Table). The mean numbers of Aedes eggs collected using metallic-ovitrap were significant different between the oil palm monoculture and the rainforest (Z = -2.04, p = 0.041) (S3 Table), and between the polyculture and the rainforest (Z = -3.45, p = 0.001) (S4 Table).
Table 6. Aedes mosquito abundance patterns in macrohabitats, and the study area in southeastern Côte d’Ivoire between January and December 2014.
| Term | Macrohabitat | Study area | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainforest | Polyculture | Oil palm monoculture | Rural housing area | ||||||||||||
| n1 | n2 | Mean ± SE | n1 | n2 | Mean ± SE | n1 | n2 | Mean ± SE | n1 | n2 | Mean ± SE | n1 | n2 | Mean ± SE | |
| Bamboo-ovitrap1 | 346 | 1,018 | 1.28 ± 0.06 | 350 | 1,899 | 2.32 ± 0.07 | 343 | 0 | 0 | 339 | 1,319 | 1.73 ± 0.06 | 1,378 | 4,236 | 1.13 ± 0.03 |
| Metallic-ovitrap2 | 344 | 1,198 | 1.44 ± 0.06 | 344 | 2,830 | 4.18 ± 0.07 | 349 | 4 | 0.01 ± 0.004 | 340 | 2,027 | 2.72 ± 0.07 | 1,377 | 6,059 | 1.61 ± 0.03 |
| Microhabitat3 | 607 | 671 | 0.36 ± 0.03 | 2,117 | 5,339 | 0.60 ± 0.02 | 0 | 0 | NA | 1,497 | 3,338 | 0.63 ± 0.03 | 4,221 | 9,348 | 0.57 ± 0.02 |
| Naturally-occurring microhabitat3 | 607 | 671 | 0.36 ± 0.03 | 435 | 1,537 | 0.80 ± 0.06 | 0 | 0 | NA | 191 | 53 | 0.09 ± 0.03 | 1,233 | 2,261 | 0.45 ± 0.03 |
| Natural tree hole3 | 92 | 372 | 1.87 ± 0.12 | 82 | 688 | 2.40 ± 0.18 | 0 | 0 | NA | 46 | 8 | 0.05 ± 0.05 | 220 | 1,068 | 1.48 ± 0.09 |
| Bamboo hole3 | 189 | 257 | 0.48 ± 0.06 | 89 | 377 | 0.95 ± 0.14 | 0 | 0 | NA | 56 | 18 | 0.11 ± 0.06 | 334 | 652 | 0.52 ± 0.05 |
| Natural plant leaf3 | 283 | 33 | 0.05 ± 0.02 | 111 | 54 | 0.14 ± 0.05 | 0 | 0 | NA | 28 | 0 | 0 | 422 | 87 | 0.07 ± 0.02 |
| Other natural microhabitat3 | 43 | 9 | 0.08 ± 0.06 | 153 | 418 | 0.69 ± 0.09 | 0 | 0 | NA | 61 | 27 | 0.15 ± 0.07 | 257 | 454 | 0.43 ± 0.06 |
| Agriculturally-occurring microhabitat3 | 0 | 0 | NA | 1,118 | 1,001 | 0.22 ± 0.02 | 0 | 0 | NA | 275 | 51 | 0.05 ± 0.02 | 1,393 | 1,052 | 0.19 ± 0.02 |
| Crop fruit husk3 | 0 | 0 | NA | 338 | 556 | 0.41 ± 0.05 | 0 | 0 | NA | 98 | 51 | 0.14 ± 0.06 | 436 | 607 | 0.35 ± 0.04 |
| Crop flower3 | 0 | 0 | NA | 266 | 16 | 0.02 ± 0.01 | 0 | 0 | NA | 54 | 0 | 0 | 320 | 16 | 0.02 ± 0.01 |
| Crop leaf3 | 0 | 0 | NA | 360 | 75 | 0.06 ± 0.02 | 0 | 0 | NA | 89 | 0 | 0 | 449 | 75 | 0.05 ± 0.01 |
| Cultivated plant hole3 | 0 | 0 | NA | 154 | 354 | 0.69 ± 0.08 | 0 | 0 | NA | 34 | 0 | 0 | 188 | 354 | 0.54 ± 0.07 |
| Man-made microhabitat3 | 0 | 0 | NA | 564 | 2,801 | 1.50 ± 0.06 | 0 | 0 | NA | 1,031 | 3,234 | 0.98 ± 0.03 | 1,595 | 6,035 | 1.15 ± 0.03 |
| Crop collection container3 | 0 | 0 | NA | 141 | 454 | 0.83 ± 0.10 | 0 | 0 | NA | 39 | 5 | 0.07 ± 0.05 | 180 | 459 | 0.63 ± 0.08 |
| Husbandry watering container3 | 0 | 0 | NA | 63 | 303 | 1.99 ± 0.16 | 0 | 0 | NA | 272 | 1,362 | 2.47 ± 0.07 | 335 | 1,665 | 2.37 ± 0.06 |
| Discarded container3 | 0 | 0 | NA | 360 | 2,044 | 1.74 ± 0.07 | 0 | 0 | NA | 360 | 1,560 | 1.20 ± 0.07 | 720 | 3,604 | 1.46 ± 0.05 |
| Household water container3 | 0 | 0 | NA | 0 | NA | 0 | 0 | NA | 360 | 307 | 0.24 ± 0.04 | 360 | 307 | 0.24 ± 0.04 | |
| Double-net trap4 | 144 | 228 | 0.71 ± 0.7 | 144 | 7,166 | 26.01 ± 0.12 | 144 | 0 | 0 | 144 | 1,239 | 4.89 ± 0.10 | 576 | 8,633 | 3.06 ± 0.07 |
n1: number of recovered bamboo-ovitraps1, or number of recovered metallic-ovitraps2, or microhabiats3, or double-net trap4; n2: number of eggs, larvae, or adults of Aedes collected; SE: standard error of the mean numbers. Mean was mean number of Aedes eggs per bamboo-ovitrap1, mean number of Aedes eggs per metallic-ovitrap2, mean number of Aedes larvae per microhabitat3; or mean number of Aedes adults per double-net trap4. The units are egg/bamboo-ovitrap/week for bamboo-ovitraps1, egg/metallic-ovitrap/week for metallic-ovitraps2, larvae/microhabitat for microhabitats3, and adult/trap/day for double-net traps4.
GLMM revealed that the mean numbers of Aedes eggs were significantly lower in oil palm monoculture than the other macrohabitats (p <0.05) (S5 Table). The rural housing areas (0.63 ± 0.03 larvae/microhabitat) and the polyculture (0.60 ± 0.02 larvae/microhabitat) showed higher means of Aedes larvae compared with the other macrohabitats. In the rainforest, the tree holes were the most Aedes-inhabited habitats, with 1.87 ± 0.12 larvae/microhabitat. The rainforest was free of any agricultural and man-made microhabitats, while the polyculture macrohabitat hosted all types of microhabitats, except for household water containers. In the rural housing areas, the water containers were the most important producers of Aedes larvae with a mean of 2.47 ± 0.07 larvae/microhabitat. In the study area, the discarded containers also exhibited high ability to harbor Aedes immatures, with a mean number of 1.46 ± 0.05 larvae/microhabitat (Table 6).
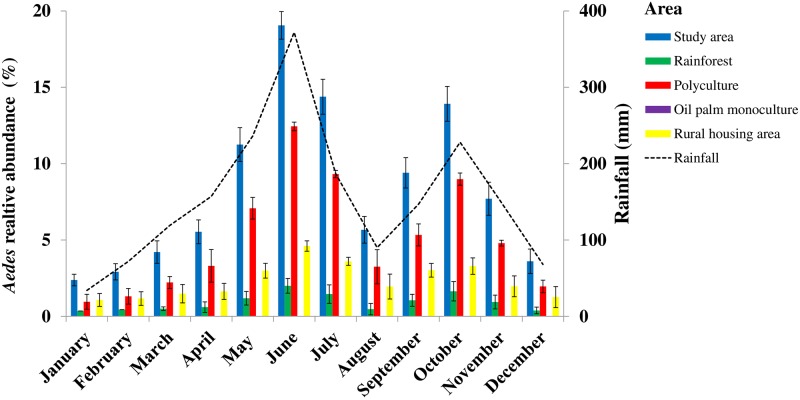
Fig 3 shows the seasonal dynamics of whole Aedes species populations, sampled as eggs, larvae, pupae, and adults, over time among the macrohabitats in the study area. In the study area and macrohabitats, Aedes species abundance varied as a function of precipitation over time. Aedes abundance reached the first series of peaks in June, during the long rainy season, proportions of 19.1% (n = 28,276) in the study area, 12.4% in the polyculture, 4.6% in the rural housing areas, 2.0% in the rainforest, and 0.01% in oil palm monoculture. The second series of peaks occurred in October, during the short rainy season, with 13.9% in the study area, 9.0% in the polyculture, 3.3% in the rural housing areas, and 1.6% in the rainforest.
Fig 3. Monthly variations in the abundance of Aedes mosquitoes in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to December 2014.
Error bars represent the standard error (SE).
Adult Aedes females’ host-seeking behaviors
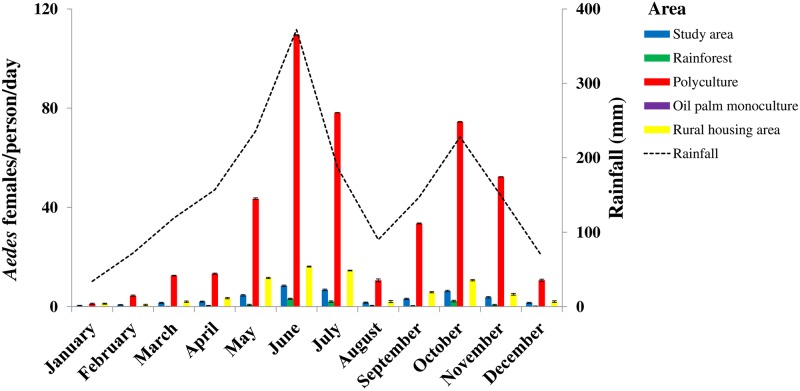
The mean biting rate of Aedes females was estimated at 2.76 ± 0.07 females/person/day in the study area (S6 Table). The highest mean biting rates were found in the polyculture macrohabitat (21.48 ± 0.12 females/person/day), followed by the rural housing areas (4.48 ± 0.10 females/person/day), and the rainforest (0.62 ± 0.60 females/person/day). Hence, the polyculture, the rural housing areas, and the whole study area increased Aedes vector biting rate by factors of 34.6 (21.48/0.62), 7.2 (4.48/0.62), and 4.5 (2.76/0.62) compared with the rainforest, respectively. However, no biting Aedes females were collected in the oil palm monoculture. GLMM revealed significant differences in the mean biting rates comparing rainforest with polyculture (Z = 2.47, p = 0.014), and rainforest with housing areas (Z = 2.37, p = 0.018) (S3 Table). Over 93.0% (n = 7,241) of biting was inflicted by Ae. aegypti. Conversely, no females of several other species such as Ae. fraseri, Ae. lilii, Ae. luteocephalus, Ae. metallicus, and Ae. opok were found in the human-baited double-net device (Table 2).
Fig 4 presents the seasonal dynamics of Aedes host-seeking in the study area and the macrohabitats. GLMM indicated that the biting rates of Aedes females significantly varied over time (p <0.05) (S5 Table) with a peak observed in June during the long rainy season and in October during the short rainy season across all macrohabitats, except for the oil palm monoculture (Fig 3). The major biting rate peaks of Aedes females averaged 109.54 ± 0.07 females/person/day in the polyculture, 16.14 ± 0.17 females/person/day in the rural housing area, 8.44 ± 0.30 females/person/day in the study area, and 3.18 ± 0.24 females/person/day in the rainforest in June. The second most important biting rates occurred in October with 74.5 ± 0.10 females/person/day in the polyculture, 10.7 ± 0.27 females/person/day in the rural-housing areas, 6.33 ± 0.29 females/person/day in the study area, and 2.27 ± 0.32 females/person/day in the rainforest.
Fig 4. Monthly variations in Aedes mosquito females’ host-seeking activities in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to December 2014.
Error bars represent the standard error (SE).
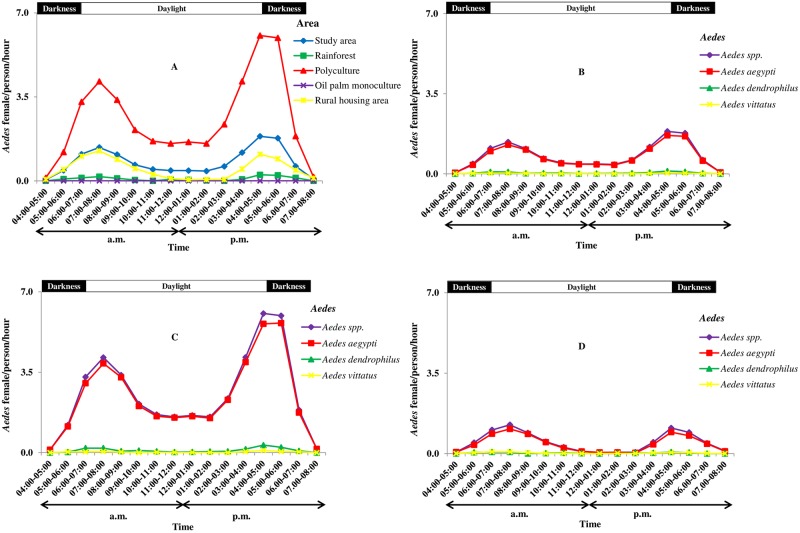
Fig 5 shows the daily host-seeking activity cycles of Aedes mosquito females in the study area and across the different macrohabitats. Aedes females fed from 04:00 a.m. to 08:00 p.m., covering daytime (06:00 a.m. to 6:00 p.m.), and darkness (04:00 a.m. to 06:00 a.m. and 06:00 p.m. to 08:00 p.m.) in all macrohabitats, except in the oil palm monoculture (Fig 5A). The biting cycles showed two peaks, with the main peak observed between 04:00 p.m. and 05:00 p.m. and a lower peak between 07:00 a.m. and 08:00 a.m. Ae. aegypti, Ae. dendrophilus, and Ae. vittatus followed the same host-seeking patterns (Fig 5A) with stronger human biting intensity in Ae. aegypti in the study area (Fig 5B), the polyculture (Fig 5C), and the rural housing areas (Fig 5D). In contrast to these similarities, there was also some dissimilarity in that host-biting activity was interrupted from 11:00 a.m. to 02:00 p.m. in the rural housing areas but continued in polyculture macrohabitat (Fig 5A).
Fig 5. Nycthemeral dynamics of Aedes mosquito females’ host-seeking activities in oil palm-dominated landscapes in southeastern Côte d’Ivoire from January to December 2014.
A: All species in all the macrohabitats and the study area, B: Prevalent Aedes species (> 1%) in the study area, C: Prevalent Aedes species (> 1%) in the polyculture, D: Prevalent Aedes species (> 1%) in the rural-housing areas.
Discussion
Our study revealed no Aedes-positive microhabitats and only four specimens of Ae. aegypti in oil palm monocultures, coupled with high Aedes species richness in the rainforest, and high biting rates in polyculture and rural housing areas over a 12-month period in southeastern Côte d’Ivoire. As identifying priority areas for IVM is of considerable importance for public health [3, 27], this study examined–for the first time–the effects of land-use changes on Aedes mosquito abundance, distribution, and human host seeking behavior in oil palm-dominated landscapes of yellow fever and dengue foci in the southeastern part of Côte d’Ivoire. Our data showed that Aedes mosquito species displayed several significant differences in community composition, distribution, and host-seeking behavior across different land-covers, with the highest species richness observed in rainforest, highest species numbers in the polyculture macrohabitats, the lowest species richness and numbers in oil palm monoculture, and stronger anthropophagic behaviors in the polyculture and rural housing areas (Fig 6 and S6 Table). Such distributional differences in Aedes vectors are likely to shape the distributions of arboviral disease transmission risks between landscapes, with low-risk and high-risk areas (Fig 7).
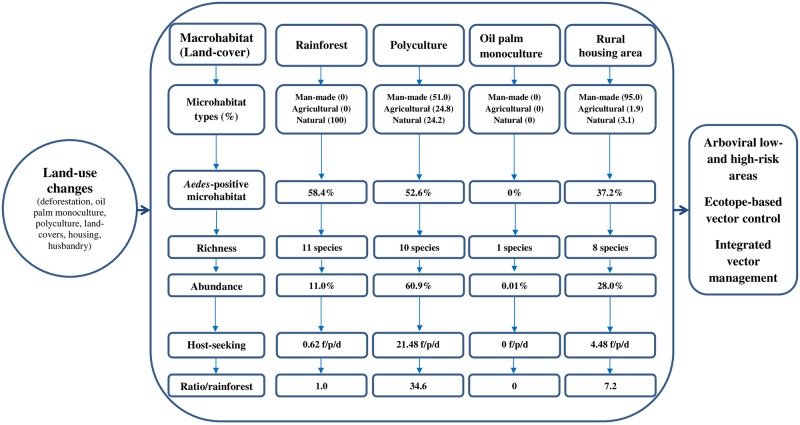
Fig 6. Synthesis of how agricultural land-use changes affect the dynamics of Aedes mosquitoes in oil palm-planted areas in southeastern Côte d’Ivoire.
f/p/d: female/person/day. Overall, there was a lack of Aedes microhabitats and species in the oil palm monoculture. In contrast, the highest abundance of Aedes mosquitoes was found in the polyculture. The rural housing area also hosted substantial numbers of Aedes mosquitoes. Conversely, the highest Aedes species richness was observed in the rainforest where the preference of Aedes females to feed on humans was very little. As a result, the polyculture and the rural areas increased Aedes vectors’ biting rates by 34.6 and 7.2 times compared with the original rainforest, respectively.
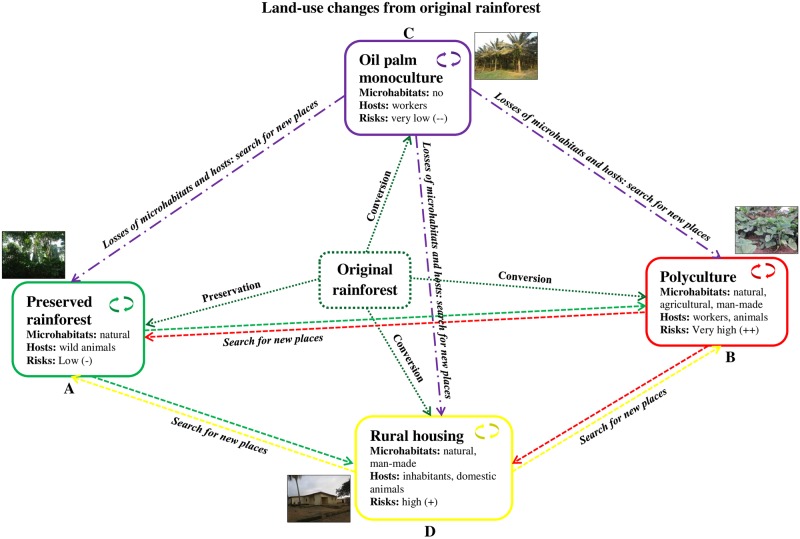
Fig 7. Effects of land-use changes on distribution of Aedes mosquitoes and arboviruses’ transmission risks in oil palm-dominated landscapes in southeastern Côte d’Ivoire.
Human-induced land-use changes into the original tropical rainforests for their conversion in large industrial oil palm plantations have resulted in changes in land-covers creating four ecologically distinct macrohabitats: preserved rainforest (A), polyculture (B), oil palm monoculture (C), and rural housing area (D). The conversion of the original rainforests into large oil palm monoculture has led to the losses of the microhabitats and hosts of forest-dwelling Aedes mosquitoes thus increasing ecological pressure for searching alternative microhabitats and hosts in the three other macrohabitats, preserved rainforest, polyculture, and rural housing areas. Aedes mosquitoes found new microhabitats as anthropogenic containers abundantly encountered in the rural housing area and polyculture where humans (inhabitants and workers) were usually present thus resulting in higher abundance of vectors and high-risks of arboviruses’ transmission in these areas. In contrast, the arboviral transmission risks were very low in the oil palm monoculture due to the lack Aedes mosquitoes, and low in the rainforest due to the low anthropophagy of forest-dwelling Aedes species.
The following points are offered for discussion. First, holistically, our study yielded high species richness and high numbers of mosquitoes, with the dominance of medically important Aedes species in areas that have undergone anthropogenic land-use changes due to oil palm plantations. Several Aedes species (e.g., Ae. aegypti, Ae. africanus, Ae. furcifer, Ae. luteocephalus, Ae. opok, and Ae. vittatus) are known vectors for viral infections, including yellow fever, dengue, chikungunya, and Zika in Côte d’Ivoire [15, 16] and Senegal [7, 28, 29]. The high Aedes species diversity is consistent with previous studies conducted in distinct landscapes in rural areas of Senegal [7, 28, 29]. This could be due to the heterogeneity of landscapes (rainforest, polyculture, oil palm monoculture, and housing areas) that possibly provide a wide range of larval habitats, resting and mating places, and nectar and blood-food sources [7, 28].
Second, we used diverse sampling methods (i.e., bamboo-ovitraps, metallic-ovitraps, larval surveys, and human-baited double-net traps) targeting different development stages (i.e., egg, larvae, pupae, and adults) of Aedes mosquitoes during the dry and rainy seasons. Due to logistic limitations, our study only focused on Aedes mosquito dwelling up to 2 m above ground, and the anthropophagic populations that are active between 04:00 a.m. and 08:00 p.m. Some canopy-dweller [29], nighttime-biter [30, 31], and zoophilic [32] Aedes species were probably missed by the current sampling techniques. A vertical stratification study, circadian (24-hour period) sampling design, and animal-baited trapping could possibly provide deeper insight into the ecology of Aedes mosquitoes living in the canopies, darkness-dependent biting, and zoophagic behaviors, respectively.
Third, from a reductionist view, we found compositional differences in Aedes species among the landscape covers, suggesting ecologically filtering effects of land-use changes on Aedes mosquito communities, as observed in arthropods [33]. Bernues-Baneres et al. [34] have observed variations in faunistic diversity of mosquitoes according to the typology of land-covers in Spain. Because of their high sensitivity to environmental changes, mosquitoes have been suggested as bio-indicators of forest degradation level in Brazil [35]. In our study area, Aedes species were absent in oil palm monocultures, while they were abundantly present in polyculture environment and rural housing areas. This may suggest the displacement of Aedes mosquitoes vectors primarily from the forested areas transformed into oil palm plantations toward preserved rainforest, the polyculture, and rural housing areas for searching alternative breeding sites [36, 37], and blood-food sources [21]. In the first possible scenario, under the increased pressure exerted by Aedes mosquito populations, they become highly abundant during the rainy season on the hosts and breeding sites available in the preserved rainforest. The ecologic Aedes-rainforest balance is probably interrupted, and hence, leading to the diffusion of forest-dwelling anthropozoophilic Aedes species toward the rural human-inhabited areas. Similar findings have been reported in rural areas of Senegal, where Aedes vectors have invaded villages from surrounding landscapes and the risk of arboviral infection became highest at the edges of the villages [29]. These wild Aedes species that have both horizontal/oral and vertical/transovarial transmission competences for arbovirus probably transmit viruses that they have previously taken from forest-dwelling animals to villagers thus linking the jungle/sylvatic cycles to emergence/rural cycles [12, 20, 21]. Alternatively, the second scenario is that people working in polyculture could be bitten by a virus-infected Aedes mosquito, which might carry the virus to rural housing areas that are already colonized by potential competent vectors [20]. These competent vectors may disseminate viruses among the populations. Both scenarios are expected to increase yellow fever and dengue emergence and re-emergence risks, especially since they do not exclude mutually [20], because people live in close proximity to wildlife.
Fourth, Aedes mosquitoes still appear to show diverse and atypical breeding patterns across macro- and microhabitats leading to horizontal stratification among species with lack of Aedes mosquitoes in the oil palm monocultures and strong colonization of the other macrohabitats (i.e., rainforest, polyculture, and rural housing areas). These findings corroborate previous results showing that land-use changes affect the ecology of immature Aedes mosquitoes in the United States of America [2] and in rural areas of Senegal [7]. Ferraguti et al. [3] have reported that mosquito richness is higher in natural areas compared to anthropized areas. Polyculture areas have more positive effects on the abundance and species richness of terrestrial arthropod than monocultures in oil palm production landscapes in Peninsular Malaysia [5, 38]. Indeed, oil palm plantations alter ecosystem functioning [39], and reduce species richness and abundance compared with forested areas [40] due to the losses of habitats and hosts [5, 6]. Moreover, the drastic decline in Aedes species in oil palm monocultures could probably be exacerbated by multiple and intense uses of chemical products such as insecticides and herbicides for crop protection [19]. Aedes species have adapted alternatively their oviposition and blood-feeding behaviors to anthropogenic habitats and hosts that are available in the polyculture and rural housing areas [7]. Polyculture still had naturally-occurring microhabitats (i.e., tree and bamboo holes), developed multiple agriculturally-occurring microhabitats (i.e., crop fruit husks, flower, sheathing leaf axils, and cultivated plant holes), and received several man-made containers (i.e., crop collection containers, and discarded containers). Indeed, people discarded high numbers of containers such as old tires, parts of vehicles and machines in the maintenance of oil palm plantations, tarps, cans, and other worn items in surrounding polycultures since people live in close proximity to their smallholdings. Additionally, urbanized housing areas are incriminated to replace natural microhabitats (e.g., tree holes, bamboo) by artificial microhabitats (e.g., tires, discarded containers, and water storage containers), increase in the number of microhabitats expose breeding sites to a higher magnitude of solar radiation and enhance the population size of Aedes mosquitoes [41]. In such areas, containers used to provide water for poultry husbandry during the dry season were found to be highly infested with Ae. aegypti larvae, as observed in bird cages in Malaysia [9]. Anthropogenic environments also act as limiting factors for Aedes mosquito predators (e.g., Eretmapodites spp. and Toxorhynchites spp.) [4]. Hence, Aedes species that uniquely oviposit in natural containers (e.g., tree holes), may lay more fragile and desiccation-sensitive eggs. Rainwater is needed for hatching eggs, thus influencing oviposition behaviors [4, 7]. Of note, Aedes species need microbial inputs from predation as food sources for their offspring [2], and wild animal hosts as blood-meals for the adult females [32]. These features probably restricted certain Aedes species to the rainforest [4, 7]. Indeed, the specialists that are strictly ecologic demanding remain confined to particular ecotopes (e.g., rainforest), while the generalists (i.e., Ae. aegypti) might spread and colonize more diverse environments [4, 7]. However, Ae. aegypti mosquitoes seem to prefer anthropically altered areas rather than natural landscapes [4]. All these biotic and abiotic factors interact with rainfalls that habitually ensure the flooding of breeding sites to induce significant variations in the abundance and distribution of Aedes mosquito species, all of which may link the different possible arbovirus transmission cycles and increase exposure of human populations to arbovirus-risks [12].
Finally, Aedes mosquito females seem to exhibit similarities and dissimilarities in host-seeking behaviors between the types of land-cover that acted as a series of ecologic filters [33]. Aedes mosquitoes were seeking for humans in every land-cover type studied here, except for the oil palm monoculture. Moreover, the vectors displayed low preference for feeding on humans in the rainforest. Host-seeking activities were higher in both polyculture and rural housing areas, and biting activity showed one peak in the morning and one peak in the evening. However, biting cycles were interrupted between 10:00 a.m. and 02:00 p.m. in the rural housing areas and maintained in the polyculture. The unexpected ecologic variations in Aedes biting behavior suggest a complex pattern of arbovirus transmission in the large-scale development of oil palm-planted landscapes. Such outstanding spillovers might be attributable to the adaptation of Aedes species to land-use patterns, and human activities and movements. In fact, the absence of aggressive Aedes females in oil palm monoculture could be explained by the losses of their habitats and animal hosts [6], while the disinterest of rainforest-dwelling vectors into feeding on humans could be due to their preference to feed on wild animals [32]. When the vector aggressiveness peaked, in the early morning and in the evening, humans are generally within housing areas suggesting that high exposures to arboviruses occur in the villages [21, 28]. The interruption of host-seeking activities of Aedes females coincided with the migration of workers to the industrial oil palm farming and other people to their own smallholdings. Such an accordance of malaria vector behaviors to human movements has been reported in rubber plantations in Thailand [42]. The gap observed in host-seeking activities also corresponded to the sunlight intensity in the rural housing areas that are directly exposed to solar radiation due to the lack of natural vegetation coverage. As observed in poikilothermic animals, including insects [43], Aedes host-seeking behavior was probably most affected by the sun in the housing area. Conversely, the continuous biting cycles of Aedes females in polyculture could be explained by the permanent presence of workers that may habitually serve as blood-food sources [42], and the shade provided by the abundance of vegetation coverage that probably reduces the negative effects of sunlight radiation on host-searching activities. The surprising darkness-biting activities could be interpreted as residual biting activities of Aedes mosquitoes that feed at night on wild animals [21, 29, 32]. The nocturne biting activities of the well-known daytime Aedes mosquitoes has been reported on Ae. aegypti in Côte d’Ivoire [30] and Ae. albopictus in Cameroon [31]. The extent of such atypical host-seeking activity rhythm observed in our study region could have important epidemiologic implications, and needs to be analyzed in greater depth, over longer times and larger scales.
We conclude that in the southeastern part of Côte d’Ivoire, agricultural land-use has changed as a result of transforming rainforest into oil palm monocultures, which significantly influences the composition, distribution, oviposition patterns, and host-seeking behavior of Aedes mosquito species. In turn, there is a lack of Aedes mosquitoes in oil palm monocultures and a strong colonization of polyculture and rural housing areas. Hence, humans are increasingly exposed to Aedes bites and arbovirus risk around their homes and farming plots. The polyculture and the rural housing ecotopes thus represent priority areas for vector control and surveillance. In oil palm-planted areas, arboviral disease control strategy should encompass integrated approaches, including landscape ecology and epidemiology, and ecotope-based vector control.
Supporting information
Potential habitats of Aedes mosquitoes are stratified into two habitat types: macrohabitats (A-D), and microhabitats (E-P). The habitat type often reflects the name of the habitats and the categories include habitats that provide comparable Aedes mosquito habitats. The macrohabitats are divided into four ecological blocks: A: Rainforest that was preserved dense forest hosting several plant species of trees, creepers, and bamboo, and animals; B: Polyculture that covered a mixture of cultivated plants such as oil palm tree, rubber, taro, banana, coconuts, and native trees; C: Oil palm monoculture that was covered uniquely with industrial oil palm trees; and D: rural-housing areas that are characterized by human-inhabited space. The microhabitats (E-P) were summarized into: Naturally-occurring microhabitats (E-H) that comprised E: Natural tree hole, F: Bamboo hole, G: Natural plant leaf, and H: Other natural microhabitats; Agriculturally-occurring microhabitats (I-L) that were composed of: I: Crop fruit husk, J: Crop flower, K: Crop leaf, and L: Cultivated plant hole; and Man-made microhabitats (M-P) that represented: M: Crop collection container, N: Husbandry watering container, O: Discarded container, and P: Household water container. Containers were categorized as “other natural microhabitats”, such as snail shells and rock holes.
(TIF)
A: Bamboo-ovitrap, B: Metallic-ovitrap, C: Larval survey, D: Human-baited double net trap.
(TIF)
Error bars represent the standard error (SE). NOM: naturally-occurring microhabitat, AOM: agriculturally-occurring microhabitat, MMM: man-made microhabitat.
(TIF)
Error bars represent the standard error (SE). NOM: naturally-occurring microhabitat, AOM: agriculturally-occurring microhabitat, MMM: man-made microhabitat.
(TIF)
Error bars represent the standard error (SE).
(TIF)
Error bars represent the standard error (SE).
(TIF)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Result are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
—: very low risk,—: low risk, +: high risk, ++: very high risk; %: percentage; SE: standard error of the mean. Host-seeking activity is expressed as the mean numbers of Aedes females collected per human-baited double-net trap. The unit of host-seeing activity is female/person/day. Overall, there was a lack of Aedes microhabitats and species in the oil palm monoculture resulting in very low arbovirus risk. In contrast, the highest abundance of Aedes mosquitoes was found in the polyculture where arbovirus risk is expected to be very high. The highest species richness was observed in the rainforest where the preference of Aedes females to feed on humans was low. The rural housing areas and the whole study area hosted substantial numbers of Aedes mosquitoes and arbovirus risk is expected to be high in rural housing area and moderate in the whole study area.
(DOCX)
Acknowledgments
We are grateful to the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (Abidjan, Côte d’Ivoire), the Swiss Tropical and Public Health Institute (Basel, Switzerland); the Swiss government, through the Federal Commission for Scholarships for Foreign Students (Bern, Switzerland; no 2014.0567), which funded the study and supported its execution. The authors would also like to extend their thanks to PALMCI staff, health authorities, local authorities, and residents in the study areas and the mosquito collection teams.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was funded by the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Côte d’Ivoire; Swiss Tropical and Public Health Institute, Basel, Switzerland; Swiss Government, through the Federal Commission for Scholarships for Foreign Students (FCS), Bern, Switzerland (no 2014.0567). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitation for their control. Emerg Microbes Infect. 2015;4: e18 doi: 10.1038/emi.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leisnham P, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. Ecohealth. 2012;9: 217–228. doi: 10.1007/s10393-012-0773-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraguti M, Puente JM, Roiz D, Ruiz S, Soriguer R, Figuerola J. Effects of landscape anthropization on mosquito community composition and abundance. Sci Rep. 2016;6: 29002 doi: 10.1038/srep29002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahouli JBZ, Utzinger J, Adja MA, Müller P, Malone D, Tano Y, et al. Oviposition ecology and species composition of Aedes spp. and Aedes aegypti dynamics in variously urbanized settings in arbovirus foci in southeastern Côte d’Ivoire. Parasit Vectors. 2016;9: 523 doi: 10.1186/s13071-016-1778-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghazali A, Asmah S, Syafiq M, Yahya MS, Aziz N, Peng T, et al. Effects of monoculture and polyculture farming in oil palm smallholdings on terrestrial arthropod diversity. J Asia Pac Entomol. 2016;19: 415–421. [Google Scholar]
- 6.Vijay V, Pimm SL, Jenkins CN, Smith SJ. The impacts of oil palm on the recent deforestation and biodiversity loss. PLoS One. 2016;7: e0159668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diallo D, Diagne C, Hanley KA, Sall AA, Buenemann M, Ba Y, et al. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern Senegal. Parasit Vectors. 2012;5: 286 doi: 10.1186/1756-3305-5-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangena JAA, Thammavong P, Wilson AL, Brey PT, Lindsay SW. Risk and control of mosquito-borne diseases in southeast Asian rubber plantations. Trends Parasitol. 2016;32: 402–415. doi: 10.1016/j.pt.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 9.Dieng H, Hassan RB, Hassan AA, Ghani IA, Abang TB, Satho T, et al. Occurrence of a mosquito vector in bird houses: development consequences and potential epidemiological implications. Acta Trop. 2015;145: 68–78. doi: 10.1016/j.actatropica.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Rochlin I, Faraji A, Ninivaggi DV, Barker CM, Kilpatrick AM. Anthropogenic impacts on mosquito populations in North America over the past century. Nat Commun. 2016;7: 13604 doi: 10.1038/ncomms13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasilikas N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9: 532–541. doi: 10.1038/nrmicro2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YJS, Higgs S, Horne KMcE, Vanlandingham DL. Flavivirus-mosquito interactions. Viruses. 2014;6: 4703–4730. doi: 10.3390/v6114703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiger DBM, Ritchie SA, Laurance SGW. Mosquito communities and disease risk influenced by land use change and seasonality in the Australian tropics. Parasit Vectors. 2016;9: 387 doi: 10.1186/s13071-016-1675-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komono BD. La fièvre jaune en Côte d’Ivoire. Historique, actualité et perspectives de recherche pour la lutte. Med Afr Noire. 2012;5910: 459–469. [Google Scholar]
- 15.Cordellier R, Bouchite B, Roche JC, Monteny N, Diaco B, Akoliba P. Circulation selvatique du virus dengue 2 en 1980, dans les savanes sub-soudaniennes de Côte d’Ivoire. Données entomologiques et considérations épidémiologiques. Cah ORSTOM Ser Ent. Med Parasitol. 1983;21: 165–179. [Google Scholar]
- 16.Akoua-Koffi C, Diarrassouba S, Bénié VB, Ngbichi JM, Bozoua T, Bosson A, et al. Investigation autour d’un cas mortel de fièvre jaune en Côte d’Ivoire en 1999. Bull Soc Pathol Exot. 2001;94: 227–230. [PubMed] [Google Scholar]
- 17.Palmafrique. Palm oil in the Ivoirian economy. http://www.palmafrique.com/en/palm-oil-in-the-ivorian-economy/; accessed on: 5 November 2017.
- 18.N’go AY, Ama-Abina JT, Kouadio AZ, Kouassi HK, Savané I. Environmental change in agricultural land in southwest Côte d’Ivoire: driving forces and impacts. J Environ Prot. 2013;4: 1373–1382. [Google Scholar]
- 19.Sadia-Kacou CMA, Alou LPA, Edi AVC, Yobo CM, Adja MA, Ouattara AF, et al. Presence of susceptible wild strains of Anopheles gambiae in large industrial palm farm located in Aboisso, south-eastern of Côte d’Ivoire. Malar J. 2017;16: 157 doi: 10.1186/s12936-017-1804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordellier R, Germain M, Hervy JP, Mouchet J. Guide pratique pour l’étude des vecteurs de fièvre jaune en Afrique et méthodes de lutte. 33rd ed Paris: ORSTOM; 1977. [Google Scholar]
- 21.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc’h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010;10: 249–258. doi: 10.1089/vbz.2009.0026 [DOI] [PubMed] [Google Scholar]
- 22.Tangena J-AA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS One. 2015;10: e0138735 doi: 10.1371/journal.pone.0138735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harbach R. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 2007;1668: 1–766. [Google Scholar]
- 24.van Strien AJ, Soldaat LL, Greory RD. Desirable mathematical properties of indicators for biodiversity change. Ecol Indic. 2012;14: 202–208. [Google Scholar]
- 25.Protectus US. Student handout 1A: how to calculate biodiversity. http://entnemdept.ifas.ufl.edu/hodges/ProtectUs/lp_webfolder/9_12_grade/Student_Handout_1A.pdf; assessed on: 5 November 2017.
- 26.Repeated measures analysis with Stata. http://www.ats.ucla.edu/stat/stata/seminars/repeated_measures/repeated_measures_analysis_stata.htm; accessed on: 5 November 2017.
- 27.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29: 460–468. doi: 10.1016/j.pt.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diallo D, Sall AA, Buenemann M, Chen R, Faye O, Diagne CT, et al. Landscape ecology of sylvatic Chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis. 2012;6: e1649 doi: 10.1371/journal.pntd.0001649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diallo D, Sall AA, Diagne CT, Faye O, Fay O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9: e109442 doi: 10.1371/journal.pone.0109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diarrassouba S, Dossou-Yovo J. Rythme d’activité atypique chez Aedes aegypti en zone de savane sub-soudanienne de Côte d’Ivoire. Entomol. Med. 1997;5: 361–363. [PubMed] [Google Scholar]
- 31.Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroun. Parasit Vectors. 2012;5: 57 doi: 10.1186/1756-3305-5-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diallo D, Chen R, Diagne CT, Ba Y, Dia I, Sall AA, et al. Bloodfeeding patterns of sylvatic arbovirus vectors in southeaster Senegal. Trans R Soc Trop Med Hyg. 2013;107: 200–203. doi: 10.1093/trstmh/trs095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkhofer K, Gossner MM, Diekötter T, Drees C, Ferlian O, Maruan M, et al. Land-use type and intensity differentially filter traits in above- and below-ground arthropod communities. J Anim Ecol. 2017;86: 551–520. [DOI] [PubMed] [Google Scholar]
- 34.Bernues-Baneres A, Jimerez-Peydro R. Diversity of mosquitoes (Diptera Culicidae) in protected natural parks form Valencia Autonomous Region (eastern Spain). Biodivers J. 2013;4: 335–342. [Google Scholar]
- 35.Dorville LFM. Mosquitoes as bioindicators of forest degradation in southeastern Brazil, a statistical evaluation of published data in the literature. Stud Neotrop Fauna Environ. 2010;31: 68–78. [Google Scholar]
- 36.Abreu FVS, Morais MM, Ribeiro SP, Eiras AE. Influence of breeding site availability on the oviposition behavior of Aedes aegypti. Mem Inst Oswaldo Cruz. 2015;110: 669–676. doi: 10.1590/0074-02760140490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong J, Morrison AC, Stoddard ST, Astete H, Chu YC, Baseer I, et al. Linking oviposition site choice to offspring fitness in Aedes aegypti: consequences for targeted larval control of dengue vectors. PLoS Negl Trop Dis. 2012;6: e1632 doi: 10.1371/journal.pntd.0001632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syafiq M, Atiqah ARN, Ghazali A, Asmah S, Yahya MS, Aziz N, et al. Responses of tropical fruit bats to monoculture and polyculture farming in oil palm smallholdings. Acta Oecol. 2016;74: 11–18. [Google Scholar]
- 39.Dislich C, Keyel AC, Salecker J, Kisel Y, Meyer KM, Auliya M, et al. A review of the ecosystem functions in oil palm plantations, using forests as a reference. Biol Rev Camb Philos Soc. 2017;92: 1539–1569. doi: 10.1111/brv.12295 [DOI] [PubMed] [Google Scholar]
- 40.Savilaakso S, Garcia C, Garcia-Ulloa J, Ghazoul J, Groom M, Guariguata MR, et al. Systematic review of effects on biodiversity form oil palm production. Environ Evid. 2014;3: 4. [Google Scholar]
- 41.Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in south-eastern Côte d’Ivoire. PLoS Negl Trop Dis. 2017;11: e0005751 doi: 10.1371/journal.pntd.0005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaewwaen W, Bhumiratana. Landscape ecology and epidemiology of malaria associated with rubber plantations in Thailand: integrated approaches to malaria ecotyping. Interdiscip Perspect Infect Dis. 2015;9009106: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barton M, Porter W, Kearney M. Behavioural thermoregulation and the relative roles of convection and radiation in basking butterfly. J Therm Biol. 2014;41: 65–71. doi: 10.1016/j.jtherbio.2014.02.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential habitats of Aedes mosquitoes are stratified into two habitat types: macrohabitats (A-D), and microhabitats (E-P). The habitat type often reflects the name of the habitats and the categories include habitats that provide comparable Aedes mosquito habitats. The macrohabitats are divided into four ecological blocks: A: Rainforest that was preserved dense forest hosting several plant species of trees, creepers, and bamboo, and animals; B: Polyculture that covered a mixture of cultivated plants such as oil palm tree, rubber, taro, banana, coconuts, and native trees; C: Oil palm monoculture that was covered uniquely with industrial oil palm trees; and D: rural-housing areas that are characterized by human-inhabited space. The microhabitats (E-P) were summarized into: Naturally-occurring microhabitats (E-H) that comprised E: Natural tree hole, F: Bamboo hole, G: Natural plant leaf, and H: Other natural microhabitats; Agriculturally-occurring microhabitats (I-L) that were composed of: I: Crop fruit husk, J: Crop flower, K: Crop leaf, and L: Cultivated plant hole; and Man-made microhabitats (M-P) that represented: M: Crop collection container, N: Husbandry watering container, O: Discarded container, and P: Household water container. Containers were categorized as “other natural microhabitats”, such as snail shells and rock holes.
(TIF)
A: Bamboo-ovitrap, B: Metallic-ovitrap, C: Larval survey, D: Human-baited double net trap.
(TIF)
Error bars represent the standard error (SE). NOM: naturally-occurring microhabitat, AOM: agriculturally-occurring microhabitat, MMM: man-made microhabitat.
(TIF)
Error bars represent the standard error (SE). NOM: naturally-occurring microhabitat, AOM: agriculturally-occurring microhabitat, MMM: man-made microhabitat.
(TIF)
Error bars represent the standard error (SE).
(TIF)
Error bars represent the standard error (SE).
(TIF)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Result are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
Results are the outputs of the generalized linear mixed model (GLMM) procedures. Results are considered significant for p-values <0.05.
(DOCX)
—: very low risk,—: low risk, +: high risk, ++: very high risk; %: percentage; SE: standard error of the mean. Host-seeking activity is expressed as the mean numbers of Aedes females collected per human-baited double-net trap. The unit of host-seeing activity is female/person/day. Overall, there was a lack of Aedes microhabitats and species in the oil palm monoculture resulting in very low arbovirus risk. In contrast, the highest abundance of Aedes mosquitoes was found in the polyculture where arbovirus risk is expected to be very high. The highest species richness was observed in the rainforest where the preference of Aedes females to feed on humans was low. The rural housing areas and the whole study area hosted substantial numbers of Aedes mosquitoes and arbovirus risk is expected to be high in rural housing area and moderate in the whole study area.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.