Abstract
Transient receptor potential channel M5 (Trpm5)-expressing cells, such as sweet, umami, and bitter taste cells in the oropharyngeal epithelium, solitary chemosensory cells in the nasal respiratory epithelium, and tuft cells in the small intestine, that express taste-related genes function as chemosensory cells. Previous studies demonstrated that Skn-1a/Pou2f3, a POU homeodomain transcription factor is expressed in these Trpm5-expressing chemosensory cells, and is necessary for their generation. Trpm5-expressing cells have recently been found in trachea, auditory tube, urethra, thymus, pancreatic duct, stomach, and large intestine. They are considered to be involved in protective responses to potential hazardous compounds as Skn-1a-dependent bitter taste cells, respiratory solitary chemosensory cells, and intestinal tuft cells are. In this study, we examined the expression and function of Skn-1a/Pou2f3 in Trpm5-expressing cells in trachea, auditory tube, urethra, thymus, pancreatic duct, stomach, and large intestine. Skn-1a/Pou2f3 is expressed in a majority of Trpm5-expressing cells in all tissues examined. In Skn-1a/Pou2f3-deficient mice, the expression of Trpm5 as well as marker genes for Trpm5-expressing cells were absent in all tested tissues. Immunohistochemical analyses demonstrated that two types of microvillous cells exist in trachea, urethra, and thymus, Trpm5-positive and Trpm5-negative cells. In Skn-1a/Pou2f3-deficient mice, a considerable proportion of Trpm5-negative and villin-positive microvillous cells remained present in these tissues. Thus, we propose that Skn-1a/Pou2f3 is the master regulator for the generation of the Trpm5-expressing microvillous cells in multiple tissues.
Introduction
The transient receptor potential channel M5 (Trpm5) was first identified in sweet, bitter, and umami taste cells [1], and plays a critical role in taste signaling as a non-elective monovalent cation channel [2–4]. Interestingly, Trpm5-expressing cells have been also identified in several specialized cells in the extraoral tissues. For example, solitary chemosensory cells in the nasal respiratory epithelium and brush cells in the tracheal epithelium characterized by an apical tuft of microvilli exhibit taste cell-like molecular characteristics by expressing taste receptors and their downstream signaling molecules such as Gnat3, Plcb2, and Trpm5 [5–8]. These cells respond to classical bitter substances and bacterial signaling molecules (acyl-homoserine lactones), inducing protective respiratory reflexes, a neurogenic local proinflammatory response, and reduction of chemical access to the vomeronasal organ [7–12]. In the urethral epithelium, brush cells were identified to express Trpm5 as well as canonical taste receptors and taste signaling molecules. Instillation of denatonium into the urethral lumen of rats induced contraction of bladder detrusor muscle by activating Trpm5-expressing urethral brush cells to exclude noxious substrates [13]. Recent works showing that intestinal tuft cells expressing Trpm5 release interleukin-25 to initiate type II immune response and clear the gut from the parasites such as helminthes [5, 14–16] and protozoa [16] further attest that Trpm5-expressing cells function as a gatekeeper of biophylactic reactions.
Skn-1a, a POU homeodomain transcription factor also known as Pou2f3, is involved in the generation of sweet, umami, and bitter taste cells [17]. Its loss-of-function mutation expanded the sour taste cells population at the expense of sweet, umami, and bitter taste cells. Thus, Skn-1a functions as an early determinant of the differentiation to these taste cells. In addition to taste system, we previously demonstrated that Skn-1a is expressed in solitary chemosensory cells in the nasal respiratory epithelium, Trpm5-expressing microvillous cells in the main olfactory epithelium, and tuft cells in the intestine and is necessary for their generation [15,18–20]. Although Trpm5-expressing cells were also found in auditory tube [21], thymic medulla [22], pancreatic duct [23,24], and stomach and large intestine [5] functions of these cells are not well understood. Given the similarity of molecular characteristics and cellular morphology among Trpm5-expressing cells in the multiple tissues, it is presumed that Trpm5-expressing cells function as chemosensory cells in multiple tissues. Because Skn-1a is involved in the generation of all Trpm5-expressing cells examined, we hypothesize that Skn-1a is a master regulator for the generation of Trpm5-expressing chemosensory cells.
Here, we examined the expression and function of Skn-1a in Trpm5-expressing cells in multiple tissues using Skn-1a-deficient mice. Our results reveal that Skn-1a is expressed in the Trpm5-expressing cells in all tissues examined and is essential for generation of these cells.
Materials and methods
Animals
Skn-1a-deficient mice (Skn-1a-/-) were generated as described elsewhere [17]. All mice used in this study were C57BL/6 background, and 7–10 weeks old mutant and wild-type mice of either sex were used. All mouse studies were approved by the institutional animal experiment committees of the Tokyo Institute of Technology, and were performed in accordance with institutional and governmental guidelines.
In situ hybridization
Probes for Skn-1a, Trpm5, Plcb2, Gnat3, Tas1r3, Tas2r105, Tas2r108, Tas2r131, and Dclk1 were prepared as described previously [15,19,25]. Single-color in situ hybridization and two-color in situ hybridization were carried out as described previously [6,19]. Briefly, tissues were dissected from mice euthanized by CO2 inhalation for fresh-frozen preparation or from mice euthanized by isoflurane inhalation and transcardially perfused with 4% paraformaldehyde in PBS for fixative preparation, and were embedded in FSC22 Frozen Section Media (Leica). For single-color in situ hybridization, fresh-frozen tissue samples were sectioned at 8 μm thickness. Sections were fixed with 4% paraformaldehyde in PBS, treated with 0.1% diethylpyrocarbonate, prehybridized with salmon sperm DNA for 2 h at 58°C, and hybridized with antisense riboprobes for 40 h at 58°C. After hybridization, the sections were washed in 5× and 0.2× saline sodium citrate at hybridized temperature, and blocked in blocking solution containing 1.0% blocking reagent (Roche Diagnostics). Sections were then incubated with alkaline phosphatase-conjugated anti-digoxigenin antibody (1:500, Roche Diagnostics). After washing, signals were visualized with 4-nitrotetrazolium blue chloride / 5-bromo-4-chloro-3-indolyl-phosphate (Roche Diagnostics) at room temperature. For two-color in situ hybridization, paraformaldehyde-fixed frozen tissue samples were sectioned at 10–12 μm thickness. Sections were treated with proteinase K (3 μg/ml, Invitrogen) for 10 min at room temperature, postfixed with 4% paraformaldehyde, acetylated with acetic anhydride, and hybridized with antisense riboprobes for 40 h at 58°C. After hybridization, the sections were washed in 2×, 0.2×, 0.1× saline-sodium citrate at 58°C and blocked in blocking solution containing 0.5% blocking reagent (Roche Diagnostics). For fluorescent double labeling, the tyramide signal amplification dinitrophenyl system (PerkinElmer) was used [19]. The images were taken on an Olympus BX51 microscope with a DP71 digital CCD camera for bright-field images, and a Leica SPE confocal microscope for fluorescent images.
Immunohistochemistry
Immunohistochemistry was performed according to a previously described method using cryosections of 10 μm thickness [19]. Tissues were dissected from mice anesthetized by isoflurane inhalation and transcardially perfused with 4% paraformaldehyde in PBS for fixative preparation, and were embedded in FSC22 Frozen Section Media (Leica). The following primary antibodies and dilutions were used: rabbit anti-Skn-1a antibody (1:500; #sc-330, Santa Cruz Biotechnology), goat anti-villin antibody (1:500; #sc-7672, Santa Cruz Biotechnology), rabbit anti-Trpm5 antibody (1:5000; #ACC-045, Alomone Labs), goat anti-ChAT antibody (1:100; #AP144P, Millipore). The following appropriate secondary antibodies were used: Alexa-488-conjugated donkey anti-rabbit IgG antibody (1:500; #A21206, Invitrogen), and Alexa-555-conjucated donkey anti-goat IgG antibody (1:500; #A11056, Invitrogen), biotin-conjugated goat anti-rabbit IgG antibody (1:500; #BA-1000, Vector Laboratories), biotin-conjugated donkey anti-goat IgG antibody (1:500; #605-706-125, Rockland).
Prior to immunostaining, we performed antigen-retrieval pretreatments in Target Retrieval Solution, pH 9.0 (Dako) for 20 min at 80°C. Following antigen-retrieval, sections were rinsed in phosphate buffered saline with 0.01% tween 20 (PBST) and blocked in 5% skimmed milk (Megmilk Snow Brand Co., Ltd.) for 1 h at room temperature, and incubated with primary antibodies overnight at 4°C. For fluorescent double labeling, sections were washed in PBST and incubated with Alexa Fluor conjugated secondary antibodies for 1h at room temperature. The sections were coverslipped with Fluomount-G including DAPI for nuclear staining (Southern Biotechnology). The fluorescent images were taken on a Leica SPE confocal microscope. For 3,3’-diaminobenzidine (DAB)-chromogenic immunostaining with streptavidin-horse radish peroxidase, sections incubated with primary antibody overnight at 4°C were washed in PBST and incubated with biotin-conjugated secondary antibodies for 1h at room temperature. The sections were rinsed in PBST and incubated in ABC solution (Vectastain ABC elite kit, Vector Laboratories) for 30 minutes according to the manufacture’s instruction. After washing, signals were visualized with 0.05% DAB (Dojindo) and 0.01% H2O2 in PBS for 5 min at room temperature.
Reverse transcription PCR (RT-PCR)
RT-PCR was performed using tissues of wild-type and Skn-1a-/- mice. Trachea, thymus (one thymus lobe), urethra, auditory tube [21], and pancreatic duct were dissected from mice euthanized by CO2 inhalation and quickly frozen in liquid nitrogen. Total RNA was isolated from homogenized tissues individually using RNeasy mini kit (QIAGEN), and reverse transcribed using ThermoScript™ Reverse Transcriptase (Invitrogen) and oligo(dT)20 primer at 50°C for 120 min, and cDNA synthesis reaction was terminated by incubating at 85°C for 5min. Omission of reverse transcriptase during cDNA synthesis served as negative control. PCR was performed with Taq DNA polymerase (Takara) and primers: Tas1r3: 5’-catcccgtgcaacaggttc-3’ and 5’-ctggcactatagctgacctg-3’ (nucleotide 196–528, NCBI reference sequence NM031872), Tas2r105: 5’- gactggcttccttctcatcg -3’ and 5’- gcaaacaccccaagagaaaa -3’ (nucleotide 129–412, NM020501) [8], Tas2r108: 5’-tggatgcaaacagtctctgg-3’ and 5’-ggtgagggctgaaatcagaa-3’ (nucleotide 269–426, NM020502) [8], Tas2r131: 5’-gcagtatttataactggaatgctgg-3’ and 5’- aggcgctagttcttgtatggt-3’ (nucleotide 22–198, NM207030) [26], Gnat3: 5’-gtttgagcaaatcaactgccc-3’ and 5’-tcatgcattctgttcacctcc-3’ (nucleotide 71–843, NM_001081143) [26], Plcb2: 5’-aaagaagtgacagagccacag-3’ and 5’-ttctcctggaactgcttttcc-3’ (nucleotide 2755–3470, NM177568) [26], Trpm5: 5’-tcctgttcattgtgggagtcac-3’ and 5’-tggcgatcagaaggttcatg-3’ (nucleotide 2444–2926, NM020277) [27], GAPDH: 5’-accacagtccatgccatcac-3’ and 5’-atgtaggccatgaggtccac-3’ (nucleotide 520–986, NM008084) [27]. Conditions of cDNA amplification were 1 min at 98°C, followed by 25–40 cycles of 30 s at 98°C, 30 s at 58°C, 30 s at 72°C, and a final extension at 72°C for 5 min. GAPDH was used as a control for PCR.
Quantitative analyses
To quantify the number of villin- and Trpm5-positive cells, every 20th transverse section (10 μm thickness) throughout the trachea (24 sections from individual mice) was collected. Urethra was transversely sectioned from bladder side at 10 μm thickness, and every 20th section was collected, and 10 sections from individual mice were used for quantitative analyses. Thymus, small intestine, and large intestine were sectioned at 10 μm thickness, and 8 sections from individual mice were used. For quantitative analyses, sections were stained using the DAB-chromogenic method as described above, and the number of signals was counted. For thymus, the densities of villin- and Trpm5-positive cells were quantified by dividing the number of positive cells by the area of sections measured by using NIH Image J. Experiments were conducted using three mice at P50–64 of each genotype, and the populations were calculated as means ± SEM (standard error of the mean), and Student’s t-test was used to determine the statistical significance.
For other tissues of stomach, pancreatic duct, and auditory tube, the total number of Trpm5-positive cells was counted. Three sections of stomach (gastric corpus) were obtained from individual animals of each genotype (n = 4), eight sections of auditory tube were obtained from individual animals (4 wild-type and 3 Skn-1a-/- mice), and four sections of pancreatic duct were obtained from individual animals (3 wild-type and 4 Skn-1a-/- animals). These sections were stained using the fluorescent labeling method as described above.
Results
The expression of Skn-1a in tracheal brush cells
First, we examined whether Skn-1a is expressed in brush cells in the tracheal epithelium. These cells share a common gene expression pattern with solitary chemosensory cells, including taste receptor genes and taste signaling genes (Gnat3, Plcb2, and Trpm5), but they are distinct from solitary chemosensory cells by apical ultrastructure and connections to nerve fibers [5–8]. To examine whether Skn-1a is expressed in tracheal brush cells, we carried out double-label immunostaining against Skn-1a combined with villin, a marker for tracheal brush cells. All Skn-1a positive cells were immunoreactive to villin, but a subset of villin positive cells was not immunoreactive to Skn-1a (Fig 1A). Because tracheal brush cells were divided into two types based on the presence or absence of choline acetyltransferase (ChAT) and ChAT-positive brush cells express Trpm5 [8], we carried out two-color in situ hybridization using probes for Skn-1a and Trpm5 to determine which type(s) of brush cells Skn-1a is expressed in. The scattered signals of Skn-1a mRNA were observed in the tracheal epithelium and almost all signals of Skn-1a mRNA were co-labeled with Trpm5 mRNA (Fig 1B). Those results indicate that Skn-1a is expressed in Trpm5-positive tracheal brush cells, but not in Trpm5-negative brush cells.
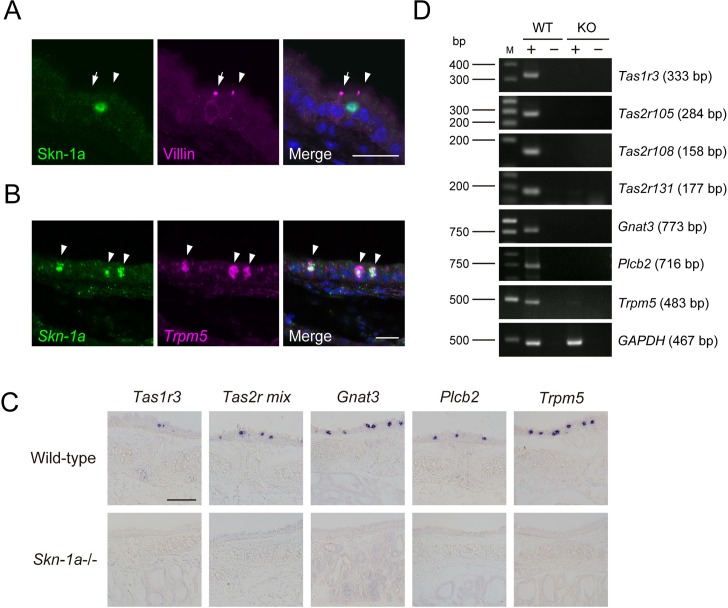
Fig 1. Effect of Skn-1a deficiency on the functional differentiation of Trpm5-expressing brush cells in trachea.
A: Skn-1a-expressing cells were characterized using immunohistochemistry with anti-Skn-1a and anti-villin antibodies. Villin-positive brush cells were divided into two types, Skn-1a-positive (arrowhead) and Skn-1a-negative brush cells (arrow). B: Skn-1a-expressing cells were characterized by two-color in situ hybridization with RNA probes for Skn-1a and Trpm5. Skn-1a-positive brush cells were co-labeled with Trpm5 riboprobe (arrowheads). Scale bars, 20 μm. C: The impact of Skn-1a deficiency on the functional differentiation of Trpm5/Skn1a-positive brush cells in the tracheal epithelium was examined by in situ hybridization using probes for taste signaling molecules of Tas1r3, Tas2rs (Tas2r105, Tas2r108, Tas2r131), Gnat3, Plcb2 and Trpm5. The mRNA signals of taste signaling molecules observed in wild-type mice were completely absent in the Skn-1a-/- mice, indicating that Skn-1a is required for the functional differentiation of Trpm5-positive brush cells. Scale bar, 100 μm. D: The expression of taste signaling molecules (Tas1r3, Tas2r105, Tas2r108, Tas2r131, Gnat3, Plcb2, and Trpm5) in wild-type (WT) and Skn-1a-/- (KO) trachea was examined by RT-PCR. The expression of taste signaling molecule genes was not detected in Skn-1a-/- trachea. A housekeeping gene, GAPDH was used as a positive control.
Impact of Skn-1a deficiency on brush cells in trachea
To investigate the function of Skn-1a in the Trpm5-expressing tracheal brush cells, we studied the impact of loss of Skn-1a function on the gene expression of the Trpm5-expressing tracheal brush cells that express the genes encoding taste receptors (Tas1r3 and Tas2rs) and taste signaling molecules (Gnat3, Plcb2 and Trpm5) [8,28]. In situ hybridization revealed that the signals for Tas1r3, Tas2rs (Tas2r105, Tas2r108 and Tas2r131), Gnat3, Plcb2 and Trpm5 mRNA detected in wild-type mice were absent in Skn-1a-/- trachea (Fig 1C). RT-PCR analyses demonstrate the loss of the expression of these taste-related marker genes in Skn-1a-/- mice (Fig 1D). These results indicate that Skn-1a is required for the generation and/or functional differentiation of Trpm5-positive brush cells.
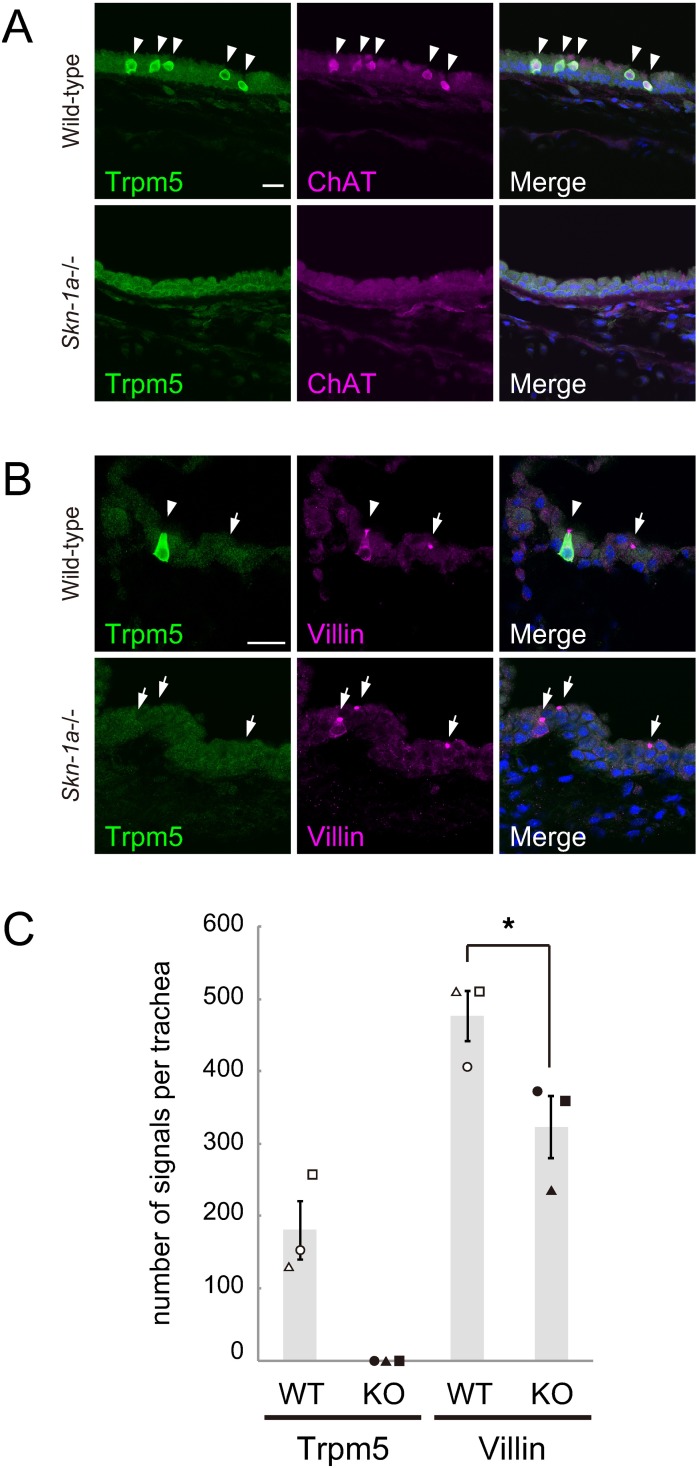
To elucidate whether Skn-1a is required for the generation of Trpm5-positive brush cells, we quantified the number of Trpm5 and villin-double positive cells in the tracheal epithelium of wild-type and Skn-1a-/- mice by immunohistochemistry. Double label immunostaining showed no immunoreactivity to Trpm5 and ChAT in Skn-1a-/- mice (Fig 2A), whereas immunoreactivity to villin remained present in Skn-1a-/- trachea (Fig 2B). Quantitative analysis of brush cell population showed that the numbers of Trpm5- and villin-positive cells were 180 ± 41 cells and 476 ± 35 cells (mean ± SEM, n = 3), respectively, in the wild-type mice. Trpm5-positive brush cells account for about 38% of the brush cells, and approximately 62% of brush cells belong to Trpm5-negative brush cells. In Skn-1a-/- mice, Trpm5-positive cells were absent and the number of villin-positive cells was 323 ± 43 cells (mean ± SEM, n = 3). Compared with wild-type mice, the number of villin-positive cells in Skn-1a-/- mice was significantly decreased by 32% (Student’s t-test, p < 0.05). Because this reduction is comparable to the percentage of Trpm5-positive brush cells in all brush cells of wild-type trachea, it is conceivable that Skn-1a knockout diminishes the population of Trpm5-positive brush cells in the tracheal epithelium and have little effect on Trpm5-negative brush cells. These results indicate that Skn-1a functions as the critical regulator for the generation of Trpm5-positive brush cells in trachea.
Fig 2. Skn-1a is required for the functional differentiation of Trpm5-positive tracheal brush cells.
A: Immunostaining of Trpm5 and ChAT on coronal sections of the trachea of wild-type and Skn-1a-/- mice. Trpm5-positive brush cells were ChAT positive in the wild-type trachea (arrows), whereas no immunoreactive signals for Trpm5 and ChAT was observed in the Skn-1a-/- trachea. B: Immunostaining of Trpm5 and villin on coronal sections of the trachea of wild-type and Skn-1a-/- mice. In wild-type mice, both Trpm5 and villin-double positive (arrowhead) and villin-single positive (arrow) brush cells were observed. In Skn-1a-/- mice, Trpm5-positive brush cells were absent and only villin-single positive brush cells (arrows) were observed. Scale bars, 20 μm. C: Quantification of the number of immunosignals for Trpm5 and villin in the wild-type and Skn-1a-/- tracheal epithelium. The signals of Trpm5 were completely absent in the Skn-1a-/- tracheal epithelium, and the number of villin-single positive cells was significantly decreased in Skn-1a-/- mice. Each symbol represents an individual mouse. The error bars represent the mean ± SEM (n = 3, *P < 0.05, Student’s t-test).
The expression and function of Skn-1a in tuft cells in digestive tracts
We next examined the expression and function of Skn-1a in Trpm5-expressing cells known as tuft cells in stomach and large intestine in addition to previously reported small intestine [15, 20]. Two-color in situ hybridization analysis revealed that Trpm5-expressing tuft cells express Skn-1a in stomach, small intestine, and large intestine (Fig 3A). Immunohistochemical analysis showed that immunoreactivity to Skn-1a was observed in villin-positive cells (Fig 3B). These results indicate that Skn-1a is expressed in Trpm5-positive tuft cells in these digestive tracts.
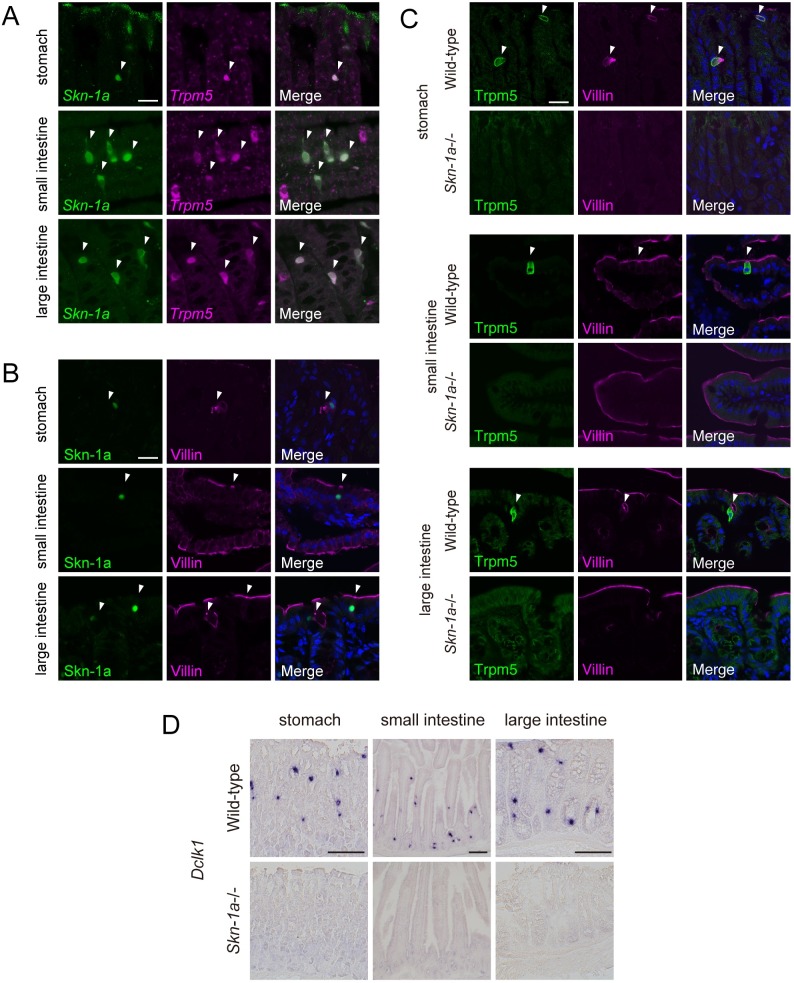
Fig 3. Impact of Skn-1a deficiency on Trpm5-positive tuft cells in digestive tracts.
A: Two-color in situ hybridization of Skn-1a (green) and Trpm5 (magenta) on sections of digestive tracts of stomach, small intestine, and large intestine of wild-type adult mice. The mRNA signals of Skn-1a were co-labeled with Trpm5 signals (arrowheads) in all tissues examined. Scale bar, 20 μm. B: Co-immunostaining using antibodies against Skn-1a (green) and villin (magenta) on sections of stomach, small intestine, and large intestine of wild-type adult mice. Skn-1a-positive cells were overlapped with villin-positive cells (arrowheads). Scale bar, 20 μm. C: The impact of Skn-1a deficiency on Trpm5-positive tuft cells was examined by double-label immunohistochemistry of Trpm5 and villin using sections of stomach, small intestine, and large intestine of wild-type (top) and Skn-1a-/- mice. Trpm5-positive cells were co-labeled with anti-villin antibody (arrowheads) in wild-type mice, whereas the expression of Trpm5 was abolished in all tested tissues in Skn-1a-/- mice. Scale bars, 20 μm. The immunoreactive signals for villin detected in wild-type mice (arrows) were not observed in Skn-1a-/- mice. D: The signals of intestinal tuft cells marker gene, Dclk1 mRNA were observed in wild-type digestive tracts, whereas no signals of Dclk1 mRNA were observed in Skn-1a-/-. Scale bars, 100 μm.
To investigate the function of Skn-1a, the impact of loss of Skn-1a function on Trpm5-positive tuft cells was examined by immunohistochemistry using antibodies against Trpm5 and villin. Trpm5 and villin-double positive tuft cells observed in wild-type mice were absent in Skn-1a-/- mice (Fig 3C). The numbers of Trpm5 and villin-double positive tuft cells are as follows; stomach: 30 ± 4.2 cells in wild-type (mean ± SEM, n = 4), 0 in Skn-1a-/- (n = 4), small intestine: 563 ± 18.9 cells in wild-type (n = 3), 0 in Skn-1a-/- (n = 3), large intestine: 199 ± 2.72 cells in wild-type (n = 3), 0 in Skn-1a-/- (n = 3). To further confirm the loss of tuft cells in digestive tracts, we examined the expression of a tuft cell marker gene, Doublecortin-like kinase 1 (Dclk1) [29–31]. In situ hybridization analysis showed that signals of Dclk1 detected in the wild-type digestive tract were completely abolished in Skn-1a-/- mice (Fig 3D).
Skn-1a acts as a master regulator for the generation of Trpm5-expressing cells
Because Skn-1a is a critical regulator for the generation of Trpm5-positive chemosensory cells in the oropharyngeal epithelium (i.e., sweet, umami, and bitter taste receptor cells), in the nasal respiratory epithelium (i.e., solitary chemosensory cells), in the main olfactory epithelium (i.e., microvillous cells), in the tracheal epithelium (i.e., brush cells), and in the stomach and intestine (i.e., tuft cells), Skn-1a may also be involved in the generation of the Trpm5-expressing cells in other tissues. To test this hypothesis, we extended our expression and functional analyses of Skn-1a to the tissues where Trpm5-expressing cells have been found, including auditory tube [21], urethra [13], thymus [22], and pancreatic duct [23,24].
Two-color in situ hybridization analysis revealed that Skn-1a was expressed in almost all Trpm5-expressing cells in these tissues (Fig 4A). We also examined whether Trpm5-expressing cells in these tissues express villin, a marker for microvilli, that is expressed in taste receptor cells, solitary chemosensory cells, tuft cells, and brush cells. Immunosignals of Skn-1a were co-labeled with those of villin (Fig 4B). Most Skn-1a-positive cells were co-labeled with anti-villin antibody in auditory tube and pancreatic duct as well as in alimentary tract, whereas villin-single positive cells were present in urethra and thymus as well as tracheal epithelia (Fig 4B).
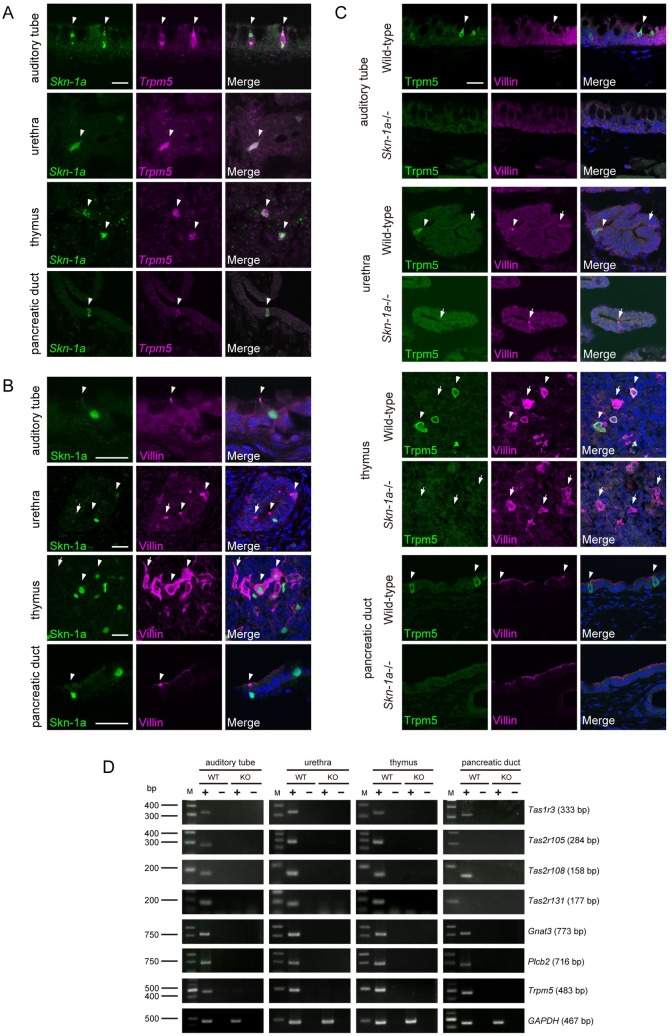
Fig 4. Loss of the Trpm5-positive chemosensory cells in multiple tissues in Skn-1a-/- mice.
A: Two-color in situ hybridization of Skn-1a (green) and Trpm5 (magenta) in various tissues of auditory tube, urethra, thymus, and pancreatic duct of wild-type adult mice. The mRNA signals of Skn-1a were co-labeled with Trpm5 signals (arrowheads) in all tissues examined. Scale bar, 20 μm. B: Co-immunostaining using antibodies against Skn-1a (green) and villin (magenta) on cryosections of auditory tube, urethra, thymus, and pancreatic duct of wild-type adult mice. Skn-1a-positive cells were overlapped with villin-positive cells (arrowheads). The arrows indicate Skn-1a negative and villin-single positive cells. Scale bar, 20 μm. C: Double-label immunohistochemistry of Trpm5 and villin on sections of auditory tube, urethra, thymus, and pancreatic duct of wild-type (top) and Skn-1a-/- mice (bottom) was carried out to examine the impact of Skn-1a deficiency on Trpm5-positive chemosensory cells. Trpm5-positive cells were co-labeled with anti-villin antibody (arrowheads) in wild-type mice, whereas the expression of Trpm5 was abolished in all tested tissues in Skn-1a-/- mice. The immunoreactive signals for villin were detected in Skn-1a-/- urethral epithelium and thymic medulla (arrows), but not in auditory tube and pancreatic duct. Scale bar, 20 μm. D: The expression of taste signaling molecules (Tas1r3, Tas2r105, Tas2r108, Tas2r131, Gnat3, Plcb2, and Trpm5) in auditory tube, urethra, thymus, and pancreatic duct was examined by RT-PCR in wild-type (WT) and Skn-1a-/- (KO) mice. The expression of taste signaling molecules observed in wilt-type mice was not detected in Skn-1a-/- mice. A housekeeping gene, GAPDH was used as a positive control.
To further test the hypothesis, we analyzed the effects of Skn-1a deficiency on Trpm5-expressing cells by immunohistochemistry. Immunostaining using antibodies against Trpm5 and villin showed that Trpm5 and villin-double positive cells were observed in wild-type mice, whereas those were completely absent in Skn-1a-/- mice (Fig 4C and S1 Fig) In urethra and thymus, where Skn-1a-negative and villin-single positive cells are located, only immunosignals to villin were detected in Skn-1a-/- mice. The numbers of Trpm5 and villin-double positive cells are as follows; auditory tube: 9.3 ± 4.3 cells in wild-type (mean ± SEM, n = 4), 0 in Skn-1a-/- (n = 3), pancreatic duct: 47 ± 2.6 cells in wild-type (n = 3), 0 in Skn-1a-/- (n = 4), and quantitative data of urethra and thymus are summarized in S1 Fig1. These results strongly support our hypothesis that Skn-1a act as a master regulator of Trpm5-expressing microvillous cells.
In several tissues, Trpm5-expressing microvillous cells express genes encoding taste receptors and taste signaling molecules [6–9,13,16,21,22]. We examined the expression of such taste-related genes of Tas1r3, Tas2r105, Tas2r108, Tas2r131, Gnat3, Plcb2, and Trpm5 in the auditory tube, urethra, thymus, and pancreatic duct of Skn-1a-/- mice by RT-PCR (Fig 4D). Taste receptor genes as well as taste signaling genes were expressed in these tissues of wild-type mice, but no taste-related genes were expressed in the Skn-1a-/- mice, suggesting that Trpm5-expresing microvillous cells function as chemosensory cells, and Skn-1a is required for the generation of functional Trpm5-expressing microvillous cells in these tissues.
Discussion
In this study, we demonstrated that Skn-1a is expressed in Trpm5-expressing cells in all tissues examined, i.e., in trachea, urethra, auditory tube, thymus, pancreatic duct, stomach, and large intestine, in addition to sweet, umami, and bitter taste cells in the oropharyngeal epithelium [17], solitary chemosensory cells in the nasal respiratory epithelium [18], Trpm5-expressing microvillous cells in the main olfactory epithelium [19], and tuft cells in the small intestinal epithelium [15,20]. Comprehensive analyses of the loss-of-function mutation of Skn-1a across these cells revealed a crucial role of Skn-1a in generating a variety of closely related Trpm5-expressing cells in different tissues. Thus, we propose that Skn-1a functions as the master regulator for the generation of functional Trpm5-expressing chemosensory cells.
Consistent with previous observation [8], our studies showed that there are at least two types of brush cells in trachea, Trpm5 and Skn-1a double-positive brush cells and Trpm5 and Skn-1a double-negative brush cells. Trpm5 and Skn-1a double-positive brush cells have taste cell-like molecular characteristics, indicating their function as chemosensory cells. Currently, molecular characterization and function of Trpm5 and Skn-1a double-negative brush cells remain unknown. Because two types of brush cells have same cellular morphology of apical tuft of microvilli expressing villin, it is possible to speculate that they may be differentiated from a common progenitor. However, the deficiency of Skn-1a caused loss of Trpm5-positive brush cells and did not affect the population of Trpm5-negative brush cells, suggesting that Skn-1a is involved in the generation of Trpm5-positive brush cells but not Trpm5-negative cells, and two types of brush cells are generated from distinct cell lineages or separate from each other at earlier stage of differentiation when Skn-1a exerts its function to proceed differentiation. This is similar to the case in the main olfactory epithelium, where Skn-1a is involved in the generation of Trpm5-positive microvillous cells but not Trpm5-negative microvillous cells [19]. Trpm5-positive and Trpm5-negative microvillous cells were also observed in urethra and thymus [13, 22], and only Trpm5-negative populations remained in Skn-1a-/- mice. Although the numbers of villin-positive microvillous cells (Trpm5-positive and Trpm5-negative cells) in urethra and thymus were decreased in Skn-1a-/- mice, these reductions are not comparable to the percentages of Trpm5-positive microvillous cells in all microvillous cells of wild-type mice, suggesting the loss of Skn-1a function may affect Trpm5-nagetive microvillous cell lineages in these tissues. Identification of progenitors for Trpm5-positive brush cells and lineage-tracing experiments will provide insights into molecular mechanisms underlying Skn-1a-dependent generation of Trpm5-positive brush cells and the duality of brush cells in trachea, urethra, thymus, and main olfactory epithelium [19].
Beginning from the identification of Trpm5-expressing taste receptor cells in the oropharyngeal epithelium, function of Trpm5-expressing cells as chemosensory cells have gradually emerged in several tissues. For example, in the airway and urethral epithelium, Trpm5-expressing cells function in protective responses to potentially harmful substances (bitter substance) and bacterial products by using taste receptors [7–13]. In the intestine, Trpm5-expressing tuft cells are required for initiation of type 2 immune response to expel intruding parasites [14–16]. Although Trpm5-expressing microvillous cells in the main olfactory epithelium do not express taste-related genes except for Trpm5, these Trpm5-expressing cells are reported to function as chemo- and thermo-sensor [32], and play a protective role in maintaining the olfactory function against long-term exposure of high concentration odor mixtures [33]. The functions of Trpm5-expressing cells in auditory tube, thymus, pancreatic duct, stomach, and large intestine are currently not well understood. However, considering commonalities of molecular characteristics of Trpm5-expressing cells, it is conceivable that Trpm5-expressing cells in other tissues may also function as a gatekeeper of biophylactic reactions. Because Skn-1a-/- mice lack the Trpm5-expressing cells in these tissues, further studies of Skn-1a-/- mice would elucidate the physiological roles of Trpm5-expressing cells in mice.
Supporting information
A: Quantification of the number of Trpm5- and villin-positive cells in the wild-type and Skn-1a-/- urethral epithelium. Trpm5-positive cells were completely absent in the Skn-1a-/- urethral epithelium (8.0 ± 0.6 cells in wild-type and 0 cells in Skn-1a-/-), and the number of villin-positive cells tended to decrease in Skn-1a-/- mice compared to wild-type mice (57 ± 11 cells in wild-type and 34 ± 0.9 cells in Skn-1a-/-). Each symbol represents an individual mouse. The error bars represent the mean ± SEM (n = 3, P = 0.058, Student’s t-test). B: Densities of Trpm5- and villin-positive cells in the sections of wild-type and Skn-1a-/- thymus. The signals of Trpm5 were completely absent in the Skn-1a-/- thymus (59 ± 1.8 cells/mm2 in wild-type and 0 cells/mm2 in Skn-1a-/-), and the density of villin positive cells decreased significantly in Skn-1a-/- mice compared to wild-type mice (91 ± 3.8 cells/mm2 in wild-type and 64 ± 1.9 cells/mm2 in Skn-1a-/-). Each symbol represents an individual mouse. The error bars represent the mean ± SEM (n = 3, *P < 0.05, Student’s t-test).
(TIF)
Acknowledgments
We are grateful to Dr. H. Miura for probe for Tas2r131.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by grant support from MEXT KAKENHI (Grant Number JP20570208 and JP16K07366 to J.H.), JSPS KAKENHI, Grant-in-Aid for JSPS Fellows (JP15J05301 to J.Y.), the Yamazaki Spice Promotion Foundation (131 to J.H.), an NIH grant (R01DC015491 to IM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5: 1169–1176. doi: 10.1038/nn952 [DOI] [PubMed] [Google Scholar]
- 2.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 Is a Voltage-Modulated and Ca2+-Activated Monovalent Selective Cation Channel. Curr Biol. 2003;13: 1153–1158. doi: 10.1016/S0960-9822(03)00431-7 [DOI] [PubMed] [Google Scholar]
- 3.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100: 15166–15171. doi: 10.1073/pnas.2334624100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100: 15160–15165. doi: 10.1073/pnas.2334159100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, et al. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49 doi: 10.1186/1471-2202-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 2008;38: 505–517. doi: 10.1016/j.mcn.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107: 3210–3215. doi: 10.1073/pnas.0911934107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci. 2011;108: 9478–9483. doi: 10.1073/pnas.1019418108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100: 8981–8986. doi: 10.1073/pnas.1531172100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. Chemoreception regulates chemical access to mouse vomeronasal organ: Role of solitary chemosensory cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91: 992–996. doi: 10.1016/j.lfs.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 12.Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A. 2014;111: 6075–6080. doi: 10.1073/pnas.1402251111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci U S A. 2014;111: 8287–8292. doi: 10.1073/pnas.1402436111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Moltke J, Ji M, Liang H-E, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature. 2015;529: 221–225. doi: 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529: 226–230. doi: 10.1038/nature16527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howitt M.R., Lavoie S., Michaud M., Blum A.M., Tran S.V., Weinstock J.V., et al. Tuft cells, taste-chemosensory cells,orchestrate parasite type 2 immunityin the gut. Science. 2016;351: 1329–1333. doi: 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14: 685–687. doi: 10.1038/nn.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmoto M, Yamaguchi T, Yamashita J, Bachmanov AA, Hirota J, Matsumoto I. Pou2f3/Skn-1a is necessary for the generation or differentiation of solitary chemosensory cells in the anterior nasal cavity. Biosci Biotechnol Biochem. 2013;77: 2154–2156. doi: 10.1271/bbb.130454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Yamashita J, Ohmoto M, Aoudé I, Ogura T, Luo W, et al. Skn-1a / Pou2f3 is required for the generation of Trpm5-expressing microvillous cells in the mouse main olfactory epithelium. BMC Neurosci. 2014;15:13 doi: 10.1186/1471-2202-15-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ushiama S, Ishimaru Y, Narukawa M, Yoshioka M, Kozuka C, Watanabe N, et al. Catecholamines Facilitate Fuel Expenditure and Protect Against Obesity via a Novel Network of the Gut-Brain Axis in Transcription Factor Skn-1-deficient Mice. EBioMedicine. 2016;8: 60–71. doi: 10.1016/j.ebiom.2016.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, et al. Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol. 2012;137: 483–497. doi: 10.1007/s00418-012-0911-x [DOI] [PubMed] [Google Scholar]
- 22.Panneck AR, Rafiq A, Schütz B, Soultanova A, Deckmann K, Chubanov V, et al. Cholinergic epithelial cell with chemosensory traits in murine thymic medulla. Cell Tissue Res. 2014;358: 737–748. doi: 10.1007/s00441-014-2002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schütz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6: 1–14. doi: 10.3389/fphys.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146: 233–244.e5. doi: 10.1053/j.gastro.2013.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomonari H, Miura H, Nakayama A, Matsumura E, Ooki M, Ninomiya Y, et al. Gα-gustducin is extensively coexpressed with sweet and bitter taste receptors in both the soft palate and fungiform papillae but has a different functional significance. Chem Senses. 2012;37: 241–251. doi: 10.1093/chemse/bjr098 [DOI] [PubMed] [Google Scholar]
- 26.Prandi S, Bromke M, Hübner S, Voigt A, Boehm U, Meyerhof W, et al. A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Negro CA Del. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preB otzinger complex. J Physiol. 2007;3: 1047–1058. doi: 10.1113/jphysiol.2007.134577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11: 3 doi: 10.1186/1471-2466-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 Expression Identifies Tuft Cells Rather Than Stem Cells in the Adult Mouse Intestinal Epithelium. Gastroenterology. 2009;137: 2179–2180. doi: 10.1053/j.gastro.2009.06.072 [DOI] [PubMed] [Google Scholar]
- 30.Gerbe F, Van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192: 767–780. doi: 10.1083/jcb.201010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. 2011;136: 191–204. doi: 10.1007/s00418-011-0831-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogura T, Szebenyi S a, Krosnowski K, Sathyanesan A, Jackson J, Lin W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol. 2011;106: 1274–1287. doi: 10.1152/jn.00186.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemons K, Fu Z, Aoude I, Ogura T, Sun J, Chang J, et al. Lack of TRPM5-Expressing Microvillous Cells in Mouse Main Olfactory Epithelium Leads to Impaired Odor-Evoked Responses and Olfactory-Guided Behavior in a Challenging Chemical Environment. eNeuro. 2017; 12;4 (3). doi: 10.1523/ENEURO.0135-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Quantification of the number of Trpm5- and villin-positive cells in the wild-type and Skn-1a-/- urethral epithelium. Trpm5-positive cells were completely absent in the Skn-1a-/- urethral epithelium (8.0 ± 0.6 cells in wild-type and 0 cells in Skn-1a-/-), and the number of villin-positive cells tended to decrease in Skn-1a-/- mice compared to wild-type mice (57 ± 11 cells in wild-type and 34 ± 0.9 cells in Skn-1a-/-). Each symbol represents an individual mouse. The error bars represent the mean ± SEM (n = 3, P = 0.058, Student’s t-test). B: Densities of Trpm5- and villin-positive cells in the sections of wild-type and Skn-1a-/- thymus. The signals of Trpm5 were completely absent in the Skn-1a-/- thymus (59 ± 1.8 cells/mm2 in wild-type and 0 cells/mm2 in Skn-1a-/-), and the density of villin positive cells decreased significantly in Skn-1a-/- mice compared to wild-type mice (91 ± 3.8 cells/mm2 in wild-type and 64 ± 1.9 cells/mm2 in Skn-1a-/-). Each symbol represents an individual mouse. The error bars represent the mean ± SEM (n = 3, *P < 0.05, Student’s t-test).
(TIF)
Data Availability Statement
All relevant data are within the paper.