Abstract
Sexual reproduction is critical for successful evolution of eukaryotic organisms in adaptation to changing environments. In the opportunistic human fungal pathogens, the Cryptococcus pathogenic species complex, C. neoformans primarily undergoes bisexual reproduction, while C. deneoformans undergoes both unisexual and bisexual reproduction. During both unisexual and bisexual cycles, a common set of genetic circuits regulates a yeast-to-hyphal morphological transition, that produces either monokaryotic or dikaryotic hyphae. As such, both the unisexual and bisexual cycles can generate genotypic and phenotypic diversity de novo. Despite the similarities between these two cycles, genetic and morphological differences exist, such as the absence of an opposite mating-type partner and monokaryotic instead of dikaryotic hyphae during C. deneoformans unisexual cycle. To better understand the similarities and differences between these modes of sexual reproduction, we focused on two cellular processes involved in sexual reproduction: cell-cell fusion and karyogamy. We identified orthologs of the plasma membrane fusion protein Prm1 and the nuclear membrane fusion protein Kar5 in both Cryptococcus species, and demonstrated their conserved roles in cell fusion and karyogamy during C. deneoformans α-α unisexual reproduction and C. deneoformans and C. neoformans a-α bisexual reproduction. Notably, karyogamy occurs inside the basidum during bisexual reproduction in C. neoformans, but often occurs earlier following cell fusion during bisexual reproduction in C. deneoformans. Characterization of these two genes also showed that cell fusion is dispensable for solo unisexual reproduction in C. deneoformans. The blastospores produced along hyphae during C. deneoformans unisexual reproduction are diploid, suggesting that diploidization occurs early during hyphal development, possibly through either an endoreplication pathway or cell fusion-independent karyogamy events. Taken together, our findings suggest distinct mating mechanisms for unisexual and bisexual reproduction in Cryptococcus, exemplifying distinct evolutionary trajectories within this pathogenic species complex.
Author summary
Sexuality is ubiquitous in eukaryotic systems, but it is present in diverse forms, ranging from distinct sexual individuals to parthenogenic organisms in both animals and plants. Consequently, different organisms have evolved different reproduction strategies in which cell-cell fusion and nuclear fusion (karyogamy) play fundamental roles. The opportunistic human fungal pathogen Cryptococcus neoformans can undergo both bisexual reproduction between a and α cells and selfing unisexual reproduction, which offsets the cost of finding a mating partner, coinciding with the observation that 99% of clinical and environmental isolates are mating type α. It has been a central interest to elucidate the similarities and differences between these two sexual cycles. Here, we identified and characterized two genes in the Cryptococcus species complex, PRM1 and KAR5, which play conserved roles in plasma membrane fusion and karyogamy in fungi. We showed that unisexual reproduction is largely independent from cell-cell fusion and is mechanistically different from bisexual reproduction. We also demonstrated that karyogamy takes place at different stages during bisexual reproduction between two sister species, exemplifying distinct evolutionary trajectories within the pathogenic species complex.
Introduction
Sexual reproduction is ubiquitous in eukaryotic systems and promotes genetic diversity important for successful evolutionary adaptation to ever-changing environments [1]. In addition to bisexual reproduction between mating partners of opposite sexes, many eukaryotic systems, including fish, amphibians, and reptiles, can undergo unisexual reproduction, termed parthenogenesis, often in the absence of the opposite sex [2]. During bisexual reproduction, parental gametes undergo cell fusion and nuclear fusion to produce recombinant progeny, whereas during parthenogenesis, the maternal genome undergoes reduplication through either cell-cell fusion or endoreplication to produce clonal offspring of the mother [2]. Analogous to parthenogenesis, several human fungal pathogens have been reported to undergo both unisexual and bisexual reproduction [3, 4]. In Candida albicans bisexual reproduction, a/a and α/α cells first undergo white-opaque switching to become mating competent and then form tetraploid cells via cell fusion and nuclear fusion. These cells then undergo a parasexual cycle to return to the diploid state. During C. albicans unisexual reproduction, loss of the Bar1 protease in a/a cells enables auto-response to MFα pheromone and promotes cell and nuclear fusion producing tetraploid cells [5]. During bisexual reproduction in the Cryptococcus species complex, cell fusion triggers a dramatic yeast-hyphal morphological transition, producing dikaryotic hyphae. The growing tips of these hyphae differentiate into basidia, in which two nuclei undergo nuclear fusion to produce basidiospores through meiosis [6]. During the unisexual cycle, α or a cells initiate hyphal growth and form monokaryotic hyphae, during which the haploid nucleus undergoes a ploidy increase through either cell-cell fusion followed by nuclear fusion, nuclear fusion between mother and daughter cells, or an endoreplication pathway, and the diploid nucleus inside the basidium then undergoes meiosis and produces haploid spore progeny [7, 8].
Sexual reproduction has only been observed under laboratory conditions In the Cryptococcus species complex. However, spore-like cells have been harvested from the environment, suggesting the sexual cycle may occur in natural environments [9, 10]. Unisexual reproduction has been documented for C. neoformans, C. deneoformans, and C. gattii [7, 11, 12]. Based on evidence from population genetics studies, natural isolates also recombine through unisexual reproduction, which may be of ecological significance because more than 99% of environmental and clinical isolates are the α mating type [13–16]. Of note, the unisexual cycle generates genotypic and phenotypic diversity de novo, similar to the bisexual cycle [17]. A common set of genetic circuits govern both unisexual and bisexual reproduction, [8, 18–20] and both sexual cycles involve similar meiotic recombination mechanisms [21]. The recombining nature of the unisexual cycle can enable a clonal population to reverse Muller’s ratchet and avoid an evolutionary dead end [22].
Despite similar regulatory genetic circuits, fundamental differences are obvious between the two modes of sexual reproduction [23–25]. Genetically, the unisexual cycle is initiated in the absence of an opposite-mating type partner, whereas the bisexual cycle is initiated upon a-α cell-cell fusion. Morphologically, the unisexual cycle produces monokaryotic hyphae with unfused clamp cells, while the bisexual cycle produces dikaryotic hyphae with fused clamp cells, which allow a nucleus to migrate between adjacent hyphal compartments to maintain dikaryotic hyphae [24, 25]. While diploidization is achieved through karyogamy in the bisexual cycle, it is not yet clear how diploidization is achieved during the unisexual cycle. Three hypotheses have been proposed, including 1) cell fusion followed by karyogamy; 2) karyogamy between mitotically dividing mother-daughter cells followed by either mis-segregation of the nucleus or cytokinesis arrest; and 3) endoreplication during hyphal growth [25, 26].
In all bisexually reproducing organisms, gamete fusion is a fundamental process requiring a set of dedicated fusion proteins [27]. In the fungal kingdom, Prm1 (Pheromone regulated multi-spanning membrane protein 1) is a conserved plasma membrane protein required for plasma membrane fusion during cell-cell fusion [28–30]. In Saccharomyces cerevisiae and Neurospora crassa, deletion of PRM1 reduces fusion frequency by approximately half and leads to cell lysis. The mutant phenotype is alleviated in the presence of a high calcium and exacerbated upon calcium depletion [31, 32]. Prm1 is also required for asexual hyphal fusion in N. crassa [29]. In Schizosaccharomyces pombe, deletion of PRM1 causes a 95% reduction in cell fusion frequency independent of extracellular calcium concentration, but does not lead to a cell lysis phenotype [30].
Cell fusion has been well studied in Cryptococcus sexual cycles. During bisexual reproduction, a-α cell-cell fusion is required for hyphae induction and clamp cell-hyphal fusion is required for proper nuclear migration between adjacent hyphal compartments to maintain dikaryotic hyphal growth [6, 33]. During unisexual reproduction, α-α cell-cell fusion occurs at a low frequency whereas the presence of a cells can enhance α-α cell fusion ~1000 fold in a ménage à trois fashion [7]. G proteins in the pheromone response pathway are required for cell-cell fusion [34], and the master transcription factor Mat2 governs the yeast-hyphal morphological transition [18]. An evolutionarily conserved Ire1 kinase/endoribonuclease in the unfolded protein response pathway has been shown to negatively regulate the pheromone response pathway and is required for cell-cell fusion [35]. However, genes that are directly involved in plasma membrane fusion during cell-cell fusion have not been identified. A transcriptomic study showed that expression of the S. cerevisiae PRM1 homolog in C. deneoformans is highly upregulated during hyphal growth, suggesting it may function in the sexual cycle, but its involvement in cell-cell fusion had yet to be determined [18].
Karyogamy is an essential step for intermixing of parental genetic information during sexual reproduction. Two sets of genes regulate karyogamy in S. cerevisiae. The class I genes, including KAR1, KAR3, KAR4, and KAR9, regulate nuclear congression, while the class II genes, including KAR2, KAR5, KAR7, KAR8, and PRM3, mediate inner and outer nuclear membrane fusion [36, 37]. Lee and Heitman identified the Cryptococcus karyogamy genes KAR2, KAR3, KAR4, KAR7, and KAR8 based on homology to S. cerevisiae [38]. While homologs of KAR2 and KAR7 were identified in Cryptococcus with roles in filamentation and meiosis, respectively, homologs of KAR3, KAR4, and KAR8 did not show karyogamy defects during unisexual or bisexual reproduction. This suggests that these genes are either rewired in Cryptococcus compared with S. cerevisiae or are functionally redundant in regulating nuclear fusion. KAR2, an ER-resident chaperone protein, is essential in Cryptococcus, and its overexpression partially rescues the filamentation defect of the ire1 mutant [35, 39]. KAR7 maintains a conserved role in mediating nuclear membrane fusion during both Cryptococcus unisexual and bisexual reproduction. However, a diploid strain without KAR7 produced hyphae and basidia but failed to undergo sporulation, suggesting KAR7 may play additional roles in meiotic processes. In S. cerevisiae, Kar5 localizes to both inner and outer nuclear membranes at the spindle pole body, and coordinates the outer and inner nuclear membrane, facilitating the inner nuclear membrane fusion step during karyogamy [40–42]. However, a KAR5 homolog was not identified in Cryptococcus. A study on the Chlamydomonas nuclear fusion gene GEX1 by Ning and colleagues [43] showed that protist and plant GEX1 genes and fungal KAR5 genes belong to an ancient cysteine rich domain (CRD) containing protein family that is conserved throughout eukaryotes, suggesting that they may share a conserved role in nuclear membrane fusion. In that same study, a KAR5 ortholog was identified for a basidiomycetous fungus, Puccinia graminis [43].
In this study, we identified PRM1 and KAR5 orthologs in both C. neoformans and C. deneoformans and investigated their conserved functions in mediating plasma membrane and nuclear membrane fusion. Utilizing these two genes, we studied cell fusion and nuclear fusion in the C. neoformans bisexual cycle and the C. deneoformans unisexual and bisexual cycles. C. neoformans and C. deneoformans bisexual cycles were dependent on cell and nuclear fusion at different stages during sexual development, whereas, cell fusion was largely dispensable in the solo unisexual cycle of C. deneoformans and the ploidy duplication during unisexual reproduction is dependent on either endoreplication or cell fusion-independent karyogamy events. Our results provide mechanistic insights relevant to studies of mating mechanisms of unisexual reproduction and parthenogenesis in other eukaryotic systems.
Results
Identification of PRM1 and KAR5 in Cryptococcus
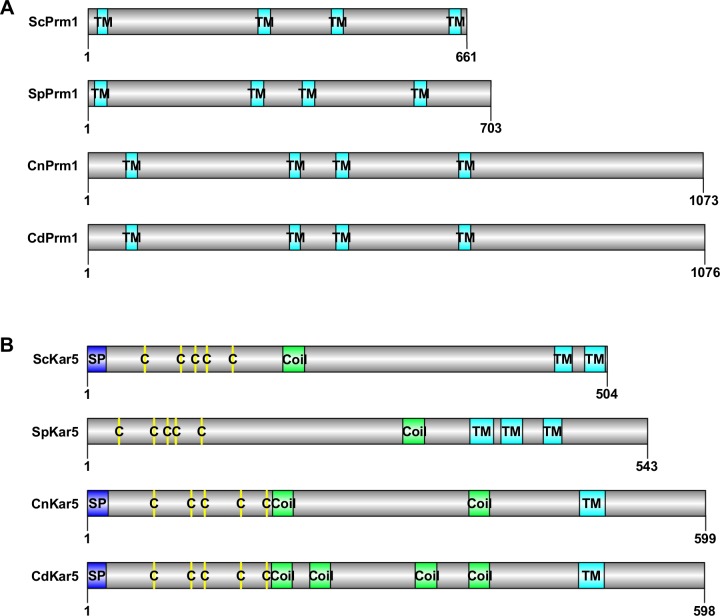
To study cell-cell fusion during the Cryptococcus sexual cycles, we performed BLASTP searches to identify plasma membrane fusion protein, Prm1, known to orchestrate cell-cell fusion during mating in other fungi. BLASTP searches using S. cerevisiae, C. albicans, Aspergillus fumigatus, S. pombe, and N. crassa Prm1 protein sequences [28–30] identified CNAG_05866 (Cn Prm1) and CNF01070 (CdPrm1) as candidate PRM1 genes in C. neoformans and C. deneoformans, respectively (S1A and S1B Fig). The CnPrm1 and CdPrm1 proteins share 91% sequence identity and are the only candidate proteins that shared significant sequence similarity with Prm1 proteins from other fungal organisms. Reciprocal BLASTP searches confirmed the orthologous nature of these fungal PRM1 genes. Both CnPrm1 and CdPrm1 are predicted to share a similar protein topology with ScPrm1 and SpPrm1, and contain four transmembrane domains based on Phobius prediction [44]. However, the Cryptococcus Prm1 proteins have a long C-terminal tail following the last transmembrane domain (Fig 1A).
Fig 1. Schematic diagrams of Cryptococcus Prm1 and Kar5 proteins.
(A) The Prm1 proteins from S. cerevisiae, S. pombe, C. neoformans, and C. deneoformans are drawn to scale. The four Prm1 proteins contain four transmembrane domains (TM) indicated by cyan boxes. In contrast to ScPrm1 and SpPrm1, CnPrm1 and CdPrm1 have a long C-terminal tail following the last transmembrane domain. (B) The Kar5 proteins from S. cerevisiae, S. pombe, C. neoformans, and C. deneoformans are drawn to scale. CnKar5 and CdKar5 protein domain structures are conserved with ScKar5 and SpKar5. All four proteins contain a cysteine-rich domain (C) indicated by yellow lines, a coiled-coil domain (Coil) indicated by green boxes, and C-terminal transmembrane domains (TM) indicated by cyan boxes. ScKar5, CnKar5, and CdKar5 contain an N-terminal signal peptide (SP) indicated by a blue box.
Another crucial cellular process during sexual reproduction is karyogamy, the fusion of nuclei. One of the karyogamy proteins in S. cerevisiae, Kar5, facilitates nuclear membrane fusion during mating [40, 42]. We identified CNAG_04850 as the KAR5 gene in C. neoformans using the Kar5 protein sequence of Puccinia graminis [43], which belongs to the same phylum (Basidiomycota) as Cryptococcus. The same BLASTP search failed to identify the CdKAR5 gene, but using the CnKAR5 genomic sequence we identified an unannotated region on chromosome 10 from bp 790071 to 792560 that encodes the KAR5 ortholog in C. deneoformans. BLASTP searches and phylogenetic analyses of Kar5 proteins from several fungal organisms suggested that Kar5 protein sequences are divergent across different fungal species (S1C and S1D Fig). Multiple sequence alignment and topology predictions by Phobius prediction and COILS/PCOILS confirmed that CnKar5 and CdKar5 share a similar protein topology with ScKar5 and SpKar5, with an N-terminal signal peptide and a CRD domain, followed by coiled-coiled domains and a C-terminal transmembrane domain, except that SpKar5 does not have the N-terminal signal peptide (Fig 1B and S1E Fig) [44, 45].
PRM1 is required for cell-cell fusion during C. neoformans bisexual reproduction
Deletion of PRM1 caused a significant filamentation delay during C. neoformans bisexual reproduction (Fig 2A). However, abundant hyphal production and sporulation were still observed after 10 days (S2A Fig). To evaluate the overall impact of PRM1 deletion on overall mating progress, we quantified the relative spore production of prm1 mutants compared to the wild type at 7 days by Percoll gradient centrifugation. Deletion of PRM1 caused a mild reduction in spore production (87.3 ± 9% of wild type, p = 0.207) (S3A Fig).
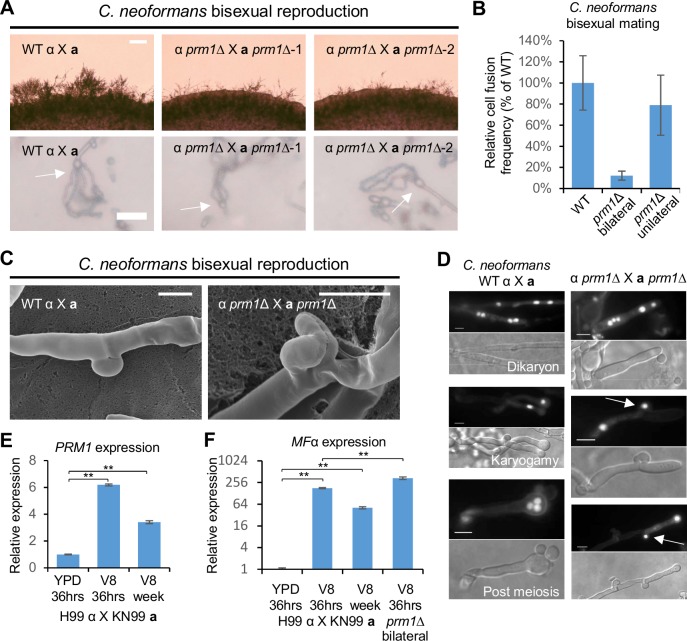
Fig 2. Deletion of PRM1 blocks cell-cell fusion and clamp cell fusion during Cryptococcus neoformans bisexual reproduction.
(A) Mating phenotypes for a wild type cross between H99α and KN99a and two independent prm1 bilateral mutant crosses between CF30 and CF448, and between CF56 and CF562. All mating patches were spotted on MS medium and incubated in the dark at room temperature. The top row shows hyphal growth on the edge of mating patches five days after inoculation. The scale bar is 100 μm. The bottom row shows the basidium and spore chain morphology (indicated by arrows)10 days after inoculation. The scale bar equals 20 μm. (B) Unilateral and bilateral prm1 mutant cell fusion frequency compared to wild type. (C) Scanning electron microscopy of clamp cell morphology of wild type cross (H99α X KN99a) and prm1 bilateral mutant cross (CF56 X CF562). The scale bar is 5 μm. (D) DAPI staining of mature hyphae and nuclei inside hyphae and basidia of C. neoformans wild type (left panel) and prm1 (right panel) bisexual crosses. Arrows in the prm1 column indicate nuclei trapped in unfused clamp cells. The scale bar is 5 μm. Gene expression patterns for (E) PRM1 and (F) MFα were examined by RT-PCR (* indicates p <0.05 and ** indicates p <0.005 for each pairwise comparison). A wild type cross (H99α X KN99a) was grown on YPD medium for 36 hours, and on V8 medium for 36 hours or one week. The prm1 bilateral mutant cross (CF56 X CF562) was grown on V8 medium for 36 hours. The Y axis for panel F is in base-2 log scale. The error bars represent the standard deviation of the mean for the three biological replicates.
We conducted a wild type mating between CF757 (JEC20a URA5-NAT) and CF762 (JEC21α ADE2-NEO) as a control. A total of 47 spore derived colonies were randomly chosen and analyzed (S4A Fig). Among the 47 progeny, all eight genotypes of Mendelian inheritance were recovered at a distribution of frequency ranging from 2.1% to 23.4% (17% parental genotype MATa URA5-NAT, 2.1% for parental genotype MATα ADE2-NEO, 12.8% for MATα URA5-NAT, 23.4% for MATa ADE2-NEO, 6.4% for MATa URA5-NAT ADE2-NEO, 6.4% for MATα URA5-NAT ADE2-NEO, 17% for MATa, and 14.7% for MATα) (S4B and S4C Fig). This provides evidence that the cells isolated by Percoll gradient centrifugation are indeed spores.
To address the involvement of Prm1 in cell-cell fusion, we performed cell fusion assays using two genetically marked mating partners. prm1 mutants showed a bilateral (prm1Δ X prm1Δ) cell fusion defect with a fusion frequency of 12% ± 4% relative to the wild type level (Fig 2B), but no defect in unilateral (prm1Δ X WT) cell fusion. The basal level of cell fusion activity may allow prm1 mutants to produce abundant hyphae after a 10-day incubation on mating inducing medium (S2A Fig).
During C. neoformans bisexual reproduction, the dikaryotic hyphae generate clamp cells, which fuse with adjacent hyphal compartments to allow a nucleus to translocate between hyphal compartments and maintain the dikaryon status [6]. To test whether Prm1 plays a role in clamp cell-hyphal fusion, we examined hyphae by scanning electron microscopy (SEM). The clamp cell and a peg from the adjacent hyphal compartment both exhibited elongated tubular morphology in prm1 mutants compared to clamp cell connections in the wild type (Fig 2C), suggesting that these clamp cells and peg protrusions failed to undergo cell fusion. Transmission election microscopy (TEM) showed that the plasma membranes failed to undergo fusion in the clamp cells (S5 Fig). DAPI staining of hyphal nuclei showed that a single nucleus was trapped in the prm1 mutant clamp cells, resulting in an abnormal number of nuclei in a single hyphal compartment (Fig 2D).
Clamp cell fusion is regulated by the pheromone signaling pathway, both PRM1 and MFα expression were maintained at a significantly high level after mating for seven days on mating inducing V8 medium compared to non-mating inducing YPD medium (3.4-fold increase for PRM1, p <0.005; and 50.8-fold increase for MFα, p <0.005) (Fig 2E and 2F). prm1 mutants exhibited a significant increase in MFα expression compared to wild type (1.9-fold increase, p <0.005), suggesting that the cell fusion defect dampens MFα repression that occurs in response to SXI1α-SXIa repression following nuclear pairing (Fig 2F). These results indicate that Prm1 plays a role in both cell-cell fusion and clamp cell-hyphal fusion during C. neoformans bisexual reproduction (Fig 3G).
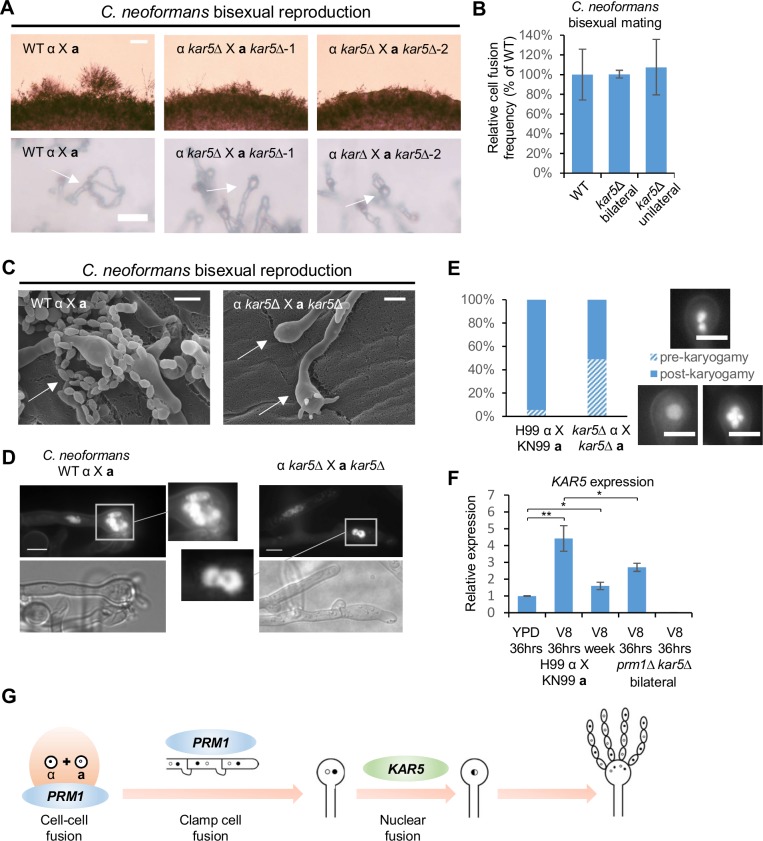
Fig 3. Deletion of KAR5 causes a sporulation defect during C. neoformans bisexual reproduction.
(A) Mating phenotypes for a wild type cross between H99α and KN99a and two independent kar5 bilateral mutant crosses between CF57 and CF549, and CF208 and CF305. The scale bars are 100 μm and 20 μm for top row and bottom row, respectively. (B) Unilateral and bilateral kar5 mutant cell fusion frequency compared to wild type. (C) Scanning electron microscopy of basidium morphology and sporulation patterns (indicated by arrows) for wild type cross (H99α X KN99a) and kar5 bilateral mutant cross (CF57 X CF549). The scale bar is 5 μm. (D) DAPI staining of nuclei inside basidia from C. neoformans wild type (left panel) and kar5 mutant (right panel) bisexual crosses. Basidia indicated by white boxes were magnified to show nuclei morphology. The scale bar is 5 μm. (E) Quantification of pre-karyogamy and post-karyogamy events for wild type and kar5 mutant crosses based on DAPI staining of nuclei inside basidia. Representative pre-karyogamy (two nuclei) and post-karyogamy (one nucleus and post meiosis) events were shown on the right. The scale bar is 5 μm. (F) Gene expression patterns for KAR5 were examined by RT-PCR (* indicates p <0.05 and ** indicates p <0.005 for each pairwise comparison). Wild type cross (H99α X KN99a) was grown on YPD medium for 36 hours, and on V8 medium for 36 hours or one week. prm1 bilateral mutant cross (CF56 X CF562) and kar5 bilateral mutant cross (CF57 X CF549) were grown on V8 medium for 36 hours. The error bars represent the standard deviation of the mean for the three biological replicates. (G) Proposed C. neoformans bisexual reproduction model. PRM1 is required for cell-cell fusion and clamp cell fusion, and KAR5 is required for karyogamy inside the basidium.
KAR5 is required for karyogamy during C. neoformans bisexual reproduction
Like prm1 mutants, kar5 mutants showed a significant delay in filamentation during C. neoformans bisexual reproduction (Fig 3A); the mutants produced abundant hyphae after 10 days (S2B Fig). In contrast to other prm1 mutant phenotypes, kar5 mutants were not defective in cell fusion but exhibited sporulation defects (Fig 3A and 3B). SEM studies showed that the abnormal basidia were either bald or had more than four budding sites compared to the four sites in the wild type (Fig 3C). However, the wild type phenotype (four spore chains) was observed in kar5 mutants after longer mating incubation periods. Similar to prm1 mutants, deletion of KAR5 caused a mild reduction in spore production (77.2% ± 8.8% p <0.05) (S3B Fig), suggesting that deletion of KAR5 did not completely block sporulation. We stained the nuclei within the abnormal basidia generated by kar5 mutants with DAPI and found two nuclei in close contact within the kar5 mutant bald basidia in contrast to either one nucleus or four meiotic nuclei present in wild type basidia (Fig 3D and 3E). Quantification of 129 wild type basidia and 131 kar5 mutant basidia stained with DAPI showed that 5.7% wild type basidia versus 48.9% kar5 mutant basidia contained two nuclei, suggesting that deletion of KAR5 inhibited, but did not completely block karyogamy inside the basidia (Fig 3E). The nuclear morphology of the C. neoformans kar5 mutant was similar to the kar5 mutant karyogamy phenotype in S. cerevisiae [42], supporting the hypothesis that KAR5 plays a conserved role in mediating karyogamy during C. neoformans bisexual reproduction. KAR5 expression was upregulated upon mating induction and maintained at a significantly high level after mating for a week compared to non-mating inducing conditions (1.6-fold increase, p <0.05). Deletion of PRM1 significantly reduced KAR5 expression (1.6-fold decrease, p <0.05), suggesting control of gene expression following cell-cell fusion during C. neoformans bisexual reproduction (Fig 3F and 3G).
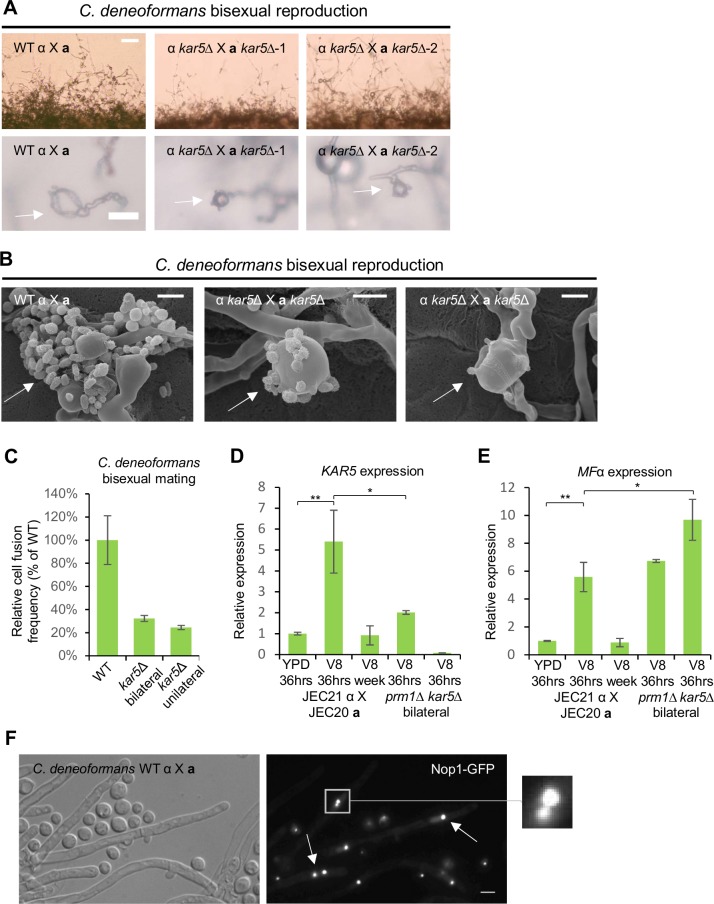
PRM1 plays a central role during C. deneoformans bisexual reproduction
In contrast to C. neoformans, C. deneoformans prm1 mutants showed a mild delay in hyphal production (Fig 4A), and exhibited a significant reduction in spore production compared to wild type C. deneoformans (27% ± 2.2%) bisexual reproduction (S3A Fig). PRM1 deletion caused both bilateral and unilateral cell fusion defects with fusion frequencies of 6.9% ± 2.6% and 8.2% ± 1.8% of the wild type levels, respectively (Fig 4B). To understand the mechanistic requirement for Prm1 in cell-cell fusion during C. deneoformans bisexual reproduction, we monitored cell-cell fusion of prm1 mutants with confocal microscopy. In the same prm1 mutant cell fusion sample, both fused and unfused cells were detected by the presence and absence of inter-cellular mixing of fluorescent signals between the Nop1-GFP and mCherry labeled fusion pairs (Fig 4C, S1 and S2 Movies). Based on quantification of fluorescent signal intermixing, the wild type cell fusion frequency was 90.6%, while the prm1 mutant unilateral cell fusion and bilateral cell fusion frequencies were 51.7% and 12.8%, respectively (Fig 4D and S6 Fig). The unilateral cell fusion defect suggests that the Prm1 plays a more important role during bisexual reproduction in C. deneoformans compared to C. neoformans; in agreement with this observation, PRM1 expression level was maintained at a similar high level after mating for seven days compared with 36 hours (Fig 4E).
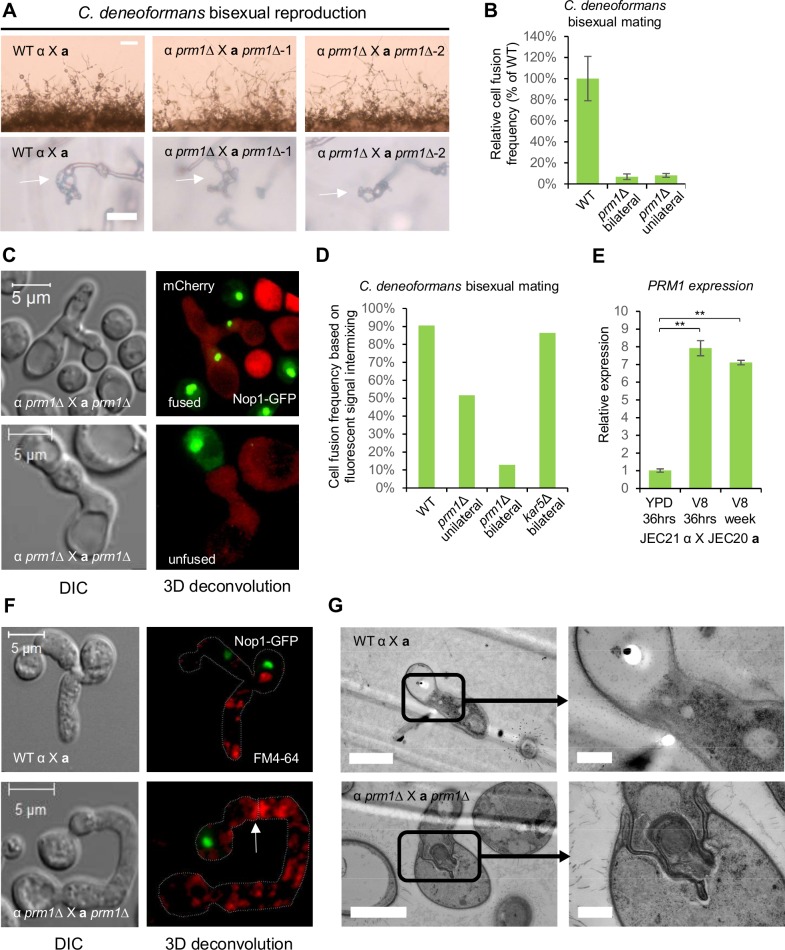
Fig 4. prm1 mutants are defective in plasma membrane fusion during C. deneoformans bisexual reproduction.
(A) Mating phenotypes for a wild type cross between JEC21α and JEC20a and two independent prm1 bilateral mutant crosses between CF1 and CF313, and CF316 and CF517. The scale bars are 100 μm and 20 μm for top row and bottom row, respectively. (B) Unilateral and bilateral prm1 mutant cell fusion frequency compared to wild type. (C) CF712 (JEC21α prm1Δ::NAT mCherry-NEO) was mated with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) on V8 medium for 24 hours. Confocal microscopy showed that both fused and unfused cell fusion pairs were present during bilateral prm1 mutant mating based on the presence or absence of fluorescent signal intermixing between fusion partners. The scale bar is 5 μm. (D) Wild type mating between CF830 (JEC21α NOP1-GFP-NAT) and JEC20a, unilateral mating between JEC21α and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), bilateral mating between CF1 (JEC21α prm1Δ::NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and bilateral mating between CF487 (JEC21α kar5Δ::NEO) and CF723 (JEC20a kar5Δ::NEO NOP1-GFP-NAT) were conducted and the cell fusion frequency was determined based on GFP fluorescence signal intermixing between fusion partners. (E) Gene expression patterns for PRM1 were examined by RT-PCR (* indicates p <0.05 and ** indicates p <0.005 for each pairwise comparison). Wild type cross (JEC21α X JEC20a) was grown on YPD medium for 36 hours, and on V8 medium for 36 hours or one week. The error bars represent the standard deviation of the mean for the three biological replicates. (F) Fusion pairs between CF830 (JEC21α NOP1-GFP-NAT) and JEC20a, and between CF1 (JEC21α prm1Δ::NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) were stained with FM4-64 to show the plasma membrane structures at the conjugation sites. The scale bar is 20 μm. (G) Fused wild type cell fusion pair (top panel) between CF830 (JEC21α NOP1-GFP-NAT) and CF1076 (JEC20a H3-mCherry-NAT) and unfused cell fusion pair (bottom panel) between CF712 (JEC21α prm1Δ::NAT mCherry-NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) were examined by transmission electron microscopy. Membrane structures at the conjugation sites were further examined at higher magnification. In the left panels, the scale bars are 2 μm, and in the right panels, the scale bars are 0.5 μm.
To visualize the structures of the plasma membrane at the conjugation sites between the fusion pairs, we stained both wild type and prm1 mutant fusion pairs with the lipophilic dye FM4-64. The prm1 mutant fusion pairs exhibited robust staining of the plasma membrane boundaries at the conjugation site (Fig 4F). Compared to the fused wild type cells, the plasma membrane of the prm1 mutant fusion pairs failed to undergo membrane fusion at the conjugation sites, and a layer of cell wall material was present between the plasma membranes (Fig 4G and S7A Fig). In 2 out of 20 observed fusion pairs by TEM, the plasma membranes formed extensive invaginations into the opposite cytosolic compartments without membrane fusion (Fig 4G). Similar to C. neoformans bisexual reproduction, prm1 mutants also exhibited a clamp cell-hyphal fusion defect during C. deneoformans bisexual reproduction (S7B Fig). However, both wild type and prm1 mutant crosses produced hyphae with unfused clamp cells, which are characteristics of monokaryotic hyphae (S7B and S8A Figs). Overall, these results suggest that PRM1 plays a more significant role in C. deneoformans bisexual reproduction in comparison to C. neoformans.
Karyogamy occurs early during C. deneoformans bisexual reproduction
Like prm1 mutants, kar5 mutants showed a mild delay in hyphal production, and produced significantly fewer spores compared to wild type (9.5% ± 2.7%) (Fig 5A and S3B Fig). SEM studies demonstrated that C. deneoformans kar5 mutants produced basidia with abnormal sporulation patterns during bisexual reproduction, similar to C. neoformans kar5 mutants (Fig 5B). Deletion of KAR5 caused both bilateral and unilateral cell fusion defects with fusion frequencies of 32.3% ± 6% and 24.5% ± 2.7% compared to the wild type level, respectively (Fig 5C). To test whether KAR5 is directly involved in cell-cell fusion, we quantified cell-cell fusion events for kar5 mutants based on fluorescent signal intermixing, and found that kar5 mutant cell-cell fusion frequency was 86.4%, similar to wild type (Fig 4D and S6D Fig), suggesting that Kar5 plays a role in post-fusion survival mechanisms for cell fusion products, and that the function of CdKar5 in bisexual reproduction is diverged from CnKar5 (Fig 3B, and 5C). Furthermore, CdKAR5 and CdMFα expression were upregulated upon mating induction, but returned to basal level after mating for seven days, which is distinct from CnKAR5 and CnMFα expression patterns (Figs 2F, 3F, 5D and 5E). However, CdKAR5 expression was significantly reduced for Cdprm1 mutants compared to wild type (2.7-fold decrease, p <0.05) (Fig 5D), similar to C. neoformans (Fig 3F).
Fig 5. kar5 mutants are defective in cell fusion and basidium sporulation during C. deneoformans bisexual reproduction.
(A) Mating phenotypes for a wild type cross between JEC21α and JEC20a and two independent kar5 bilateral mutant crosses between CF226 and CF364, and CF487 and CF464. The scale bars are 100 μm and 20 μm for top row and bottom row, respectively. (B) Scanning electron microscopy of basidia morphology and sporulation patterns (indicated by arrows) for wild type cross (JEC21α X JEC20a) and kar5 bilateral mutant cross (CF226 X CF364). The scale bar is 5 μm. (C) Unilateral and bilateral kar5 mutant cell fusion frequency compared to wild type. Gene expression patterns for (D) KAR5 and (E) MFα were examined by RT-PCR (* indicates p <0.05 and ** indicates p <0.005 for each pairwise comparison). Wild type cross (JEC21α X JEC20a) was grown on YPD medium for 36 hours, and on V8 medium for 36 hours or one week. prm1 bilateral mutant cross (CF1 X CF313) and kar5 bilateral mutant cross (CF226 X CF364) were grown on V8 medium for 36 hours. The error bars represent the standard deviation of the mean for the three biological replicates. (F) The GFP labeled nucleolar marker Nop1 was used to study C. neoformans bisexual cross (CF830 α NOP1-GFP-NAT X JEC20a) hyphal nuclear morphology. Arrows indicate instances of both monokaryotic and dikaryotic hyphae during early stages of bisexual reproduction, and the area within the box was magnified to highlight two nuclei in close contact.
To elucidate the phenotypic differences of prm1 and kar5 mutants during bisexual reproduction between C. neoformans and C. deneoformans, we stained wild type and mutant hyphal nuclei with DAPI. In contrast to the dikaryotic hyphae produced by C. neoformans (Fig 2D), C. deneoformans bisexual reproduction produced monokaryotic hyphae (S8A Fig), similar to those produced during C. deneoformans unisexual reproduction (S8B Fig). To dissect the involvement of Prm1 and Kar5 in monokaryotic hyphae formation during C. deneoformans bisexual mating, we tracked nuclear dynamics using the nucleolar marker Nop1-GFP [38]. During early bisexual mating at 48 hours, wild type produced both monokaryotic and dikaryotic hyphae (Fig 5F and S9A Fig), whereas prm1 mutants mainly produced monokaryotic hyphae (S9A Fig). In both wild type and kar5 mutant hyphae, pairs of congressed nuclei were observed, resembled the C. neoformans kar5 mutant karyogamy phenotype inside basidia during bisexual reproduction (Figs 3D and 5F and S9A Fig). After 10 days, monokaryotic and dikaryotic hyphae were present in the wild type cross, while prm1 mutants mainly produced monokaryotic hyphae and kar5 mutants mainly produced dikaryotic hyphae (S9B Fig). After six weeks, wild type and prm1 mutants mainly produced monokaryotic hyphae, whereas, kar5 mutants produced both monokaryotic and dikaryotic hyphae (S9C Fig). Live cell imaging of hyphal nuclear morphology suggests the following: 1) karyogamy may take place early in bisexual reproduction in C. deneoformans; 2) deletion of PRM1 leads to monokaryotic hyphae formation; and 3) deletion of KAR5 blocks early karyogamy in fused cells and could explain the observed post-fusion survival defect for the fused cells, which in turn promoted dikaryon hyphae formation.
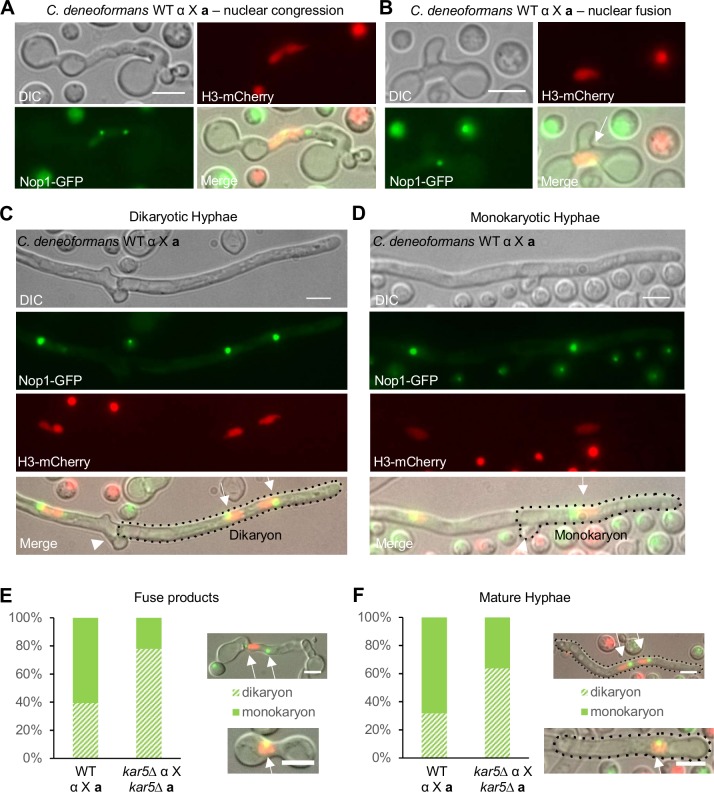
To confirm that karyogamy occurs early during C. deneoformans bisexual reproduction, we followed the hyphal nuclear dynamics between mating partners labeled with fluorescent markers (nucleolar marker Nop1-GFP and nuclear marker H3-mCherry). We observed nuclear congression in fused a-α cells (Fig 6A); and a single nucleus labeled with both fluorescent protein markers was observed, confirming that karyogamy can occur immediately after cell fusion (Fig 6B). We also observed both dikaryotic hyphae with fused clamp cells and monokaryotic hyphae with unfused clamp cells during the early mating process (Fig 6C and 6D). These hyphae expressed both parental fluorescent markers, indicating that karyogamy can occur at different stages during C. deneoformans bisexual reproduction. To test whether Kar5 functions in karyogamy immediately after cell fusion and deletion of KAR5 leads to dikaryon formation, we quantified monokaryon and dikaryon fusion products and mature hyphae labeled with both nuclear fluorescent markers in wild type and kar5 mutant crosses (Fig 6E and 6F). Among 125 wild type and 126 kar5 mutant cell fusion products, 60.8% wild type versus 22.2% kar5 mutant fused cells were monokaryotic (Fig 6E). Among 133 wild type and 132 kar5 mutant mature hyphae, 68.4% wild type versus 36.4% kar5 mutant mature hyphae were monokaryotic (Fig 6F). These results confirmed that deletion of KAR5 inhibited, but did not completely block karyogamy in early cell fusion products, and promoted dikaryon formation.
Fig 6. Karyogamy occurs at different stages during C. deneoformans bisexual reproduction.
GFP labeled nucleolar marker Nop1 and mCherry labeled nuclear marker Histone H3 protein were used to study the formation of monokaryotic hyphae during C. denoeformans bisexual mating (CF830 α NOP1-GFP-NAT X CF1076 a H3-mCherry-NAT). (A) Nuclear congression occurs in a-α fused cells. (B) Nuclear fusion occurs in a-α fused cells. Arrow points to the fused nucleus, as indicated by the mixing of the fluorescent signals. (C-D) C. deneoformans bisexual reproduction produces both (C) dikaryotic hyphae with fused clamp cells and (D) monokaryotic hyphae with unfused clamp cells. Single hyphal compartments are marked with dotted circles. Arrows point to nuclei labeled with both GFP and mCherry. Arrowheads point to a fused clamp cell in panel C and an unfused clamp cell in panel D. The scale bar is 5 μm. (E-F) Quantification of monokaryon and dikaryon fusion products (E) or mature hyphae (F) for wild type (CF830 α NOP1-GFP-NAT X CF1076 a H3-mCherry-NAT) and kar5 mutant (CF1185 α kar5Δ::NEO H3-mCherry-NAT X CF723 a kar5Δ::NEO NOP1-GFP-NAT) crosses. Representative dikaryon and monokaryon fusion products are shown on the right. Single hyphal compartments are marked with dotted circles, and each arrow points to one nucleus. The scale bar is 5 μm.
PRM1 and KAR5 are largely dispensable for C. deneoformans solo unisexual reproduction
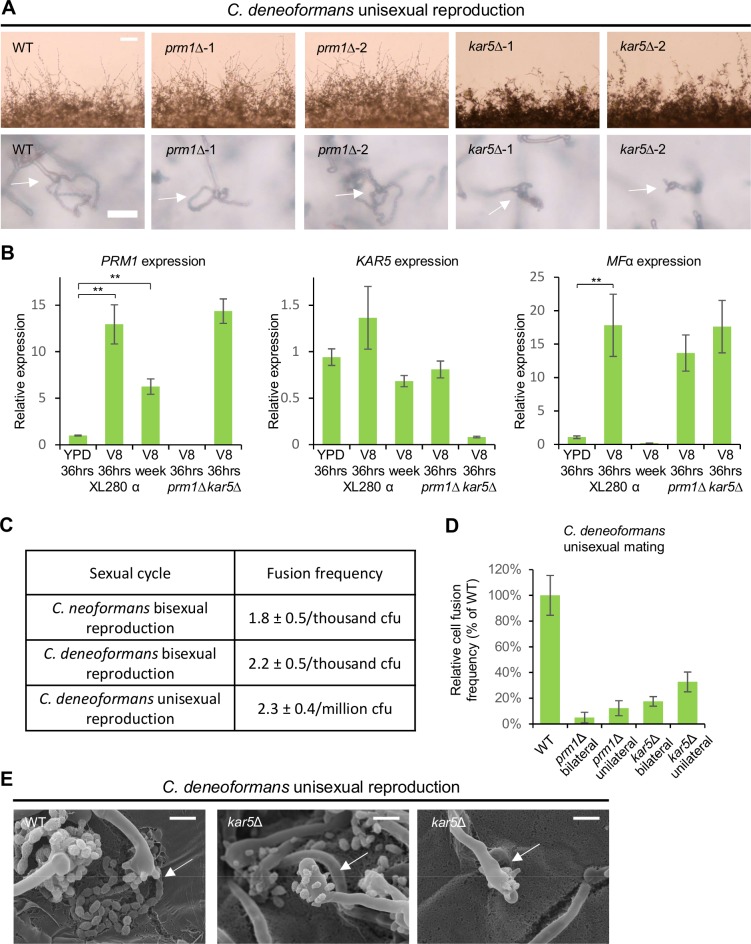
In contrast to bisexual reproduction, deletion of PRM1 and KAR5 in C. deneoformans did not impact filamentation during solo unisexual reproduction (Fig 7A), and caused less reduction in spore production relative to the wild type level (57.3% ± 7.2% and 52.8% ± 4.6%, respectively) (Fig 7A and S3 Fig). PRM1 and MFα expression were upregulated upon mating induction, but, KAR5 expression was maintained at a low level and was not affected by pheromone induction (Fig 7B), suggesting KAR5 may play a less important role in solo unisexual reproduction. Similar to what is seen during bisexual reproduction in C. deneoformans, PRM1 expression was maintained at a significantly high level after mating for seven days compared to non-mating inducing conditions (6.2-fold increase, p <0.005), whereas pheromone signaling subsided to basal level, indicating that PRM1 expression is not tightly coordinated with the pheromone signaling pathway in C. deneoformans (Fig 7B). Although PRM1 expression was significantly upregulated, cell fusion occurred at a 1000-fold lower frequency during unisexual reproduction in C. deneoformans compared to both C. neoformans and C. deneoformans bisexual reproduction (Fig 7C). Among cells that underwent cell-cell fusion during unisexual reproduction, deletion of PRM1 and KAR5 caused both bilateral and unilateral cell fusion defects (Fig 7D), and deletion of KAR5 produced basidia with abnormal sporulation patterns (Fig 7E), which were also observed during bisexual reproduction. These results suggest that during unisexual reproduction, a minority of cells undergo α-α cell fusion followed by karyogamy, similar to C. deneoformans bisexual reproduction.
Fig 7. PRM1 and KAR5 are largely dispensable for unisexual reproduction.
(A) Mating phenotypes for wild type XL280α, two independent prm1 mutants (CF317 and CF659), and two independent kar5 mutants (CF150 and CF260) during C. deneoformans unisexual reproduction. The scale bars are 100 μm and 20 μm for top row and bottom row, respectively. (B) Gene expression patterns for PRM1, KAR5, and MFα were examined by RT-PCR (* indicates p <0.05 and ** indicates p <0.005 for each pairwise comparison). Wild type (XL280α) was grown on YPD medium for 36 hours, and on V8 medium for 36 hours or one week. prm1 mutant (CF317) and kar5 mutant (CF150) were grown on V8 medium for 36 hours. The error bars represent the standard deviation of the mean for the three biological replicates. (C) Comparison of wild type cell-cell fusion frequency among three different sexual cycles between C. neoformans and C. deneoformans. (D) Unilateral and bilateral prm1 mutant and kar5 mutant cell fusion frequency compared to wild type. (E) Scanning electron microscopy of basidium morphology and sporulation patterns (indicated by arrows) of the wild type (XL280α) and the kar5 mutant (CF150). The scale bar is 5 μm.
Although cell fusion is largely dispensable for solo unisexual reproduction, karyogamy may function independently of cell fusion between mother and daughter cells or inside basidium. Deletion of KAR7 has been indicated to block nuclear congression inside the basidium during unisexual reproduction [38]. To test whether KAR5 has similar functions, we stained wild type, kar5 mutant, and kar7 mutant basidia with DAPI. Interestingly, all strains produced basidia with one, two, or more than two nuclei, which may represent three different stages of meiosis inside basidia (one nucleus as pre-meiosis, two nuclei as post meiosis I, and more than two nuclei as post meiosis II) (S10A Fig). Among 114 wild type, 116 kar5 mutant, and 115 kar7 mutant basidia, only 1.8% wild type, 4.3% kar5 mutant, and 1.7% kar7 mutant basidia contained two nuclei (S10B Fig), which is different from the cnkar5 mutant with 48.9% basidia containing two pre-karyogamy nuclei during bisexual reproduction (Fig 3E), suggesting that nuclear fusion occurs differently during unisexual reproduction of strain XL280α. If KAR5 and KAR7 were required for karyogamy in the basidia, we would have expected to see a higher population of basidia with 2 nuclei trapped at a pre-karyogamy stage compared to wild type. However, wild type and kar5 mutants exhibited similar basidia nuclear morphology with few two nuclei basidia (S10B Fig), indicating that KAR5 is not required for a later stage of unisexual reproduction, and nuclear fusion is not occurring inside the basidium. The kar7 mutant produced 24.3% basidia versus 60.5% basidia in wild type with more than two nuclei (S10B Fig), suggesting that KAR7 plays a role in meiosis during unisexual reproduction, supporting the previous observation that a diploid kar7/kar7 mutant has a defect in sporulation [38]. To validate these results, we examined basidia nuclear morphology based on nuclear fluorescent signals of wild type (CF836), kar5 mutant (CF718), and kar7 mutant (CF1442) cells labeled with Nop1-GFP, and observed similar results (S11 Fig), supporting the hypothesis that karyogamy occurs at a low frequency and karyogamy defects do not impact basidia nuclear morphology during solo unisexual reproduction.
Given that cell fusion is dispensable in solo unisexual reproduction, and kar5 is not required for meiotic basidia formation, we aimed to confirm that meiosis was involved during spore production. We generated prm1 spo11 and kar5 spo11 double mutants and observed two short spore chains compared to the four long spore chains produced by prm1 and kar5 single mutants (S12 Fig). The lack of normal spore chains confirms that spore production in unisexual reproduction is indeed dependent on the key meiotic gene SPO11 as shown previously [8].
Diploidization is achieved early in hyphae during unisexual reproduction in C. deneoformans
It is unclear how diploidization occurs during solo unisexual reproduction. By following mating partners of the same mating type labeled with different fluorescent markers (nucleolar marker Nop1-GFP and nuclear marker H3-mCherry), we showed that hyphae frequently originated from single cells rather than as of α-α cell fusion products (S3 Movie), which further confirmed that cell fusion is dispensable for the solo yeast-hyphal morphological transition.
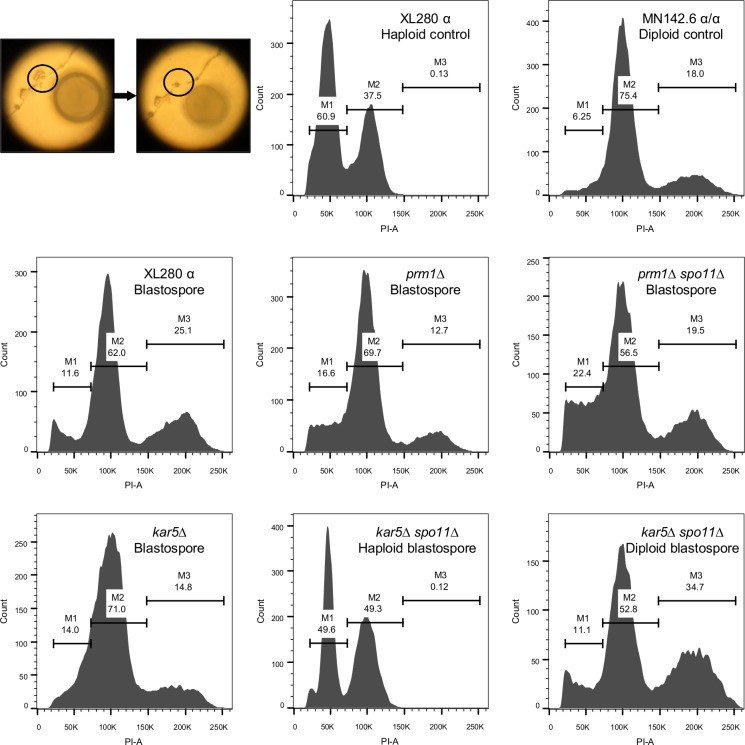
To understand when and where diploidization takes place during solo unisexual reproduction, we dissected nascent blastospores from the growing hyphae (Fig 8) and analyzed their ploidy by FACS (Table 1 and S3 Table). In the wild type, 66 out of 71 blastospores dissected from eight budding sites germinated with a survival rate of 93%. FACS analysis of 16 blastospore derived colonies, including two from each budding site, showed that all were diploid (Fig 8). prm1 and kar5 mutants exhibited blastospore germination defects with survival rates of 19.2% and 62.1% respectively. prm1 spo11 and kar5 spo11 double mutants exhibited blastospore survival rates of 63.3% and 92.5% respectively. FACS analysis revealed all blastospores of the prm1 mutant, 12 blastospores from 6 budding sites (four blastospores from two budding sites failed to germinate) of the prm1 spo11 double mutant, and 14 blastospores from seven budding sites (two blastospores from one budding site failed to germinate) of the kar5 mutant were diploid. In analysis of 19 blastospores from 10 budding sites in the kar5 spo11 double mutant, 6 blastospores were haploid, and 13 were diploid (Fig 8). The six haploid blastospores were dissected from three budding sites, suggesting that blastospores originating from the same budding site may have the same ploidy composition. To infer whether the observed single nucleus in the kar7 mutant basidia might be a product of karyogamy (S10 and S11 Figs), we dissected 248 blastospores from 46 budding sites for the kar7 mutant, and only 16 blastospores from 10 budding sites germinated with a survival rate of 6.45%, suggesting Kar7 is required for wild type blastospore survival (Table 1 and S3 Table). Among 15 blastospores analyzed, 9 were diploid, 3 were haploid, and 3 were aneuploid (Table 1 and S13 Fig), suggesting that the nuclei inside kar7 mutant basidia are likely largely diploid and that diploidization occurs earlier and outside of the basidium. The limited sample size of dissected blastospores presented here may explain why a few haploid blastospores were only recovered from the kar5 spo11 double mutant and kar7 mutant but not from wild type or the other mutant strains. That 74 out of a total of 86 (86%) tested blastospores were diploid suggests that diploidization occurs early in the hyphae during unisexual reproduction, and this process may be dependent on an endoreplication pathway or early karyogamy events between mother and daughter cells or inside the growing hyphae. Prm1 and Kar5 were dispensable for diploidization, but may contribute to blastospore survival, implying that Prm1 and Kar5 could have additional cellular functions.
Fig 8. Ploidy determination by FACS for blastospores produced during unisexual reproduction.
The upper left panel is the diagram for dissection of blastospores. The circle at left is before and the circle at right is after the blastospores were removed for dissection. The upper middle and right panels are FACS results for haploid control XL280α and diploid control MN142.6 α/α. The middle and lower panels are representative FACS results for blastospores produced by the indicated strains. Wild type XL280α, prm1Δ, prm1Δ spo11Δ, and kar5Δ produced diploid blastospores, whereas, kar5Δ spo11Δ produced both haploid and diploid blastospores.
Table 1. Ploidy determination by FACS for blastospores produced during unisexual development.
| Strain | Budding sites dissected (n) | Blastospores dissected (n) | Blastospores germinated (n) | Germination rate | Blastospores tested for ploidy* (n) | Diploid (n) | Haploid (n) | Aneuploid (n) |
|---|---|---|---|---|---|---|---|---|
| XL280α | 8 | 71 | 66 | 93.0% | 16 | 16 | 0 | 0 |
| prm1Δ | 8 | 52 | 10 | 19.2% | 10 | 10 | 0 | 0 |
| prm1Δ spo11Δ | 8 | 60 | 38 | 63.3% | 12 | 12 | 0 | 0 |
| kar5Δ | 8 | 58 | 36 | 62.1% | 14 | 14 | 0 | 0 |
| kar5Δ spo11Δ | 10 | 40 | 37 | 92.5% | 19 | 13 | 6 | 0 |
| kar7Δ | 46** | 248 | 16 | 6.45% | 15 | 9 | 3 | 3 |
* For each budding site, no more than two blastospores were chosen for FACS determination of ploidy.
** Out of the 46 budding sites dissected for kar7Δ mutant, only 10 budding sites yielded germinated blastospores.
Discussion
Without an obligate requirement for a mating partner, unisexual reproduction mitigates the two-fold cost of bisexual reproduction in finding an opposite mate. However, lacking genome diversity, clonal unisexual reproduction could be considered an evolutionary dead-end. In Cryptococcus, this assumption is challenged, as unisexual reproduction can generate genotypic and phenotypic diversity de novo by forming aneuploid progeny through meiosis [17]. Given that more than 99% of the natural isolates are α mating type, the presence of a unisexual cycle allows a clonal population to adapt to changing environments, which provides ecological significance to the Cryptococcus pathogenic species complex [46]. In this study, we demonstrated that a small population of cells undergo cell-cell fusion and nuclear fusion during unisexual reproduction, which enables recombination between cells of the same mating type. In response to selection pressures in the environment, the cell fusion dependent unisexual reproduction could facilitate selection of beneficial alleles in a large same sex population and reverse Muller’s ratchet [22]. Same sex cell-cell fusion can be further stimulated by the presence of small population of the opposite mating type [7]. Besides the similar ecological benefits conferred by unisexual and bisexual reproduction, many studies have shown that both modes of sexual cycles share a common signaling network that regulates the yeast-to-hyphal morphological transition and meiotic recombination [25, 26, 47]. Despite the similarities, there are key mechanistic differences between the two. In this study, we focused on two key cellular processes involved in sexual reproduction, cell-cell fusion and nuclear fusion, and studied their involvement in unisexual and bisexual reproduction in two sister Cryptococcus species harboring different sexual cycles.
Cryptococcus orthologs of the S. cerevisiae cell fusion gene PRM1 perform conserved roles during Cryptococcus sexual reproduction. Prm1 facilitates cell fusion between a and α mating partner cells, cell fusion between α-α cells, and clamp cell-hyphal fusion during dikaryotic hyphal growth. During C. neoformans bisexual reproduction, deletion of PRM1 caused a bilateral (prm1Δ X prm1Δ) cell fusion defect, which is similar to what has been observed in S. cerevisiae and N. crassa [29, 31]. However, during C. deneoformans bisexual reproduction, deletion of PRM1 caused both unilateral (prm1Δ X WT) and bilateral (prm1Δ X prm1Δ) cell fusion defects, suggesting that Prm1 plays a more significant role in C. deneoformans.
Cell-cell fusion and clamp cell-hyphal fusion in Cryptococcus is analogous to cell fusion between conidial anastomosis tubes and hyphal fusion in filamentous fungi [48, 49]. Like in S. cerevisiae, N. crassa, and S. pombe, deletion of PRM1 resulted in plasma membrane curvature at the membrane merger site (Fig 4D), but these membranes were separated by a layer of cell wall (Fig 4G and S6 Fig), similar to the prm1 mutant phenotype in S. pombe. Although deletion of PRM1 caused a cell fusion defect, it did not completely block cell fusion in Cryptococcus, suggesting that Prm1 is not the sole membrane fusion protein. Additional candidate cell fusion genes have been identified in S. cerevisiae and N. crassa, including FIG1, LFD1, and LFD2; but BLASTP searches failed to identify homologs of these genes in Cryptococcus [31, 32]. Prm1 may be the evolutionary conserved core component for cell fusion in the fungal kingdom, and species-specific plasma membrane fusion machinery may have evolved independently.
Similarly, the Cryptococcus karyogamy machinery has been previously shown to function differently than that of S. cerevisiae [38]. Deletion of KAR5 did not completely block either unisexual or bisexual reproduction, suggesting that additional karyogamy genes may have redundant functions with KAR5. The nuclear morphology inside cnkar5 mutant basidia and cdkar5 mutant early fusion products is similar to the kar5 mutant karyogamy defect phenotype in S. cerevisiae, indicating KAR5 plays a conserved role in nuclear fusion between Saccharomyces and Cryptococcus [41]. During C. neoformans bisexual reproduction, deletion of KAR5 blocked nuclear fusion inside basidia, whereas, during C. deneoformans bisexual reproduction, deletion of KAR5 blocked nuclear fusion at an early developmental step and caused growth arrest for the cell fusion products leading to an apparent cell fusion defect. Early karyogamy in C. deneoformans wild type relieved the requirement for pheromone signaling for directing clamp cell-hyphal fusion during dikaryotic hyphal growth, and the pheromone expression level was rapidly reduced to a basal level in the wild type. However, deletion of KAR5 promoted dikaryotic hyphal growth, and as a consequence the pheromone signaling pathway in kar5 mutants was significantly upregulated compared to the wild type. The pheromone expression patterns validated KAR5’s function in karyogamy.
During C. neoformans and C. deneoformans bisexual reproduction, the involvement of KAR5 in nuclear fusion revealed that karyogamy machinery takes place at different sexual development stages between these two closely related sister species. As reported by Ning and colleagues, the Kar5 protein belongs to a divergent nuclear fusion protein family [43]. Neither CnKar5 nor CdKar5 share sequence similarities outside of the conserved CRD domain with Kar5 proteins from other ascomycetous fungi. Interestingly, the CnKar5 and CdKar5 protein sequences share 85% identity, compared to the average of 93% identity for the 5569 orthologs shared by these two sister species [50]. This suggests that the KAR5 gene has undergone more rapid divergent evolution. The divergence of these proteins may contribute to the mechanistic differences in the karyogamy machinery and may represent a barrier for inter-species nuclear fusion (Fig 9). Several diploid or aneuploid environmental and clinical hybrid isolates of the two Cryptococcus species have been reported, but the few that produce spores have a <10% germination rate [51]. Incompatibility in components of the karyogamy machinery may help to generate a physical barrier for mating and drive speciation events within the Cryptococcus species complex.
Fig 9. Sexual cycles in Cryptococcus.
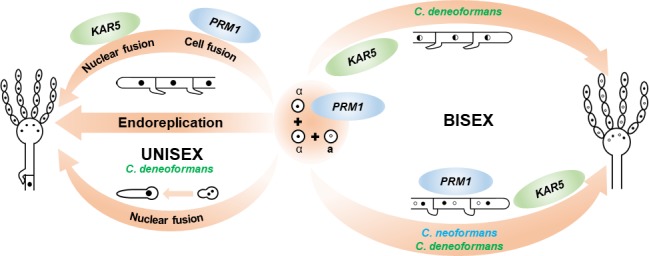
During C. neoformans bisexual reproduction, a-α cell-cell fusion generates dikaryotic hyphae and karyogamy occurs inside the basidia. During C. deneoformans bisexual reproduction, karyogamy takes place at different stages and generates both dikaryotic and monokaryotic diploid hyphae. During C. deneoformans unisexual reproduction, diploidization in the hyphae is achieved early during differentiation through either endoreplication or cell fusion-independent karyogamy events. Cell fusion plays less significant roles during solo unisexual reproduction.
Although we validated the conserved roles for PRM1 and KAR5, neither is the sole fusion protein for plasma membrane fusion or nuclear membrane fusion; and deletion of these two factors caused different impacts on bisexual cycles in Cryptococcus (Fig 9). In C. neoformans, Prm1 participates in cell-cell fusion during the initial mating process and mediates clamp cell-hyphal fusion, which is required for maintaining dikaryotic hyphal growth, and Kar5 functions in karyogamy inside the basidia during bisexual reproduction. whereas, in C. deneoformans, Prm1 plays a more significant role in cell-cell fusion, and Kar5 can function in karyogamy immediately following cell fusion, which produces monokaryotic diploid hyphae (Fig 9). However, the observed monokaryotic hyphae could be derived from unisexual reproduction, as pheromone produced by cells of the opposite mating type can promote unisexual reproduction [7]. To address this, we used GFP- and mCherry-labeled nuclear markers to show that the nuclei inside of the monokaryotic hyphae are indeed karyogamy products labeled with both fluorescent markers and thus the products of bisexual reproduction (Fig 6). Collectively, these results demonstrated that there are major differences in both the cell fusion machinery and the karyogamy program during bisexual reproduction between these two closely related sister species (Fig 9).
In contrast to bisexual reproduction, deletion of PRM1 did not cause a significant phenotypic defect during solo unisexual reproduction in C. deneoformans. Although PRM1 was highly upregulated during the unisexual cycle, α-α cell fusion occurred at a 1000-fold lower frequency compared to a-α cell fusion. Furthermore, live cell imaging of yeast cell germination during unisexual reproduction provided compelling evidence that the yeast-hyphal morphological transition is largely independent of cell-cell fusion. It is likely PRM1 may be a fortuitous transcriptional target during unisexual reproduction. However, it is worth noting that those cells that undergo cell-cell fusion do complete the unisexual cycle follow a pathway similar to the bisexual mating mechanism in C. deneoformans, and both PRM1 and KAR5 mediate cell-cell and nuclear fusion during modes of unisexual reproduction that results from α-α cell fusion as detected with genetically marked strains.
In bisexual reproduction, pheromone expression is dampened by the formation of the transcription factor complex Sxi1α-Sxi2a after a-α cell fusion [52]. Interestingly, pheromone expression was also dampened quickly during unisexual reproduction, but the transcriptional downregulation trigger must differ from bisexual reproduction because the opposite mating type was absent. During bisexual reproduction in both C. neoformans and C. deneoformans, KAR5 expression was upregulated and dampened by PRM1 deletion. KAR5 expression was maintained at a basal level and was not affected by the deletion of PRM1 during unisexual reproduction. Furthermore, deletion of KAR5 did not change basidia nuclear morphology compared to wild type, demonstrating that KAR5 is not required for unisexual reproduction. The fact that wild type, the kar5 mutant, and the kar7 mutant produced very few basidia with the two nuclei, indicating either that karyogamy does not occur inside the basidia during unisexual reproduction or that karyogamy occurs transiently and it is hard to capture by DAPI staining or nucleolar fluorescent marker Nop1-GFP. Interestingly, FACS analyses showed that the majority of blastospores produced along the hyphae from unisexual reproduction were diploid, supporting the hypothesis that nuclear fusion does not occur inside the basidium. Despite the fact that deletion of KAR5 does not impact unisexual reproduction and nuclear fusion does not occur inside basidium, we can not entirely rule out that karyogamy could occur during unisexual reproduction, as deletion of KAR5 did not completely block karyogamy during bisexual reproduction, and karyogamy genes in Cryptococcus share redundant functions [38]. Karyogamy occurs early during C. deneoformans bisexual reproduction, and it could also occur early in mother and daughter cells or growing hyphae, which leads to ploidy duplication. However, we favor the interpretation that karyogamy is dispensable for solo unisexual reproduction and an endoreplication pathway, which has been implicated in the formation of polyploid titan cells during Cryptococcus animal infection, contributes to ploidy duplication [53, 54] (Fig 9), which must be differentially controlled compared to titan cell formation, as titan cells reach a much higher ploidy [53].
With the ability to undergo both unisexual and bisexual reproduction, Cryptococcus serves as a model system to study the mating mechanisms for different sexual cycles. Our findings reveal the evolutionary differences in bisexual reproduction within the Cryptococcus species complex and suggest that the unisexual mating mechanism is plastic and complex, providing mechanistic insights to studies of mating mechanisms of unisexual reproduction and parthenogenesis in other eukaryotic systems.
Materials and methods
Strains, media, and growth conditions
Strains and plasmids used in this study are listed in S1 Table. All strains used to study bisexual reproduction in C. neoformans were generated in the congenic MATα H99 and MATa KN99 strain backgrounds [33]. All strains used to study bisexual reproduction in C. deneoformans were generated in the congenic MATα JEC21 and MATa JEC20 strain backgrounds [55]. All strains used to study unisexual reproduction in C. deneoformans were generated in the MATα XL280 strain background [7]. Yeast cells were grown at 30°C on Yeast extract Peptone Dextrose (YPD) medium. Strains harboring dominant selectable markers were grown on YPD medium supplemented with nourseothricin (NAT) or G418 (NEO). Mating assays were performed on either 5% V8 juice agar medium (pH = 5.0 for C. neoformans and pH = 7.0 for C. deneoformans) or Murashige and Skoog (MS) medium minus sucrose (Sigma-Aldrich) in the dark at room temperature for the designated time period.
Bioinformatics and phylogenetic analysis
To identify the PRM1 orthologs in C. neoformans and C. deneoformans, BLASTP searches using the S. cerevisiae, S. pombe, C. albicans, N. crassa, and A. fumigatus Prm1 protein sequences were conducted against C. neoformans H99 and C. deneoformans JEC21 genomes on FungiDB (www.fungidb.org) [56]. This approach identified CNAG_05866 in C. neoformans and CNF01070 for C. deneoformans as candidate PRM1 genes. Reciprocal BLAST searches confirmed that these two genes are PRM1 orthologs in Cryptococcus spp. Phobius prediction suggested that both CdPrm1 and CnPrm1 have four transmembrane domains at the same amino acid positions (67–87, 352–371, 433–455, and 647–688) [44].
To identify the KAR5 othologs in C. neoformans and C. deneoformans, a BLASTP search using the P. graminis Kar5 protein sequence against the C. neoformans H99 genome identified CNAG_04850 as a candidate KAR5 gene for C. neoformans. However, the same BLASTP search failed to identify a candidate KAR5 gene for C. deneoformans. A subsequent BLASTP search using the C. neoformans KAR5 gene sequence against the C. deneoformans JEC21 genome identified a region from bp 790071 to 792560 on chromosome 10 encoding a protein that shares 85% identity with the C. neoformans candidate Kar5 protein sequence. Multiple sequence alignment of candidate Cryptococcus Kar5 protein sequences with predicted Kar5 protein sequences from other fungal species using the MUSCLE program confirmed they contain Cysteine Rich Domain (CRD) [43, 57]. Phylogenetic analyses for Prm1 and Kar5 were tested with 1000 bootstrap replicas by using the maximum likelihood method in MEGA7 [58, 59]. Phobius prediction predicted that both CdKar5 and CnKar5 have an N-terminal signal peptide and a C-terminal transmembrane domain at amino acid positions 1–16 and 476–501 for CdKar5, and 1–21 and 477–502 for CnKar5 [44]. The COILS/PCOILS program predicted that CdKar5 has four coiled-coil domains at amino acid positions 179–199, 216–236, 318–339, and 368–389, and that CnKar5 has two coiled-coil domains at amino acid positions 180–200 and 370–390 [45].
Gene disruption and fluorescent protein expression
S1 Table and S2 Table lists the plasmids and primers, respectively, used in this study. To generate deletion mutants for genes of interest, deletion constructs consisting of the 5’ and 3’ regions of the targeted genes flanking an appropriate selection marker (NAT or NEO cassette) were generated by overlap PCR as previously described [60]. The deletion constructs were introduced into the respective strains via biolistic transformation as previously described [61]. Stable transformants were selected on YPD medium supplemented with NAT (100 mg/L) or G418 (200 mg/L). Gene replacements by homologous recombination were confirmed by PCR and Southern hybridization. To generate C. deneoformans wild type strains with dominant selectable markers for cell fusion assays, an analogous method was used to insert a dominant selectable marker (NAT cassette) into the intergenic region immediately downstream of the URA5 gene (CNG03730) and a dominant selectable marker (NEO cassette) into the intergenic region between CNE02520 and CNE02530, which is downstream of the ADE2 gene (CNE02500).
To visualize the cytosol in Cryptococcus, a plasmid encoding the cytosolic mCherry gene and containing a dominant selectable marker (NEO cassette) was generated. The mCherry coding sequence was amplified from pLKB25 [62] and inserted into pXL1 after the GPD1 promoter using the Gibson assembly method, which assembles multiple DNA fragments with 20 to 40 bp overlap sequences in a single reaction containing exonuclease, DNA polymerase, and ligase [63], resulting in pCF1. To monitor nuclear morphology and dynamics during Cryptococcus sexual reproduction, plasmid pSL04 encoding a GFP-tagged nucleolar protein Nop1 from a previous study [38] and a plasmid encoding an mCherry-tagged histone H3 were used. To express the H3-mCherry chimera, the 1075-bp 5’UTR and the 683-bp 3’UTR of the H3 gene were used as promoter (P) and terminator (T), respectively. The H3 promoter and coding sequences before the stop codon and the H3 terminator sequence were amplified from JEC21α genomic DNA, and the mCherry coding sequence was inserted between the H3 coding sequence and H3 terminator by overlap PCR. The chimera expression cassette H3P-H3-mCherry-H3T was then inserted into pAI3 using the Gibson assembly method [63], resulting in pCF9. C. deneoformans strains were biolistically transformed with the pCF1, pSL04, and pCF9 plasmids, and the fluorescent protein expression cassettes were randomly inserted into the genomes. Stable transformants were screened based on fluorescent signals and the selectable markers.
Cell-cell fusion assay
In C. neoformans bisexual reproduction, YSB119 (H99α aca1Δ::NAT ura5 ACA1-URA5) and YSB121 (KN99a aca1Δ::NEO ura5 ACA1-URA5) were used as genetically marked wild type strains to study the fusion competency of prm1 (CF56 and CF562) and kar5 (CF57 and CF549) mutants. In C. deneoformans bisexual reproduction, CF757 (JEC20a URA5-NAT) and CF762 (JEC21α ADE2-NEO) were used as wild type strains to study the fusion competency of prm1 (CF1 and CF313) and kar5 (CF487 and CF364) mutants. InC. deneoformans unisexual reproduction, CF750 (XL280α URA5-NAT) and CF752 (XL280α ADE2-NEO) were used as wild type strains to study the fusion competency of prm1 (CF317 and CF659) and kar5 (CF150 and CF260) mutants. Strains for each fusion pair were grown overnight in YPD liquid medium at 30°C. Cells were washed twice with ddH2O and diluted to a final density of OD600 = 2. Then, 50 μl of equal-volume mixed cells were spotted on V8 medium and incubated for 48 hours (for bisexual reproduction) or 72 hours (for unisexual reproduction) in the dark at room temperature. The cells were then removed, washed with ddH2O, and plated in serial dilution on both YPD medium and YPD medium supplemented with both NAT and G418. The cells were incubated for five days at room temperature. Cell-cell fusion frequency was measured by counting the average number of double drug resistant cfu/total cfu.
To quantify the cell-cell fusion frequency during C. deneoformans bisexual reproduction based on fluorescent signal mixing, CF830 (JEC21α NOP1-GFP-NAT) was mated with JEC20a for wild type fusion frequency, CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) was mated with either JEC21α for prm1 mutant unilateral cell fusion frequency or with CF1 (JEC21α prm1Δ::NEO) for prm1 mutant bilateral cell fusion frequency, and CF723 (JEC20a kar5Δ::NEO NOP1-GFP-NAT) was mated with CF487 (JEC21α kar5Δ::NEO) for kar5 mutant bilateral cell fusion frequency. Cells were prepared as described above and collected for direct fluorescence microscopic observation after 24 hours of incubation. Approximately 100 fusion events were recorded for each mating and were identified by the presence of conjugation tubes connecting the fusion pairs. Fusion frequency was determined by the number of fusion pairs with Nop1-GFP labeled nuclei in both cellular compartments/total fusion events.
Percoll gradient purification of spores
To determine whether prm1 and kar5 mutants were defective in spore production, spores were isolated by Percoll gradient centrifugation as previously described [64]. For C. neoformans bisexual reproduction, CF56 (H99α prm1Δ::NAT) crossed with CF562 (KN99a prm1Δ::NEO) and CF57 (H99α kar5Δ::NAT) crossed with CF549 (KN99a kar5Δ::NEO) were compared to the wild type cross between H99α and KN99a. For C. deneoformans bisexual reproduction, CF1 (JEC21α prm1Δ::NEO) crossed with CF313 (JEC20a prm1Δ::NAT) and CF487 (JEC21α kar5Δ::NEO) crossed with CF364 (JEC20a kar5Δ::NAT) were compared to wild type cross between JEC21α and JEC20a. For C. deneoformans unisexual mating, CF317 (XL280α prm1Δ::NEO) and CF260 (XL280α kar5Δ::NEO) were compared to the wild type XL280α. For each mating, triplicates were performed for statistical analysis. Strains were grown overnight in YPD liquid medium. Cells were washed twice with ddH2O and diluted to a final cell density of OD600 = 0.5. Then, 10 μl of equal-volume mixed cells were spotted on V8 medium and incubated for seven days in the dark at room temperature. The entire mating patch was suspended in 60% Percoll (GE Health) in PBS with 0.1% Triton X100. After centrifugation at 10,000 X g for 30 mins in an SW41Ti ultracentrifuge rotor (Beckman-Coulter), a band of spores near the bottom of the Percoll gradient was recovered with a 1-ml tuberculin syringe and transferred into an Eppendorf tube. The total spore production was determined by multiplying the spore density, measured by hemocytometer, with the final volume.
Wild type matings between CF757 (JEC20a URA5-NAT) and CF762 (JEC21α ADE2-NEO) were conducted as controls. The isolated cells were serially diluted and plated on YPD medium and allowed to recover for five days at 30°C. A total of 47 colonies were randomly chosen and grown on YPD medium supplemented with either NAT or G418 to assess growth phenotypes (S2 Fig). Mating type specific primer pairs were used to determine the MAT locus for the progeny.
RNA extraction and RT-PCR
For all three modes of sexual reproduction studied, prm1 and kar5 mutant strains and wild type strains were grown overnight in YPD liquid medium. Cells were washed twice with ddH2O and diluted to OD600 = 2. Then 250 Δl of an equal-volume mixture of cells were spotted on V8 medium or YPD medium and incubated for 36 hours (YPD and V8) or one week (V8), as the pheromone pathway has been shown to be upregulated upon mating induction on V8 medium and the expression levels are maintained at relatively high levels between 24 and 48 hours [8]. Mating patches were harvested and flash frozen in liquid nitrogen. RNA was extracted using TRIzol reagent (Thermo) following the manufacturer’s instructions. RNA was treated with Turbo DNAse (Ambion), and single-stranded cDNA was synthesized by AffinityScript RT-RNAse (Stratagene). For each sample, cDNA synthesized without the RT/RNAse block enzyme mixture was used as a “no RT control” to control for genomic DNA contamination. The relative expression level of target genes was measured by quantitative real-time PCR using Brilliant III ultra-fast SYBR green QPCR mix (Stratagene) in an Applied Biosystems 7500 Real-Time PCR System. For each target, a “no template control” was performed to analyze melting curves to exclude primer artifacts. Technical triplicates and biological triplicates were performed for each sample. Gene expression levels were normalized using the endogenous reference gene GPD1 and determined by using the comparative ΔΔCt method. The primers used for RT-PCR are listed in S2 Table. The Student’s t-test was used to determine if the relative gene expression levels between different strains exhibited statistically significant differences (P <0.05).
Nuclear and plasma membrane staining
To visualize the nuclei during sexual reproduction, cells were stained with DAPI as previously described [62]. In brief, a 1-mm3 MS agar block containing hyphae on the edge of mating patches was excised and transferred to a small petri dish. The agar block was fixed in 3.7% formaldehyde and permeabilized in 1% Triton X100. The agar block was stained with 2 Δg/ml DAPI (Sigma) and transferred to a glass slide and covered with a cover slip for fluorescent microscopic observation.
To visualize the plasma membrane of the conjugation tubes during C. deneoformans prm1 mutant bisexual reproduction, strain CF1 (JEC21α prm1Δ::NEO) was crossed with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). After incubation on V8 medium for 24 hours, cells were harvested and resuspended in cold YPD liquid medium on ice. FM4-64 (Thermo) was added at a final concentration of 10 μM and the cells were stained on ice for 15 mins. The cells were then washed with cold YPD medium and fixed in 3.7% formaldehyde in PBS for 10 mins. After a final wash with PBS, the stained cells were examined immediately by confocal microscopy.
Microscopy
Hyphal growth on the edge of mating patches, basidia, and spore chains were captured using a Nikon Eclipse E400 microscope equipped with a Nikon DXM1200F camera.
For fluorescence imaging of hyphae, an agar block supporting hyphal growth was excised and transferred onto a glass slide and covered with a coverslip. For fluorescence imaging of short early hyphae and fusion pairs, early mating patches were harvested and suspended in ddH2O and cells were placed on a glass slide containing a 2% agar patch and covered with a coverslip. Fluorescent images were obtained using a Deltavision system (Olympus IX-71 base) equipped with a Coolsnap HQ2 high resolution SSD camera. Images were processed using the software FIJI.
Confocal fluorescent images were captured by confocal laser scanning microscopy using a Zeiss LSM 710 Confocal Microscope at the Duke Light Microscopy Core Facility. Plan-Apochromat 63X/1.40 Oil DIC M27 objective lenses were used for imaging, and a smart setup was used for image acquisition configuration. Confocal fluorescent images and movies were processed using the ZEN software.
SEM and TEM were performed at the North Carolina State University Center for Electron Microscopy, Raleigh, NC, USA. Samples were prepared for SEM as previously described [8]. In brief, 1-mm3 MS agar blocks containing hyphae on the edge of mating patches were excised and fixed in 0.1 M sodium cacodylate buffer, pH = 6.8, containing 3% glutaraldehyde at 4°C for several weeks. Before viewing, the agar block was rinsed with cold 0.1 M sodium cacodylate buffer, pH = 6.8 three times and post-fixed in 2% osmium tetroxide in cold 0.1 M cacodylate buffer, pH = 6.8 for 2.5 hours at 4°C. Then the block was critical-point dried with liquid CO2 and sputter coated with 50 Å of gold/palladium using a Hummer 6.2 sputter coater (Anatech). The samples were viewed at 15KV with a JSM 5900LV scanning electron microscope (JEOL) and captured with a Digital Scan Generator (JEOL) image acquisition system. For TEM, conjugation tubes were prepared by crossing strain CF712 (JEC21α prm1Δ::NAT mCherry-NEO) with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). After incubation on V8 medium for 24 hours, cells were harvested and analyzed with a B-C Astrios Sorter to enrich fusion pairs that were positive for both GFP and mCherry fluorescence at the Duke Cancer Institute Flow Cytometry Shared Resource. Hyphae were prepared by crossing strain CF56 (H99α prm1Δ::NAT) with CF562 (KN99a prm1Δ::NEO). After incubation on V8 medium for four weeks, hyphae on the edge of the mating patches were harvested for observation of clamp cell morphology. Upon harvest, cells or hyphae were immediately fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH = 6.8, at 4°C for several weeks. The sample preparation was performed as previously described [62]. In brief, cells were post-fixed with 4% KMnO4 and pre-embedded in 2% agarose. After dehydration with an increasing gradient of ethanol solutions and filtration with Spurr’s resin, the agarose block was embedded in 100% Spurr’s in BEEF capsules. Thin sections were cut and collected on 200-mesh grids, followed by staining with 4% aqueous uranyl acetate and Reynold’s lead citrate. Grids were viewed using a Philips 400T transmission electron microscope. TEM images were processed with Photoshop (Adobe).
Flow cytometry
Ploidy of blastospores was determined by Fluorescence Activated Cell Sorting (FACS) analysis as previously described [65]. XL280α and MN142.6 (XL280α/α ura5Δ::NAT/ura5Δ::NEO) were used as haploid and diploid controls respectively. Dissected blastospores were grown on YPD medium between three and five days at 30°C to yield colonies. Cells were harvested and washed with PBS buffer. After fixation in 70% ethanol at 4°C overnight, cells were washed once with 1 ml of NS buffer (10 mM Tris-HCl, pH = 7.2, 250 mM sucrose, 1 mM EDTA, pH = 8.0, 1 mM MgCl2, 0.1 mM CaCl2, 0.1 mM ZnCl2, 0.4 mM phenylmethylsulfonyl fluoride, and 7 mM β-mercaptoethanol), and stained in 180 μl NS buffer with 20 μl 10 mg/ml RNase and 5 μl 0.5 mg/ml propidium iodide at 4°C overnight. Then, 50 μl stained cells were diluted in 2 ml of 50 mM Tris-HCl, pH = 8.0 and sonicated for 1 min. For each sample, 10,000 cells were analyzed on the FL1 channel on the Becton-Dickinson FACScan at Duke Cancer Institute Flow Cytometry Shared Resource. Data analysis was performed using the software FlowJo.
Supporting information
(A) We identified PRM1 homologs for C. neoformans (CNAG_05866) and C. deneoformans (CNF01070) using BLASTP searches of Prm1 protein sequences from S. cerevisiae, S. pombe, C. albicans, N. crassa, and A. fumigatus against the C. neoformans and C. deneoformans protein databases. BLASTP and reciprocal BLASTP E-values for CNAG_05866 are listed. (B) Phylogenetic analysis of Prm1 protein sequences based on the maximum likelihood method in MEGA7. The percentage of trees in which the associated taxa clustered together is shown at each split. Branch length indicates the number of substitutions per site. (C) Identification of the KAR5 genes for C. neoformans (CNAG_04850) and C. deneoformans by BLASTP searches of Kar5 protein sequences from S. cerevisiae, S. pombe, C. albicans, N. crassa, A. fumigatus, and P. graminis against the C. neoformans and C. deneoformans protein databases. BLASTP and reciprocal BLASTP E-values for CNAG_04850 are listed. Only the P. graminis Kar5 protein sequence showed sequence similarity with C. neoformans and C. deneoformans Kar5 protein sequences. (D) Phylogenetic analysis of Kar5 protein sequences from eight fungal species based on the maximum likelihood method in MEGA7. Node and branch annotations for Kar5 tree are the same as for the Prm1 tree. (E) Multiple sequence alignment of Kar5 protein sequences using the MUSCLE alignment program revealed the conserved Cysteine Rich Domain (CRD) for the distantly related Kar5 proteins in the eight fungal species included.
(TIF)
(A) A wild type cross between H99α and KN99a and two independent prm1 bilateral mutant crosses (between CF30 and CF448, and between CF56 and CF562) were incubated on MS medium in the dark at room temperature for 10 days. (B) A wild type cross between H99α and KN99a and two independent kar5 bilateral mutant crosses (between CF57 and CF549, and between CF208 and CF305) were incubated on MS medium in the dark at room temperature for 10 days. The scale bar is 100 μm.
(TIF)
(A) Relative spore production of prm1 mutants and (B) Relative spore production of kar5 mutants compared to wild type after 7-days incubation on V8 medium.
(TIF)
Spores from a wild type cross CF757 (JEC20a URA5-NAT) and CF762 (JEC21α ADE2-NEO) were isolated following seven days of co-incubation. To test recombination among F1 progeny, 47 spore-derived colonies were randomly chosen for phenotypic and genetic analysis. (A) The 47 progeny were grown on YPD medium supplemented with NAT or G418 to test for selectable marker inheritance. (B) Mating type for each progeny was determined by MAT locus specific primer sets. Genotypes for each progeny are provided in the grid in the same order as the progeny were grown on the YPD medium. (C) Parental and non-parental genotypes are summarized in the graphical table.
(TIF)
CF56 (H99α prm1Δ::NAT) was mated with CF562 (KN99a prm1Δ::NEO) on V8 medium for four weeks and hyphae on the edge of the mating patch were collected for TEM. (A) Cross section of a fused clamp cell morphology is provided on the left, and the diagram is shown on the right. The scale bar is 2 μm. (B) Plasma membrane structures at three unfused conjugation sites were further examined at higher magnification. The diagram for the clamp cell cross section is provided on the right. In the left panels, the scale bars are 2 μm, and in the middle panels, the scale bars are 0.5 μm.
(TIF)
Equal number of cells for each fusion pair were mixed and incubated on V8 medium for 24 hours. Cells were harvested and examined under fluorescent microscope to determine cell fusion frequency based on Nop1-GFP fluorescent signal intermixing between each fusion pair. (A) Wild type cell fusion between CF830 (JEC21α NOP1-GFP-NAT) and JEC20a. (B) Unilateral cell fusion between JEC21α and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). (C) Bilateral mating between CF1 (JEC21α prm1Δ::NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). (D) Bilateral mating between CF487 (JEC21α kar5Δ::NEO) and CF723 (JEC20a kar5Δ::NEO NOP1-GFP-NAT). Closed shapes (left panels) and arrows (right panels) indicate successful cell fusion pairs, whereas open shapes (left panels) and arrowheads (right panels) indicate unfused cell fusion pairs. The scale bars are 20 μm.
(TIF)
(A) Plasma membrane structures of unfused yeast cells during C. deneoformans bisexual reproduction as visualized by transmission electron microscopy. Unfused cell fusion pairs between CF712 (JEC21α prm1Δ::NAT mCherry-NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) were examined by transmission electron microscopy. Plasma membrane structures at the conjugation sites were further examined at higher magnification. The diagram for the fusion pair cross section is provided on the right. For the left panels, the scale bars are 2 μm, and for the middle panels, the scale bars are 0.5 μm. (B) prm1 mutants are defective in clamp cell fusion during C. deneoformans bisexual reproduction. SEM of the unfused clamp cell morphology for the wild type cross (JEC21α X JEC20a) and of the defective clamp cell fusion morphology for the prm1 mutant cross (CF1 X CF313). The scale bar is 5 μm.
(TIF)
(A) Wild type cross between JEC20a and JEC21α, prm1 mutant cross between CF1 and CF313, and kar5 mutant cross between CF226 and CF364 for bisexual reproduction, and (B) Wild type strain XL280α, prm1 mutant CF659, and kar5 mutant CF260 were incubated on V8 medium in the dark at room temperature for four weeks to generate hyphae and basidia from unisexual reproduction. DAPI staining showed wild type, prm1 mutants, and kar5 mutants all produced monokaryotic hyphae during both unisexual and bisexual reproduction. The scale bar is 5 μm.
(TIF)
Wild type cross CF830 (JEC21α NOP1-GFP-NAT) X JEC20a, prm1 bilateral mutant cross CF1 (JEC21α prm1Δ::NEO) X CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and kar5 bilateral mutant cross CF487 (JEC21α kar5Δ::NEO) X CF723 (JEC20a kar5Δ::NAT NOP1-GFP-NAT) were examined by direct fluorescence microscopy to track hyphal nuclear morphology at different stages of sexual reproduction. (A) At 48 hours, wild type and prm1 mutants produced both monokaryotic and dikaryotic hyphae (arrows point to monokaryotic hyphae, and arrowhead points to mitotically dividing dikaryotic wild type hyphae). kar5 mutants produce hyphae with two nuclei in close contact (arrows). (B) At 10 days, the wild type cross produced both monokaryotic and dikaryotic hyphae, prm1 mutants mainly produced monokaryotic hyphae, and kar5 mutants mainly produced dikaryotic hyphae. (C) At six weeks, wild type and prm1 mutants mainly produced monokaryotic hyphae, and kar5 mutants produced both monokaryotic and dikaryotic hyphae. The scale bar is 5 μm.
(TIF)
(A) Representative basidia containing one nucleus (left panel), or two nuclei (middle panel), or more than two nuclei (right panel) with DAPI staining are shown for wild type XL280α, kar5 mutant (CF260), and kar7 mutant (SL277). Arrows point to DAPI stained nuclei inside basidia. The scale bars are 5 μm. (B) Basidia containing one nucleus, or two nuclei, or more than four post-meiotic nuclei were quantified for the above strains.
(TIF)
(A) Representative basidia containing one nucleus (left panel), or two nuclei (middle panel), or more than two nuclei (right panel) with the nucleolar marker Nop1-GFP fluorescent signals are shown for wild type XL280α (CF836), kar5 mutant (CF718), and kar7 mutant (CF1442). Arrows point to Nop1-GFP signal inside basidia. The scale bars are 5 μm. (B) Basidia containing one nucleus, or two nuclei, or more than two nuclei were quantified for the above strains.
(TIF)
(A) prm1 mutant (CF317) and two independent prm1 spo11 double mutants (CF894 and CF901), and (B) kar5 mutant (CF260) and two independent kar5 spo11 double mutants (CF883 and CF884) were incubated on V8 medium in the dark at room temperature for four weeks. prm1 spo11 double mutants and kar5 spo11 double mutants produced two spore chains compared to the four spore chains produced by prm1 or kar5 mutants. Schemes showing wild type and mutant sporulation patterns were provided at the lower left corner of each image. The scale bar equals 10 μm.
(TIF)
The upper panels are FACS results for haploid control XL280α and diploid control MN142.6 α/α. The lower panels are representative FACS results for haploid, aneuploid, and diploid blastospores produced by the kar7 mutant.
(TIF)
CF712 (JEC21α prm1Δ::NAT mCherry-NEO) was mated with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and the cytosolic mCherry fluorescence signal and nucleolar marker Nop1-GFP signal were intermixed in the fusion pair.
(AVI)
CF712 (JEC21α prm1Δ::NAT mCherry-NEO) was mated with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and the cytosolic mCherry fluorescence signal and nucleolar marker Nop1-GFP signal were restricted to distinct cellular compartments.
(AVI)
Cells from CF836 (XL280α NOP1-GFP-NAT) and CF1091 (XL280α H3-mCherry-NAT) were mixed and co-incubated on V8 medium for 24 hours at room temperature. Early hyphae were examined for nuclear fluorescence under confocal microscope for 30 minutes.
(AVI)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are indebted to Soo Chan Lee for help with identification of KAR5 and valuable discussions. We would like to acknowledge Sheng Sun and R. Blake Billmyre for insightful discussions, and Anna Floyd-Averette, Shelly Clancey, Sheng Sun, and Marianna Feretzaki for technical support. We thank Connie Nichols, Sheng Sun, and Shelby Priest for critical reading of the manuscript. We are also grateful to Valerie Knowlton and the NC State EM core laboratory for SEM and TEM analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by NIH/NIAID grants R37 AI39115-20 and R01 AI50113-13 to JH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Valen L. A new evolutionary law. Evol Theory 1973;1:1–30. [Google Scholar]
- 2.Neaves WB, Baumann P. Unisexual reproduction among vertebrates. Trends Genet. 2011;27(3):81–8. doi: 10.1016/j.tig.2010.12.002 . [DOI] [PubMed] [Google Scholar]
- 3.Ene IV, Bennett RJ. The cryptic sexual strategies of human fungal pathogens. Nat Rev Microbiol. 2014;12(4):239–51. doi: 10.1038/nrmicro3236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitman J. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe. 2010;8(1):86–99. doi: 10.1016/j.chom.2010.06.011 ; PubMed Central PMCID: PMCPMC2916653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature. 2009;460(7257):890–3. doi: 10.1038/nature08252 ; PubMed Central PMCID: PMC2866515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976;68(4):821–33. . [PubMed] [Google Scholar]
- 7.Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434(7036):1017–21. Epub 2005/04/23. doi: 10.1038/nature03448 . [DOI] [PubMed] [Google Scholar]
- 8.Feretzaki M, Heitman J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLOS Genet. 2013;9(8):e1003688 doi: 10.1371/journal.pgen.1003688 ; PubMed Central PMCID: PMC3744442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz A, Bulmer GS. Particle size of airborn Cryptococcus neoformans in a tower. Appl Environ Microbiol. 1981;41(5):1225–9. ; PubMed Central PMCID: PMCPMC243893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz A, Fromtling RA, Bulmer GS. Distribution of Cryptococcus neoformans in a natural site. Infect Immun. 1981;31(2):560–3. ; PubMed Central PMCID: PMCPMC351344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phadke SS, Feretzaki M, Clancey SA, Mueller O, Heitman J. Unisexual reproduction of Cryptococcus gattii. PLOS ONE. 2014;9(10):e111089 doi: 10.1371/journal.pone.0111089 ; PubMed Central PMCID: PMC4206507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, Patel S, Litvintseva AP, Floyd A, Mitchell TG, Heitman J. Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLOS Pathog. 2009;5(1):e1000283 doi: 10.1371/journal.ppat.1000283 ; PubMed Central PMCID: PMC2629120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriconde F, Gilgado F, Arthur I, Ellis D, Malik R, van de Wiele N, et al. Clonality and alpha-a recombination in the Australian Cryptococcus gattii VGII population—an emerging outbreak in Australia. PLOS ONE. 2011;6(2):e16936 doi: 10.1371/journal.pone.0016936 ; PubMed Central PMCID: PMCPMC3044715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saul N, Krockenberger M, Carter D. Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single eucalyptus tree hollows. Eukaryot Cell. 2008;7(4):727–34. doi: 10.1128/EC.00020-08 ; PubMed Central PMCID: PMCPMC2292630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui T, Lin X, Malik R, Heitman J, Carter DA. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, α mating type populations. Eukaryot Cell. 2008;7(10):1771–80. doi: 10.1128/EC.00097-08 ; PubMed Central PMCID: PMC2568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lengeler KB, Wang P, Cox GM, Perfect JR, Heitman J. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc Natl Acad Sci USA. 2000;97(26):14455–60. doi: 10.1073/pnas.97.26.14455 ; PubMed Central PMCID: PMCPMC18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni M, Feretzaki M, Li W, Floyd-Averette A, Mieczkowski P, Dietrich FS, et al. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLOS Biol. 2013;11(9):e1001653 doi: 10.1371/journal.pbio.1001653 ; PubMed Central PMCID: PMC3769227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLOS Genet. 2010;6(5):e1000953 doi: 10.1371/journal.pgen.1000953 ; PubMed Central PMCID: PMC2869318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mead ME, Stanton BC, Kruzel EK, Hull CM. Targets of the sex inducer homeodomain proteins are required for fungal development and virulence in Cryptococcus neoformans. Mol Microbiol. 2015;95(5):804–18. doi: 10.1111/mmi.12898 ; PubMed Central PMCID: PMCPMC4339537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alspaugh JA, Nichols CB, Xue C, Shen W-C, Wang P. G-protein signaling pathways: Regulating morphogenesis and virulence of Cryptococcus In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, editors. Cryptococcus. Washington, DC: ASM Press; 2011. p. 153–65. [Google Scholar]
- 21.Sun S, Billmyre RB, Mieczkowski PA, Heitman J. Unisexual reproduction drives meiotic recombination and phenotypic and karyotypic plasticity in Cryptococcus neoformans. PLOS Genet. 2014;10(12):e1004849 doi: 10.1371/journal.pgen.1004849 ; PubMed Central PMCID: PMCPMC4263396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roach KC, Heitman J. Unisexual reproduction reverses Muller's ratchet. Genetics. 2014;198(3):1059–69. doi: 10.1534/genetics.114.170472 ; PubMed Central PMCID: PMCPMC4224152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc Natl Acad Sci USA. 1996;93(14):7327–31. ; PubMed Central PMCID: PMC38983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin X. Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol. 2009;9(4):401–16. doi: 10.1016/j.meegid.2009.01.013 . [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Lin X. Mechanisms of unisexual mating in Cryptococcus neoformans. Fungal Genet Biol. 2011;48(7):651–60. doi: 10.1016/j.fgb.2011.02.001 . [DOI] [PubMed] [Google Scholar]
- 26.Fu C, Sun S, Billmyre RB, Roach KC, Heitman J. Unisexual versus bisexual mating in Cryptococcus neoformans: Consequences and biological impacts. Fungal Genet Biol. 2015;78:65–75. doi: 10.1016/j.fgb.2014.08.008 ; PubMed Central PMCID: PMCPMC4344436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, et al. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29(7):427–37. doi: 10.1016/j.tig.2013.01.011 ; PubMed Central PMCID: PMCPMC4022042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiman MG, Walter P. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J Cell Biol. 2000;151(3):719–30. ; PubMed Central PMCID: PMCPMC2185589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleissner A, Diamond S, Glass NL. The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions. Genetics. 2009;181(2):497–510. doi: 10.1534/genetics.108.096149 ; PubMed Central PMCID: PMC2644943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curto MA, Sharifmoghadam MR, Calpena E, De Leon N, Hoya M, Doncel C, et al. Membrane organization and cell fusion during mating in fission yeast requires multipass membrane protein Prm1. Genetics. 2014;196(4):1059–76. doi: 10.1534/genetics.113.159558 ; PubMed Central PMCID: PMCPMC3982680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar PS, Engel A, Walter P. The plasma membrane proteins Prm1 and Fig1 ascertain fidelity of membrane fusion during yeast mating. Mol Biol Cell. 2007;18(2):547–56. doi: 10.1091/mbc.E06-09-0776 ; PubMed Central PMCID: PMC1783792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma-Guerrero J, Leeder AC, Welch J, Glass NL. Identification and characterization of LFD1, a novel protein involved in membrane merger during cell fusion in Neurospora crassa. Mol Microbiol. 2014;92(1):164–82. doi: 10.1111/mmi.12545 . [DOI] [PubMed] [Google Scholar]
- 33.Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect Immun. 2003;71(9):4831–41. doi: 10.1128/IAI.71.9.4831-4841.2003 ; PubMed Central PMCID: PMCPMC187335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsueh YP, Xue C, Heitman J. G protein signaling governing cell fate decisions involves opposing Gα subunits in Cryptococcus neoformans. Mol Biol Cell. 2007;18(9):3237–49. doi: 10.1091/mbc.E07-02-0133 ; PubMed Central PMCID: PMC1951760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung KW, So YS, Bahn YS. Unique roles of the unfolded protein response pathway in fungal development and differentiation. Sci Rep. 2016;6:33413 doi: 10.1038/srep33413 ; PubMed Central PMCID: PMCPMC5024300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurihara LJ, Beh CT, Latterich M, Schekman R, Rose MD. Nuclear congression and membrane fusion: two distinct events in the yeast karyogamy pathway. J Cell Biol. 1994;126(4):911–23. ; PubMed Central PMCID: PMCPMC2120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ydenberg CA, Rose MD. Yeast mating: a model system for studying cell and nuclear fusion. Methods Mol Biol. 2008;475:3–20. doi: 10.1007/978-1-59745-250-2_1 . [DOI] [PubMed] [Google Scholar]
- 38.Lee SC, Heitman J. Function of Cryptococcus neoformans KAR7 (SEC66) in karyogamy during unisexual and opposite-sex mating. Eukaryot Cell. 2012;11(6):783–94. doi: 10.1128/EC.00066-12 ; PubMed Central PMCID: PMC3370462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung KW, Kang HA, Bahn YS. Essential roles of the Kar2/BiP molecular chaperone downstream of the UPR pathway in Cryptococcus neoformans. PLOS ONE. 2013;8(3):e58956 doi: 10.1371/journal.pone.0058956 ; PubMed Central PMCID: PMCPMC3590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beh CT, Brizzio V, Rose MD. KAR5 encodes a novel pheromone-inducible protein required for homotypic nuclear fusion. J Cell Biol. 1997;139(5):1063–76. ; PubMed Central PMCID: PMCPMC2140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melloy P, Shen S, White E, Rose MD. Distinct roles for key karyogamy proteins during yeast nuclear fusion. Mol Biol Cell. 2009;20(17):3773–82. doi: 10.1091/mbc.E09-02-0163 ; PubMed Central PMCID: PMCPMC2735476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers JV, Rose MD. Kar5p is required for multiple functions in both inner and outer nuclear envelope fusion in Saccharomyces cerevisiae. G3. 2014;5(1):111–21. doi: 10.1534/g3.114.015800 ; PubMed Central PMCID: PMCPMC4291462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning J, Otto TD, Pfander C, Schwach F, Brochet M, Bushell E, et al. Comparative genomics in Chlamydomonas and Plasmodium identifies an ancient nuclear envelope protein family essential for sexual reproduction in protists, fungi, plants, and vertebrates. Genes Dev. 2013;27(10):1198–215. doi: 10.1101/gad.212746.112 ; PubMed Central PMCID: PMCPMC3672651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kall L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338(5):1027–36. doi: 10.1016/j.jmb.2004.03.016 . [DOI] [PubMed] [Google Scholar]
- 45.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–25. . [DOI] [PubMed] [Google Scholar]
- 46.Kwon-Chung KJ, Bennett JE. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108(4):337–40. [DOI] [PubMed] [Google Scholar]
- 47.Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev. 2012;36(1):78–94. doi: 10.1111/j.1574-6976.2011.00286.x ; PubMed Central PMCID: PMC3318972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read ND, Fleissner A, Roca MG, Glass NL. Hyphal fusion In: Borkovich KA, Ebbole DJ, editors. Cellular and Molecular Biology of Filamentous Fungi. Washington, DC: ASM Press; 2010. p. 260–73. [Google Scholar]
- 49.Gabriela Roca M, Read ND, Wheals AE. Conidial anastomosis tubes in filamentous fungi. FEMS Microbiol Lett. 2005;249(2):191–8. doi: 10.1016/j.femsle.2005.06.048 . [DOI] [PubMed] [Google Scholar]
- 50.Janbon G, Ormerod KL, Paulet D, Byrnes EJ 3rd, Yadav V, Chatterjee G, et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLOS Genet. 2014;10(4):e1004261 doi: 10.1371/journal.pgen.1004261 ; PubMed Central PMCID: PMCPMC3990503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lengeler KB, Cox GM, Heitman J. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect Immun. 2001;69(1):115–22. doi: 10.1128/IAI.69.1.115-122.2001 ; PubMed Central PMCID: PMC97862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hull CM, Boily MJ, Heitman J. Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot Cell. 2005;4(3):526–35. doi: 10.1128/EC.4.3.526-535.2005 ; PubMed Central PMCID: PMCPMC1087792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol. 2013;16(4):409–13. doi: 10.1016/j.mib.2013.03.006 ; PubMed Central PMCID: PMC3723695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLOS Pathog. 2010;6(6):e1000953 doi: 10.1371/journal.ppat.1000953 ; PubMed Central PMCID: PMCPMC2887476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon-Chung KJ, Edman JC, Wickes BL. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60(2):602–5. ; PubMed Central PMCID: PMCPMC257671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stajich JE, Harris T, Brunk BP, Brestelli J, Fischer S, Harb OS, et al. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 2012;40(Database issue):D675–81. doi: 10.1093/nar/gkr918 ; PubMed Central PMCID: PMCPMC3245123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340 ; PubMed Central PMCID: PMCPMC390337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. doi: 10.1093/molbev/msw054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25(7):1307–20. doi: 10.1093/molbev/msn067 . [DOI] [PubMed] [Google Scholar]
- 60.Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, et al. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology. 2002;148(Pt 8):2607–15. doi: 10.1099/00221287-148-8-2607 . [DOI] [PubMed] [Google Scholar]
- 61.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175(5):1405–11. ; PubMed Central PMCID: PMCPMC193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozubowski L, Heitman J. Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans. Mol Microbiol. 2010;75(3):658–75. doi: 10.1111/j.1365-2958.2009.06983.x ; PubMed Central PMCID: PMCPMC3699866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–5. doi: 10.1038/nmeth.1318 . [DOI] [PubMed] [Google Scholar]
- 64.Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot Cell. 2009;8(4):595–605. doi: 10.1128/EC.00352-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka R, Taguchi H, Takeo K, Miyaji M, Nishimura K. Determination of ploidy in Cryptococcus neoformans by flow cytometry. J Med Vet Mycol. 1996;34(5):299–301. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) We identified PRM1 homologs for C. neoformans (CNAG_05866) and C. deneoformans (CNF01070) using BLASTP searches of Prm1 protein sequences from S. cerevisiae, S. pombe, C. albicans, N. crassa, and A. fumigatus against the C. neoformans and C. deneoformans protein databases. BLASTP and reciprocal BLASTP E-values for CNAG_05866 are listed. (B) Phylogenetic analysis of Prm1 protein sequences based on the maximum likelihood method in MEGA7. The percentage of trees in which the associated taxa clustered together is shown at each split. Branch length indicates the number of substitutions per site. (C) Identification of the KAR5 genes for C. neoformans (CNAG_04850) and C. deneoformans by BLASTP searches of Kar5 protein sequences from S. cerevisiae, S. pombe, C. albicans, N. crassa, A. fumigatus, and P. graminis against the C. neoformans and C. deneoformans protein databases. BLASTP and reciprocal BLASTP E-values for CNAG_04850 are listed. Only the P. graminis Kar5 protein sequence showed sequence similarity with C. neoformans and C. deneoformans Kar5 protein sequences. (D) Phylogenetic analysis of Kar5 protein sequences from eight fungal species based on the maximum likelihood method in MEGA7. Node and branch annotations for Kar5 tree are the same as for the Prm1 tree. (E) Multiple sequence alignment of Kar5 protein sequences using the MUSCLE alignment program revealed the conserved Cysteine Rich Domain (CRD) for the distantly related Kar5 proteins in the eight fungal species included.
(TIF)
(A) A wild type cross between H99α and KN99a and two independent prm1 bilateral mutant crosses (between CF30 and CF448, and between CF56 and CF562) were incubated on MS medium in the dark at room temperature for 10 days. (B) A wild type cross between H99α and KN99a and two independent kar5 bilateral mutant crosses (between CF57 and CF549, and between CF208 and CF305) were incubated on MS medium in the dark at room temperature for 10 days. The scale bar is 100 μm.
(TIF)
(A) Relative spore production of prm1 mutants and (B) Relative spore production of kar5 mutants compared to wild type after 7-days incubation on V8 medium.
(TIF)
Spores from a wild type cross CF757 (JEC20a URA5-NAT) and CF762 (JEC21α ADE2-NEO) were isolated following seven days of co-incubation. To test recombination among F1 progeny, 47 spore-derived colonies were randomly chosen for phenotypic and genetic analysis. (A) The 47 progeny were grown on YPD medium supplemented with NAT or G418 to test for selectable marker inheritance. (B) Mating type for each progeny was determined by MAT locus specific primer sets. Genotypes for each progeny are provided in the grid in the same order as the progeny were grown on the YPD medium. (C) Parental and non-parental genotypes are summarized in the graphical table.
(TIF)
CF56 (H99α prm1Δ::NAT) was mated with CF562 (KN99a prm1Δ::NEO) on V8 medium for four weeks and hyphae on the edge of the mating patch were collected for TEM. (A) Cross section of a fused clamp cell morphology is provided on the left, and the diagram is shown on the right. The scale bar is 2 μm. (B) Plasma membrane structures at three unfused conjugation sites were further examined at higher magnification. The diagram for the clamp cell cross section is provided on the right. In the left panels, the scale bars are 2 μm, and in the middle panels, the scale bars are 0.5 μm.
(TIF)
Equal number of cells for each fusion pair were mixed and incubated on V8 medium for 24 hours. Cells were harvested and examined under fluorescent microscope to determine cell fusion frequency based on Nop1-GFP fluorescent signal intermixing between each fusion pair. (A) Wild type cell fusion between CF830 (JEC21α NOP1-GFP-NAT) and JEC20a. (B) Unilateral cell fusion between JEC21α and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). (C) Bilateral mating between CF1 (JEC21α prm1Δ::NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT). (D) Bilateral mating between CF487 (JEC21α kar5Δ::NEO) and CF723 (JEC20a kar5Δ::NEO NOP1-GFP-NAT). Closed shapes (left panels) and arrows (right panels) indicate successful cell fusion pairs, whereas open shapes (left panels) and arrowheads (right panels) indicate unfused cell fusion pairs. The scale bars are 20 μm.
(TIF)
(A) Plasma membrane structures of unfused yeast cells during C. deneoformans bisexual reproduction as visualized by transmission electron microscopy. Unfused cell fusion pairs between CF712 (JEC21α prm1Δ::NAT mCherry-NEO) and CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT) were examined by transmission electron microscopy. Plasma membrane structures at the conjugation sites were further examined at higher magnification. The diagram for the fusion pair cross section is provided on the right. For the left panels, the scale bars are 2 μm, and for the middle panels, the scale bars are 0.5 μm. (B) prm1 mutants are defective in clamp cell fusion during C. deneoformans bisexual reproduction. SEM of the unfused clamp cell morphology for the wild type cross (JEC21α X JEC20a) and of the defective clamp cell fusion morphology for the prm1 mutant cross (CF1 X CF313). The scale bar is 5 μm.
(TIF)
(A) Wild type cross between JEC20a and JEC21α, prm1 mutant cross between CF1 and CF313, and kar5 mutant cross between CF226 and CF364 for bisexual reproduction, and (B) Wild type strain XL280α, prm1 mutant CF659, and kar5 mutant CF260 were incubated on V8 medium in the dark at room temperature for four weeks to generate hyphae and basidia from unisexual reproduction. DAPI staining showed wild type, prm1 mutants, and kar5 mutants all produced monokaryotic hyphae during both unisexual and bisexual reproduction. The scale bar is 5 μm.
(TIF)
Wild type cross CF830 (JEC21α NOP1-GFP-NAT) X JEC20a, prm1 bilateral mutant cross CF1 (JEC21α prm1Δ::NEO) X CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and kar5 bilateral mutant cross CF487 (JEC21α kar5Δ::NEO) X CF723 (JEC20a kar5Δ::NAT NOP1-GFP-NAT) were examined by direct fluorescence microscopy to track hyphal nuclear morphology at different stages of sexual reproduction. (A) At 48 hours, wild type and prm1 mutants produced both monokaryotic and dikaryotic hyphae (arrows point to monokaryotic hyphae, and arrowhead points to mitotically dividing dikaryotic wild type hyphae). kar5 mutants produce hyphae with two nuclei in close contact (arrows). (B) At 10 days, the wild type cross produced both monokaryotic and dikaryotic hyphae, prm1 mutants mainly produced monokaryotic hyphae, and kar5 mutants mainly produced dikaryotic hyphae. (C) At six weeks, wild type and prm1 mutants mainly produced monokaryotic hyphae, and kar5 mutants produced both monokaryotic and dikaryotic hyphae. The scale bar is 5 μm.
(TIF)
(A) Representative basidia containing one nucleus (left panel), or two nuclei (middle panel), or more than two nuclei (right panel) with DAPI staining are shown for wild type XL280α, kar5 mutant (CF260), and kar7 mutant (SL277). Arrows point to DAPI stained nuclei inside basidia. The scale bars are 5 μm. (B) Basidia containing one nucleus, or two nuclei, or more than four post-meiotic nuclei were quantified for the above strains.
(TIF)
(A) Representative basidia containing one nucleus (left panel), or two nuclei (middle panel), or more than two nuclei (right panel) with the nucleolar marker Nop1-GFP fluorescent signals are shown for wild type XL280α (CF836), kar5 mutant (CF718), and kar7 mutant (CF1442). Arrows point to Nop1-GFP signal inside basidia. The scale bars are 5 μm. (B) Basidia containing one nucleus, or two nuclei, or more than two nuclei were quantified for the above strains.
(TIF)
(A) prm1 mutant (CF317) and two independent prm1 spo11 double mutants (CF894 and CF901), and (B) kar5 mutant (CF260) and two independent kar5 spo11 double mutants (CF883 and CF884) were incubated on V8 medium in the dark at room temperature for four weeks. prm1 spo11 double mutants and kar5 spo11 double mutants produced two spore chains compared to the four spore chains produced by prm1 or kar5 mutants. Schemes showing wild type and mutant sporulation patterns were provided at the lower left corner of each image. The scale bar equals 10 μm.
(TIF)
The upper panels are FACS results for haploid control XL280α and diploid control MN142.6 α/α. The lower panels are representative FACS results for haploid, aneuploid, and diploid blastospores produced by the kar7 mutant.
(TIF)
CF712 (JEC21α prm1Δ::NAT mCherry-NEO) was mated with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and the cytosolic mCherry fluorescence signal and nucleolar marker Nop1-GFP signal were intermixed in the fusion pair.
(AVI)
CF712 (JEC21α prm1Δ::NAT mCherry-NEO) was mated with CF768 (JEC20a prm1Δ::NEO NOP1-GFP-NAT), and the cytosolic mCherry fluorescence signal and nucleolar marker Nop1-GFP signal were restricted to distinct cellular compartments.
(AVI)
Cells from CF836 (XL280α NOP1-GFP-NAT) and CF1091 (XL280α H3-mCherry-NAT) were mixed and co-incubated on V8 medium for 24 hours at room temperature. Early hyphae were examined for nuclear fluorescence under confocal microscope for 30 minutes.
(AVI)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.