Abstract
Despite the evolution of the total knee and hip arthroplasty surgery, high postoperative complication rates in the short and long term still persist. Infection is one of the most challenging complications; due to its gravity and treatment difficulties, prophylaxis protocols have been created to decrease its incidence. The objective of this study was to evaluate the impact of the prophylaxis protocol for methicillin-resistant Staphylococcus aureus decolonization of the nares in patients previously identified by swab cultures, who were to be submitted to a total joint arthroplasty. A systematic review with meta-analysis was conducted, following the PRISMA-2015 protocol, using the descriptors: “arthroplasty” and “nasal decolonization,” or “joint arthroplasty” and “decolonization,” or “joint arthroplasty” and “nasal decolonization,” for final selection of four observational studies from 79 references identified. This study included a total sample of 10,179 patients, divided in two groups: the control group (4788 patients) and intervention group (5391 patients). It was observed that the intervention group, in which prophylaxis with nasal decolonization was used, 59 (1.09%) of the patients developed a surgical site infection, while in the control group there were 86 cases of surgical site infection (1.79%). This trend repeated itself in all articles, showing no publication biases, forming a homogeneous sample. The use of a prophylaxis protocol for decolonization of methicillin-resistant Staphylococcus aureus, reduced surgical site infection cases by approximately 39%.
Keywords: Arthroplasty, Prophylaxis, Infection, Decontamination
Resumo
Apesar da evolução dos resultados após a artroplastia total de joelho e quadril, a infecção ainda é uma das causas mais desafiadoras para o cirurgião. Em virtude da gravidade e dificuldade do tratamento da infecção articular periprotética, foram criados protocolos de profilaxia para esse tipo de complicação. O objetivo deste estudo foi avaliar a profilaxia infecciosa com a descolonização nasal prévia contra Staphylococcus aureus resistente à meticilina, identificados por meio da coleta de material da nasofaringe por swabs em pacientes com programação cirúrgica de artroplastia total de joelho e artroplastia total de quadril. Foi elaborado um estudo de revisão sistemática com metanálise que usou o protocolo PRISMA-2015, no qual foram utilizados os descritores: arthroplasty e nasal decolonization ou joint arthroplasty e decolonization ou joint arthroplasty e nasal decolonization na língua inglesa. Foram selecionados quatro estudos observacionais dentre as 79 referências identificadas. A amostra total foi de 10.179 pacientes, divididos em dois grupos: controle (4.788 pacientes) e intervenção (5.391 pacientes). Foi observado que, no grupo de intervenção, no qual a profilaxia com descolonização nasal foi aplicada, 59 (1,09%) dos pacientes desenvolveram infecção do sitio cirúrgico, enquanto a infecção do sitio cirúrgico foi observada em 86 (1,79%) dos pacientes no grupo controle. Essa tendência se repetiu em todos os artigos estudados, não sendo observador viés de publicação, constituindo em uma amostra homogênea. A profilaxia pré-operatória com descolonização nasal para Staphylococcus aureus resistente àmeticilina, reduz em 39% os casos de infecção pós-artroplastias do joelho, devendo ser considerada como um protocolo complementar pelos cirurgiões.
Palavras-chave: Artroplastia, Profilaxia, Infecção, Descontaminação
Introduction
Total knee (TKA) and hip (THA) arthroplasties are surgical procedures that aim to improve quality of life, promoting pain relief, functional gain, and correction of deformities of the affected joint.1, 2
Each year 600,000 TKAs are performed in the United States; by 2030, a 673% increase in demand is expected worldwide. In Brazil, the number of TKAs is estimated to range between 60,000 and 70,000 per year.3, 4
Despite the evolution of arthroplasty results, complications in the postoperative period, both in short- and long-term, still persist. Post arthroplasty infection is one of the most challenging causes of complication for the surgeon.5, 6 The rate of post-TKA surgical site infections (SSI) can vary between 0.5% and 23%, and has an impact of roughly U$ 300 million in North American countries.7 SSI is one of the main infection types associated with health care, accounting for 17% of those in the United States and 37% worldwide, according to the World Health Organization (WHO).7, 8, 9, 10
Due to the seriousness and difficulty of treatment of periprosthetic infections (PI), the development of effective measures to minimize these rates is necessary; prophylactic measures in the preoperative period of TKA have been demonstrated in the literature.2, 10
Parvizi et al.2 have developed a protocol that summarizes the most effective and proven prophylactic measures. These measures include the assessment of nasal colonization by Staphylococcus aureus and its methicillin-resistant strain (MRSA). However, there is still no consensus for recommending universal screening, despite the fact that the decolonization of MRSA carriers decreases the SSI rate.10
Among the postoperative hospital infections, S. aureus has been reported as the main pathogen isolated in culture exams. High levels of nasal colonization by MRSA strains may be a risk factor for SSI onset.11 The nasal epithelium stands out as the site of greatest colonization; its prevalence reaches, on average, 40% in the adult population. As part of the human microbiota, said bacterium does not constitute a risk and can be carried for a long period without damage to the health of individuals.12
Collecting samples from the nostrils with the swab technique allows the identification of MRSA by culture or by polymerase chain reaction (PCR) test; both present high positive predictive value and specificity.13 This method is indicated for MRSA screening from the nasal region of patients who are candidates for TKA and THA.14
Topical antibiotics, which act on S. aureus strains, are indicated as a prophylactic method. Topical mupirocin is the most frequently used antibiotic and recommended for preoperative nasal decolonization (ND), and should be considered as one of the pillars of anti-infection prophylaxis.15
Thus, prophylaxis with ND for MRSA may be indicated as an important prophylactic method for periprosthetic joint infection.16, 17 However, studies that analyzed this subject did not reach a consensus in the validation of prophylaxis through universal MRSA assessment in TKA and THA candidates.2
This study is aimed at assessing whether infection prophylaxis through ND for MRSA in TKA and THA candidates, identified through nasopharyngeal swab collection, is a risk-reducing factor for SSI.
Methods
The systematic review study with meta-analysis was elaborated according to the PRISMA statement protocol (2015).18
The literature was searched to identify studies that evaluated prophylaxis as a risk-reducing factor for SSI through ND for MRSA in colonized patients, identified through nasopharyngeal swab collection.
The inclusion criteria for the selection of studies evaluating prophylactic treatment after MRSA colonization assessment in the preoperative period of patients who would undergo TKA and THA were cohort studies in Portuguese and English.
Studies with an inadequate description and those in which the clinical outcome was not the one proposed by the authors were excluded. Case reports, case series studies, or descriptive reviews were not included. Incomplete articles or those that did not provide data regarding MRSA colonization in patients submitted to TKA and THA were also excluded. Articles with a score lower than 7 in the Newcastle-Ottawa Quality Assessment Scale (NOS),19 which determines the quality of the study, and those that did not fit the required Level of Scientific Evidence by Type of Study (Oxford Center for Evidence-based Medicine) to determine the study's publication value were also excluded.20
The inclusion criteria, presented in Table 1, and the exclusion criteria were determined in accordance with the objective of this study, so that only articles that evaluated the efficacy of surgical prophylaxis with ND for MRSA were considered. These criteria are shown in Table 1 and were stratified according to the PICO strategy (Patient, Intervention, Comparison, Objectives),21 in order to provide internal validity to the study.
Table 1.
Inclusion criteria according to PICO strategy.
| PICO inclusion criteria | |
|---|---|
| Indicators | Results according to PICO |
| Project | Cohort studies |
| Population | Patients in surgical planning for hip and knee arthroplasty |
| Intervention | Prophylaxis: decolonization for S. aureus MRSA in identified patients |
| Comparisons | No prophylaxis |
| Results | Infection rates (incidence) |
The databases used for the research were MEDLINE, SciELO, Science Direct, PubMed, Scopus, and Google Scholar.
The search was performed with the following descriptors: “arthroplasty” and “nasal decolonization,” or “joint arthroplasty” and “decolonization,” or “joint arthroplasty” and “nasal decolonization” in English; “arthroplasty” and “nasal decolonization,” or “arthroplasty” and “decolonization,” “joint arthroplasty” and “nasal decolonization” in Portuguese.
The results were presented through an organization chart for the selection of articles; the dispersion data was compiled into funnel charts and forest plots to better understand the meta-analysis. The percentage of SSI in patients who underwent arthroplasty was expressed as a relative risk (RR) in 95% confidence interval (CI). The results from different studies were combined into a random effect model, since not all studies presented the same methodology. The heterogeneity was analyzed by the chi-squared, I2, and tau2 tests in order to identify differences that could cause bias in the study. These analyzes were carried out with the appropriate software: Review Manager, version 5.2 (The Cochrane Collaboration, 2012).
Results
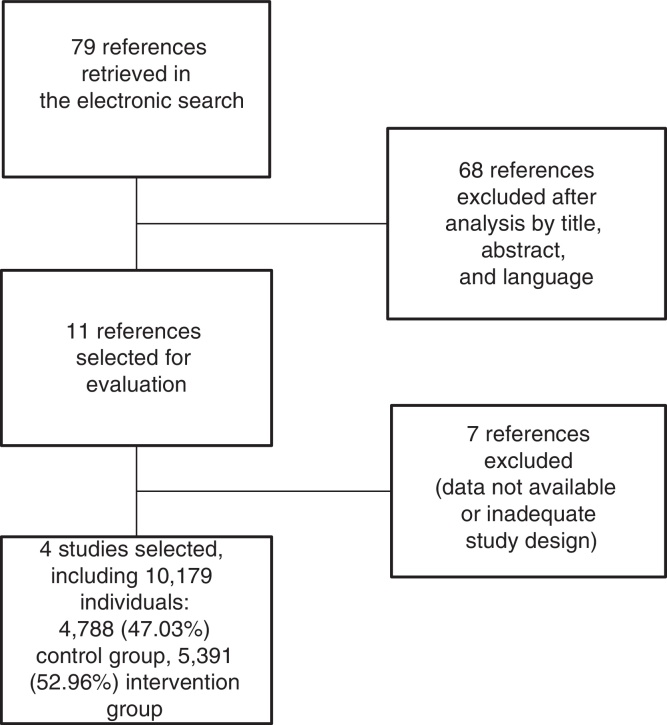
Fig. 1 shows the organization chart of article selection, in which 79 references were identified as potentially relevant during the search. After analysis of the title, abstract, and language of publication, 68 studies did not meet the inclusion criteria. Of the 11 remaining references, seven were discarded according to the exclusion criteria, based on lack of data or inadequate study design.
Fig. 1.
Organization chart of article selection. PRISMA Protocol – 2015.
After the selection of four studies,22, 23, 24, 25 the total sample of 10,179 patients was divided into two groups for statistical analysis: no prophylaxis, 4788 (47.03%) and use of prophylaxis, 5391 (52.96%; Fig. 1).
Table 2 shows the data collected that characterize the four selected studies.22, 23, 24, 25 All selected studies presented cohort design, were considered suitable for systematic review through meta-analysis based on the Level of Scientific Evidence by Type of Study (Oxford Centre for Evidence-based Medicine),21 and presented a minimum value of 7 in the NOS.20 All studies identified patients with MRSA through nasal swab collections. The use of topical mupirocin applied to the nostrils as a prophylactic method was evaluated for five days in three of the studies.22, 23, 25 The exception was the study by Baratz et al.,24 who evaluated the use of cephalosporin for two weeks. Patients were followed-up for the outcome for two years in the studies of Baratz et al.24 and Hacek et al.25; in the studies by Barbero Allende et al.,23 and Rao et al.,22 patients were followed-up for one year.
Table 2.
Characteristics of selected studies.
| Type of study | Total patients | Follow-up time | Screening method | Prophylaxis method | Prophylaxis time | |
|---|---|---|---|---|---|---|
| Allende et al. (2015) | Cohort study | 793 | 1 year | Nasal swab | Mupirocin | 5 days |
| Baratz et al. (2015) | Cohort study | 6514 | 2 years | Nasal swab | Cephalosporin | 2 weeks |
| Hacek et al. (2008) | Cohort study | 1495 | 2 years | Nasal swab | Mupirocin | 5 days |
| Rao et al. (2008) | Cohort study | 1377 | 1 year | Nasal swab | Mupirocin | 5 days |
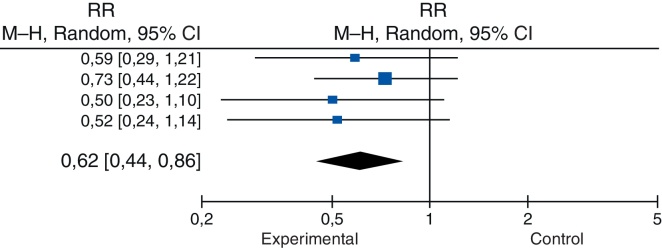
In the group where MRSA prophylaxis was performed, 59 (1.09%) of the patients developed SSI, whereas postoperative infection was observed in 86 (1.79%) of the patients, which reflects a reduction of 39% in the cases of this complication (Table 3). The following reductions were observed: 40.7% in the study by Barbero Allende et al.23; 27.2% in the study by Baratz et al.24; 50% in the study by Hacek et al.25; and 47.6% in the study by Rao et al.22
Table 3.
Characteristics and review of studies.
| Study or subgroup | Prophylaxis used |
Prophylaxis not used |
Weight | Relative risk H-M, Random, 95% CI |
||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| Allende et al. (2015) | 12 | 409 | 19 | 384 | 21.7% | 0.59 (0.29; 1.21) |
| Baratz et al. (2015) | 27 | 3434 | 33 | 3080 | 42.6% | 0.73 (0.44, 1.22) |
| Hacek et al. (2008) | 11 | 912 | 14 | 583 | 17.8% | 0.50 (0.23; 1.10) |
| Rao et al. (2008) | 9 | 636 | 20 | 741 | 18% | 0.53 (0.24; 1.14) |
| Total (95% CI) | 59 | 5391 | 86 | 4788 | 100% | 0.62 (0.44; 0.86) |
Heterogeneity: tau2 = 0.0; chi2 = 0.9; df = 3 (p = 0.83); I2 = 0%.
Overall test effect: Z = 2.87 (p = 0.004).
In the study by Baratz et al.,24 in which the lowest infection rates were observed, the intervention group presented 0.78% cases and the control group, 1.07% (Table 3).
The final result of the weight attributed to each study, reported in Table 3, was based on the algorithm of the random effect model, which takes into account the fact that there are considerable methodological differences among the studies and therefore makes a differentiated calculation based on variance, and not only on the number of patients.
Table 3 shows that the confidence interval did not exceed 1, which indicates that the intervention group, in which prophylaxis had been performed, presented a lower risk of infection. This was confirmed by the final statistical test, which presented p = 0.004.
Regarding the heterogeneity tests, which seek to identify variations among the studies that may suggest conflicting data, whether due to differences in design, methodology, sampling, and bias, among others, the first parameter, tau2, quantitatively evaluates the heterogeneity of the results among the studies, and demonstrated that they overlapped in a very orderly way (Fig. 2), converging in a low value. The chi-squared test was then applied, with its p-value, again seeking to identify evidence of heterogeneity. Finally, the I2 parameter was used to explain how much of the variability among the results was associated with heterogeneity; the value of 0 (zero) indicated that inconsistency among the studies should not be an important factor (Table 3).
Fig. 2.
Forest Plot. RR, risk ratio; CI, confidence interval.
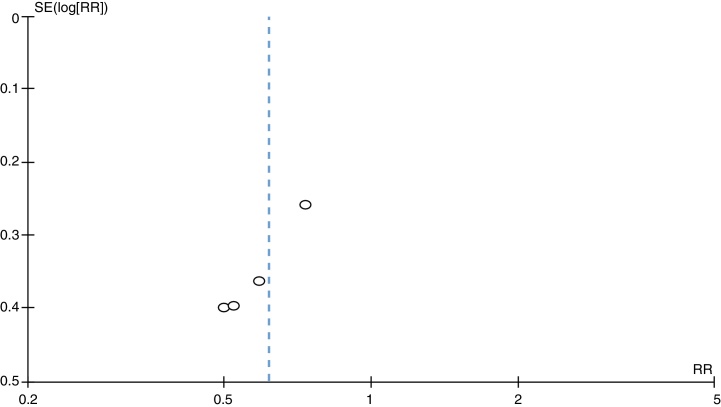
Heterogeneity, represented by the funnel plot (Fig. 3), was non-significant (tau2 = 0.0; I2 = 0%) and the occasional estimate of relative risk was 0.62 (95% CI = 0.44–0.86). Therefore, no statistically significant differences were observed between the studies (Fig. 2 and Table 3).
Fig. 3.
Funnel plot of the four independent samples, examining the relationship between surgical site infection risk and intervention.
In Fig. 3, all studies presented data favorable to intervention, with a slight tendency toward a better result in the smaller groups, as can be observed in the comparison with the value obtained in the compilation of the results.
Discussion
Despite the progress in infection control after TKA or THA, especially in the short term, the treatment of established infections in prosthetic joints remains complex and costly in terms of time and resources. Therefore, careful attention to prevent infections is required.
In this context, this meta-analysis evaluated MRSA prophylaxis as an SSI risk-reducing factor in colonized patients, identified by nasopharyngeal swabs in TKA and THA candidates in four cohort studies.
The study demonstrated that prophylaxis with ND is a protective factor. This model accounted for a 39% reduction in the incidence of SSI; the rate decreased from 1.79% in the control group to 1.09% in the group in which the intervention was performed. This trend was observed in all four studies, arranged in an orderly manner, presenting statistically irrelevant differences, which suggest the absence of heterogeneity.
The results obtained are reflected in the relative risk (0.62) and its confidence interval (0.44–0.86), which indicate that MRSA prophylaxis is a protective factor. This probably arises from the prevalence of these pathogens and their strains, recognized as a major cause of SSI in the general and in-hospital population.13, 26 Infection in this group of patients may be associated with colonization of the nostrils, which justifies ND with topical muporicin or an antibiotic drug that acts on this pathogen.27, 28
The importance of preoperative prophylaxis with investigation of MRSA colonization is observed in studies such as those by Barbero Allende et al.,23 who performed preoperative prophylaxis in TKA and THA patients with topical mupirocin, applied in the nostrils, and chlorhexidine skin wash. When comparing the groups in which prophylaxis was used or not used, a significant decrease in SSI rates was observed, with a reduction of 40.7%; the incidence was 4.94% in the group in which prophylaxis was not performed, vs. 2.93% in the intervention group.
Baratz et al.24 compared the incidence of SSI in patients submitted to TKA and THA, considering patients who had been treated with ND for MRSA and methicillin-sensitive S. aureus (MSSA) and those who did not receive prophylaxis. It was observed that the intervention group, treated prior to surgery with first generation cephalosporin, presented an incidence of 0.79%, a 27.3% reduction in comparison with the control group, which presented an incidence of 1.07%.
In the same context, Hacek et al.25 demonstrated that 1.2% of the patients treated with ND developed SSI vs. 2.4% of those in the group that was not treated with ND, that resulted in a 50% reduction in SSI incidence.
Rao et al.22 compared the results of patients who had or not undergone ND with nasal mupirocin and chlorhexidine body wash in the preoperative period. The SSI rate was 1.41% in the prophylaxis group and 2.69% in the control group. This data suggests an SSI reduction of 47.6%.
To evaluate the percentage of patients submitted to TKA who remained colonized by S. aureus despite prophylactic ND, Economedes et al.,29 in a pilot study, evaluated 634 patients who underwent the procedure by the same surgeon and who completed an ND protocol before surgery. The authors demonstrated that 33% (19 of 58) of the patients who underwent ND continued to present positive S. aureus culture despite preoperative decolonization, inferring that ND is not able to ensure that patients will remain decolonized throughout the postoperative period. The effects of persistent S. aureus colonization in the postoperative period require further studies in order to assert that this group of patients is at greater risk of acquiring late infection.29
In the same line of reasoning, the International Consensus on Periprosthetic Joint Infections, conducted by Parvizi et al.,2 indicated that patients colonized by MRSA, especially in the anterior portion of the nostrils, have a potential source of hospital and postoperative infections, and that ND with topical mupirocin applied to the nostrils for five days prior to surgery was an effective measure in reducing the incidence of SSI.
The reduction of SSI rate after MRSA screening and prophylaxis corroborates the clinical and pathological reasoning, in which this pathogen stands out as one of the main causes of infection due to its high presence in the in-hospital and general populations. The concern with the MRSA strain is higher due to its difficult treatment and control, since it does not respond to beta-lactam antibiotics, including synthetics, as in the case of methicillin, which implies the need for adequate preoperative prophylaxis.30 Topical mupirocin for ND is the treatment of choice.31 Although some first-generation cephalosporins are efficient against S. aureus, these are not the first-choice antimicrobial, either because they have little or no activity against MRSA, or because their routine indiscriminate use can contribute to the emergence of drug-resistant bacteria.32
Failure to perform prophylaxis against SSI in the preoperative period offers evidence for the clinical and pathological reasoning regarding this complication by demonstrating the increase in the risk factor for its occurrence.33
In general, the following pathogens can also cause periprosthetic infection: Coagulase-negative staphylococci, Streptococcus, and Gram-negative and anaerobic bacilli.34
In the studies by Baratz et al.,24 Hacek et al.,25 and Rao et al.,22 it was possible to infer that, in spite of the significant reduction in SSI due to the S. aureus screening and prophylaxis protocol, infections were also caused by other pathogens, such as Pseudomonas, Enterobacter, and Streptococcus, among others.
The variation in the incidence of S. aureus is related to the existence of associated risk factors of infection, such as obesity, early age, and diabetes.35 Thus, populations with different characteristics may present different SSI incidence rates.35
Due to its inherent characteristic of grouping several studies, combining the individual samples and results into a synthesis, some factors could not be approached in the present study, such as the quantitative and qualitative impact of the profile of different populations on the rate of SSI in patients submitted the TKA and THA, the long-term efficacy of the systematic use of the suggested prophylactic method, as well as the spectrum of action of the antibiotic used and possible associations. Therefore, new studies are required to perform a multivariate analysis of these factors.
This systematic review with meta-analysis, as others retrieved in the literature, is based on secondary data extracted from original cohort studies. However, this characteristic is a limitation for the model, since it does not allow the assessment of all the data necessary for multivariate analyses and is subject to possible biases.
The inherent characteristics of the study confer it a level of evidence 2 according to the Level of Scientific Evidence by Type of Study (Oxford Centre for Evidence-based Medicine): systematic review of homogenous cohort studies.18
Although the diagnostic possibilities have improved with the development of new immunological and imaging techniques, the devastating consequences of an infection, even when early diagnosed, remain a major challenge for the orthopedic surgeon. Thus, the development of additional prophylaxis techniques should be investigated in order to achieve an even greater reduction of this serious complication. Nonetheless, ND screening for S. aureus and its MRSA strain through nasopharyngeal swab collection can be presented as an effective measure in the reduction of SSI in TKA and THA that should be implemented.
Final considerations
The investigation of nasopharyngeal swab material to detect colonization by Staphylococcus aureus and its methicillin-resistant variant in patients with indication for TKA and THA, followed by decolonization with topical antibiotic therapy with muporicin, presents data that confirm the effectiveness of its use as a prophylactic method in order to reduce the rates of postoperative infection.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at the Hospital Manoel Victorino, Departamento de Ortopedia e Traumatologia, Salvador, BA, Brazil.
References
- 1.Lima A.L.L.M., Pécora J.R., Albuquerque R.M., Paula A.P., D’Elia C.O., Santos A.L.G. Infection following total knee joint arthroplasty: considerations and treatment. Acta Ortop Bras. 2004;12(4):236–241. [Google Scholar]
- 2.Parvizi J, Gehrke T. Consenso Internacional em Infecções Articulares Periprotéticas. Available from: http://www.rbo.org.br/pdf/consensos/consensos_ciiap.pdf [accessed 11.01.16].
- 3.Almeida R.F., Queiroz A.A., Belloti J.C., Castro Filho J.M., Cohen M., Navarro R.D. Approach towards total knee arthroplasty in Brazil: cross-sectional study. Sao Paulo Med J. 2009;127(4):190–197. doi: 10.1590/S1516-31802009000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Elia C.O., Santos A.L.G., Leonhardt M.C., Lima A.L.L.M., Pécora J.R., Camanho G.L. Treatment of infections following total knee arthroplasty: 2-year follow-up outcomes. Acta Ortopédica Bras. 2007;15(3):158–162. [Google Scholar]
- 5.Carvalho Júnior L.H., Temponi E.F., Badet R. Infecção em artroplastia total de joelho: diagnóstico e tratamento. Rev Bras Ortop. 2013;48(5):389–396. doi: 10.1016/j.rboe.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi H-R., von Knoch F., Zurakowski D., Nelson S.B., Malchau H. Can implant retention be recommended for treatment of infected TKA? Clin Orthop Relat Res. 2011;469(4):961–969. doi: 10.1007/s11999-010-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradella J.G.D.P., Bovo M., Salles M.J.C., Klautau G.B., Camargo O.A.P., Cury R.P.L. Artroplastia primária de joelho infectada: fatores de risco para falha na terapia cirúrgica. Rev Bras Ortop. 2013;48(5):432–437. doi: 10.1016/j.rboe.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organização Mundial da Saúde . Organização Pan-Americana da Saúde; Ministério da Saúde; Agência Nacional de Vigilância Sanitária; Rio de Janeiro: 2009. Segundo desafio global para a segurança do paciente: Cirurgias seguras salvam vidas (orientações para cirurgia segura da OMS) Available form: http://bvsms.saude.gov.br/bvs/publicacoes/seguranca_paciente_cirurgia_salva_manual.pdf. [Google Scholar]
- 9.Centers for Disease Prevention and Control (CDC) CDC; Atlanta, GA, USA: 2009. The National Healthcare Safety Network (NHSN) Manual. Healthcare personnel safety component protocol. Available from: http://www.cdc.gov/nhsn/pdfs/hspmanual/hps_manual.pdf. [Google Scholar]
- 10.Anderson D.J., Podgorny K., Berríos-Torres S.I., Bratzler D.W., Dellinger E.P., Greene L. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezapoor M., Parvizi J. Prevention of periprosthetic joint infection. J Arthroplasty. 2015;30(6):902–907. doi: 10.1016/j.arth.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista S.S., Oliveira A.C., Evangelista S.S., Oliveira A.C. Community-acquired methicillin-resistant Staphylococcus aureus: a global problem. Rev Bras Enferm. 2015;68(1):136–143. doi: 10.1590/0034-7167.2015680119p. [DOI] [PubMed] [Google Scholar]
- 13.Hombach M., Pfyffer G.E., Roos M., Lucke K. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth-enriched culture in an area with a low prevalence of MRSA infections. J Clin Microbiol. 2010;48(11):3882–3887. doi: 10.1128/JCM.00670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parvizi J., Ghanem E., Sharkey P., Aggarwal A., Burnett R.S.J., Barrack R.L. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop. 2008;466(11):2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coia J.E., Duckworth G.J., Edwards D.I., Farrington M., Fry C., Humphreys H. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006;63(Suppl. 1):S1–S44. doi: 10.1016/j.jhin.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva Pinto C.Z., Alpendre F.T., Stier C.J.N., Maziero E.C.S., de Alencar P.G.C., de Almeida Cruz E.D. Characterization of hip and knee arthroplasties and factors associated with infection. Rev Bras Ortop. 2015;50(6):694–699. doi: 10.1016/j.rboe.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laudermilch D.J., Fedorka C.J., Heyl A., Rao N., McGough R.L. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067–2073. doi: 10.1007/s11999-010-1304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottawa Hospital Research Institute. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 11.08.16].
- 20.Phillips B., Ball C., Sackett D., Badenoch D., Straus S., Haynes B. 1998. Oxford centre for evidence-based medicine levels of evidence. Available from: http://www.cebm.net/levels_of_evidence.asp. [Google Scholar]
- 21.Bernardo W.M., Nobre M.R.C., Jatene F.B. Evidence based clinical practice: part II – searching evidence databases. Rev Assoc Médica Bras. 2004;50(1):104–108. doi: 10.1590/s0104-42302004000100045. [DOI] [PubMed] [Google Scholar]
- 22.Rao N., Cannella B., Crossett L.S., Yates A.J., McGough R. A preoperative decolonization protocol for Staphylococcus aureus prevents orthopaedic infections. Clin Orthop. 2008;466(6):1343–1348. doi: 10.1007/s11999-008-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbero Allende J.M., Romanyk Cabrera J., Montero Ruiz E., Vallés Purroy A., Melgar Molero V., Agudo López R. Eradication of Staphylococcus aureus in carrier patients undergoing joint arthroplasty. Enferm Infec Microbiol Clin. 2015;33(2):95–100. doi: 10.1016/j.eimc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Baratz M.D., Hallmark R., Odum S.M., Springer B.D. Twenty percent of patients may remain colonized with methicillin-resistant Staphylococcus aureus despite a decolonization protocol in patients undergoing elective total joint arthroplasty. Clin Orthop. 2015;473(7):2283–2290. doi: 10.1007/s11999-015-4191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacek D.M., Paule S.M., Thomson R.B., Robicsek A., Peterson L.R. Implementation of a universal admission surveillance and decolonization program for methicillin-resistant Staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S. aureus isolates reported by the clinical laboratory. J Clin Microbiol. 2009;47(11):3749–3752. doi: 10.1128/JCM.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levent T., Vandevelde D., Delobelle J-M., Labourdette P., Létendard J., Lesage P. Infection risk prevention following total knee arthroplasty. Orthop Traumatol Surg Res. 2010;96(1):49–56. doi: 10.1016/j.rcot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Matar W.Y. Preventing infection in total joint arthroplasty. J Bone Jt Surg Am. 2010;92(Suppl. 2):36. doi: 10.2106/JBJS.J.01046. [DOI] [PubMed] [Google Scholar]
- 28.Nasser S. Prevention and treatment of sepsis in total hip replacement surgery. Orthop Clin North Am. 1992;23(2):265–277. [PubMed] [Google Scholar]
- 29.Economedes D.M., Deirmengian G.K., Deirmengian C.A. Staphylococcus aureus colonization among arthroplasty patients previously treated by a decolonization protocol: a pilot study. Clin Orthop. 2013;471(10):3128–3132. doi: 10.1007/s11999-013-2856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel H., Khoury H., Girgenti D., Welner S., Yu H. Burden of surgical site infections associated with arthroplasty and the contribution of Staphylococcus aureus. Surg Infect. 2016;17(1):78–88. doi: 10.1089/sur.2014.246. [DOI] [PubMed] [Google Scholar]
- 31.Chandrananth J., Rabinovich A., Karahalios A., Guy S., Tran P. Impact of adherence to local antibiotic prophylaxis guidelines on infection outcome after total hip or knee arthroplasty. J Hosp Infect. 2016;93(4):423–427. doi: 10.1016/j.jhin.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Duplessis C., Crum-Cianflone N.F. Ceftaroline a new cephalosporin with activity against methicillin-resistant Staphylococcus aureus (MRSA) Clin Med Rev Ther. 2011;3 doi: 10.4137/CMRT.S1637. pii:a2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C.T., Chen I.L., Wang J.W., Ko J.Y., Wang C.J., Lee C.H. Surgical site infection after total knee arthroplasty: risk factors in patients with timely administration of systemic prophylactic antibiotics. J Arthroplast. 2016;31(7):1568–1573. doi: 10.1016/j.arth.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal V.D. International nosocomial infection control consortium (INICC) resources: INICC multidimensional approach and INICC surveillance online system. Am J Infect Control. 2016;44(6):e81–e90. doi: 10.1016/j.ajic.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Malinzak R.A., Ritter M.A., Berend M.E., Meding J.B., Olberding E.M., Davis K.E. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplast. 2009;24(6 Suppl.):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]