Abstract
INTRODUCTION
About 5% of GISTs originate in the rectum and historically radical resection was commonly performed. Little is known about the outcome of rectal GIST in the era of imatinib.
METHODS
Using a prospectively maintained database, we retrospectively analyzed 47 localized, primary rectal GISTs treated at our center from 1982 to 2016, stratified by when imatinib became available in 2000. Overall, disease-specific, and recurrence-free survival (OS, DSS, and RFS) were analyzed by the Kaplan-Meier method.
RESULTS
Rectal GISTs represented 7.1% of 663 primary GISTs. There were 17 patients in the pre-imatinib era and 30 in the imatinib era. The 2 groups had similar follow-up, age, gender, Miettinen risk, and distance to the anal verge. In the imatinib era, tumors were smaller at diagnosis (median 4.0 vs. 5.0 cm, p=0.029) and 24 of 30 patients received perioperative imatinib. In high-risk patients, organ-preservation and negative margins were more common in the 13 patients treated with neoadjuvant imatinib compared to the 21 treated directly with surgery. High-risk patients who received perioperative imatinib (n=15) had greater (or nearly significantly greater) 5yr OS, DSS, local RFS, and distant RFS than those (n=19) who did not (91, 100, 100, and 71% compared to 47, 65, 74, and 41%, p=0.049, 0.052, 0.077, 0.051, respectively). In the imatinib era, no patient has had a local recurrence or death due to GIST.
CONCLUSIONS
The use of imatinib is associated with organ-preservation and improved oncologic outcome in rectal GIST.
Keywords: rectal GIST, imatinib, organ-preserving surgery, neoadjuvant
INTRODUCTION
GIST is the most common sarcoma of the GI tract and is now thought to be the most common subtype of sarcoma overall1. A recent population-based study of histologically confirmed GIST in the United States found an annual age-adjusted incidence of 0.78/100,000 per year in 2011, or roughly 2500 cases per year2. However, the actual incidence may in fact be much higher, with up to one third of people having <1cm microGISTs, yet many of these are not clinically relevant3. GIST can arise anywhere in the gastrointestinal tract, although it is most commonly found in the stomach, followed by the small intestine4. For unknown reasons, colonic GIST is extraordinarily rare, but rectal GIST is the third most common site, representing 1.6–5%2,5–8.
The treatment of rectal GIST is particularly challenging due to the anatomical constraints of the bony pelvis. Historically, rectal GIST was often treated with radical operations, including abdominoperineal resection or total pelvic exenteration. In the modern era, however, rectal GIST is sometimes resected by local excision via the transanal, transvaginal, or transabdominal approach or by low anterior resection.
Most GISTs are driven by activating mutations in the KIT (75%) or PDGFRα (10%) oncogenes and are usually responsive to the oral tyrosine kinase inhibitor imatinib mesylate (Gleevec™)4. After resection of primary GIST at moderate to high-risk of recurrence, adjuvant imatinib has become the standard of care, based on large phase III randomized trials showing improved oncologic outcome9,10. Neoadjuvant imatinib is sometimes employed in an attempt to downsize locally advanced GISTs and may allow R0 resection in up to 83% of patients.11 The benefit of neoadjuvant imatinib in rectal GIST is uncertain, although small, retrospective series have been reported12–15 Here, we present the largest single-institution experience with rectal GIST.
METHODS
Patient and methods
Using a prospectively maintained institutional database, we identified 55 patients with rectal GIST treated at our institution between July 1982 and April 2016. The diagnosis was confirmed with histology and CD117 (KIT) and/or DOG-1 immunohistochemistry. Tumor mutation analysis became routine in the mid-2000s. Risk of recurrence was classified using the Miettinen criteria16,17. The imatinib era was defined as starting in October 2000 when the first patients were treated with the drug at our institution. Institutional Review Board approval was obtained and research was done in compliance with Health Insurance Portability and Accountability Act regulations. Survival and recurrence status was last updated in September 2016.
Statistics
OS, DSS, and RFS were measured from the time of surgery and analyzed by the Kaplan-Meier method. Groups were compared using the log rank test. Continuous variables were compared using the Student’s t test and categorical variables were analyzed with the Fisher’s exact or the Chi-square test, with the level of significance set at 0.05. Analysis was performed using GraphPad Prism 6.0 (San Diego, CA).
RESULTS
Patients characteristics
We excluded 8 patients who presented with metastasis or recurrence after having undergone surgery for their primary tumor at another institution. The remaining 47 patients underwent surgery for primary rectal GIST, which represented 7.1% of 663 primary GISTs treated at our institution. Thirty patients underwent surgery in the imatinib era and 17 in the older era (Table 1). The 2 groups had similar median follow-up, sex, age, distance from the anal verge, high-risk classification, and mutation type. In the imatinib era, tumors were smaller at diagnosis and less often symptomatic.
Table 1.
Clinical and genomic characteristics of rectal GIST patients depending on when imatinib (IM) became available.
| Pre-IM era | IM era | p-value | |
|---|---|---|---|
| Total number | 17 | 30 | |
|
| |||
| Follow-up (yrs) | |||
| Median | 4.5 | 3.7 | 0.32 |
| Range | 0.7 – 16.4 | 0.1 – 13.0 | |
|
| |||
| Sex | |||
| Male | 10 | 22 | 0.34 |
| Female | 7 | 8 | |
|
| |||
| Age at resection (yrs) | |||
| Median | 58 | 57 | 0.37 |
| Range | 43 – 78 | 23 – 82 | |
|
| |||
| Tumor size (cm)* | |||
| Median | 5 | 4 | 0.03 |
| Range | 0.6 – 21.0 | 0.8 – 9.5 | |
|
| |||
| Distance to AV (cm) | |||
| Median | 4 | 4 | 0.3 |
| Range | 1.0 – 8.0 | 0.0 – 6.0 | |
|
| |||
| Miettinen high-risk** | |||
| Yes | 15 | 19 | 0.16 |
| No | 2 | 9 | |
|
| |||
| Presentation | |||
| Symptoms | 13 | 16 | 0.11 |
| Exam or endoscopy | 3 | 14 | |
|
| |||
| Mutation | |||
| KIT exon 9 | 1 (5.9%) | 0 (0.0%) | |
| KIT exon 11 del | 8 (47.1%) | 8 (26.7%) | |
| KIT exon 11 pm | 0 (0.0%) | 4 (13.3%) | 0.35 |
| KIT exon 13 | 0 (0.0%) | 1 (3.3%) | |
| KIT exon 11 and 17 | 0 (0.0%) | 1 (3.3%) | |
| PDGFRα | 0 (0.0%) | 0 (0.0%) | |
| Wild type | 3 (17.6%) | 5 (16.7%) | |
| Not tested | 5 (29.4%) | 11 (36.7%) | |
For tumors receiving neoadjuvant imatinib, size was based on pretreatment imaging.
Some patients lacked necessary clinicopathologic data to determine Miettinen risk.
del=deletion, pm=point mutation
Perioperative radiation and imatinib therapy
In the imatinib era, perioperative radiation was not used, but 24 patients (80%) received perioperative imatinib therapy – 21 (70%) received neoadjuvant imatinib, 12 patients (40%) received adjuvant imatinib, and 9 (30%) received both (Table 2). Neoadjuvant imatinib was administered for a median of 7.7mo (3–62) and the median size change was −28% (−55 to +18%; Figure 1a). This equated to RECIST partial response and stable disease rates of 42 and 58% respectively. Median residual viable tumor (when quantified) was 20% (0–100%; Figure 1b). Only one patient demonstrated an appreciable increase in tumor size after neoadjuvant imatinib (+18%), but the tumor was <1% viable at pathologic analysis. Another tumor was 100% viable, yet had stable size (−5%) and contained KIT exon 11 and 17 mutations. The median mitotic rate in the resected specimens was 0, compared to 8 (0–50) in the 12 patients for whom pre-treatment mitotic rate was available (Figure 1c). Twelve patients were treated with adjuvant imatinib for a median of 2.8yrs (0.1–6.5), of whom 8 remained on therapy at the last follow-up.
Table 2.
Therapy for rectal GIST depending on when imatinib (IM) became available.
| Pre-IM era (n=17) |
IM era (n=30) |
p-value | |
|---|---|---|---|
| Perioperative radiation* | |||
| Yes | 9 | 0 | <0.0001 |
| No | 8 | 30 | |
|
| |||
| Perioperative IM** | |||
| Any perioperative | 0 | 24 | |
| Any neoadjuvant | 0 | 21 | |
| Any adjuvant | 0 | 12 | |
| Neoadjuvant + adjuvant | 0 | 9 | |
|
| |||
| Surgical procedure | |||
| APR/TPE | 10 | 1 | <0.0001 |
| LAR | 2 | 11 | |
| LE | 5 | 18 | |
|
| |||
| Margin status | |||
| R0 | 12 | 21 | 0.12 |
| R1 | 3 | 9 | |
| R2 | 2 | 0 | |
|
| |||
| 30-day complications | |||
| Grade 1 | N/A*** | 5a | |
| 2 | 0 | ||
| 3 | 3b | ||
| 4 | 0 | ||
| 5 | 0 | ||
APR, abdominoperineal resection; TPE, total pelvic exenteration; LAR, low anterior resection; LE, local excision.
Includes external beam radiation and brachytherapy.
Perioperative IM includes neoadjuvant only (n=12), adjuvant only (3), or both (9)
30-day complication data was only available for the IM era.
Wound infection (1), urinary tract infection (1), urinary retention (1), deep venous thrombosis (1), and clostridium difficile infection (1) all were treated with standard therapy.
Among 18 patients undergoing LE, complications related to the rectal repair occurred in 3 (none received Neo-IM): dehiscence of rectal repair (2; treated with temporary diverting stoma), and bleeding from rectal repair (1; controlled in the operating room).
Figure 1.
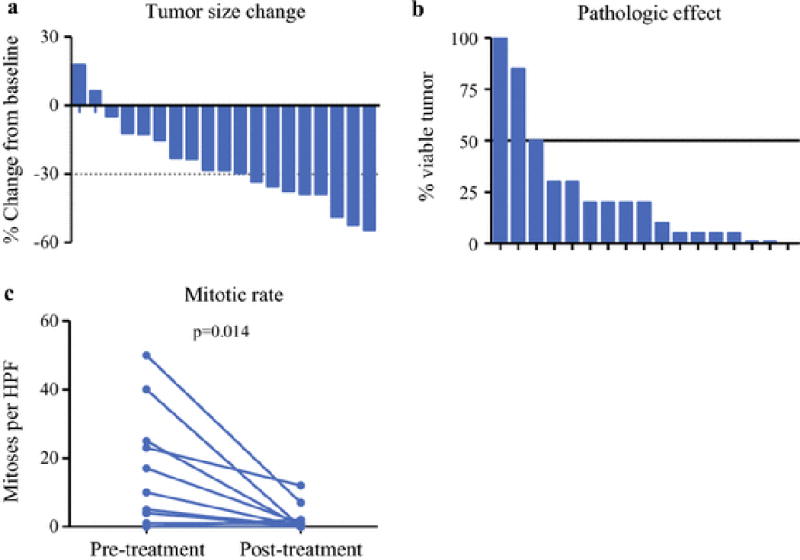
Response to neoadjuvant imatinib in rectal GIST.
Waterfall plots are shown representing tumor size change (A) and pathologic response (B) after neoadjuvant imatinib treatment. Each bar represents a single patient. Of 21 patients undergoing neoadjuvant imatinib, 19 had pre- and post-treatment tumor size measurements available. For pathologic response, there was an assessment of tumor viability available for 17 patients. Among patients undergoing neoadjuvant imatinib, 12 patients had pre- and post-treatment mitotic rate available, expressed as mitoses per 50 high-power fields (C). Mitotic rate post-treatment was available for an additional 7 patients, ranging from 0–2.
Surgery
Only 3% of patients underwent radical surgery with abdominoperineal resection or total pelvic exenteration in the imatinib era compared to 59% in the pre-imatinib era (p<0.0001; Table 2). Instead, low anterior resection (37 vs. 12%) and local excision (60 vs 29%) were more common. Despite less radical surgery, the R0 margin rate was similar (Table 2). Given the trend toward more Miettinen high-risk patients in the pre-imatinib era (Table 1), we also separately analyzed 34 high-risk patients, of whom 13 received neoadjuvant imatinib (patient characteristics in Supplemental Table 1). Only 4 of the high-risk neoadjuvant imatinib patients (31%) had positive margins, compared to 15 of 21 (71%) who did not receive neoadjuvant imatinib (p=0.03). Among high-risk patients, low anterior resection and local excision were more common after neoadjuvant imatinib (12 of 13, 92%) than without it (10 of 21, 48%; p=0.02).
Outcome of treatment
5yr OS, DSS, and RFS were significantly higher in the imatinib era at 91, 100, and 82%, compared to 47, 61, and 44% (Figure 2a–c). There were 15 patients who developed recurrence at a median of 2.3yrs (0.5–6.1) after the initial surgery, including 8 with isolated distant recurrences, 1 with isolated local recurrence, and 6 with both. There have been no local recurrences in the imatinib era, while there were 5 in the pre-imatinib era (excludes two R2 resections; 5yr local RFS of 100 vs. 63%; p=0.002; Figure 2d). Likewise, distant RFS was also greater in the imatinib era (Figure 2e). Among high-risk patients (Figure 3), we found that those who received any perioperative imatinib therapy (n=15) had longer 5yr OS (91% vs 47%, p=0.049) compared to those who did not (n=19), with a trend for better 5yr DSS (100 vs. 65%, p=0.051), 5yr local RFS (100 vs. 74%; p=0.077), and 5yr distant RFS (71 vs. 41%, p=0.051).
Figure 2.
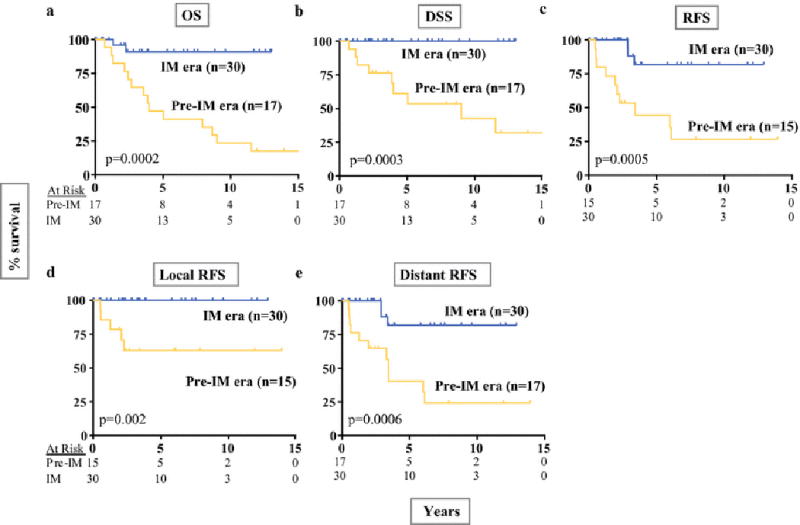
Oncologic outcome in rectal GIST by imatinib era.
Kaplan-Meier curves are shown depicting OS (A), DSS (B), RFS (C), local RFS (D), and distant RFS (E) for patients in the imatinib era compared to the pre-imatinib era. The number of patients at risk is listed for each time point. Distant recurrence was defined as recurrence outside of the pelvis, typically in the liver or peritoneal cavity. For RFS and local RFS, R2 resections (n=2) were excluded.
Figure 3.
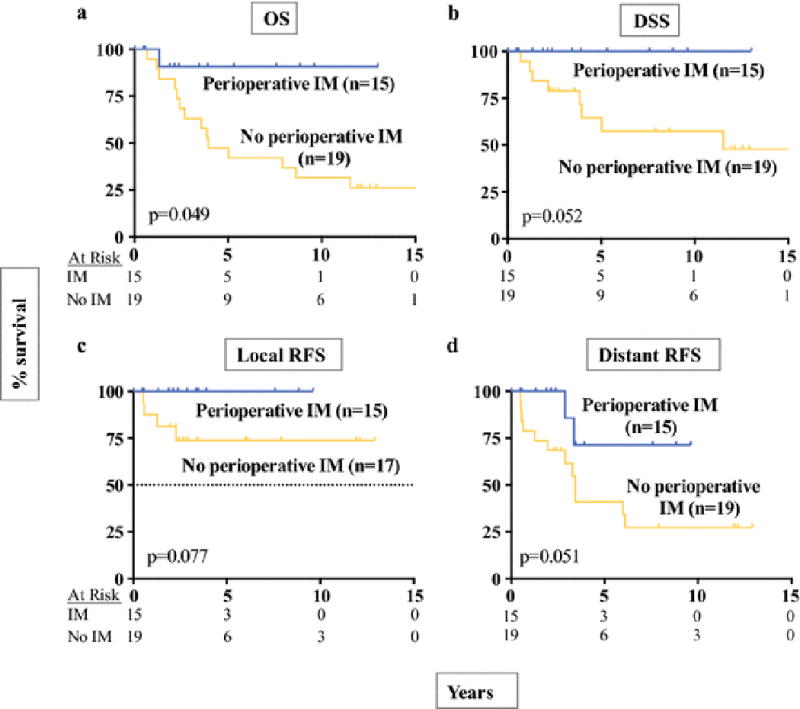
Oncologic outcome in high-risk rectal GIST after perioperative imatinib therapy.
Kaplan-Meier curves are shown depicting OS (A), DSS (B), local RFS (C), and distant RFS (D) for Miettinen high-risk patients who received perioperative imatinib compared to no perioperative imatinib. The number of patients at risk is listed for each time point. For local RFS, R2 resections (n=2) were excluded.
Three patients experienced distant recurrence in the imatinib era approximately three years after surgery (Supplemental Table 2). One had not been treated with imatinib and the other two had received 1.2 and 1.4yrs of perioperative imatinib. All three underwent metastasectomy, with preoperatively initiated imatinib continued to last follow-up in two. All three were alive and disease-free at 5.3, 12.6 and 13.0 yrs at last follow-up. In contrast, in the pre-imatinib era there were five patients with isolated distant recurrences, one with isolated local recurrence, and six with both. Four patients eventually received imatinib once the drug became available. Only two patients underwent metastasectomy, of whom only one remained alive without disease at last follow-up on imatinib at 16.4yrs. In total, 9 of 12 patients who developed recurrence in the pre-imatinib era patients died of GIST, while there have not been any deaths due to GIST in the imatinib era.
We tested the association of survival with other surgical factors. There was no difference in OS, DSS, or distant RFS in patients with positive margins (not shown). Local recurrence was seen in the pre-imatinib era in both margin-positive and margin-negative patients, but not in imatinib era even in margin-positive patients (Supplemental Figure 1). Multivariate analysis to assess the individual contribution of clinicopathologic risk factors, era, perioperative imatinib, and margins was not possible due to the small number of patients and events.
Discussion
Our report is distinct from other modern series13–15,18–20. First, our data represent the largest single-institution experience, coming from a prospectively maintained database with mature follow-up, as opposed to multiple institutions where treatment strategies and follow-up patterns vary. Our series spans the historical and imatinib eras, while some publications were only in the recent era14,15,18,19. Some included patients who did not receive imatinib either due to availability (pre-imatinib) or because it was not felt to be indicated clinically; often these patients were analyzed as one group or without clear designation13,18,20. Finally, we were able to classify the Miettinen risk groups for 96% of our patients, while in many other reports the risk status was often unclear or there was no comparison group. Thus, we were able to compare the outcome of high-risk patients (76% of our cohort) by whether or not they received perioperative imatinib.
For primary GISTs, neoadjuvant imatinib can induce tumor shrinkage, although complete response is rare11,18,21. In six retrospective series comprising 83 patients with rectal GIST who were treated with neoadjuvant imatinib, there were 60 (72.3%) partial responses, 18 (21.7%) with stable disease, 3 (4.8%) complete responses, and 1 (1.2%) with progressive disease by RECIST13–15,18–20. Our study adds another 21 patients treated with neoadjuvant imatinib, however, our partial response rate was lower at 42%. Nevertheless, size decrease is not always a reliable indicator of response in GIST, with density on CT often a better indicator22. Our patients had a median of only 20% residual viable tumor and median mitotic index of 0 at surgery, consistent with robust treatment effects. Lack of treatment response should prompt tumor mutation analysis, as one patient with 100% viable tumor at surgery had an imatinib-resistant secondary mutation.
The imatinib era was associated with more rectal and sphincter preservation. While it has become clear that GIST does not require regional lymphadenectomy23, neoadjuvant imatinib contributed to smaller operations. Among 34 high-risk patients, 12 of 13 (92%) who received neoadjuvant imatinib underwent low anterior resection or local excision compared to only 10 of 21 (48%) who did not. This high rate of organ-preservation was consistent with some13–15,18, but not all19,20, previous reports.
Historically, radical surgery for rectal GIST was associated with less local recurrence24. Remarkably, in our series, regardless of one-third having positive margins in each era, there were no local recurrences in the imatinib era. Similarly, among high-risk patients, there were no local recurrences in patients who received perioperative imatinib compared to 26% with local recurrence at 5 years in those who did not receive imatinib (Figure 3c). Although the rates of positive margins were similar globally by era, in high-risk patients who received neoadjuvant imatinib only 31% had positive margins compared to 71% in those who did not. Taken together, these data suggest that neoadjuvant imatinib is associated with more frequent complete resection and lack of local recurrence, although adjuvant imatinib likely also contributed the latter. Five of six retrospective series had similar findings with regards to margins; 46 of 51 patients (90%) who underwent neoadjuvant imatinib and surgery in those studies had negative margins compared to 33 of 63 (52%) patient who did not13–15,18,19. Another study20 showed no difference, although both groups had high rates of negative margins.
In patients undergoing local excision in the absence of perioperative imatinib treatment, high local recurrence rates have been reported compared to radical resection18,24,25. However, our data suggest this does not hold true in the modern era. Because lymph node involvement is extremely rare23, lower rectal GISTs can often be removed by full-thickness local excision either transanally or transvaginally26,27. In our experience, local excision can also be accomplished by a transabdominal approach, which is facilitated by the high-resolution optics of robotic surgery, allowing precise minimally invasive, local excision that may include the prostatic capsule, vaginal wall, portions of the levator ani, or muscular wall of rectum. For a rectal GIST requiring transabdominal resection, we prefer the low anterior resection as opposed to local excision, when a majority of the circumference of the rectum is compromised. However, a formal total mesenteric excision is not necessary. In our experience, in the imatinib era, abominoperineal resection with colostomy was necessary only in one patient (3%) due to direct involvement of the anal sphincters. In general, we consider all distal rectal GISTs for local excision provided a negative margin can be obtained and the anal sphincter mechanism can be preserved. Based on our experience, we would accept a close or microscopically positive (R1) margin if the patient had been responsive to imatinib. However, if sacrifice of the sphincter would be necessary for tumor removal, or if the patient would be at high risk for fecal incontinence postoperatively (poor baseline sphincter function), we would consider abdominoperineal resection. Supplemental Figure 2 shows a proposed algorithm for the surgical management of rectal GIST.
Interpretation of oncologic outcome in previous studies is limited by lack of clear risk stratification and appropriate comparison groups, and in some cases, unclear reasons for lack of imatinib treatment. Huyhn. et al. saw longer disease-free survival (DFS) and local RFS with imatinib, but no difference in OS in 16 patients who received perioperative imatinib compared to 29 patients who did not, yet detailed risk stratification was not reported by group13. Jakob et al. reported longer OS, DFS, and local RFS in 21 patients who received perioperative imatinib compared to 15 who did not, again without reporting the risk categories18. Tielen et al. showed longer DFS in 22 high-risk patients receiving perioperative imatinib compared to 10 undesignated patients, with no difference in OS20. Here we show dramatically higher OS, DSS, local and distant RFS in the imatinib era compared to the pre-imatinib era. Likewise, in high-risk patients alone (76% of our cohort), these findings remained consistent. We believe this represents the best-controlled evidence to date supporting the association of perioperative imatinib with reduced local and distant recurrence, and improved DSS and OS.
Unlike local recurrences, distant recurrences were reduced but not prevented (Figure 3c–d). However, distant recurrences were found only after stopping adjuvant imatinib. Based on prospective trials of adjuvant imatinib9,28, it is our practice to prescribe at least 3 years of adjuvant imatinib after resection of high-risk rectal GIST. While there was no difference in recurrence in the Z9000/Z9001 studies with positive margins regardless of imatinib treatment29, we did not see any local recurrences in the imatinib era even with positive margins, while local recurrences were seen with positive margins in the pre-imatinib era (Supplemental Figure 1). This suggests that adjuvant imatinib may contribute to suppressing local recurrence after positive margins. Thus, in the face of positive microscopic margins, we generally prescribe adjuvant imatinib instead of more extensive re-resection (Supplemental Table 3).
Tyrosine kinase inhibitors have changed the natural history of GIST4 and many other solid tumors30. A guiding principle has been matching genomic analysis to treatment. In GIST, KIT exon 11 deletions may benefit most from imatinib31. Other mutations, such as KIT exon 9, may require a higher dose, while a common subset of PDGFRα mutations (D842V) do not respond to imatinib4. We did not detect any PDGFRα mutations in 31 tumors. Among 66 tumors tested in the six retrospective series, only one (1.5%) PDGFRα mutation was found, even though the rate is 10% in the overall GIST population4.
The 30-day complication rate was available from our prospectively maintained record for the IM era. Grade 1 complications occurred in 5 of 30 patients (17%; Table 2), all of which were managed with bedside and/or oral medical therapy. Grade 3 complications related to the rectal repair developed in 3 of 18 (17%) patients from the local excision group, none of whom had undergone neoadjuvant or adjuvant imatinib. Two of these patients developed dehiscence of the repair after transanal excision. Both were treated successfully by diverting stomas that were later reversed. Another patient developed suture line bleeding after a transanal excision, which required control in the operating room. Among patients who received any perioperative imaitinib (n=24), the only complications were grade 1 (n=4; 17%). Overall, we believe this is a low and acceptable complication rate for perioperative imatinib. It is our recommendation to clearly discuss with patients the risk of complications related to transanal local excision, in the context of the organ preservation that is achieved.
Although this study represents the largest single institution experience of rectal GIST, there are several limitations. While this is the largest series, the numbers are still small, and were collected over a longer period of time than the other series (34 years). The data are overall quite complete, however, complication data are not available for the pre-imatinib era. Finally, these data are retrospective in nature, and as a result may be subject to undetected bias.
In conclusion, in high-risk rectal GIST, neoadjuvant imatinib treatment was associated with higher rates of organ and sphincter preservation and lower rates of positive margins. Despite positive margins in up to one third, there were no local recurrences in those who received perioperative imatinib. Imatinib treatment was associated with reduced distant recurrence, and in those who did experience distant recurrence, salvage was possible with further imatinib treatment and surgery, resulting in no disease-related deaths in the imatinib era so far. Overall, we believe this approach yields better organ preservation without compromising (and possibly improving) oncologic outcome. In this rare subset of an uncommon disease where prospective study is difficult, we believe our data support perioperative imatinib and organ-preservation as the preferred approach in most rectal GIST.
Supplementary Material
Supplemental Figure 1: Oncologic outcome in rectal GIST patients by margin status.
Kaplan-Meier curve is shown depicting local RFS stratified by IM era for all patients. The number of patients at risk is listed for each time point. R2 resections (n=2) were excluded.
Supplemental Figure 2: Algorithm for management of rectal GIST.
aFor small GISTs (<3cm) for which local excision (transanal or transabdominal) would be readily possible, we recommend proceeding directly to surgery, with adjuvant imatinib therapy applied as in Supplemental Table 3. For larger rectal GISTs or small ones in which a size reduction would reduce the extent of surgery, we recommend 6 months of neoadjuvant imatinib. Workup includes a CT abdomen/pelvis to rule out synchronous metastasis, although in our institutional experience of 1,000 GISTs, among 128 presenting with synchronous metastasis, none were from a rectal primary (data not shown). MRI of rectum provides more precise assessment of the tumor in relation to adjacent organs, including the anal sphincter muscles.
bHigh rectal GISTs are above the peritoneal reflection or above the second rectal fold. approach, transanal minimally invasive surgery (TAMIS), or transanal endoscopic microsurgery (TEM), depending on the surgeon’s preference and expertise.
cFinal determination of local excision and risk of lumen compromise is made at the time of surgery.
dSphincter involvement is determined by digital rectal exam, rectal MRI, or proctoscopy.
eTransabdominal local excision can be accomplished open, laparoscopically, or robotically depending on the surgeon’s expertise. Wide margins are not necessary; instead, a negative margin of 5mm grossly should the goal of the resection.
fLow anterior resection (LAR) should be considered if the tumor involves 50% or more of the rectal circumference. Formal lymphadenectomy with total mesorectal excision is not necessary as lymph node metastasis is extremely rare.
gTransanal local excision can be accomplished with traditional methods, TAMIS, or TEM depending on the surgeon’s preference and expertise. The goal should be a negative margin although a close or even R1 margin may be acceptable in a patient responding to imatinib, if it allows sphincter preservation.
hRadical resection with APR or TPE may be necessary if sphincter involvement or other involvement of other pelvic organs (respectively) persists after neoadjuvant imatinib. Also, in a patient with poor sphincter function, low LAR or local excision close to sphincters may lead to significant fecal incontinence such that APR would be more appropriate from a quality of life perspective.
SYNOPSIS.
In high-risk rectal GIST, neoadjuvant imatinib was associated with greater preservation of the rectum and sphincters, and perioperative imatinib was associated with lower recurrence and longer survival.
Acknowledgments
We thank Heidi Trenholm and Christina Curtin from the Memorial Sloan Kettering Cancer Center sarcoma database for their efforts extracting data.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PloS one. 2011;6(8):e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(1):298–302. doi: 10.1158/1055-9965.EPI-14-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Human pathology. 2006;37(12):1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annual review of medicine. 2012;63:247–258. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mucciarini C, Rossi G, Bertolini F, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC cancer. 2007;7:230. doi: 10.1186/1471-2407-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 7.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. The American journal of gastroenterology. 2005;100(1):162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason G, Gislason HG, Magnusson MK, Jonasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. International journal of cancer. 2005;117(2):289–293. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 9.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(3):244–250. doi: 10.1200/JCO.2015.62.9170. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Annals of surgical oncology. 2013;20(9):2937–2943. doi: 10.1245/s10434-013-3013-7. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto Y, Akiyoshi T, Konishi T, Nagayama S, Fukunaga Y, Ueno M. Laparoscopic sphincter-preserving surgery (intersphincteric resection) after neoadjuvant imatinib treatment for gastrointestinal stromal tumor (GIST) of the rectum. International journal of colorectal disease. 2014;29(1):111–116. doi: 10.1007/s00384-013-1769-7. [DOI] [PubMed] [Google Scholar]
- 13.Huynh TK, Meeus P, Cassier P, et al. Primary localized rectal/pararectal gastrointestinal stromal tumors: results of surgical and multimodal therapy from the French Sarcoma group. BMC cancer. 2014;14:156. doi: 10.1186/1471-2407-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Yan Z, Liao G, Yin H. Treatment strategy of rectal gastrointestinal stromal tumor (GIST) Journal of surgical oncology. 2014;109(7):708–713. doi: 10.1002/jso.23562. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson MJ, Fitzgerald JE, Strauss DC, et al. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. The British journal of surgery. 2015;102(8):965–971. doi: 10.1002/bjs.9818. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Archives of pathology & laboratory medicine. 2006;130(10):1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Seminars in diagnostic pathology. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Annals of surgical oncology. 2013;20(2):586–592. doi: 10.1245/s10434-012-2647-1. [DOI] [PubMed] [Google Scholar]
- 19.Pai VD, Demenezes JL, Patil PS, Saklani AP. Multimodality therapy of rectal gastrointestinal stromal tumors in the era of imatinib-an Indian series. Journal of gastrointestinal oncology. 2016;7(2):262–268. doi: 10.3978/j.issn.2078-6891.2015.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tielen R, Verhoef C, van Coevorden F, et al. Surgical management of rectal gastrointestinal stromal tumors. Journal of surgical oncology. 2013;107(4):320–323. doi: 10.1002/jso.23223. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. Journal of surgical oncology. 2009;99(1):42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(13):1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 23.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Annals of surgery. 1993;217(1):72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Changchien CR, Wu MC, Tasi WS, et al. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Diseases of the colon and rectum. 2004;47(11):1922–1929. doi: 10.1007/s10350-004-0687-8. [DOI] [PubMed] [Google Scholar]
- 25.Agaimy A, Vassos N, Markl B, et al. Anorectal gastrointestinal stromal tumors: a retrospective multicenter analysis of 15 cases emphasizing their high local recurrence rate and the need for standardized therapeutic approach. International journal of colorectal disease. 2013;28(8):1057–1064. doi: 10.1007/s00384-013-1655-3. [DOI] [PubMed] [Google Scholar]
- 26.Centonze D, Pulvirenti E, Pulvirenti D’Urso A, Franco S, Cinardi N, Giannone G. Local excision with adjuvant imatinib therapy for anorectal gastrointestinal stromal tumors. Techniques in coloproctology. 2013 doi: 10.1007/s10151-013-0976-0. [DOI] [PubMed] [Google Scholar]
- 27.Hara M, Takayama S, Arakawa A, Sato M, Nagasaki T, Takeyama H. Transvaginal resection of a rectal gastrointestinal stromal tumor. Surgery today. 2012;42(9):909–912. doi: 10.1007/s00595-012-0215-8. [DOI] [PubMed] [Google Scholar]
- 28.Joensuu H, Eriksson M, Hatrmann J, et al. Twelve versus 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: Final results of a randomized trial (SSGXVIII/AIO) Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(18_suppl) LBA1. [Google Scholar]
- 29.McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. Journal of the American College of Surgeons. 2012;215(1):53–59. doi: 10.1016/j.jamcollsurg.2012.05.008. discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen NA, Kim TS, DeMatteo RP. Principles of Kinase Inhibitor Therapy for Solid Tumors. Annals of surgery. 2017;265(2):311–319. doi: 10.1097/SLA.0000000000001740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(15):1563–1570. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Oncologic outcome in rectal GIST patients by margin status.
Kaplan-Meier curve is shown depicting local RFS stratified by IM era for all patients. The number of patients at risk is listed for each time point. R2 resections (n=2) were excluded.
Supplemental Figure 2: Algorithm for management of rectal GIST.
aFor small GISTs (<3cm) for which local excision (transanal or transabdominal) would be readily possible, we recommend proceeding directly to surgery, with adjuvant imatinib therapy applied as in Supplemental Table 3. For larger rectal GISTs or small ones in which a size reduction would reduce the extent of surgery, we recommend 6 months of neoadjuvant imatinib. Workup includes a CT abdomen/pelvis to rule out synchronous metastasis, although in our institutional experience of 1,000 GISTs, among 128 presenting with synchronous metastasis, none were from a rectal primary (data not shown). MRI of rectum provides more precise assessment of the tumor in relation to adjacent organs, including the anal sphincter muscles.
bHigh rectal GISTs are above the peritoneal reflection or above the second rectal fold. approach, transanal minimally invasive surgery (TAMIS), or transanal endoscopic microsurgery (TEM), depending on the surgeon’s preference and expertise.
cFinal determination of local excision and risk of lumen compromise is made at the time of surgery.
dSphincter involvement is determined by digital rectal exam, rectal MRI, or proctoscopy.
eTransabdominal local excision can be accomplished open, laparoscopically, or robotically depending on the surgeon’s expertise. Wide margins are not necessary; instead, a negative margin of 5mm grossly should the goal of the resection.
fLow anterior resection (LAR) should be considered if the tumor involves 50% or more of the rectal circumference. Formal lymphadenectomy with total mesorectal excision is not necessary as lymph node metastasis is extremely rare.
gTransanal local excision can be accomplished with traditional methods, TAMIS, or TEM depending on the surgeon’s preference and expertise. The goal should be a negative margin although a close or even R1 margin may be acceptable in a patient responding to imatinib, if it allows sphincter preservation.
hRadical resection with APR or TPE may be necessary if sphincter involvement or other involvement of other pelvic organs (respectively) persists after neoadjuvant imatinib. Also, in a patient with poor sphincter function, low LAR or local excision close to sphincters may lead to significant fecal incontinence such that APR would be more appropriate from a quality of life perspective.


