SUMMARY
Temporary and permanent facial nerve dysfunctions can be observed after parotidectomy for benign and malignant lesions. Intraoperative nerve monitoring is a recognised tool for the preservation of the nerve, while the efficacy of the operative microscope has been rarely stated. The authors report their experience on 198 consecutive parotidectomies performed on 196 patients with the aid of the operative microscope and intraoperative nerve monitoring. 145 parotidectomies were performed for benign lesions and 53 for malignancies. Thirteen patients treated for benign tumours experienced temporary (11 cases) or permanent facial palsy (2 cases, both of House-Brackmann grade II). Ten patients with malignant tumour presented with preoperative facial nerve weakness that did not improve after treatment. Five and 6 patients with malignant lesion without preoperative facial nerve deficit experienced postoperative temporary and permanent weakness respectively (the sacrifice of a branch of the nerve was decided intraoperatively in 2 cases). Long-term facial nerve weakness after parotidectomy for lesions not directly involving or originating from the facial nerve (n = 185) was 2.7%. Patients treated for benign tumours of the extra facial portion of the gland without inflammatory behaviour (n = 91) had 4.4% facial nerve temporary weakness rate and no permanent palsy. The combined use of the operative microscope and intraoperative nerve monitoring seems to guarantee facial nerve preservation during parotidectomy.
KEY WORDS: Parotid tumours, Facial weakness, Intraoperative nerve monitoring, Microscope-assisted parotidectomy, Salivary glands
RIASSUNTO
I pazienti sottoposti ad intervento chirurgico di parotidectomia per lesioni benigne e maligne possono presentare disfunzioni temporanee o permanenti del nervo facciale. Il monitoraggio intraoperatorio della motilità facciale è uno strumento ampiamente riconosciuto per la sua utilità nella preservazione del nervo, mentre l'efficacia del microscopio operatorio è stata raramente discussa. Gli autori riportano la loro esperienza su 198 parotidectomie consecutive eseguite su 196 pazienti con l'ausilio del microscopio operatorio e del monitoraggio intraoperatorio del nervo facciale. Centoqurantacinque interventi sono stati eseguiti per lesioni benigne e 53 per neoplasie maligne. Tredici pazienti operati per lesioni benigne hanno presentato un deficit della funzionalità del nervo facciale: 11 hanno sofferto di paralisi temporanea e 2 di paralisi permanente (entrambe di secondo grado). Dieci pazienti affetti da patologia maligna presentavano un interessamento preoperatorio del nervo facciale. Cinque e sei pazienti affetti da patologia maligna senza interessamento preoperatorio del nervo hanno presentato un deficit rispettivamente temporaneo e definitivo (in 2 casi il sacrificio di un ramo del nervo macroscopicamente infiltrato dalla neoplasia fu deciso solo durante la procedura chirurgica). L'incidenza di paralisi definitiva di una singola branca del nervo facciale dopo interventi eseguiti per lesioni che non originavano dal nervo facciale o che non lo infiltravano macroscopicamente (n = 185) è stata del 2,7%. I pazienti trattati per tumori benigni non flogistici del lobo superficiale della ghiandola parotide (n = 91) hanno presentato una paralisi facciale postoperatoria temporanea nel 4,4% dei casi e nessun deficit permanente. L'uso combinato del microscopio operatorio e del monitoraggio intraoperatorio del nervo sembra garantire la preservazione del nervo facciale nei pazienti sottoposti a parotidectomia.
Introduction
Salivary gland neoplasms represent 3% of all head and neck tumours, the majority of which originate from the parotid 1. Surgical treatment of parotid neoplasms is focused on complete removal of the tumour together with the extra-petrosal dissection of the facial nerve. Although parotidectomy is a well-known and safe procedure if correctly performed, immediate postoperative facial nerve weakness may occur in 10% to 40% of patients treated for a benign neoplasm 2, whereas permanent postoperative facial weakness has been described in 1% to 7.1% of patients 2-8. Malignant lesions are generally related to a higher rate of postoperative palsy.
Intraoperative nerve monitoring (NIM) has been associated with a lower risk of immediate postoperative facial nerve palsy in primary cases of parotidectomy 2, while the efficacy of the operative microscope during the parotidectomy has been rarely evaluated. The authors report their experience on the surgical management of parotid gland tumours with microscope assisted parotidectomy (MAP) combined with NIM, focusing on postoperative facial nerve function and clinical/oncologic outcomes according to histology.
Materials and methods
This is a prospective study involving a consecutive cohort of 196 patients treated at our Department with MAP coupled with NIM from November 2010 to January 2016. Medical history was collected before admission. High-resolution ultrasonography (US), computerised tomography (CT), magnetic resonance imaging (MRI) and fine needle aspiration cytology (FNAC) were used for preoperative evaluation, although not always together. Facial nerve function was evaluated preoperatively, on the first day after surgery, after one month and at least at three months after surgery using the House-Brackmann scale 9 completed with the description of the function of all the facial nerve branches (frontal, zygomatic, buccal, mandibular). Patients underwent superficial (removal of all the parotid tissue above the facial nerve) or total parotidectomy (extended to all parotid tissue including the deep lobe of the gland), according to the extension of the tumour. We never performed enucleations or extra-capsular dissections. Neck dissection (levels II-V) and adjuvant therapies were performed in case of malignant lesions, according to the Oncology Guidelines approved by our Institutional Review Board. Facial nerve dissection was always performed coupling the intraoperative microscope (ZEISS S7, focal length 250 mm) with NIM (Medtronic NIM Response® 3.0-4 channels). Typical parameters used at our institution are: stimulus intensity of 0.5-0.7 mA, duration of the stimulus of 100 μsec, rate of the stimuli of 4 bursts/sec and event threshold of 100 μV. Antibiotic prophylaxis (ceftriaxone 1 g IV/day) was given at least 30 min preoperatively; corticosteroids (dexamethasone, 4 mg/day for 7 days) were used when immediate postoperative facial nerve weakness was present. Patients were reviewed during this study for the purpose of facial nerve dysfunction and oncologic outcome as well as for development of symptomatic Frey's syndrome and numbness of the pinna following the sectioning of the great auricular nerve (GAN). This study also considered the correlation between facial nerve dysfunction, histology and surgical complexity (total parotidectomy and superficial parotidectomy, benign neoplasms of the superficial and deep lobe, inflammatory lesions of the superficial and deep lobe, facial nerve schwannoma, primitive malignant tumours of the superficial and deep lobe, lymphomas, normal parotid gland removed during management of skin malignant neoplasms, metastatic tumours of the parotid without skin infiltration or preoperative facial nerve involvement, metastatic tumours of the parotid with skin infiltration or preoperative facial nerve involvement). Patients with a diagnosis of lymphoma were referred for long-term oncologic follow-up to the Department of Onco-haematology with autonomous follow-up. Survival time was assessed from the date of surgery to the date of the last followup visit. 5-year overall survival (OS) and disease-specific survival (DSS) rates were calculated using the Kaplan- Meier method. The statistical significance of the different patient populations was tested with the chi-square test, considering significance when p was less than 0.05.
Results
During the period of the study, 198 parotidectomies were performed on 97 men and 99 women (sex ratio M/F of 0.98, mean age 56.1 years, range 15-88). The procedure was performed on the right side in 95 patients, on the left side in 99 patients and bilateral in 2 cases (1 patient presented a primary benign neoplasm of both the parotid glands and 1 patient underwent bilateral parotidectomy for suspicious bilateral metastasis of skin malignancy of the scalp). 145 parotidectomies (73.2%) were performed for benign lesions and 53 (26.8%) for malignant lesions (Table I). 169 patients were evaluated for a chronic swelling of a parotid mass previously confirmed with US, 6 patients were treated for local recurrence after parotidectomy for benign lesion performed elsewhere, 11 patients were treated for possible parotid involvement of cutaneous tumours, and 10 patients presented with an advanced malignant neoplasm with clinical involvement of the parotid area. 143 patients (73%) were preoperatively evaluated with MRI with contrast medium; 53 patients (27%) were evaluated only with CT with contrast medium (28 patients affected by malignant lesions were investigated only with CT). MRI (41.2%) allowed a preoperative diagnosis in 59 cases: 70.9% (44/62) of MRI for pleomorphic adenomas allowed pre-operative diagnosis, while only 13.3% (4/30) of MRI were reliable in pre-operative diagnosis of Warthin's tumour. CT and/or MRI allowed preoperative diagnosis of malignant lesion of the parotid in 18 cases (34%), in 24 cases (45.3%) it confirmed the parotid lesion but could not predict the benign or malignant nature, and was negative in 11 cases (20.7%) whose definitive histological analysis confirmed the presence of carcinoma. FNAC was performed in 37 patients (18.9%), and was predictive in 22/37 lesions (accuracy of 59.5%). FNAC diagnosis of pleomorphic adenoma was predictive in 100% of cases (n = 9), while FNAC was diagnostic in 50% of cases of Warthin's tumour (2/4 patients). Patients with a cytological diagnosis of benign tumour of the parotid (n = 19) was benign in 18 cases, while 1 was malignant. Patients with FNAC suspicious for malignancy (n = 5) underwent neck dissection during the same procedure after intraoperative histological confirmation (4/5 resulted pN+), patients with non-diagnostic FNAC (n = 13) were submitted in 11 cases to parotidectomy alone (definitive diagnosis showed 2 primary malignancies, 1 lymphoma and 8 benign lesions), in 1 case to a delayed neck dissection after histological diagnosis of primitive parotid malignancy, and in 1 case with obvious clinical malignant behaviour, to parotidectomy with neck dissection performed during the same general anaesthesia after intraoperative histological confirmation of the malignant nature of the lesion. Trans-nasal intubation was always performed to avoid the retrograde move of the angle of the mandible due to the oro-tracheal tube, improving the space for nerve identification. A superficial parotidectomy was performed in 140 cases (70.7%) and a total parotidectomy in 58 cases (29.3%). Thirty-eight tumours (19.2%) involved the deep lobe of the parotid gland (28 benign neoplasms, 3 malignant lesions and 7 inflammatory lesions). The sacrifice of single branches of the nerve involved by the tumour was necessary in 13 cases: in 10 patients, the involvement of the nerve was preoperatively obvious, in 2 cases it was evident only intraoperatively (neural invasion was definitively confirmed by histology as shown in Fig. 1) and in 1 case the tumour was originating from the nerve; in 4 cases GAN grafting was performed in the attempt to restore the nerve continuity. Two parotidectomies were performed during management of a head and neck melanoma. In 28 cases the parotidectomy was associated with a selective or modified radical neck dissection, in 3 cases it was associated with the removal of the pinna, and in 8 cases it was widely extended to the facial skin and required a reconstructive procedure (2 pectoralis major pedicled flaps, 2 platysma pedicled flaps, 3 free flaps of rectus abdominis, and 1 forearm free flap). Three patients underwent delayed neck dissection after histological diagnosis of parotid malignancy.
Table I.
Parotid tumours.
| Histologic types | No. of parotidectomies | % |
|---|---|---|
| Pleomorphic adenoma | 70 | 35.4 |
| Warthin's tumour1 | 42 | 21.2 |
| Salivary cyst | 5 | 2.6 |
| Lymph epithelial cyst | 2 | 1 |
| Epidermoid cyst | 2 | 1 |
| Branchial cyst | 1 | 0.5 |
| Mucopapillary cyst | 1 | 0.5 |
| Inflammatory lymphonode | 2 | 1 |
| Sjogren's syndrome | 1 | 1 |
| Lithiasis | 1 | 0.5 |
| Inflammatory degeneration | 1 | 0.5 |
| Masson's tumour | 1 | 0.5 |
| Cystadenoma | 3 | 1.5 |
| Lymph node | 2 | 1 |
| Basal cell adenoma | 1 | 0.5 |
| Haemangioma | 1 | 0.5 |
| Lymphangioma | 1 | 0.5 |
| Fibrosis | 1 | 0.5 |
| Follicular hyperplasia | 1 | 0.5 |
| Chronic cystic hyperplasia | 2 | 0.5 |
| Schwannoma | 1 | 0.5 |
| Oncocytoma | 1 | 0.5 |
| Lipoma | 1 | 0.5 |
| Connective substitution | 1 | 0.5 |
| TOTAL BENIGN LESIONS | 145 | 73.2 |
| Metastasis of skin malignancy 15 squamocellular carcinomas | 172 | 8.7 |
| 1 melanoma 1 Merkel's tumour | ||
| Metastasis of renal malignancy | 1 | 0.5 |
| Adenocarcinomas | 113 | 5.6 |
| Carcinoma on pleomorphic adenoma | 3 | 1.5 |
| Mucoepidermoid carcinoma | 44 | 2 |
| Oncocytic carcinoma | 1 | 0.5 |
| Myoepithelial carcinoma | 1 | 0.5 |
| Neuroendocrine carcinoma | 1 | 0.5 |
| Lymphoma | 6 | 3 |
| Normal parotid gland (associated with neck dissection) | 85 , 6 , 7 | 4 |
| TOTAL MALIGNANT LESIONS | 53 | 26.8 |
| ALL | 198 | 100 |
1 patient underwent bilateral parotidectomy for bilateral Warthin's tumour
Facial nerve neoplastic infiltration was evident preoperatively in 7 cases.
Facial nerve neoplastic infiltration was evident preoperatively in 1 case.
Facial nerve neoplastic infiltration was evident preoperatively in 1 case.
1 patient was submitted to bilateral parotidectomy for suspicious bilateral metastasis of squamous cell carcinoma; histology revealed the carcinoma in only one parotid gland.
1 patient was submitted to parotidectomy with radical neck dissection for head and neck melanoma; histology did not reveal the melanoma in the parotid gland.
A facial nerve deficit was preoperatively due to the neoplastic involvement of the nerve from the zygomatic skin malignancy in 1 case.
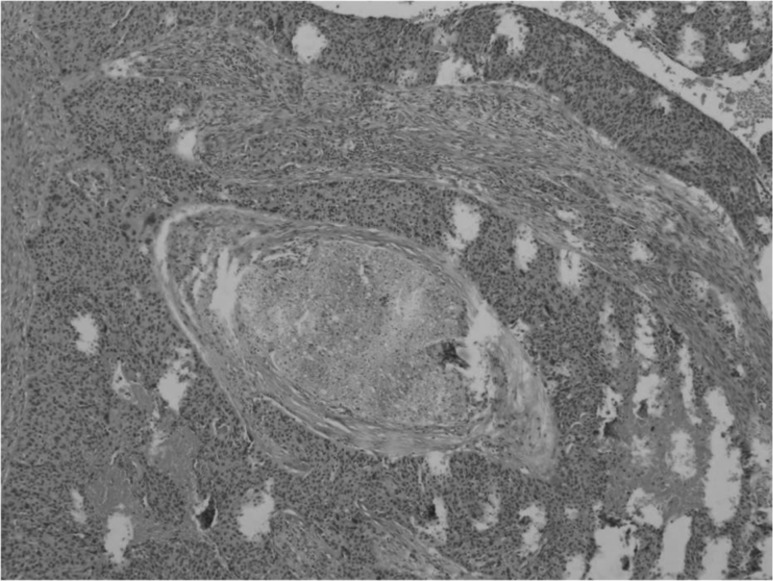
Fig. 1.
H&E (20X): squamous cell carcinoma perineural involvement. The patient did not show any preoperative facial weakness, but intraoperatively the mass showed important adherences to a branch of the nerve that was resected.
Average operation time for benign lesion was 221 min (range of 120-350). Mean hospitalisation time was 4.3 days (2.4 days for patients submitted to parotidectomy alone and 7.7 days for patients submitted to more invasive procedures). Postoperative complications occurred in 17 patients (Table II). Bleeding always required surgical revision of the haemostasis, while other complications like seroma, salivary fistula and infection were managed by antibiotics and conservative procedures.
Table II.
Postoperative complications.
| Complications | Benign tumour | vs. | Malignant tumour | Superficial parotidectomy | vs. | Total parotidectomy |
|---|---|---|---|---|---|---|
| Bleeding | 5 | 4 | 6 | 3 | ||
| Wound infection | 1 | - | - | 1 | ||
| Salivary fistula | - | 1 | 1 | - | ||
| Seroma | 5 | 1 | 6 | - | ||
| TOTAL | 11 | 6 | 13 | 4 |
Among the benign pathologies (n = 145), 11 showed a temporary (7.6%) and 2 a permanent weakness (1.4%) of the facial nerve. The 11 temporary weaknesses were grade II in 8 cases and grade III in 3 cases, with a mean recovery time of 2.3 months (0.2-6 months); in 3/11 cases the tumour involved the deep lobe of the gland and in 4/11 cases the lesion (inflammatory) presented multiple adherences to the nerve. The 2 permanent palsies were of grade II and involved one single branch of the nerve: the first was observed after total parotidectomy for an inflammatory cystic lesion of the para-pharyngeal portion of the parotid gland adhering to the marginal mandibular branch, and the second after superficial parotidectomy for a schwannoma originating from the zygomatic branch of the facial nerve. Among the malignancies, 10/53 procedures were performed in patients with preoperative facial nerve weakness that did not improve after treatment; patients without preoperative facial nerve deficit experienced a temporary weakness in 5/43 cases (11.6%), while in 6/43 cases (14%) presented a permanent deficit (in 2 cases the palsy was due to the surgical removal of a peripheral branch of the nerve macroscopically involved by the tumour, and in 3 cases it was observed after parotidectomy associated with selective neck dissection).
Temporary and permanent facial nerve weakness rates after superficial and total parotidectomy in patients without preoperative deficit were 7.1% (10/140) and 3.6% (5/140) vs. 12.5% (6/48) and 6.25% (3/48) respectively. Patients treated for benign tumours of the extra-facial portion of the gland without inflammatory behaviour (n = 91) had 4.4% facial nerve temporary weakness rate and no permanent palsy. Patients without preoperative facial weakness undergoing superficial or total parotidectomy associated with neck dissection showed a 11.1% rate (2/18) of definitive deficit of the mandibular branch of the nerve; permanent facial nerve weakness after parotidectomy for benign and malignant lesions not directly involving or originating from the facial nerve was 2.7% (5/185). The degrees and branches involved are detailed in Table III. After surgery, 15 patients treated for malignancy underwent adjuvant radiotherapy (50–60 Gy, associated with chemotherapy in 5 patients). Two patients with melanoma underwent adjuvant chemotherapy.
Table III.
Postoperative outcomes according to surgical complexity.
| Histological type | Number of procedures | Immediate temporary facial nerve weakness | Permanent facial nerve weakness | Frey's syndrome | Recurrence |
|---|---|---|---|---|---|
| Benign neoplasms of the superficial lobe |
91 | 4 1 Grade II – Marginal mandibular 2 Grade II – Buccal 1 Grade III – Marginal mandibular |
0 | 18 | 0 |
| Benign tumours of the deep lobe | 28 | 3 2 Grade II – Marginal mandibular 1 Grade III – Marginal mandibular |
0 | 7 | 0 |
| Inflammatory tumours of the superficial lobe |
18 | 2 1 Grade II – Marginal mandibular 1 Grade III – Marginal mandibular |
0 | 7 | 0 |
| Inflammatory lesions of the deep lobe |
7 | 2 1 Grade II – Marginal mandibular 1 Grade III – Marginal mandibular |
1 Grade II – Marginal mandibular |
3 | 0 |
| Schwannoma | 1* | 0 | 1 Grade II – Zygomatic |
0 | 0 |
| Primary malignancy of the superficial lobe without pre-operative facial nerve involvement |
16** | 3 2 Grade II – Marginal mandibular 1 Grade III – Marginal mandibular |
3 2 Grade II – Marginal mandibular 1 Grade III – Frontal |
3 | 0 |
| Primitive malignancy of the deep lobe without pre-operative facial nerve involvement |
3 | 0 | 2 1 Grade III – Marginal mandibular 1 Grade III – Zygomatic |
0 | 0 |
| Lymphomas | 6 | 0 | 1 Grade III – Zygomatic |
1 | - |
| Normal parotid gland removed during the management of skin malignant neoplasms |
7 | 1 Grade II – Marginal mandibular |
0 | 2 | 0 |
| Parotid metastasis of renal malignancy without skin infiltration or preoperative facial nerve involvement |
1 | 0 | 0 | 0 | 0 |
| Parotid metastasis of skin malignancies without skin infiltration or preoperative facial nerve involvement |
10 | 1 Grade II – Marginal mandibular |
0 | 2 | 0 |
| Primitive or metastatic parotid malignancies with skin infiltration and preoperative facial nerve weakness |
10 | - | 10 | 0 | 2 |
| TOTAL | 198 | 16 | 18 | 43 | 2 |
The tumour originated from the nerve and required the sacrifice of a minor branch.
2 procedures required sacrifice of a peripheral branch of the nerve that was directly involved by the neoplasm.
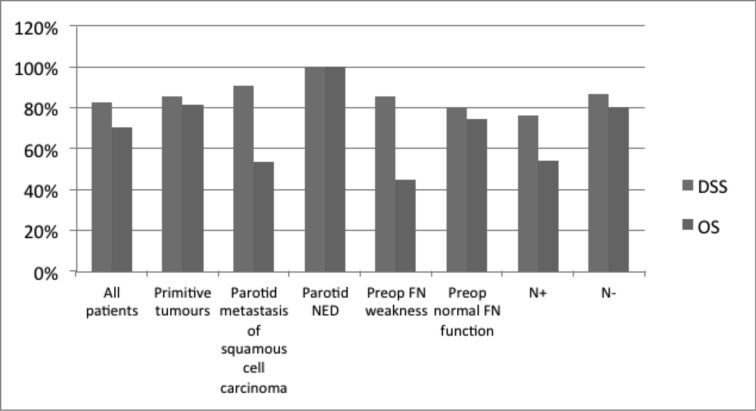
No recurrences have occurred after surgery for benign lesions to date. Among the malignancies, 46 patients were included in regular oncologic follow-up at our Department; mean follow-up period was 20 months (range of 3 month-5 years). Two patients experienced local recurrence and underwent chemoradiotherapy, 2 patients (1 diagnosis of melanoma and 1 Merkel carcinoma) experienced neck skin relapse and underwent salvage surgery followed by chemotherapy and radiotherapy, respectively, 3 patients experienced distant metastasis and underwent chemotherapy. Thirty-five patients were alive with no evidence of parotid tumour-related disease at the last followup, while 5 patients died of disease and 6 patients died for other causes. DSS and OS for the cohort of patients followed-up in our Department (n = 46) were 83% and 70.6%; patients with primary malignancy of the parotid (n = 22) showed DSS and OS of 85.6% and 81.3%; patients with metastatic squamous cell carcinoma involving the parotid (n = 15) showed DSS and OS of 90.9% and 53.8%; patients submitted to parotidectomy for suspicious metastasis of squamous cell carcinoma, but negative at histology (n = 6) presented DSS and OS of 100%; patients with preoperative facial nerve weakness/skin infiltration (n = 10) presented DSS and OS of 85.7% and 45%; patients without preoperative facial nerve weakness/skin infiltration (n = 36) presented DSS and OS of 80.3% and 74.6%; patients with node metastasis (n = 17) presented DSS and OS of 76.4% and 54.3%; patients without node metastasis (n = 29) presented DSS and OS of 87% and 80.4% (Fig. 2).
Fig. 2.
Survival rates according to different risk factors in malignancies.
All patients experienced postoperative auricular numbness; at present (or at the last examination for patients died during the follow-up), 133 patients still report numbness in correspondence of the inferior lobe of the pinna: 44.7% who underwent parotidectomy in 2011, 65.9% of those treated in 2012, 67.5% of those treated in 2013, 90.2% of those treated in 2014 and 97.2% of those treated in 2015. Thirty-five patients experienced a Frey's syndrome after parotidectomy performed for benign tumour (n = 145). No patients experienced first bite syndrome.
Discussion
A large variety of benign and malignant tumours can arise from the salivary glands 10, therefore, when a localised parotid mass is detected, it requires a specific diagnostic work-up focusing on evaluating the risk of malignancy, since it changes the prognosis and the attitude toward the facial nerve 11. High-resolution US, conventional MRI and CT with contrast medium are commonly used for evaluating parotid masses 12 13. We used, when possible, MRI for preoperative evaluation, but in patients previously evaluated with adequate CT scan images, we did not ask for further imaging. Preoperative diagnostic assessment also takes advantage of FNAC, a minimally invasive technique with a recognised role in cytological diagnosis of patients with salivary gland tumours, with reasonable sensitivity and specificity 14. Nevertheless, the interpretation of FNAC of salivary gland lesions could be a great challenge for cytologists: the material provided by FNAC may contain poor diagnostic elements and there are some rare tumours that can cause confusion 15 16. Piccioni et al 17 reported sensitivity and specificity rates of 81% and 99%, respectively, excluding non-diagnostic procedures; however, the analysis of all the procedures of the same study, diagnostic and non-diagnostic, shows an accuracy rate of 77.3% (136 reliable diagnoses in 176 procedures). FNAC has been employed in our Department since 2010, with an accuracy of 59.5%, in preoperative differential diagnosis of benign to malignant neoplasms. The experience of the pathologist is considered fundamental in FNAC 15 18. According to the literature, we consider that FNAC contribute to, but cannot substitute overall diagnostic assessment 19.
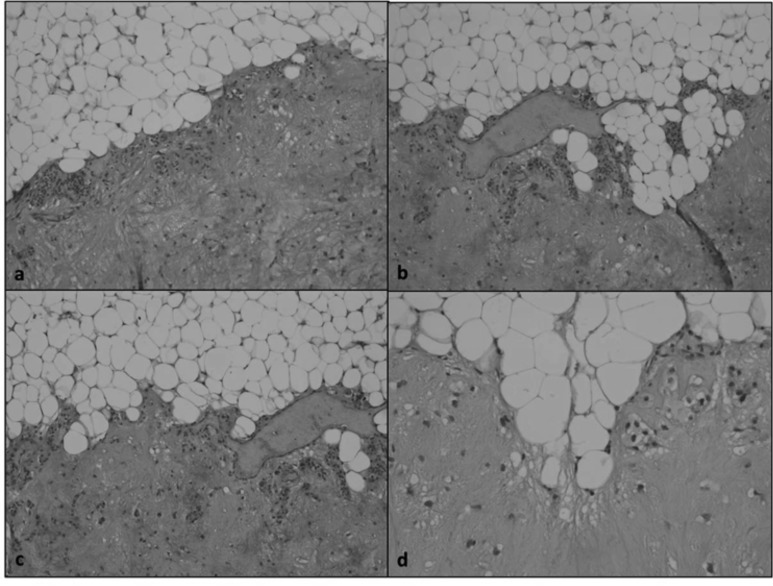
We do not use it systematically when clinical and radiologic evaluations appeared enough to diagnose a benign lesion since the FNAC would not have modified the therapeutic approach. In only one patient with definitive histology showing malignancy, non-diagnostic FNAC was considered responsible for a delayed neck dissection. In the presence of a parotid lesion, it is mandatory that complete removal of the tumour is performed in form of enucleation/ extra-capsular dissection (the removal of the entire lesion, without sacrifice of parotid tissue) and superficial or total parotidectomy with the complete excision of the gland including the tumour in healthy margins. Parotid dissection is based on the anatomical knowledge of the extra-temporal facial nerve course and its branches. The classic approach to parotidectomy is anterograde from the main trunk of the VII cranial nerve to the peripheral branches. The main trunk of the nerve is identified and isolated where it emerges from the stylo-mastoid foramen, through the three classical landmarks: mastoid tympanic sulcus, the "pointer" and the posterior belly of the digastric muscle 20. We routinely perform anterograde dissection with superficial or total parotidectomy, but in three cases of voluminous tumour arising from the superficial lobe of the gland with wide contact between the deepest aspect of the tumour and the main trunk of the nerve, we performed a retrograde dissection, starting from the identification of the frontal branch being the most superficial in the close proximity to the incision, and no postoperative facial nerve weakness was observed. Extra-capsular dissection through a standard preauricular approach is reported in the literature, abandoning the concept of formal dissection of the nerve with minimal dissection of parotid tissue 21. Mantsopopulos et al. 22 suggested that this technique may be applied in cases of a superficially located mobile lesion or in cases of pleomorphic adenoma arising from the pharyngeal portion of the parotid after digastric muscle sectioning (trans cervical approach), while a more radical procedure requiring dissection of the facial nerve and its branches should be reserved only after detection of malignancy in frozen sections of an extra-capsular specimen. He reported a 1.9% rate of postoperative permanent facial palsy after extra-capsular dissection for benign neoplasms of the superficial lobe. We observed a lower incidence of facial palsy (4.4% temporary weakness rate and no permanent palsy) after superficial parotidectomy performed for well-defined benign tumours of the superficial lobe of the parotid gland, and we do not agree with the extra-capsular dissection also on the basis of our personal histological findings of pleomorphic adenomas that lack of a capsule (Fig. 3). Superficial parotidectomy in experienced hands moderately increases surgical time compared to extra-capsular dissection, but we believe that it is rewarding in terms of facial palsy and risk for recurrences, allowing to remove intraparotid lymph nodes, avoiding asymmetry of the parotid region and reducing the chances to leave metachronous Warthin's tumours. Nevertheless, extra-capsular dissection has to be performed in the majority of tumours of the deep lobe of the parotid, where the glandular parenchyma is very limited or, when the tumour of the superficial lobe is in close contact with the nerve; in these cases a blunt dissection, usually performed by the finger of the surgeon, represents the most frequently performed technique. Spillage of the tumour after an accidentally interrupted capsule is indeed the main cause of single or multiple recurrences, while complete removal of the lesion without spillage of the tumour, leaving a boundary of parenchyma along the tumour can be obtained only after the complete visualisation of the facial nerve. Furthermore, the experience of the surgeon arises only from routine careful dissection of the nerve that may be imperative in challenging cases, such as voluminous tumours, recurrences and chronic inflammation. Witt et al. observed a higher tumour recurrence rate after extra-capsular dissection (3%) than after superficial parotidectomy (0.3%) with a p < 0.05 23. Colella et al. also reported lower recurrence rates after total or superficial parotidectomy (0.01% and 0.02% respectively) compared to those reported after extracapsular/ enucleation (0.08%) 24.
Fig. 3.
H&E: microscopic view (a & b 10X, c & d 20X) of a discontinuous capsule of a pleomorphic adenoma. The removal of the lesion by superficial parotidectomy leaving a border of glandular parenchyma along the tumour allowed complete removal of the lesion.
On the contrary, Albergotti et al. did not observe difference in tumour recurrence after superficial parotidectomy vs. extra-capsular dissection, with a significantly lower rate of transient facial nerve paresis after extra-capsular dissection and no difference in permanent facial paralysis between the two procedures 25. In the present series we did not observe spillage of the tumour as a consequence of accidental rupture of the capsule, and we did not observe recurrence of benign tumours. Facial nerve injury is the most significant complication of parotidectomy 2. Postoperative facial weakness may be due to the section of the nerve, intraoperative trauma (thermal trauma, compression or stretching), or ischaemia, when neoplasms adhere to the nerve and require a dissection with the sacrifice of the "vasa nervorum". Nerve damage is classified as neurapraxia, axonotmesis, and neurotmesis, largely based on the degree of injury, which ranges from microscopic to macroscopic 26. When the anatomy of the nerve is preserved, the facial deficit should resolve within 12 weeks 27, after this time all deficits could be considered definitive with low chance of regression. House-Brackmann is a common and reproducible tool developed to quantify facial function in six steps from normal (I) to total paralysis (VI). Since it doesn't fully correlate with facial function of each of the branches 28, in our study we added the description of the function of each single branch, with main attention to the marginal mandibular branch.
Intraoperative facial nerve monitoring is widely used in otology, neurotology, and skull base surgery 2 29 30. The goals of NIM during parotidectomy are the same as those during otology and neurotology: early facial nerve identification, warning the surgeon of unexpected facial nerve stimulation, mapping of the course of the nerve, reduction of mechanical trauma to the nerve and evaluation and prognosis of nerve function at the end of the procedure 31. Facial nerve monitoring is a safe procedure: facial nerve injury due to voluntary overstimulation is almost impossible, since electrically evoked facial nerve responses during electrophysiological facial nerve monitoring are obtained using a safe pulsed nerve probe, and the intensity of the stimulus is preoperatively established.
In our protocol, the setting of the stimulation was never greater than 0.7 mA. Other complications may be due to nerve electrodes such as skin infection and bleeding, but these are rare with sterile techniques and proper needle electrode placement and removal. There is a statistically significant reduction in postoperative facial nerve weakness in patients treated with the aid of NIM 2 32, and postoperative facial dysfunction can be presumed intraoperatively by an elevated nerve response (> 0.5 mA) 32.
In the present series, NIM was mainly used as a warning of initial stressing of the nerve inducing the surgeon to change the dissection and waiting for the silencing of the spontaneous bursting of the involved branch. We believe that such a policy can play an important role in reducing immediate postoperative facial nerve weakness as described by different authors 2 30.
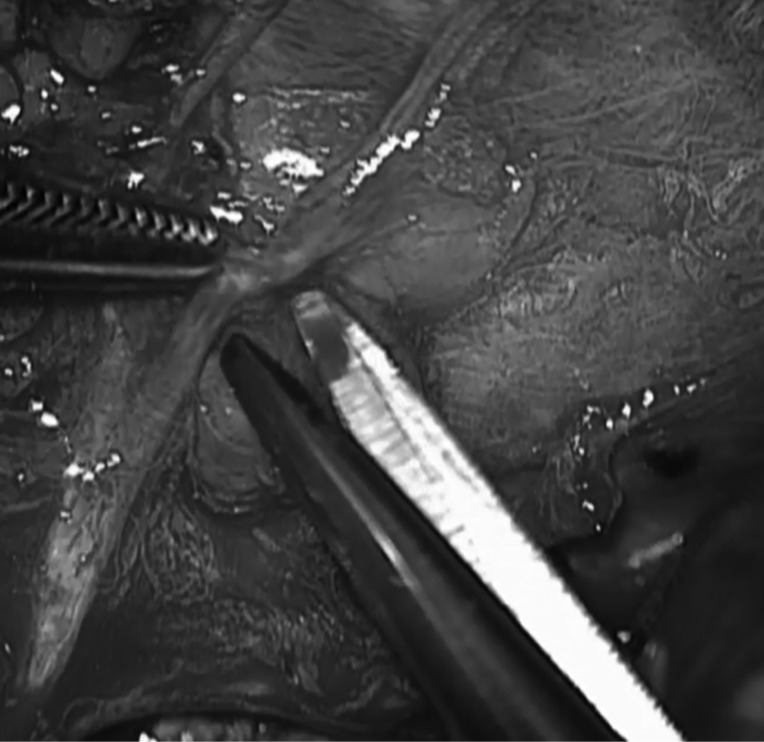
Microsurgery is a discipline of multiple ENT subspecialties requiring optical magnification and 3D view. The microscope is an important tool for the success of many of the most complex and difficult surgical procedures in medicine today 33. The interchangeable lenses (200-400 mm) allow a working distance that is appropriate to the depth of the various surgical approaches in otolaryngology 34. MAP improves the surgeon's view, with better magnification and resolution, and better and more stable light source, allowing for fine discrimination of the terminal branches of the facial nerve from the vessels and the salivary ducts, even when neoplasms deeply adhere to the nerve (Fig. 4). Consequently a more fine dissection can be performed with precise haemostasis around the vasa nervorum, with reduction of postoperative facial nerve dysfunction rate. Average operation time for benign lesion was 221 minutes (range of 120-350). In our series, the mean operative time also includes procedures in which the identification of the facial nerve was performed by surgeons in training, which explains the longest operative times observed.
Fig. 4.
Microscopic assisted dissection of the perinevrium in a malignancy.
Facial nerve preservation rate changes according to different studies (Table IV) 2 6 21 35-42. Cristofaro et al. reported a transient and definitive facial paralysis rates significantly more frequent after superficial parotidectomy than after extra-capsular dissection (20% versus 4.5%, and 2.2% versus 0%, respectively), involving mainly the mandibular branch in both techniques 35. In our series, the MAP used together with the NIM was associated with very limited postoperative facial weakness rates: after procedures performed for benign lesions, and all the definitive weaknesses were limited to one peripheral branch and of grade II according to the House-Brackman classification. Patients treated for benign tumours of the extra facial portion of the gland without inflammatory behaviour (n = 91) experienced 4.4% facial nerve temporary weakness rate and no permanent palsy. In the literature, inflammatory lesions adherent to the nerve are associated with postoperative facial weakness 43. In our study, inflammatory lesions of the deep lobe showed a higher risk of postoperative facial nerve temporary and definitive deficits (p = 0.005); nerve trauma could be due to adherences and fibrosis between the facial nerve and the gland. The 14% rate of postoperative permanent facial nerve weakness of an isolated branch in our patients treated for malignant lesions without symptomatic preoperative facial nerve involvement (see Table III) is strictly related to the following causes: when perineural involvement is intraoperatively demonstrated without preoperative facial nerve palsy, if it is a minor branch, we tend to sacrifice the branch and perform nerve grafting, and, in addition, we observed that parotidectomy associated with neck dissection is related to a higher permanent palsy of the marginal mandibular branch (11.1%), probably due to excessive manipulation of the exposed nerve during neck dissection. In addition, the extensive devascularisation of the nerve may increase the risk of postoperative weakness, as observed in our total parotidectomies.
Table IV.
Postoperative facial nerve weakness reported in the literature.
| Source | No. of cases | Temporary | Permanent |
|---|---|---|---|
| Present work (benign tumours) | 145 | 7.6% | 1.4% |
| Cristofaro (superficial parotidectomy) | 45 | 20% | 4.5% |
| Reza Nouraei | 162 | 40.3% | 1.2% |
| George and McGurk (extracapsular dissection) | 156 | 3% | 1% |
| Ciuman | 196 | 6.5% | 2% |
| Goutinas-Lichius 2006 | 937 | 25% | 6% |
| Upton | 237 | 18% | 1.2% |
| Koch | 492 | 32.7% | 2.3% |
| Goutinas-Lichius 2004 | 295 | 27% | 5% |
| Yuan | 626 | 23.16% | 4.15% |
| Laccourreye | 229 | 5.7% | 3.9% |
| Greer Albergotti | 397 | 20.4% | 1.1% |
| O'Brien 2003 | 355 | 27% | 2.5% |
| ALL SERIES | 4272 | 22.4% (N = 957) | 3.4% (N = 145) |
Abscesses are uncommon complications and rarely require surgical revision 44; we never observed this complication in our series. Bleeding and haematomas occur after 3-7% of procedures 3. In the present series, immediate bleeding requiring revision was observed after 4.5% of the parotidectomies, and it was generally due to the accidental cauterisation of the branch of the stylomastoid artery that we think should be constantly ligated. Frey's syndrome after parotidectomy has been reported in 18%- 50% of cases 6. In our series, it was observed in 24.1% of patients treated for benign pathology. Surgical and non-surgical treatments of Frey's syndrome have been proposed, but the outcomes are disappointing since only temporary relief is achieved 45.
The GAN is frequently sacrificed during parotid surgery especially if runs close to the tumour, with consequent numbness around the ear lobe but a modest impact on patient quality of life 46. Moretti et al. suggest that saving as many branches of the GAN as possible during parotid surgery could be useful in reducing hypo-dysaesthesia 47. In the present study we noticed that numbness decreases progressively over time and is not reported as a main postoperative problem by patients.
First bite syndrome is facial pain localised in the parotid area and associated with severe cramps or spasms; although it has been estimated to occur after 9.6% of all procedures 48, we did not observe this condition in our series.
Surgical incision and the removal of parotid tissue impact aesthetically on a visible area of the head and neck, but in literature only a small percentage of patients complain of scar and/or depression of the surgical area 34. In our series, scar was commonly related to the Blair's incision more frequently used in elderly or males, but in females, if requested and possible in relation to the site and size of the tumour, a modified intra-canal facelift incision was performed with optimal results. Nowadays, the facelift approach is considered a cosmetically superior approach to parotid tumours as confirmed by objective data 49.
A long follow-up is recommended even in patients treated for benign neoplasms, since the mean interval between recurrences reported in literature is 7.0 ± 5.3 years for first recurrence 50. A limit of the present series is a follow-up no longer than 6 years. The most important cause of recurrence is rupture and incomplete removal of the tumour 51. Facial nerve schwannoma can be conservatively removed keeping facial nerve function intact with an intra-capsular enucleation under the microscope 52. The schwannoma of the facial nerve of our series originated from a minor branch, as a consequence the surgeon decided to sacrifice the nerve with a subsequent minor deficit that was well accepted by the patient.
Early-stage low-grade malignant parotid gland tumours were cured by surgical resection alone, while more advanced lesions were treated by surgical resection combined with radiotherapy. Surgical management of the facial nerve in patients with parotid cancers presenting with normal facial function is focused on the preservation of the nerve unless it is adherent to, or entrenched in, a malignant tumour, since no statistically significant difference in survival rate is reported between conservative and radical treatment of the seventh cranial nerve 53. Sacrifice of the nerve could be considered when it is clinically and/or intraoperatively massively involved in a clearly malignant neoplasm 54. When the margins of resection are close to the facial nerve, adjuvant radiotherapy can improve local control of the disease 51.
Although our survival data should be considered with caution since only 21 patients of 46 had a follow-up longer than 2 years, 5-year DSS and OS rates of our patients with parotid malignancies (83% and 70.6%) were comparable to those reported in the literature (78% and 58%) 55. As observed by Xiao et al. 56, N+ patients of our series experienced worse survival rates compared to N0 patients (5 years DSS and OS of 76.4% and 54.3% vs. 87% and 80.4%, respectively).
Conclusions
The surgeon's experience is the main guarantee for facial nerve preservation and low recurrence rates during parotidectomy. In our experience, superficial parotidectomy for benign tumours not directly involving the nerve is a safe procedure with minimal risk for facial nerve injury. Inflammatory lesions requiring surgical treatment can be burdened by a moderately higher risk of temporary or permanent facial nerve dysfunction; as a consequence, precise counselling with the patient is mandatory, as well for patients undergoing surgery for malignancy. Minor complications such as numbness of GAN areas and Frey's syndrome do not usually represent major discomfort for patients compared to facial nerve damage.
References
- 1.Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834–840. doi: 10.1016/S0194-5998(99)70323-2. [DOI] [PubMed] [Google Scholar]
- 2.Sood AJ, Houlton JJ, Nguyen SA, et al. Facial nerve monitoring during parotidectomy: a systematic review and metaanalysis. Otolaryngol Head Neck Surg. 2015;152:631–637. doi: 10.1177/0194599814568779. [DOI] [PubMed] [Google Scholar]
- 3.Reza Nouraei SA, Ismail Y, Ferguson MS, et al. Analysis of complications following surgical treatment of benign parotid disease. ANZ J Surg. 2008;78:134–138. doi: 10.1111/j.1445-2197.2007.04388.x. [DOI] [PubMed] [Google Scholar]
- 4.Koch M, Zenk J, Iro H. Long-term results of morbidity after parotid gland surgery in benign disease. Laryngoscope. 2010;120:724–730. doi: 10.1002/lary.20822. [DOI] [PubMed] [Google Scholar]
- 5.Guntinas-Lichius O, Kick C, Klussmann JP, et al. Pleomorphic adenoma of the parotid gland: a 13-year experience of consequent management by lateral or total parotidectomy. Eur Arch Otorhinolaryngol. 2004;261:143–146. doi: 10.1007/s00405-003-0632-9. [DOI] [PubMed] [Google Scholar]
- 6.Guntinas-Lichius O, Klussmann JP, Wittekindt C, Stennert E. Parotidectomy for benign parotid disease at a University Teaching Hospital: outcome of 963 operations. Laryngoscope. 2006;116:534–550. doi: 10.1097/01.mlg.0000200741.37460.ea. [DOI] [PubMed] [Google Scholar]
- 7.Yuan X, Gao Z, Jiang H, et al. Predictors of facial palsy after surgery for benign parotid disease: multivariate analysis of 626 operations. Head Neck. 2009;31:1588–1592. doi: 10.1002/hed.21134. [DOI] [PubMed] [Google Scholar]
- 8.Bron LP, O'Brien CJ. Facial nerve function after parotidectomy. Arch Otolaryngol Head Neck Surg. 1997;123:1091–1096. doi: 10.1001/archotol.1997.01900100065009. [DOI] [PubMed] [Google Scholar]
- 9.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 10.Barnes LEJ, Reichart P, Sidransky D. World Health Organization classification of tumors. Pathology and genetics of head and neck tumors. Lyon, France: IARC Press; 2005. [Google Scholar]
- 11.Bussu F, Rigante M, Giglia V, et al. Clinical history, prognostic factors, and management of facial nerve in malignant tumors of the parotid gland. Clin Exp Otorhinolaryngol. 2014;7:126–132. doi: 10.3342/ceo.2014.7.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bag AK, Curé JK, Chapman PR, et al. Practical Imaging of the Parotid Gland. Curr Probl Diagn Radiol. 2015;44:167–192. doi: 10.1067/j.cpradiol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Celebi I, Mahmutoglu AS, Ucgul A, et al. Quantitative diffusion- weighted magnetic resonance imaging in the evaluation of parotid gland masses: a study with histopathological correlation. Clinical Imaging. 2013;37:232–238. doi: 10.1016/j.clinimag.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Layfield LJ, Tan P, Glasgow BJ. Fine-needle aspiration of salivary gland lesions: comparison with frozen sections and histologic findings. Arch Pathol Lab Med. 1987;111:346–353. [PubMed] [Google Scholar]
- 15.Mukunyadz P. Review of fine-needle aspiration cytology of salivary gland neoplasms, with emphasis on differential diagnosis. Am J Clin Pathol. 2002;118:S100–S115. doi: 10.1309/WVVR-30E4-13TW-494D. [DOI] [PubMed] [Google Scholar]
- 16.Tyagi R, Dey P. Diagnostic problems of salivary gland tumors. Diagn Cytopathol. 2015;43:495–509. doi: 10.1002/dc.23255. [DOI] [PubMed] [Google Scholar]
- 17.Piccioni LO, Fabiano B, Gemma M, et al. Fine-needle aspiration cytology in the diagnosis of parotid lesions. Acta Otorhinolaryngol Ital. 2011;31:1–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Tatomirovic Z, Skuletic V, Bokun R, et al. Fine needle aspiration cytology in the diagnosis of head and neck masses: accuracy and diagnostic problems. J BUON. 2009;14:653–659. [PubMed] [Google Scholar]
- 19.Alphs HH, Eisele DW, Westra WH. The role of fine needle aspiration in the evaluation of parotid masses. Curr Opin Otolaryngol Head Neck Surg. 2006;14:62–66. doi: 10.1097/01.moo.0000193184.38310.0a. [DOI] [PubMed] [Google Scholar]
- 20.Pia F, Policarpo M, Dosdegani R, et al. Centripetal approach to the facial nerve in parotid surgery: personal experience. Acta Otorhinolaryngol Ital. 2003;23:111–115. [PubMed] [Google Scholar]
- 21.George KS, McGurk M. Extracapsular dissection - minimal resection for benign parotid tumors. Br J Oral Maxillofac Surg. 2011;49:451–454. doi: 10.1016/j.bjoms.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Mantsopoulos K, Koch M, Klintworth N, et al. Evolution and changing trends in surgery for benign parotid tumors. Laryngoscope. 2015;125:122–127. doi: 10.1002/lary.24837. [DOI] [PubMed] [Google Scholar]
- 23.Witt RL, Rejto L. Pleomorphic adenoma: extracapsular dissection versus partial superficial parotidectomy with facial nerve dissection. Del Med J. 2009;81:119–125. [PubMed] [Google Scholar]
- 24.Colella G, Cannavale R, Chiodini P. Meta-analysis of surgical approaches to the treatment of parotid pleomorphic adenomas and recurrence rates. J Craniomaxillofac Surg. 2015;43:738–745. doi: 10.1016/j.jcms.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Albergotti WG, Nguyen SA, Zenk J, et al. Extracapsular dissection for benign parotid tumors: a meta-analysis. Laryngoscope. 2012;122:1954–1960. doi: 10.1002/lary.23396. [DOI] [PubMed] [Google Scholar]
- 26.Seddon HJ. Three types of nerve injury. Brain. 1943;66:237–288. [Google Scholar]
- 27.Dulguerov P, Marchal F, Lehmann W. Postparotidectomy facial nerve paralysis: possible etiologic factors and results with routine facial nerve monitoring. Laryngoscope. 1999;109:754–762. doi: 10.1097/00005537-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140:154–158. doi: 10.1016/j.otohns.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Lowry TR, Gal TJ, Brennan JA. Patterns of use of facial nerve monitoring during parotid gland surgery. Otolaryngol Head Neck Surg. 2005;133:313–318. doi: 10.1016/j.otohns.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Terrel JE, Kileny PR, Yan C. Clinical outcome of continuous facial nerve monitoring during primary parotidectomy. Arch Otolaryngol Head Neck Surg. 1997;157:1081–1087. doi: 10.1001/archotol.1997.01900100055008. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein H, Rosenberg S. Intraoperating facial nerve monitoring. Otolaryngol Clin North Am. 1991;24:709–725. [PubMed] [Google Scholar]
- 32.Brennan J, Moore EJ, Shuler KJ. Prospective analysis of the efficacy of continuous intraoperating nerve monitoring during thyroidectomy, parathyroidectomy, and parotidectomy. Otolaryngol Head Neck Surg. 2001;124:537–543. doi: 10.1067/mhn.2001.115402. [DOI] [PubMed] [Google Scholar]
- 33.Uluç K, Kujoth GC, Başkaya MK. Operating microscopes: past, present, and future. Neurosurg Focus. 2009;27:E4–E4. doi: 10.3171/2009.6.FOCUS09120. [DOI] [PubMed] [Google Scholar]
- 34.Edwards WG. The versatility of the basic microscope system in otolaryngology. J Microsurg. 1980;1:387–393. doi: 10.1002/micr.1920010510. [DOI] [PubMed] [Google Scholar]
- 35.Cristofaro MG, Allegra E, Giudice A, et al. Pleomorphic adenoma of the parotid: extracapsular dissection compared with superficial parotidectomy - a 10-year retrospective cohort study. ScientificWorld Journal. 2014;2014:564053–564053. doi: 10.1155/2014/564053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laccourreye H, Laccourreye O, Cauchois R, et al. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope. 1994;104:1487–1494. doi: 10.1288/00005537-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Ciuman RR, Oels W, Jaussi R, et al. Outcome, general, and symptom-specific quality of live after various types of parotid resection. Laryngoscope. 2012;122:1254–1261. doi: 10.1002/lary.23318. [DOI] [PubMed] [Google Scholar]
- 38.Upton DC, McNamar JP, Connor NP, et al. Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007;136:788–792. doi: 10.1016/j.otohns.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Koch M, Zenk J, Iro H. Long-term results of morbidity after parotid gland surgery in benign disease. Laryngoscope. 2010;120:724–730. doi: 10.1002/lary.20822. [DOI] [PubMed] [Google Scholar]
- 40.Yuan X, Gao Z, Jiang H, et al. Predictors of facial palsy after surgery for benign parotid disease: multivariate analysis of 626 operations. Head Neck. 2009;31:1588–1592. doi: 10.1002/hed.21134. [DOI] [PubMed] [Google Scholar]
- 41.Greer Albergotti W, Nguyen SA, Zenk J, Boyd Gillespie M. Extracapsular dissection for benign parotid tumors: a metaanalysis. Laryngoscope. 2012;122:1954–1960. doi: 10.1002/lary.23396. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien CJ. Current management of benign parotid tumors- -the role of limited superficial parotidectomy. Head Neck. 2003;25:946–952. doi: 10.1002/hed.10312. [DOI] [PubMed] [Google Scholar]
- 43.Gaillard C, Périé S, Susini B, St Guily JL. Facial nerve dysfunction after parotidectomy: the role of local factors. Laryngoscope. 2005;115:287–291. doi: 10.1097/01.mlg.0000154735.61775.cd. [DOI] [PubMed] [Google Scholar]
- 44.Zanaret M, Brasnu D, Lacau Saint Guily J, Hans S. Laccourreye O, Chabolle F, editors. Le complications de la chirurgie des tumeurs et affections benignes des glandes salivaires. Les risqué chirurgicaux en oto-rhino-laryngologie: information, prise en charge et prevention. 2008:399–407. SFORL. [Google Scholar]
- 45.Bjerkhoel A, Trobbe OJ. Frey's syndrome: treatment with botulinum toxin. Laryngol Otol. 1997;111:839–844. doi: 10.1017/s0022215100138769. [DOI] [PubMed] [Google Scholar]
- 46.Galli J, Pandolfini M, Rigante M, et al. Sensory dysfunction and quality of life after great auricular nerve sacrifice during parotidectomy: our experience. J Laryngol Otol. 2015;129:1121–1127. doi: 10.1017/S0022215115001863. [DOI] [PubMed] [Google Scholar]
- 47.Moretti A, Citraro L, Petrucci AG, et al. Great auricular nerve preservation in parotid surgery: rationale and long-term results insights. Eur Arch Otorhinolaryngol. 2015;272:3515–3520. doi: 10.1007/s00405-014-3342-6. [DOI] [PubMed] [Google Scholar]
- 48.Linkov G, Morris LGT, Shah JP, Kraus DH. First bite syndrome: incidence, risk factors, treatment, and outcomes. Laryngoscope. 2012;122:1773–1778. doi: 10.1002/lary.23372. [DOI] [PubMed] [Google Scholar]
- 49.Grover N, D'Souza A. Facelift approach for parotidectomy: an evolving aesthetic technique. Otolaryngol Head Neck Surg. 2013;148:548–556. doi: 10.1177/0194599812475221. [DOI] [PubMed] [Google Scholar]
- 50.Ghanem YA, Mizrachi A, Popovtzer A, et al. Recurrent pleomorphic adenoma of the parotid gland: Institutional experience and review of the literature. J Surg Oncol. 2016;114:714–718. doi: 10.1002/jso.24392. [DOI] [PubMed] [Google Scholar]
- 51.Witt RL, Eisele DW, Morton RP, et al. Etiology and management of recurrent parotid pleomorphic adenoma. Laryngoscope. 2015;125:888–893. doi: 10.1002/lary.24964. [DOI] [PubMed] [Google Scholar]
- 52.Rigante M, Petrelli L, Corso E, Paludetti G. Intracapsular microenucleation technique in a case of intraparotid facial nerve schwannoma. Technical notes for a conservative approach. Acta Otorhinolaryngol Ital. 2015;35:49–52. [PMC free article] [PubMed] [Google Scholar]
- 53.Magnano M, Gervasio CF, Cravero L, et al. Treatment of malignant neoplasms of the parotid gland. Otolaryngol Head Neck Surg. 1999;121:627–732. doi: 10.1016/S0194-5998(99)70070-7. [DOI] [PubMed] [Google Scholar]
- 54.Spiro JD, Spiro RH. Cancer of the parotid gland: role of 7th nerve preservation. World J Surg. 2003;27:863–867. doi: 10.1007/s00268-003-7112-7. [DOI] [PubMed] [Google Scholar]
- 55.Nagliati M, Bolner A, Vanoni V, et al. Surgery and radiotherapy in the treatment of malignant parotid tumors: a retrospective multicenter study. Tumori. 2009;95:442–448. doi: 10.1177/030089160909500406. [DOI] [PubMed] [Google Scholar]
- 56.Xiao CC, Zhan KY, White-Gilbertson SJ, Day TA. Predictors of nodal metastasis in parotid malignancies: a national cancer data base study of 22,653 patients. Otolaryngol Head Neck Surg. 2016;154:121–130. doi: 10.1177/0194599815607449. [DOI] [PubMed] [Google Scholar]