Abstract
Introduction
Antiretroviral treatment (ART) guidelines recommend lifelong ART for all HIV positive individuals. This study evaluated TB incidence on ART in a cohort of HIV positive individuals starting ART regardless of CD4 count in a programmatic setting at three clinics included in the HPTN 071 (PopART) trial in South Africa.
Methods
A retrospective cohort analysis of HIV-positive individuals aged ≥18 years starting ART, between January 2014 and November 2015, was conducted. Follow up was continued until 30 May 2016 or censored on the date of i) incident TB ii) loss to follow up from HIV care or death or iii) elective transfer out; whichever occurred first.
Results
The study included 2423 individuals. Median baseline CD4 count was 328 cells/μL (IQR 195–468), TB incidence rate was 4.41/100 PY (95% CI 3.62–5.39). The adjusted hazard ratio of incident TB was 0.27 (95% CI 0.12 – 0.62) when comparing individuals with baseline CD4 > 500cells/μL and ≤ 500cells/μL. Amongst individuals with baseline CD4 count > 500cells/μL there were no incident TB cases in the first three months of follow up. Adjusted hazard of incident TB was also higher amongst men (aHR 2.16; 95% CI: 1.41 – 3.30).
Conclusion
TB incidence after ART initiation was significantly lower amongst individuals starting ART at CD4 counts above 500cells/μL. Scale up of ART, regardless of CD4 count, has the potential to significantly reduce TB incidence amongst HIV-positive individuals. However, this needs to be combined with strengthening of other TB prevention strategies that target both HIV positive and HIV negative individuals.
Introduction
Tuberculosis (TB) remains the leading cause of morbidity and mortality amongst HIV positive individuals [1]. Recent antiretroviral treatment (ART) WHO guidelines recommend lifelong ART for all HIV positive individuals regardless of CD4 count [2]. This expanded ART access should lead to a reduction of TB and other WHO-defining illnesses and mortality amongst HIV positive individuals initiating ART [3–6].
TB at the time of ART initiation (baseline TB) is significantly higher at lower baseline CD4 counts amongst HIV positive individuals [4]. Two South African programmatic studies completed between 2002 and 2008, in patients with low median baseline CD4 counts, reported high baseline TB prevalence, between 20–30% [7, 8], and baseline TB prevalence continues to be underestimated due to the difficulties in diagnosing amongst individuals starting ART in routine settings [9].
Published TB incidence rates in the first 12 months on ART range from 7.3/100PY to 10.9/100PY and are particularly high when pre-ART (baseline) CD4 counts are below 100 cells/μL [8, 10]. TB incidence rates have been reported to be highest during the first four months on ART and approximately double in the first year of ART compared to subsequent years [9]. When measuring the association between CD4 count measured after a period of immune reconstitution on ART (‘on ART’ CD4 counts) and TB incidence, TB incidence is significantly lower among individuals with higher ‘on ART’ CD4 counts [7, 10].
While randomised controlled trials (RCTs) have shown lower TB incidence among HIV positive individuals initiating ART at baseline CD4 counts > 500cells/μL compared to CD4 ≤500 cells/μL and CD4 351 to 500cells/μL [11 , 12], there are very few published studies from programmatic settings on the impact of routine ART initiation at CD4 counts > 500cells/μL on TB incidence. This study, embedded in the HPTN 071 (PopART) trial, assessed the association between baseline CD4 count and TB incidence after ART initiation in a cohort of HIV-positive individuals starting ART regardless of CD4 count under programmatic conditions in the Western Cape, South Africa.
Study setting
The study was conducted at three primary health care (PHC) Department Of Health (DOH) clinics which offered ART regardless of CD4 count (Arm A) for the ‘Population Effect of ART to Reduce HIV Incidence’, HPTN 071 (PopART) study. Study clinics were in two sub districts in the Cape Metro district (Metro1 and Metro 2 clinics) and in one sub district in the Cape Winelands district (Rural clinic). Antenatal HIV prevalence in the Cape Metro and Cape Winelands districts is 20.3% and 14.3% respectively and routine DOH data show annual TB incidence rates are 596/100 000 and 880/100 000 population respectively [13, 14].
A full description of the HPTN 071 (PopART) study design has been previously published [15]. Communities randomly allocated to arm A of the HPTN 071 (PopART) trial received, from 1 January 2014, a combination HIV prevention package including HIV education, HIV testing, screening for TB symptoms and active linkage to care for individuals diagnosed with HIV, TB and sexually transmitted infections (STIs). Clinics allocated to arm A of the HPTN 071 (PopART) trial provided ART regardless of CD4 count to all HIV positive individuals aged ≥18 years. Clients initiating ART outside of prevailing ART guidelines signed research informed consent prior to ART initiation. All HIV positive individuals on ART were otherwise managed according to DOH ART guidelines. Routine assessment prior to ART initiation included TB symptom and pregnancy screening [16].
HIV and TB services were integrated at all three study clinics. Isoniazid (INH) TB prophylaxis (IPT) was recommended for individuals with a positive tuberculin skin test (TST) at ART initiation, to be continued for 36 months. If TST was unavailable or negative then 12 months IPT was advised for individuals starting ART [16]. HIV positive individuals with TB symptoms were investigated according to a standardised diagnostic algorithm that used GeneXpert MTB/RIF (Xpert) for first line diagnostic investigation, followed by culture if Xpert negative. HIV positive individuals diagnosed with TB at ART initiation and started on TB treatment, were recorded in the routine DOH electronic TB monitoring system (ETR.net) and stabilised on TB treatment for two to eight weeks prior to ART initiation [17]. The same TB diagnostic algorithm was utilised for diagnosis of TB in individuals already initiated on ART [18].
Cohort overview, data sources and definitions
A retrospective cohort study design was used. All data were obtained from routine DOH systems including the routine HIV monitoring system, Tier.net [19], ETR.net and routine laboratory reports from the National Health Laboratory Services (NHLS). All HIV positive individuals aged ≥18 years, recorded in Tier.net as having started ART at the three study clinics between 1 January 2014 and 30 November 2015 with a recorded baseline CD4 count were included in the study sample. Individuals were followed up until 30 May 2016 or until the date of i) incident TB ii) loss to follow up from HIV care or death (LTFU) or iii) elective transfer out (TFO); whichever occurred first.
Data linkage between Tier.net and ETR.net was conducted using an automated linkage algorithm in Microsoft SQL Server TM previously validated in other studies which utilised first name, surname and date of birth as individual identifiers and linkages were validated manually. CD4 data missing from Tier.net were, where available, extracted directly from the NHLS database and linked to Tier.net data using the DOH unique identifier. Data cleaning and validation included manual checking of automated linkages and cross referencing data of key across data elements within Tier.net and within ETR.net.
The following standardised definitions were used; i) Baseline CD4 as the most recent CD4 count completed within 6 months prior to starting ART. Baseline CD4 categories were chosen to align with previous guideline ART criteria [20]. ii) Baseline TB as recorded in ETR.net as having started on TB treatment in the 6 months prior to ART initiation. iii) Bacteriologically confirmed TB as confirmed with TB on smear microscopy, Xpert, or culture on one of sputum, lymph node tissue, pleural effusion or cerebrospinal fluid. iv)Incident TB as recorded in ETR.net as starting TB treatment after ART initiation; this included individuals on TB treatment at baseline who, after stopping TB treatment while on ART were subsequently recorded in ETR.net as restarting TB treatment for a new TB episode. v) LTFU as three months late for an antiretroviral pharmacy pickup appointment. LTFU was reported in combination with death due to significant underreporting of death in Tier.net. vi) TFO as electively transferred to another health facility.
Baseline characteristics were described using standard descriptive statistics for continuous and categorical variables. Heterogeneity of baseline characteristics across baseline CD4 count categories was assessed using Chi Squared and Kruskal-Wallis tests. Binomial confidence intervals were generated for baseline TB prevalence by baseline CD4 count category. Person time was measured from ART initiation or from the estimated date of stopping baseline TB treatment. Recording of the outcome date of baseline TB episodes in ETR.net was of poor quality, therefore the outcome date for baseline TB cases was assumed to be 6 months after the start of TB treatment in all cases. The date for incident TB was the date an individual started TB treatment recorded in ETR.net. Individuals were permanently censored at the first occurrence of either incident TB, LTFU, TFO or 30 May 2016.
Time to event analyses were completed using Kaplan Meier survival estimates. Cox regression was used for crude and adjusted modelling of the association of baseline characteristics with incident TB. Proportional hazard assumptions were tested using Schoenfeld residuals. Baseline variables for inclusion in regression analysis were selected a priori, based on clinical significance. Selection of the baseline variable category used for comparison (HR=1) was based on sample size and clinical significance. All adjusted models included the following baseline characteristics unless otherwise stated: baseline CD4 count, age, sex, pregnancy status, baseline TB, previous ART exposure of more than 3 months, clinic and year of ART initiation. Likelihood ratios were used to calculate P values in regression models for categorical independent variables with more than 2 strata. A subset analysis was conducted excluding individuals with baseline TB. A sensitivity analysis was also conducted in which incident cases of TB were restricted to bacteriologically confirmed cases. Logistic regression was used to compare baseline characteristics of individuals retained in the study sample and those combined LTFU and TFO. All analyses were performed using Stata version 13TM (StataCorp LP, College Station, TX, USA).
Ethics statement
The HPTN071 (PopART) study was approved by the Stellenbosch University Health Research Ethics Committee (SU HREC) (Ref. No. N12/11/074) and the London School of Hygiene and Tropical Medicine research ethics committee (Ref no. 6326). All individuals starting ART outside of prevailing routine guidelines signed informed consent. Further approvals for this study, including for the use of individual level routine data with a waiver of informed consent, were received from SU HREC (reference number N12/11/074A), the Western Cape Government (Ref no. WC_2015RP51_715) and the City of Cape Town (Ref no 10529) research committees.
Results
A total of 2593 individuals started ART during the study enrolment period. Baseline CD4 counts were missing for 170 individuals (6.6%) who were excluded leaving 2423 (93.4%) individuals in the analysis (Table 1). Median follow up time was 10.4 months (IQR 6.4–15.6). In total, 600 (24.7%) individuals were defined LTFU with a median follow up of 3.4 (IQR 0.9 – 7.2) months amongst those LTFU. There were 134 (5.5%) individuals with TFO recorded during the follow up period.
Table 1.
Baseline characteristics of study cohort
| Baseline CD4 cell count (cells/μl) | 0–200 | 201–350 | 351–500 | >500 | All | P value* | ||
|---|---|---|---|---|---|---|---|---|
| N (%)** | 631 (26.0) | 708 (29.2) | 582 (24.0) | 502 (20.7) | 2423 | |||
| Sex | Female | N (%) | 355 (56.3) | 463(65.4) | 421 (72.3) | 404 (80.5) | 1643 (67.8) | P<0.001 |
| Male | N (%) | 276 (43.7) | 245 (34.6) | 161 (27.7) | 98 (19.5) | 780 (32.1) | ||
| Age (years) | Median (IQR) | 33(29.0–40.0) | 31(25.0–37.0) | 31(26.0–37.0) | 30(25.0–37.0) | 31(26.0–38.0) | P<0.001 | |
| 18–25 | N% | 79 (12.5) | 179 (25.3) | 142 (24.4) | 134 (26.7) | 534 (22.0) | P<0.001 | |
| 26–35 | N | 312 (49.6) | 311 (43.9) | 272 (46.7) | 227 (45.2) | 1122 (46.3) | ||
| 36–45 | N | 167 (26.5) | 138 (19.5) | 109 (18.7) | 87 (17.3) | 501 (20.7) | ||
| 46–55 | N | 57 (9.0) | 63(8.9) | 46(7.9) | 44(8.8) | 210(8.7) | ||
| >55 | N | 17 (2.7) | 17(2.4) | 13(2.2) | 10(2.0) | 57(2.3) | ||
| Pregnant at ART start | Yes | N | 14 (3.9) | 39(8.4) | 41 (9.7) | 48 (11.9) | 142 (8.6%) | P<0.001 |
| Baseline TB | Yes | N | 162 (25.7) | 56 (7.9) | 41 (6.7) | 26 (5.2) | 285 (11.8) | P<0.001 |
| Clinic | Rural clinic | N | 88 (13.9) | 113(15.9) | 126(21.7) | 127(25.3) | 454 (18.7) | P<0.001 |
| Metro clinic 1 | N | 299 (47.4) | 301 (42.5) | 231 (39.7) | 191 (38.1) | 1022 (42.2) | ||
| Metro clinic 2 | N | 244 (38.7) | 294 (41.5) | 225 (38.7) | 184 (36.7) | 947 (39.1) | ||
| ART exposed*** | Yes | N | 27 (4.3) | 10 (1.4) | 7 (1.2) | 5 (1.0) | 49 (2.0) | P=0.005 |
| Year of ART start | 2014 | N | 161 (25.5) | 208 (29.4) | 160 (27.5) | 161 (32.1) | 690 (28.5) | P=0.091 |
| 2015 | N | 470 (74.2) | 500 (70.6) | 422 (72.5) | 341 (67.9) | 1733 (71.5) | ||
The P value measures heterogeneity across baseline CD4 categories. Chi squared and Kruskal Wallis tests were used - measure heterogeneity of baseline characteristics.
For the first row of the table the % refers - the % across CD4 categories. For all other rows the % refers - the % within the CD4 category.
ART exposed is defined as previous ART exposure of > 3 months.
Median baseline CD4 count was 328 cells/μL (IQR 195–468), median age 31 years (IQR 26–38) and 1643 (67.8%) individuals were female. The numbers of individuals initiating ART in different baseline CD4 count categories varied from 631 (26.0%) at CD4 0–200 cells/μL to 502 (20.7%) at CD4 >500 cells/μL (Table 1). Baseline TB was recorded in 285 individuals (11.8% 95% CI; 10.5–13.1%). Baseline TB prevalence ranged from 25.7% (95% CI 22.3–29.3%) at baseline CD4 counts < 200 cells/μL to 5.2% (95% CI 3.4–7.5%) at CD4 > 500 cells/μL. A small number of individuals, 49 (2.0%), ‘were recorded as having previous ART exposure of more than 3 months duration at baseline, the majority (33, 67.5%) of whom presented with a baseline CD4 count < 200cells/μL. More individuals attended Metro1 (1022, 42.2%) and Metro2 clinics (947, 39.1%) compared with the rural clinic (454, 18.7%).
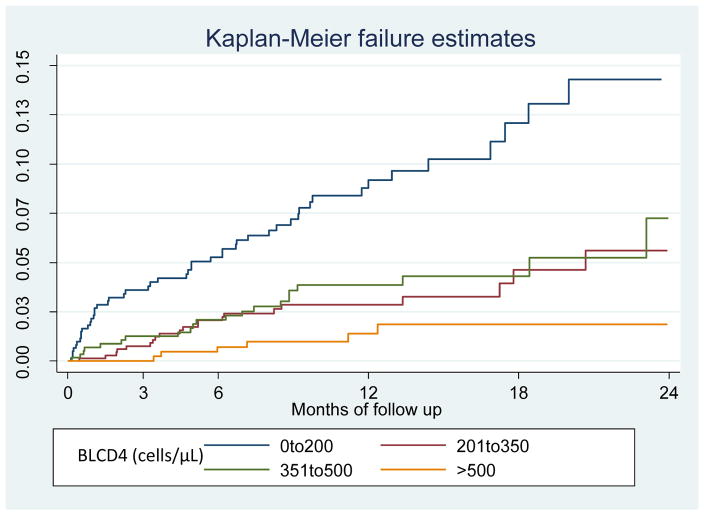
There were 97 incident TB cases during 2196 person years of follow up (Incidence rate (IR): 4.41/100 person years (PY) (95% CI 3.62–5.39) (Table 2). Eleven (11.3%) of these incident cases occurred in individuals with baseline TB. Kaplan Meier estimates showed lower rates of incident TB in individuals with baseline CD4 counts > 500 cells/μL when compared to those with CD4 < 500 cells/μL and across all CD4 categories (P<0.001) (Figure 1). TB incidence rates in different baseline CD4 count categories ranged from 9.62/100 PY (95% CI 7.27–12.73) at CD4 0–200 cells/μL to 1.26/100 PY (95% CI 0.57–2.81) at CD4 > 500 cells/μL (Table 2). There was a non-significant trend towards a decrease in TB incidence at longer follow up duration, incidence rates were 5.96 /100PY (95% CI 4.24–8.38) from 0 to 3 months, 4.73 /100PY (95% CI 3.14–7.12) from 4 to 6 months, 4.73 /100PY (95% CI 3.14–7.12) from 4 to 6 months and 3.04 /100PY (95% CI 1.79–5.13) from 13 to 24 months. When analysing TB incidence by baseline CD4 category and follow up duration there were, notably, no recorded incident TB cases during the first three months of follow up amongst individuals with baseline CD4 counts > 500cells/μL. Subset analysis excluding individuals with baseline TB showed similar TB incidence rates and with incidence rates ranging from 6.6 /100PY (95% CI 4.69–9.29) from 0 to 3 months to 2.97 /100PY (95% CI 1.72–5.12) from 13 to 24 months.
Table 2.
Incidence of TB and characteristics of incident TB cases by baseline CD4 count
| TB incidence | Bacteriologically confirmed TB | Site of TB disease | Treatment type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PY* | TB cases | IR (95% CI) | Yes | No | Pulmonary | Extra Pulmonary | New TB | Retreatment TB | |
| Baseline CD4 categories (cells/μL) | N | N | TB cases/100PY | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| 0–200 | 509 | 49 | 9.62 (7.27–12.73) | 29(59.2) | 20(40.8) | 39(79.6) | 10(20.4) | 31(63.3) | 18(36.7) |
| 201–350 | 667 | 21 | 3.15 (2.05–4.83) | 14(66.7) | 7(33.3) | 18(85.7) | 3(14.3) | 15(71.4) | 6(28.6) |
| 351–500 | 545 | 21 | 3.85 (2.51–5.91) | 14(66.7) | 7(33.3) | 18(85.7) | 3(14.3) | 17(80.9) | 4(19.1) |
| >500 | 475 | 6 | 1.26 (0.57–2.81) | 5(83.3) | 1(16.7) | 6(100.0) | 0(0.0) | 5(83.3) | 1(16.7) |
| Total | 2196 | 97 | 4.41 (3.62–5.39) | 62(63.9) | 35(36.1) | 81(83.5) | 16(16.5) | 68(70.1) | 29(29.9) |
This table includes all individuals in the study sample including those with baseline TB
PY: Person Years Person time was measured from the estimated time of end of TB treatment in individuals on TB treatment at baseline. In all other participants’ person time was measured from the date of ART start.
Figure 1.
Kaplan Meir failure estimates for incident TB stratified by baseline CD4 cell count categories
BLCD4: Baseline CD4 cell count. Log-rank test for equality of survivor functions: P<0.001
Of 97 incident TB cases, 81 (83.5%) cases were pulmonary and 16 (16.5%) extra pulmonary. (Table 2). Sixty eight (70.1%) cases were recorded as new (not previously treated) and 29 (39.9%) as having been previously treated but were not on TB treatment at initiation of ART. Sixty two (63.9%) incident TB cases were bacteriologically confirmed, most commonly on sputum using Xpert (54, 86.0%). Of the 35 (36.1%) incident TB cases not bacteriologically confirmed, diagnosis was based on X-ray for 21 (60.0%). There were no significant differences across baseline CD4 count categories in the proportions of TB cases that were pulmonary or extra-pulmonary, new or retreatment cases or bacteriologically confirmed.
Multivariate Cox regression showed a lower hazard of incident TB (aHR 0.27; 95% CI 0.12 – 0.62) amongst individuals with baseline CD4 count > 500cells/μL when compared to CD4 ≤ 500cells/μL. When stratifying by all baseline CD4 count categories; adjusted hazard ratios for incident TB were: aHR 0.15 (95% CI 0.06 – 0.36) at CD4 >500 cells/μL; aHR 0.45 (95% CI 0.27 – 0.77) at CD4 351–500 cells/μL and aHR 0.36 (95% CI 0.21 – 0.60) at CD4 201–350 cells/μL compared to CD4 0–200 cells/μL (Table 3). Adjusted hazard of incident TB was higher amongst men (aHR 2.16; 95% CI: 1.41 – 3.30), individuals with previous ART exposure more than 3 months (aHR 3.28; 95% CI: 1.49 – 7.2) and individuals attending the rural clinic (aHR 2.17; 95% CI: 1.19 – 3.97).
Table 3.
Cox regression modelling of baseline characteristics and incident TB
| Unadjusted analyses | Adjusted analyses | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P | AHR (95% CI) | P | ||
| Baseline CD4* (cells/μL) | > 500 | 0.13 (0.06 – 0.32) | <0.001 | 0.15 (0.06 – 0.36) | <0.001 |
| 350 – 500 | 0.41 (0.24 – 0.68) | 0.45 (0.27 – 0.77) | |||
| 200 – 350 | 0.33 (0.20 – 0.56) | 0.36 (0.21 – 0.60) | |||
| 0 – 200 | 1.00 | 1.00 | |||
| Gender | Male | 2.58 (1.73 – 3.85) | <0.001 | 2.16 (1.41 – 3.30) | <0.001 |
| Female | 1.00 | 1.00 | |||
| Clinic | Metro 1 | 1.00 | 0.212 | 1.00 | 0.047 |
| Metro 2 | 1.26 (0.77 – 2.05) | 1.54 (0.89 – 2.66) | |||
| Rural 1 | 1.63 (0.95 – 2.79) | 2.17 (1.19 – 3.97) | |||
| Age category (Years) | 15–25 | 0.84 (0.47 – 1.49) | 0.465 | 1.18 (0.66 – 2.13) | 0.951 |
| 26 – 35 | 1.00 | ||||
| 36–45 | 1.38 (0.84 – 2.27) | 1.28 (0.68 – 1.87) | |||
| 46–55 | 1.34 (0.69 – 2.61) | 1.22 (0.62–2.38) | |||
| >55 | 1.52 (0.47 – 4.89) | 1.34 (0.41 – 4.38) | |||
| Pregnant at baseline** | Yes | 0.51 (0.16 – 1.62) | 0.207 | 0.79 (0.23 – 2.46) | 0.727 |
| Baseline TB*** | Yes | 1.50 (0.80 – 2.82) | 0.155 | 0.83 (0.41 – 1.53) | 0.487 |
| Previous ART exposure of > 3 months | Yes | 3.94 (1.82 – 8.52) | 0.001 | 3.28 (1.49 – 7.2) | 0.003 |
| Year ART start | 2014 | 0.97 (0.62 – 1.53) | 0.906 | 0.75 (0.45 – 1.26) | 0.272 |
| 2015 | 1.00 | 1.00 | |||
All individuals were included in this analysis regardless of baseline TB status. Follow up time is therefore not equal - time on ART as follow up for individuals on TB treatment at ART initiation was delayed until the estimated end of TB treatment (6 months after TB treatment initiation). Baseline characteristics for inclusion in multivariate modelling were chosen based on clinical significance. For baseline characteristics with more than two categories likelihood ratios were used - estimate P values for hazard of incident TB. Baseline CD4 count was the most recent CD4 count completed in the 6 months prior - ART initiation.
All individuals initiating ART are screened for pregnancy at baseline. Baseline TB was defined as having started TB treatment within the 6 months prior - ART initiation.
A subset analysis which excluded the 285 individuals with baseline TB showed similar results (Table 4). Hazard of TB remained lower at higher baseline CD4 counts; aHR = 0.13 (95% CI 0.05 – 0.33) at CD4 > 500 cells/μL; aHR = 0.42 (95% CI 0.24 – 0.74) at CD4 351–500 cells/μL and aHR 0.35 (95% CI 0.20 – 0.60) at CD4 201–350 cells/μL compared to CD4 0–200 cells/μL Hazard of incident TB remained higher in men (aHR 2.31 ; 95% CI: 1.48 – 3.60), individuals with previous ART exposure more than 3 months (aHR 2.71; 95% CI: 1.08 – 6.81) and amongst individuals attending the rural clinic (aHR 2.16; 95% CI: 1.13 – 4.13) on subset analysis.
Table 4.
Subset Cox regression modelling of baseline characteristics and incident TB excluding individuals with baseline TB
| Crude hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Baseline CD4* (cells/μL) | >500 | 0.11(0.04–0.28) | <0.001 | 0.13(0.05–0.33) | <0.001 |
| 351 – 500 | 0.37(0.22–0.64) | 0.42(0.24–0.74) | |||
| 201 – 350 | 0.32(0.19–0.55) | 0.35(0.20–0.60) | |||
| 0 – 200 | 1.0 | 1.0 | |||
| Gender | Male | 2.75(1.8–4.19) | <0.001 | 2.31(1.48–3.60) | <0.001 |
| Female | 1.0 | 1.0 | |||
| Clinic | Metro 1 | 1.0 | 0.286 | 1.0 | 0.059 |
| Metro 2 | 1.35(0.8–2.26) | 1.73(0.98–3.07) | |||
| Rural 1 | 1.58(0.88–2.84) | 2.16(1.13–4.13) | |||
| Age category (Years) | 15–25 | 0.91(0.5–1.63) | 0.416 | 1.28(0.7–2.32) | 0.762 |
| 26 – 35 | 1 | 1 | |||
| 36–45 | 1.55(0.92–2.61) | 1.30(0.77–2.21) | |||
| 46–55 | 1.27(0.61–2.63) | 1.17(0.56–2.44) | |||
| >55 | 0.65(0.09–4.71) | 0.57(0.08–4.16) | |||
| Pregnant at baseline** | Yes | 0.53(0.17–1.69) | 0.285 | 0.85(0.26–2.77) | 0.785 |
| Previous ART exposure of > 3 months | Yes | 3.46(1.4–8.55) | 0.007 | 2.71(1.08–6.81) | 0.785 |
| Year ART start | 2014 | 0.93(0.57–1.52) | 0.780 | 1.36(0.79–2.36) | 0.271 |
| 2015 | 1.0 | 1.0 |
Individuals (285) with baseline TB were excluded. In this analysis follow up time equals time on ART. Baseline characteristics for inclusion in multivariate modelling were chosen based on clinical significance. For baseline characteristics with more than two categories likelihood ratios were used - estimate P values for hazard of incident TB. Baseline CD4 count was the most recent CD4 count completed in the 6 months prior - ART initiation.
All individuals initiating ART are screened for pregnancy at baseline. Baseline TB was defined as having started TB treatment within the 6 months prior - ART initiation.
Sensitivity analysis using bacteriologically confirmed incident TB as the primary outcome showed similar results with a lower hazard of bacteriologically confirmed incident TB in individuals starting ART at baseline CD4 counts > 500 cells/μL compared to CD4 ≤500 cells/μL (aHR 0.35; 95% CI 0.14–0.89). Sensitivity analyses also confirmed reduced hazard of bacteriologically confirmed incident TB at higher baseline CD4 counts when comparing across all baseline CD4 count categories; aHR: 0.21 (95%CI 0.08–0.57) at CD4 > 500 cells/μL, aHR: 0.51 (95%CI 0.26–0.98) at 350–500 cells/μL, aHR: 0.41 (95%CI 0.22–0.79) at 201–350 cells/μL compared to CD4 < 200 cells/μL.
Logistic regression analysis of the association of baseline characteristics with an endpoint that combined LTFU and TFO showed adjusted odds ratios (aORs) of LTFU and TFO at different baseline CD4 categories were 1.23 ( 95% CI 0.95 – 1.61), 0.74 (95% CI 0.56 – 0.96) and 1.05 (95% CI 0.82 – 1.33) amongst individuals with baseline CD4 counts >500cells/μL, 351 to 500cells/μL and 201 to 350 cells/μL respectively when compared to those with baseline CD4 counts ≤ 200cells/μL. Other baseline characteristics associated with combined LTFU and TFO included, age 18 to 25 (aOR = 1.36; 95% CI 1.09–1.70) and age 46 to 55 (aOR 0.65; 95% CI: 0.46–0.93) compared to age 26 to 35, being treated at Metro 2 (aOR: 1.31; 95% CI: 1.05–1.66) compared to Metro 1 and baseline TB treatment (aOR: 1.44; 95% CI: 1.09 to 1.91). Male gender was not associated with increased LTFU and TFO (aOR 1.11.; 95% CI 0.90–1.36) when compared to female gender.
Discussion
Lifelong ART is now recommended for all HIV positive individuals regardless of CD4 count [2]. In addition to improving individual level clinical outcomes and potentially reducing community HIV transmission, earlier ART initiation has the potential to reduce population level TB incidence [3, 5]. The impact of starting ART at baseline CD4 counts > 500cells/μL on subsequent TB incidence has been demonstrated in RCTs; however, there are very limited data from programmatic settings. This study has demonstrated significantly lower TB incidence for individuals starting ART at CD4 counts > 500 cells/μL (1.26/100 PY (95% CI 0.57–2.81), compared to those starting at lower CD4 counts. This rate is, however, higher than previously reported TB case notification rates for the HIV negative population, aged 15 to 60 years, in the Cape Town area (0.48/100PY) [21]. A direct comparison cannot be made because these were different studies with different methodologies and interpretation of this comparison is limited by the relatively small sample size of our study and should be the subject of further evaluation.
In contrast to previous studies amongst HIV positive individuals [7, 8, 10], the hazard of incident TB on ART in this study was higher amongst men, reasons for which are not clear from these data. This may, however, be due to lower ART adherence among men, with associated greater immunosuppression, as reported in other studies [22]. There is a reported trend toward recurrent episodes of ART non-adherence amongst individuals on ART and it is therefore possible that individuals with previous ART exposure were more likely to be non-adherent to ART which may account for the higher TB incidence in this group [23]. The higher TB incidence at the rural clinic is likely to, in part, reflect higher background annual TB incidence [17]. Previous studies have shown a strong trend toward decreasing TB incidence with increasing duration of follow up after ART initiation [8, 10]. In contrast in this study there was a trend toward decreased TB incidence at longer durations of ART, however, it was not statistically significant. This may be in part due to limited sample size, however, there were no recorded incident TB cases during the first three months on ART amongst individuals with baseline CD4 counts starting >500cells/μL, which would have reduced overall cohort incidence during early ART.
Unmasking of TB is known to contribute to TB incidence during the first three months on ART [24, 25]. The absence of incident TB cases during this period of ART in HIV positive individuals who have not spent time pre-ART at CD4 counts lower than 500cells/μL in this high TB burden setting is promising and should be the subject of future research.
Effective management of HIV and TB at PHC clinics is critical in reducing associated morbidity and mortality in high burden settings [26, 27]. Integration of HIV and TB services in PHC clinics is increasingly recommended [26, 27], but has not always been shown to lead to improved clinical outcomes. The best way to integrate services may be highly context specific [28, 29], and there remains the need for high quality data evaluating best practices for HIV and TB integration [2]. Differentiated models of care, which provide intensified care for high risk individuals in PHC clinics, may be a successful strategy for improving integrated HIV and TB care [30, 31]. To this end, studies such as this one, that have identified key baseline risk factors for TB incidence on ART, could be utilised to develop risk matrices for incorporation into differentiated models of care for improving clinical outcomes in HIV and TB co-infected individuals.
The HPTN071 (PopART) trial has provided a unique opportunity to evaluate, under programmatic conditions, a cohort of HIV-positive individuals routinely starting ART at baseline CD4 counts > 500cells/μL, prior to ART regardless of CD4 count being recommended by WHO and South African guidelines. The primary outcome of this study, TB incidence, is a topic of great public health importance and the analysis of an objective primary end point was strengthened by sensitivity analyses of microbiologically confirmed TB. Data included in this analysis were representative of the planned study cohort with only 170 (6.6%) of eligible individuals excluded due to missing baseline CD4 count. The prospective health systems support provided by HPTN 071 (PopART) to the study clinics was likely to have improved the accuracy with which TB was diagnosed and reported in ETR.net.
There were, however, limitations that require consideration. ETR.net only captures individuals starting TB treatment. Individuals diagnosed with TB but not started on TB treatment were therefore excluded along with individuals starting TB treatment who were erroneously not recorded in ETR.net. Similarly, the majority of the estimated 4 to 5 % of individuals diagnosed with drug resistant TB at the time of TB treatment start, [32] were not captured in ETR.net, but into a separate database (Electronic Drug Resistant TB register (EDR.net)) and were therefore excluded in this analysis. The authors were therefore not able to report the contribution of MDR to incident TB cases. The omission of MDR TB cases in this study may have reduced the overall reported TB incidence but given that CD4 count has not been shown to be associated with the risk of MDR TB versus drug susceptible TB, [33] there is no evidence the missing MDR data differentially affected TB incidence across different baseline CD4 categories in this study. Despite these missing data, baseline TB prevalence stratified by baseline CD4 count category in this study was similar to that reported for corresponding baseline CD4 count categories by another South African study, from a comparable area in the Cape Metro, in which data were limited to individuals with baseline CD4 counts ≤ 500cells/μL [4].
Furthermore, there was marked heterogeneity of baseline characteristics across baseline CD4 categories, and although we adjusted for some of these characteristics in the Cox regression analysis, we cannot rule out that there may be residual confounding. There were also high rates of LTFU (24.7%) and transfer out (5.5%), which are likely to have reduced the overall reported TB incidence. When analyzing LTFU and TFO across baseline CD4 categories, although there was reduced LTFU and TFO amongst individuals with baseline CD4 counts of 351 to 500cells/μL. However, LTFU and TFO amongst individuals with baseline CD4 counts of >500cells/μL, and 201 to 350 cells/μL was not statistically different to LTFU and TFO amongst individuals with baseline CD4 counts ≤ 200cells/μL and it is therefore not evident that LTFU and TFO differentially affected TB incidence across baseline CD4 categories. LTFU and TFO were similar with respect to other baseline characteristics associated with TB incidence. Non-availability of viral load data after ART initiation is a further limitation of this study. Increased viral load after ART initiation is associated with increased incidence of TB on ART [8], and inclusion of these data would have assisted in interpreting the association of key baseline characteristics with TB incidence; such as the increased hazard of TB in men who are also associated with decreased ART adherence and increased risk of increased viral load on ART [22].
IPT has been shown to decrease the risk of TB amongst individuals starting ART [11]. The non –reporting of IPT provision in this study due to missing data in ETR.net and Tier.net is a limitation. It was not apparent whether IPT uptake in this study differed across baseline CD4 categories and the non-availability of IPT data may therefore have biased the primary outcomes. Anecdotally the use of IPT was low and inconsistent in PHC clinics in the Western Cape at the time of this study. IPT has been shown to be effective in reducing TB incidence in HIV positive individuals testing TST positive [11, 34, 35]. The need for TST testing and concerns about INH resistance are thought to have contributed to low uptake of IPT [36, 37]. The need for TST testing prior to IPT is debated and recent changes to ART guidelines, with more individuals starting ART at higher CD4 counts, when TST testing is more sensitive [35], should be a critical consideration in this debate going forward.
Conclusion
The HPTN 071 (PopART) trial has provided a unique opportunity to evaluate TB incidence in the setting of universal offer of ART through programmatic clinic data. This study showed a significantly lower TB prevalence and on ART incidence among HIV positive individuals initiating ART at CD4 counts > 500 cells/μL, suggesting that scale up of ART regardless of CD4 count, has the potential to significantly reduce TB burden amongst HIV positive individuals. At the same time, scale up of other TB prevention strategies that target both HIV positive and HIV negative individuals are urgently required to contribute substantially to TB elimination in high HIV prevalence settings.
Acknowledgments
Funding Sources: HPTN 071 is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Additional funding is provided by the International Initiative for Impact Evaluation (3ie) with support from the Bill & Melinda Gates Foundation, as well as by NIAID, the National Institute on Drug Abuse (NIDA) and the National Institute of Mental Health (NIMH), all part of NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIMH, NIDA, PEPFAR, 3ie, or the Bill & Melinda Gates Foundation
GM was supported by the Wellcome Trust (098316) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) (Grant No 64787).
The authors wish to acknowledge implementing partners in South Africa, including PEPFAR partners (Kheth’ Impilo and ANOVA) and the City of Cape Town and Western Cape Government department of health colleagues, who have partnered in implementing the HPTN071 (PopART) trial and also granted access to the data used in this study. A special thanks to Ms Judy Caldwell at the City of Cape Town for her assistance with data access and interpretation. The authors also thank HPTN 071 research partners (HPTN, FHI 360 North Carolina, London School of Hygiene and Tropical Medicine, Imperial College and Zambart) whose support has been critical in completion of this manuscript.
Footnotes
Author contribution: PB, GF, HC, NB conceptualised the manuscript. All authors contributed toward development of the manuscript and reviewed drafts including the final draft in this submission.
Conflict of interest: No authors declare a conflict of interest.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2015. Url: www.who.int/tb/publications/global_report/gtbr15_main_text.pdf. [Google Scholar]
- 2.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015. Url: www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed] [Google Scholar]
- 3.Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proceedings of the National Academy of Sciences of the United States of America. 2010 Nov 09;107(45):19485–9. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Ayles H, Egwaga S, Williams B, Mukadi YD, Santos Filho ED, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011 Mar;15(3):287–95. [PubMed] [Google Scholar]
- 5.Granich R, Gupta S, Suthar AB, Smyth C, Hoos D, Vitoria M, et al. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Current HIV research. 2011 Sep;9(6):446–69. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010 May 07;5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr. 2011 Apr;56(4):349–55. doi: 10.1097/QAI.0b013e3181f9fb39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS (London, England) 2010 Jun 1;24(9):1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS (London, England) 2009 Aug 24;23(13):1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. The New England journal of medicine. 2015 Aug 27;373(9):808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. The New England journal of medicine. 2015 Aug 27;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health WCDo. Western Cape Antenatal Survey Report. Cape Town Western Cape Deaprtment of Health; 2014. [Google Scholar]
- 14.Health Systems Trust. District Health Barometer, 20115–16. Durban: Health Systems Trust; 2016. Url: www.hst.org.za/.../District%20Health%20Barometers/ [Google Scholar]
- 15.Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): Rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment - a study protocol for a cluster randomised trial. Trials. 2014;15(1):57. doi: 10.1186/1745-6215-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Western Cape Department of Health. The Western Cape Antiretroviral Treatment Guidelines. Cape Town: Western Cape Department of Health; 2015. [Google Scholar]
- 17.Western Cape Department of Health. The Western Cape Consolidated Guidelines for HIV Treatment:Prevention of Mother- to- Child Transmission of HIV (PMTCT), Children, Adolescents and Adults. Cape Town: Western Cape Department of Health; 2016. [Google Scholar]
- 18.National Department of Health of South Africa. National Tuberculosis Management Guidelines. Pretoria: National Department of Health of South Africa; 2014. Url: https://www.idealclinic.org.za/docs/National-Priority-Health-Conditions/National%20TB%20management%20guidelines%202014.pdf. [Google Scholar]
- 19.Osler M, Hilderbrand K, Hennessey C, Arendse J, Goemaere E, Ford N, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. Journal of the International AIDS Society. 2014;17:18908. doi: 10.7448/IAS.17.1.18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty MBM, Babovic T, et al. Uptake and implementation of the WHO 2015 consolidated ARV guidelines: progress towards treat all. IAS Conference; 2016; Durban. 2016. [Google Scholar]
- 21.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6(10):e25098. doi: 10.1371/journal.pone.0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulle C, Kouanfack C, Laborde-Balen G, Boyer S, Aghokeng AF, Carrieri MP, et al. Gender Differences in Adherence and Response to Antiretroviral Treatment in the Stratall Trial in Rural District Hospitals in Cameroon. J Acquir Immune Defic Syndr. 2015 Jul 01;69(3):355–64. doi: 10.1097/QAI.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 23.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011 Oct;16(10):1297–313. doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 24.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet infectious diseases. 2008 Aug;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. The Lancet infectious diseases. 2010 Apr;10(4):251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO global strategy on people-centred and integrated health services. Geneva: World Health Organization; 2015. [Google Scholar]
- 27.Bock P, Cox H. Acute care - an important component of the continuum of care for HIV and tuberculosis in developing countries. Anaesthesia. 2016 Nov 21; doi: 10.1111/anae.13604. [DOI] [PubMed]
- 28.Ndagijimana A, Rugigana E, Uwizeye CB, Ntaganira J. One-stop TB-HIV services evaluation in Rwanda: comparison of the 2001–2005 and 2006–2010 cohorts. Public health action. 2015 Dec 21;5(4):209–13. doi: 10.5588/pha.15.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan R, Caldwell J, Bekker LG, Jennings K, Lombard C, Enarson DA, et al. Integration of TB and ART services fails to improve TB treatment outcomes: comparison of ART/TB primary healthcare services in Cape Town, South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014 Mar;104(3):204–9. doi: 10.7196/samj.7696. [DOI] [PubMed] [Google Scholar]
- 30.Pathmanathan I, Pevzner E, Cavanaugh J, Nelson L. Addressing tuberculosis in differentiated care provision for people living with HIV. Bulletin of the World Health Organization. 2017 Jan 01;95(1):3. doi: 10.2471/BLT.16.187021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. Journal of the International AIDS Society. 2016;19(1):21484. doi: 10.7448/IAS.19.1.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Western Cape Department of Health. Personal communication with department of health colleagues on 6 September 2017 by Bock, PA. Cape Town: [Google Scholar]
- 33.Lim HJ, Park JS, Cho YJ, Yoon HI, Park KU, Lee CT, et al. CD4(+)FoxP3(+) T regulatory cells in drug-susceptible and multidrug-resistant tuberculosis. Tuberculosis (Edinburgh, Scotland) 2013 Sep;93(5):523–8. doi: 10.1016/j.tube.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014 Aug 23;384(9944):682–90. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerkhoff AD, Kranzer K, Samandari T, Nakiyingi-Miiro J, Whalen CC, Harries AD, et al. Systematic review of TST responses in people living with HIV in under-resourced settings: implications for isoniazid preventive therapy. PLoS One. 2012;7(11):e49928. doi: 10.1371/journal.pone.0049928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churchyard GJ, Mametja LD, Mvusi L, Ndjeka N, Hesseling AC, Reid A, et al. Tuberculosis control in South Africa: successes, challenges and recommendations. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014 Mar;104(3 Suppl 1):244–8. doi: 10.7196/samj.7689. [DOI] [PubMed] [Google Scholar]
- 37.Wood R, Bekker LG. Isoniazid preventive therapy for tuberculosis in South Africa: an assessment of the local evidence base. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014 Mar;104(3):174–7. doi: 10.7196/samj.7968. [DOI] [PubMed] [Google Scholar]