Abstract
Background
The relationship between sildenafil dosing, exposure, and systemic hypotension in infants is incompletely understood.
Objectives
Characterize the relationship between predicted sildenafil exposure and hypotension in hospitalized infants.
Methods
We extracted sildenafil dosing and clinical characteristics from electronic health records of 348 neonatal intensive care units (1997–2013), and we predicted drug exposure using a population pharmacokinetic model.
Results
We identified 232 infants receiving sildenafil at a median dose of 3.2 mg/kg/day (2.0, 6.0). The median steady-state area under the concentration time curve over 24 hours (AUC24,SS) and maximum concentration of sildenafil (CmaxSS,SIL) were 712 ng*hr/mL (401, 1561) and 129 ng/mL (69, 293). Systemic hypotension occurred in 9% of the cohort. In multivariable analysis, neither dosing nor exposure were associated with systemic hypotension (odds ratio=0.96 [95% confidence interval: 0.81, 1.14] sildenafil dose; 0.87 [0.59, 1.28] AUC24,SS; 1.19 [0.78, 1.82] Cmax,SS,SIL).
Conclusions
We found no association between sildenafil dosing or exposure with systemic hypotension. Continued assessment of sildenafil’s safety profile in infants is warranted.
Keywords: dosing, exposure, systemic hypotension, infant, safety, sildenafil
INTRODUCTION
Sildenafil is a potent type-5 phosphodiesterase inhibitor increasingly used off label in infants.1,2 Despite this increasing use, sildenafil’s safety profile in infants remains poorly defined. Anecdotally reported adverse events associated with sildenafil therapy include bleeding, ocular anomalies, changes in cerebral blood flow, and systemic hypotension.3,4
Systemic hypotension is particularly significant given the hemodynamic instability frequently present in infants receiving sildenafil.5–7 Prior studies evaluating sildenafil’s safety in infants are contradictory.5,7–10 These findings may be due to differences in dosing and drug exposure, as evidenced by the wide range of observed plasma concentrations of sildenafil in prior pharmacokinetic studies.11,12 Because of this variability, a complete investigation of the cardiovascular safety profile of sildenafil in infants will require evaluation of drug dosing and exposure.
Safety studies in infants are challenging, and evaluation of drug exposure adds to the complexity.13 Novel strategies are urgently needed to overcome limitations of traditional studies, including large sample sizes and blood draws to measure drug concentrations.14 As a potential solution, we predicted individual sildenafil exposures using a published population pharmacokinetic model and combined them with electronic health record data from a large cohort of hospitalized infants. We hypothesized that increasing sildenafil exposures will be associated with clinically significant systemic hypotension.
MATERIALS AND METHODS
Data source and patient population
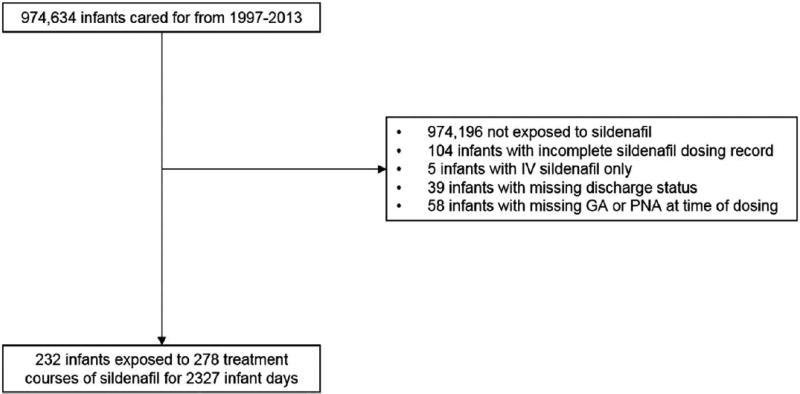
We used an electronic health record-derived database populated by admission notes, discharge summaries, and progress notes written by neonatologists caring for infants admitted to 1 of 348 Pediatrix Medical Group neonatal intensive care units in North America from 1997 to 2013. Clinical data routinely collected during hospitalization are captured in a data warehouse maintained by the Pediatrix Medical Group for quality-improvement and research purposes.15 We included all infants of 24–41 weeks gestational age at birth who received at least one day of enteral sildenafil during their hospitalization, to match the demographics of infants included in a published population pharmacokinetic model (Figure 1).11 We excluded intravenous doses of sildenafil, and infants with incomplete dosing records or missing information on survival status at discharge. This study was approved by the Duke University Institutional Review Board without the need for written informed consent.
Figure 1.
Study flow chart. GA=gestational age; PNA=postnatal age.
Definitions
The unit of observation for analysis was a course of sildenafil therapy. A course was defined as consecutive days of therapy with sildenafil at the same dose, dosing interval, and route. Any change to dosing or interruption in dosing for one or more days constituted a new course. We extracted total daily sildenafil dose in mg/kg/day and dosing interval. We defined “small for gestational age” as previously described.16 We identified severity of illness surrogates including daily use of mechanical ventilation, any inotrope (amrinone, dobutamine, dopamine, epinephrine, milrinone, norepinephrine, or phenylephrine), any other pulmonary vasodilator (inhaled nitric oxide, bosentan, or epoprostenol), and the presence of a positive blood culture with organisms not typically considered contaminants. We defined exposure to cytochrome P450 3A4 (CYP3A4) inducers as concomitant exposure to either rifampin or bosentan. We defined exposure to CYP3A4 inhibitors as concomitant exposure to either fluconazole, ketoconazole, voriconazole, clarithromycin, or erythromycin.
The primary outcome was systemic hypotension diagnosed during a sildenafil treatment course. We defined systemic hypotension as either new exposure to a vasopressor (dobutamine, dopamine, epinephrine, norepinephrine, or phenylephrine) or discontinuation of an antihypertensive (angiotensin-converting enzyme inhibitor, beta-blocker, calcium channel blocker, tolazoline, phentolamine, nitroprusside, nitroglycerin, hydralazine, or clonidine). We performed a sensitivity analysis defining systemic hypotension as new exposure to vasopressor only.
Exposure predictions
To simulate sildenafil exposure, we used a published infant population pharmacokinetic model consisting of a one-compartment structural model for sildenafil and its primary active metabolite desmethylsildenafil with combined additive and proportional residual errors.11 Using nonlinear mixed effects modeling methods, this model quantifies the different sources of variability in the dose-concentration relationship, including within- and between-subject variability and residual variability, and seeks to identify the measurable pathophysiologic factors responsible for the differences observed between subjects. When dose-concentration relationships vary between subjects, as is common in critically ill infants, the population pharmacokinetic model can predict concentrations of sildenafil based on dose received and specific clinical characteristics, rather than simply having to assume that concentrations achieved depend only on dose administered. The final model included weight (WT) as an allometrically scaled covariate for clearance (CL) and volume of distribution (V) for both parent and metabolite: sildenafil ; sildenafil V (L/70kg) = 596 * (WT/70); desmethylsildenafil ; desmethylsildenafil V(L/70kg) = 245 * (WT/70). The first-order absorption rate constant was fixed at 2.4 hr−1. Interindividual variability was included on sildenafil clearance and V, and on desmethylsildenafil clearance, with separate residual variabilities for parent and metabolite. We used infant daily weight and dosing information from the electronic health record to simulate daily sildenafil and desmethylsildenafil exposure as maximum concentration at steady-state (CmaxSS,SIL and CmaxSS,DMS) and area under the concentration versus time curve from 0 to 24 hours at steady-state using NONMEM version 7.3 (Icon Development Solutions, Ellicott City, MD) (Supplementary Table S1).
To represent total pharmacologic exposure of sildenafil, the area under the sildenafil concentration time curve from 0 to 24 hours at steady-state (AUC24,SS,SIL) was added to half the area under the desmethylsildenafil concentration versus time curve from 0 to 24 hours at steady-state (AUC24,SS,DMS) (AUC24,SS,SIL+DMS = AUC24,SS,SIL + 0.5 * AUC24,SS,DMS), as done in the original model publication.11 Due to an insufficient number of infants exposed to fluconazole, we did not include it as a covariate effect; instead, we included concomitant exposure to any CYP3A4 inducer or inhibitor in the multivariable regression.
Statistical analysis
We used summary statistics including medians (interquartile ranges) and counts (percentages) to describe continuous and categorical variables. We compared distribution of variables using Wilcoxon rank-sum, chi-square, and Fisher’s exact tests as appropriate. We used Spearman’s rank correlation to describe relationships between sildenafil dose and CmaxSS,SIL and AUC24,SS,SIL+DMS. We used multivariable logistic regression with random effects for neonatal intensive care unit to evaluate associations between systemic hypotension and sildenafil dosing, CmaxSS,SIL, and AUC24,SS,SIL+DMS, which were centered and scaled prior to inclusion in the regression, e.g., CmaxSS,SIL,center−scale = (CmaxSS,SIL − mean(Cmaxss,SIL)/standard deviation(CmaxSS,SIL). We fit separate models for total daily sildenafil dose, AUC24,SS,SIL+DMS, and CmaxSS,SIL (3 models total), all including the following as covariates: postnatal age in days, weight, gestational age, small for gestational age, mechanical ventilation, inotropes, other pulmonary vasodilators, and exposure to CYP3A4 inducers or inhibitors. Because an association between systemic hypotension and younger postnatal age became apparent in our analysis, and because younger infants treated with sildenafil may be more likely to suffer from persistent pulmonary hypertension of the newborn, we conducted a second sensitivity analysis limited to infants with this diagnosis. We reported adjusted odds ratios and 95% confidence intervals for systemic hypotension. We conducted statistical analyses using STATA SE 14.1 (College Station, TX), and considered P values <0.05 to be statistically significant.
RESULTS
We identified 232 infants exposed to 278 sildenafil courses for 2327 infant-days of therapy (Table 1). Median (interquartile range) gestational age and birth weight were 27 weeks (25, 36) and 775 g (593, 2368). Median postnatal age and postmenstrual age on the first day of sildenafil exposure were 85 days (21, 126) and 41 weeks (38, 44). Only 20% of infants were <37 weeks postmenstrual age when starting sildenafil. Over half of infants (55%) were receiving mechanical ventilation, and 30% were exposed to another pulmonary vasodilator when starting sildenafil. The most commonly used pharmacologic pulmonary vasodilator other than sildenafil was inhaled nitric oxide administered to 30% of infants, while bosentan (<1%) and epoprosterenol (1%) were administered less frequently. The median daily fraction of inspired oxygen on the first day of sildenafil therapy was 50% (35%, 100%), and 57% of infants were on >50% fraction of inspired oxygen. The median dose of sildenafil was 1.0 mg/kg/dose (0.6, 1.7) or 3.2 mg/kg/day (2.0, 6.0). The most common dosing intervals were every 6 (55%) and every 8 hours (27%). Mean (5th, 95th percentile) treatment course duration was 8.5 days (1, 46). The most common diagnoses potentially associated with sildenafil were persistent pulmonary hypertension of the newborn in 195/232 (84%) infants and bronchopulmonary dysplasia in 179/232 (77%) infants. Other diagnoses potentially associated with sildenafil use included congenital diaphragmatic hernia (24/232, 10%), pulmonary hypoplasia (20/232, 9%), and meconium aspiration syndrome (10/232, 4%).
Table 1.
Demographics
| Infants with Hypotension | |||
|---|---|---|---|
| Yes N=24 |
No N=208 |
||
| Gestational age at birth, weeks | |||
| ≤ 25 | 6 (25%) | 79 (38%) | |
| 26–28 | 3 (13%) | 46 (22%) | |
| 29–32 | 3 (13%) | 22 (11%) | |
| 33–36 | 4 (17%) | 12 (6%) | |
| ≥ 37 | 8 (33%) | 49 (24%) | |
| Birth weight, g | |||
| < 1000 | 8 (33%) | 127 (61%) | |
| 1000–1499 | 3 (13%) | 14 (8%) | |
| 1500–2499 | 2 (8%) | 25 (12%) | |
| 2500–3499 | 8 (33%) | 32 (15%) | |
| ≥ 3500 | 3 (13%) | 10 (5%) | |
| Small for gestational age | 2 (8%) | 70 (34%) | |
| Male gender | 13 (54%) | 117 (56%) | |
| Inborn status | 15 (63%) | 163 (78%) | |
| C-section | 15 (63%) | 149 (73%) | |
| 5-minute APGAR | |||
| 0–3 | 2 (9%) | 17 (8%) | |
| 4–6 | 8 (36%) | 50 (25%) | |
| 7–10 | 12 (55%) | 135 (67%) | |
| Age at first exposure, days | |||
| < 7 | 8 (33%) | 11 (5%) | |
| 7–29 | 5 (21%) | 45 (22%) | |
| 30–59 | 2 (8%) | 28 (13%) | |
| 60–119 | 5 (21%) | 58 (28%) | |
| ≥ 120 | 4 (17%) | 66 (32%) | |
Systemic hypotension occurred in 10% (24/232) of infants and 9% (25/278) of therapy courses. Of the 24 infants with hypotension, 18 were started on an inotrope or had an increase in the number of inotropes, and 6 infants had their antihypertensive medications discontinued or the number of antihypertensive medications decreased. Of the 6 infants who had a decrease in antihypertensive medications, angiotensin-converting enzyme inhibitors were discontinued in 3 infants, calcium channel blockers in 1, beta-blockers in 1, and other antihypertensives in 1. Infants with systemic hypotension had a higher median birth weight (2108 g [870, 3071] versus 759 g [585, 2278], P=0.004), but the median weight on the day of first exposure did not differ (median 3094 g [2313, 3547] versus 3081 g [2418, 3768], P=0.79). There was no significant difference in the distribution of gestational age, but infants with systemic hypotension had lower median postmenstrual ages and postnatal ages on the day of first exposure (postmenstrual age: 39 weeks [37, 42] versus 41 weeks [38, 44], P=0.03; postnatal age: 17 days [3, 96] versus 89 days [25, 129]; P=0.002). Hypotension was diagnosed early in the course: median 0.5 days (0, 4) after the start of therapy. The median dose and total daily dose did not differ between infants with systemic hypotension versus those without (1.0 mg/kg [0.5, 1.4] versus 1.0 mg/kg [0.5, 1.5], P=0.42; and 3.0 mg/kg/day [1.8, 5.8] versus 3.3 mg/kg/day [2.0, 6.2], P=0.37). On the first day of therapy, 72% of infants diagnosed with systemic hypotension were receiving mechanical ventilation, and 40% were exposed to another pulmonary vasodilator.
Median predicted AUC24,SS,SIL+DMS and CmaxSS,SIL were 712 ng*hr/mL (401, 1561) and 129 ng/mL (69, 293). None of the predicted exposures differed between courses with or without systemic hypotension (Table 2). As anticipated, we observed a significant positive correlation between total daily dose and AUC24,SS,SIL+DMS (R=0.65, P<0.001) and CmaxSS,SIL (R=0.69, P<0.001). Concomitant administration of CYP3A4 inducers was rare (<1%), while CYP3A4 inhibitors were co-administered during 5% of treatment courses. The most commonly co-administered CYP3A4 inhibitor on days of sildenafil exposure was fluconazole (3%), followed by erythromycin (1%), while the most commonly co-administered CYP3A4 inducer was rifampin (<1%).
Table 2.
Median (interquartile range) total daily dose and daily simulated exposures
| Courses With Hypotension | P | ||
|---|---|---|---|
| Yes N=25 | No N=253 | ||
| Sildenafil dose (mg/kg/day) | 3.0 (1.8, 5.8) | 3.3 (2.0, 6.2) | 0.37 |
| AUC24,SS,SIL+DMS (ng*hr/mL)a | 721 (400, 1614) | 711 (401, 1540) | 0.84 |
| Cmax,SS,SIL (ng/mL) | 101 (53, 373) | 129 (69, 287) | 0.88 |
Cmax,ss,SIL: maximal sildenafil steady state concentration.
AUC24,ss, SIL+DMS: 24-hour area under the concentration-time curve at steady-state, accounting for the contribution of the active desmethylsildenafil metabolite by adding 50% of this metabolite’s AUC to the corresponding value for the parent drug (AUC24,ss, SIL+DMS = AUC24,ss,SIL + 0.5*AUC24,ss,DMS).
In multivariable analysis, the total daily sildenafil dose was not associated with systemic hypotension (odds ratio=0.96 [95% confidence interval: 0.81, 1.14 per 1 standard deviation [SD] increase in total daily dose). We also found no association between predicted sildenafil exposures and systemic hypotension (Table 3). Results were similar in a sensitivity analysis defining systemic hypotension as a new exposure to inotropes only. Neither total daily dose (odds ratio=1.34, 95% confidence interval: 0.90, 1.99 per 1 SD increase in total daily dose), AUC24,SS,SIL+DMS (odds ratio=1.36, 95% confidence interval: 0.93, 1.97 per 1 SD increase in AUC24,SS,SIL+DMS), or CmaxSS,SIL (odds ratio=1.34, 95% confidence interval: 0.85, 2.10 per 1 SD increase in CmaxSS,SIL) were associated with increased odds of systemic hypotension. Results were also unchanged when all 3 regressions were repeated in infants with persistent pulmonary hypertension of the newborn and when stratifying the study population by gestational age into term (≥37 weeks gestational age) vs. preterm (<37 weeks gestational age) infants: odds ratio=0.36 (0.18, 2.20) per 1 SD increase in total daily dose, odds ratio=5.02 (0.59, 42.45) per 1 SD increase in AUC24,SS,SIL+DMS, odds ratio=7.12 (0.18, 285.88) per 1 SD increase in CmaxSS,SIL for full-term infants, odds ratio= 1.01 (0.85, 1.21) per 1 SD increase in total daily dose, odds ratio=1.22 (0.80, 1.85) per 1 SD increase in AUC24,SS,SIL+DMS, odds ratio=0.66 (0.27, 1.58) per 1 SD increase in CmaxSS,SIL for preterm infants.
Table 3.
Adjusted odds of hypotension
| Odds Ratiob (95% confidence interval) | |
|---|---|
| Sildenafil dose (mg/kg/day) | 0.96 (0.81, 1.14) |
| AUC24,SS,SIL+DMS (ng*hr/mL)a | 0.87 (0.59, 1.28) |
| Cmax,SS,SIL (ng/mL) | 1.19 (0.78, 1.82) |
Cmax,ss,SIL: maximal sildenafil steady state concentration.
AUC24,ss, SIL+DMS: 24-hour area under the concentration-time curve at steady-state, accounting for the contribution of the active desmethylsildenafil metabolite by adding 50% of this metabolite’s AUC to the corresponding value for the parent drug (AUC24,ss, SIL+DMS = AUC24,ss,SIL + 0.5*AUC24,ss,DMS).
adjusted for postnatal age, daily weight, gestational age, small for gestational age status, exposure to inotropes, other pulmonary vasodilators, mechanical ventilation, CYP3A4 inducers or inhibitors, and random effects for site.
DISCUSSION
In a large group of hospitalized infants, we found that neither dosing nor predicted sildenafil exposure were associated with systemic hypotension after adjusting for infant characteristics and severity of illness surrogates. While our study is retrospective, it is the largest evaluation of sildenafil safety in infants, and it is the first study to use predicted sildenafil exposure to characterize safety.17
Sildenafil induces vasodilation through inhibition of type-5 phosphodiesterase.18 Because type-5 phosphodiesterase is predominantly expressed in the lungs, sildenafil is considered a selective pulmonary vasodilator.19,20 Despite this perceived pulmonary selectivity, systemic hypotension can occur due to direct passage of sildenafil into the systemic circulation through intra- and extra-cardiac shunts, due to direct negative effects of sildenafil on the neonatal myocardium, or as a result of variable levels of type-5 phosphodiesterase expression in the lungs with potential for off-target effects.9,21 Treatment-related systemic hypotension was first reported in 5 of 36 (14%) infants >34 weeks gestational age exposed to escalating doses of sildenafil via intravenous infusion.7 These findings are comparable to the 10% prevalence of hypotension found in our cohort. Two other prospective and one retrospective study of infants receiving enteral sildenafil failed to find serious cardiovascular adverse events; however, they lacked the sample size necessary to identify rare adverse events and did not assess drug exposure.5,9,22
Systemic drug exposure mediates the relationship between dosing and its safety profile and may be variable in critically ill infants.23,24 Early clinical studies suggested that this is the case for sildenafil. In the first open-label trial of intravenous sildenafil in infants, blood concentrations varied both between and within dose levels.7 This variability in exposure was confirmed using population pharmacokinetic modeling. In a study of intravenous sildenafil in 36 term infants with persistent pulmonary hypertension of the newborn, sildenafil clearance increased from 0.84 L/h for a 1-day old infant to 2.58 L/h at 7 days of age.12 The authors concluded that sildenafil pharmacokinetics in infants were best characterized by a model that accounted for the relationship between clearance and postnatal age but noted that despite this age effect, interindividual variability in clearance remained high (>50%). Similar conclusions were drawn from a single-center pharmacokinetic study of 11 infants receiving enteral sildenafil.11 Blood concentrations and calculated AUC24,SS,SIL+DMS varied, and interindividual variability in pharmacokinetic parameters predicted using a 1-compartment model were >80% for both clearance and V. More recently, 30 blood concentrations obtained from 6 infants 24 to 39 weeks gestational age receiving a median (range) dose of 0.9 mg/kg (0.5–2.1) of sildenafil per standard of care in an opportunistic pharmacokinetic study ranged from 2.6 to 434 ng/mL.25 These findings support the notion of variability in sildenafil exposure owing to variable pharmacokinetics, and that assessments of the drug’s safety require evaluation of drug exposure.
Performing a large-scale sildenafil safety study with blood concentration measurements in infants would be challenging. Fortunately, published pharmacokinetic models allowed us to predict drug exposure using infant characteristics and dosing information collected in electronic health records. Our predicted exposures were within the range reported in one prior pharmacokinetic trial but lower than in the cohort of infants used to derive the pharmacokinetic model applied in our study.11,12 This difference may be due to the higher doses administered to the pharmacokinetic model development cohort (mean 1.9 mg/kg/dose) compared to the electronic health record cohort (mean 1.2 mg/kg/dose) or the younger postnatal age at the time of sildenafil dosing (mean 34 hours in the model development cohort versus mean 3.5 days in the electronic health record).
Despite the range of predicted sildenafil exposures in our study, we did not identify a relationship with systemic hypotension. Concerns about an exposure-safety relationship of sildenafil stem primarily from the results of the STARTS-2 trial.26 This open-label extension to the pivotal STARTS-1 study found increased mortality after 2 years of high-dose sildenafil in children >20 kg.26–28 Mortality was lower in children receiving low or medium dosing. While exposure data were not available for the long-term extension study, predicted geometric mean AUC of parent drug in children treated with high-dose sildenafil in the 16-week STARTS-1 study ranged from 941 ng*hr/mL to 2193.6 ng*hr/mL, higher than exposures in the medium- and low-dose groups (114.8 ng*hr/mL to 769.5 ng*hr/mL).29 Interpreting these findings has proven to be challenging, and their significance for infants remains debated.30,31 Even if one assumes that the observed exposure-safety relationship is true in older children, physiologic and disease-specific differences may explain why this does apply to infants. Disease states treated in infants such as persistent pulmonary hypertension of the newborn and concomitant therapy with high fractions of inspired oxygen may increase pulmonary type-5 phosphodiesterase activity and/or expression, resulting in improved on-target effects.21 Children in the long-term STARTS-2 trial were receiving outpatient sildenafil therapy for a variety of forms of pulmonary hypertension, but not persistent pulmonary hypertension of the newborn, and they were not exposed to high fractions of inspired oxygen.27 Given these differences and the potential benefits of sildenafil in infants, careful population-specific safety assessments are needed.
The strengths of our study included its novel, cost-effective, efficient, and minimal-risk design. Nevertheless, it is not without limitations. The dosing and safety data are derived from electronic health records, which have not undergone the development and scrutiny of a prospective trial database. Clinically significant hypotension was defined based on pharmacologic interventions, as blood pressure measurements were not available. This methodology likely underestimates the true prevalence of hypotension, but may serve as an acceptable surrogate of the clinically significant events. This is particularly true in the neonatal population, in which defining hypotension based on blood pressure measurements remains challenging. As a result, neonatal clinical trial experts of the NICHD-funded Pediatric Trials Network concluded that for the assessment of drug safety, hypotension should be defined based on the need for vasopressors and inotropes, as was done in our study.32 Because the reason for discontinuing drugs is not provided in the electronic health record data available, we further did not include discontinuation of sildenafil as a surrogate for hypotension but acknowledge that this may be a common first intervention for infants experiencing this complication. Overall, the limitations in hypotension definitions likely led us to underestimate the true prevalence of this complication in premature infants. In order to predict exposures, we used a previously published population pharmacokinetic model derived in neonates, but we acknowledge that this model has not been externally validated.11 Unfortunately, this limitation is common in pediatric pharmacokinetic models owing to the significant challenges of conducting prospective validation trials in this population. We further had to limit our study population to match the characteristics of the population used to develop the model, and therefore excluded infants receiving intravenous sildenafil. The original model publication does not provide information about gestational age, limiting our ability to compare it to the gestational age distribution in our cohort. However, the mean postnatal age and weight of the infants included in the model development cohort were 33 days and 3.6 kg, suggesting that they are predominantly premature. This is consistent with our study population.11 Details of administration, such as formulations, concomitant nutrition, or use of a nasogastric tube, were not available. Further, the mean treatment duration of our cohort was short, limiting our ability to report on the long-term safety. Lastly, while we were able to adjust for several characteristics in our regression analyses, other risk factors not available in the electronic health record may lead to residual bias.
In conclusion, this large, novel sildenafil exposure-safety study in hospitalized infants found that neither dosing nor predicted exposure were associated with systemic hypotension. Given the known dose-concentration variability observed in premature infants, the use of exposure predictions strengthens the lack of observed association between sildenafil use and hypotension in this population. Our findings are consistent with the majority of recently conducted, smaller, retrospective and prospective studies supporting the safety of sildenafil in hospitalized infants.
Supplementary Material
Acknowledgments
None.
Financial Support:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117.
Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117) and the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act).
Dr. Smith receives salary support for research from the NIH (1R21HD080606-01) and the Food and Drug Administration (1R18-FD005292-01) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Dr. Cohen-Wolkowiez receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Allergy and Infectious Disease (NIAID) (HHSN272201500006I and HHSN272201300017I), National Institute of Child Health and Human Development (NICHD) (HHSN275201000003I), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry (CardioDx and Durata Therapeutics) for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp).
Dr. Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act), from the NICHD (K23HD068497), from the National Heart, Lung, and Blood Institute (NHLBI) (R34HL124038), and from Pfizer, Cempra, and Astellas for work on DSMBs.
Dr. Gonzalez receives research support through 1K23HD083465-01 from the NICHD, and from the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org).
Footnotes
Conflicts of Interest: Complete disclosure information for Drs. Hornik, Smith, and Cohen-Wolkowiez can be found at https://dcri.org/about-us/conflict-of-interest/.
Drs. Laughon, Onufrak, Clark, and Gonzalez have no conflicts of interest to disclose for this study.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by Duke University Institutional Review Board.
References
- 1.Steinhorn RH. Pharmacotherapy for pulmonary hypertension. Pediatr Clin North Am. 2012;59:1129–1146. doi: 10.1016/j.pcl.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porta NF, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol. 2012;39:149–164. doi: 10.1016/j.clp.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxton N, Flannery T, Wild D, Bassi S. Sildenafil (Viagra)-induced spontaneous intracerebral haemorrhage. Br J Neurosurg. 2001;15:347–349. doi: 10.1080/02688690120072513. [DOI] [PubMed] [Google Scholar]
- 4.Samada K, Shiraishi H, Aoyagi J, Momoi MY. Cerebral hemorrhage associated with sildenafil (Revatio) in an infant. Pediatr Cardiol. 2009;30:998–999. doi: 10.1007/s00246-009-9460-z. [DOI] [PubMed] [Google Scholar]
- 5.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatr. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 6.Steinhorn RH. Diagnosis and treatment of pulmonary hypertension in infancy. Early Hum Dev. 2013;89:865–874. doi: 10.1016/j.earlhumdev.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155:841–847. e1. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–384. 384 e1–2. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limjoco J, Paquette L, Ramanathan R, Seri I, Friedlich P. Changes in mean arterial blood pressure during sildenafil use in neonates with meconium aspiration syndrome or sepsis. Am J Ther. 2015;22:125–131. doi: 10.1097/MJT.0b013e31826fc4ec. [DOI] [PubMed] [Google Scholar]
- 10.Vassalos A, Peng E, Young D, et al. Pre-operative sildenafil and pulmonary endothelial-related complications following cardiopulmonary bypass: a randomised trial in children undergoing cardiac surgery. Anaesthes. 2011;66:472–480. doi: 10.1111/j.1365-2044.2011.06702.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahsman MJ, Witjes BC, Wildschut ED, et al. Sildenafil exposure in neonates with pulmonary hypertension after administration via a nasogastric tube. Arch Dis Child Fetal Neonatal Ed. 2010;95:F109–114. doi: 10.1136/adc.2009.168336. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85:56–63. doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- 13.Laughon MM, Benjamin DK, Jr, Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughon MM, Benjamin DK., Jr Mechanisms to provide safe and effective drugs for children. Pediatr. 2014;134:e562–563. doi: 10.1542/peds.2014-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for "meaningful use" in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatr. 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 17.Samiee-Zafarghandy S, Smith PB, van den Anker JN. Safety of sildenafil in infants*. Pediatr Crit Care Med. 2014;15:362–368. doi: 10.1097/PCC.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. 26. [DOI] [PubMed] [Google Scholar]
- 19.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 20.Shekerdemian LS, Ravn HB, Penny DJ. Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatr Res. 2004;55:413–418. doi: 10.1203/01.PDR.0000112033.81970.C2. [DOI] [PubMed] [Google Scholar]
- 21.Farrow KN, Groh BS, Schumacker PT, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102:226–233. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas-Origel A, Gomez-Rodriguez G, Aldana-Valenzuela C, et al. The use of sildenafil in persistent pulmonary hypertension of the newborn. Am J Perinatol. 2010;27:225–230. doi: 10.1055/s-0029-1239496. [DOI] [PubMed] [Google Scholar]
- 23.Kearns GL. Selecting the proper pediatric dose: It is more than size that matters. Clin Pharmacol Ther. 2015;98:238–240. doi: 10.1002/cpt.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holford N. Dosing in children. Clin Pharmacol Ther. 2010;87:367–370. doi: 10.1038/clpt.2009.262. [DOI] [PubMed] [Google Scholar]
- 25.Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36:744–747. doi: 10.1038/jp.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129:1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 27.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. Revatio (sildenafil): Drug Safety Communication-Recommendation against use in children. [Accessed April 20, 2016]; Avialable at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucsm317743.htm.
- 29.US Food and Drug Administration. Medical, statistical, adn clinical pharmacology reviews of pediatric studies conducted under Section 505A and 505B of the Federal Food, Drug, and Comestic Act, as amended by the FDA Amendments Act of 2012 (FDASIA) [Accessed May 3, 2016]; Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM320473.pdf.
- 30.Dodgen AL, Hill KD. Safety and tolerability considerations in the use of sildenafil for children with pulmonary arterial hypertension. Drug Healthc Pat Saf. 2015;7:175–183. doi: 10.2147/DHPS.S65571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraemer U, Wildschuth E, Tibboel D. "Out of the blue"-safety and efficacy of pulmonary hypertension treatment in childhood*. Pediatr Crit Care Med. 2014;15:377–378. doi: 10.1097/PCC.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 32.England A, Wade K, Smith PB, Berezny K, Laughon M. Optimizing operational efficiencies in early phase trials: The Pediatric Trials Network experience. Contemp Clin Trials. 2016;47:376–382. doi: 10.1016/j.cct.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.