Abstract
Background
Adolescent girls and young women (AGYW) in sub-Saharan Africa have high HIV prevalence and incidence. We sought to understand which HIV risk factors individually and in combination contribute to risk, and whether these factors are associated with HIV worry and risk perception.
Setting
This study is ongoing at four public health centers in Lilongwe, Malawi (2016–2017).
Methods
AGYW 15-24 years old were recruited to participate in a study assessing four models of service delivery. At each health center, participants completed a baseline survey assessing socio-economic, behavioral, biomedical and partnership characteristics; self-reported HIV status; and, if HIV-uninfected, HIV risk perception (high versus low or none) and HIV worry (any versus none). We analyzed associations between baseline characteristics and HIV prevalence, risk perception, and worry.
Results
Among 1000 AGYW, median age was 19 years (IQR 17–21). Thirty-three participants reported being HIV-infected. Fifteen characteristics were associated with HIV infection. Having more risk factors was associated with higher HIV prevalence (≤4 factors, 0.5%; 5-8 factors, 6%; >8 factors, 21%). Having more risk factors was also associated with higher risk perception (p<0.001) and higher worry (p<0.001). However, among those with >8 risk factors, 52% did not consider themselves to be at high risk and 21% did not report any HIV worry.
Conclusion
Most AGYW perceive little risk of HIV acquisition, even those at highest risk. As a critical gap in the HIV prevention cascade, accurate risk perception is needed to tailor effective and sustained combination prevention strategies for this vulnerable population.
Keywords: HIV prevention, risk factors, risk perception, adolescent girls, young women, Malawi
Background
Adolescent girls and young women (AGYW) in sub-Saharan Africa (SSA) shoulder a disproportionate burden of the HIV epidemic.1 Nearly three-quarters of people aged 15–24 years living with HIV in SSA are AGYW, and they acquire HIV nearly a decade before men.1 The high prevalence among AGYW has been attributed to behavioral,2-4 biological,5-8 and socio-economic9 vulnerabilities. Although HIV incidence has declined in SSA overall,1 gains have been slower for AGYW.10-12
In Malawi, while the overall HIV prevalence has decreased from 10.6% to 8.8% from 2010 to 2015, it remains higher among women – particularly young women and adolescent girls – than among their male counterparts. Over five years, HIV prevalence in AGYW has remained at approximately 5%, whereas among young men and boys, it has decreased from 1.9% to 1.0%.13,14 In urban Malawi, the disparity is starker: 9.1% of AGYW are HIV-infected compared to 1.0% of young men and boys.13
HIV risk is not evenly distributed in all AGYW. The World Health Organization recommends pre-exposure prophylaxis (PrEP) to reduce the HIV risk in populations with incidence >3 per 100 person-years and for individuals at substantial risk.15 Determining which AGYW in SSA meet these definitions is challenging, yet important.10,16 Much of the analysis-to-date has been conducted in South Africa among trial participants,17-19 and understanding regional variation is critical.
Risk perception is a critical element in the HIV prevention cascade, and is especially important in light of new biomedical technologies. For example, despite PrEP being efficacious among adherent young women, PrEP effectiveness has varied among AGYW, largely due to adherence challenges.20-22 Ensuring sustained adherence to proven effective strategies, including PrEP, relies on accurate individual risk perception as a first step in the prevention cascade.23,24 Despite widespread education on HIV transmission and behavioral risk reduction, and an emerging focus on biomedical prevention in Malawian media and institutional discourse,25-27 only 41% of AGYW demonstrate comprehensive basic behavioral knowledge of HIV.13 Poor understanding of partnership and structural factors that may increase risk could sustain high rates of incidence in some groups and render preventive interventions ineffective. Thus, prior to scaling up new prevention programs, there is an urgent need to determine whether high-risk individuals accurately perceive their HIV risk.17
In this paper, we aimed 1) to identify the socioeconomic, behavioral, biomedical, and partnership factors associated with HIV infection among AGYW in Lilongwe, Malawi; 2) to understand the factors associated with risk perception and worry; and 3) to determine whether presence of more risk factors predicts higher prevalence of HIV infection, risk perception, and worry.
Methods
Study design, Setting, and Population
The Girl Power study was conducted at four health centers each in Lilongwe, Malawi and Western Cape, South Africa. This analysis is restricted to the Malawi sites. Girl Power is assessing four different models of service delivery for AGYW: 1) standard of care (no intervention), 2) youth-friendly health services (YFHS), 3) YFHS + empowerment sessions, 4) YFHS + empowerment sessions + cash transfer. Four comparable clinics were selected and randomly assigned to one service delivery model.
At each site, 250 AGYW were enrolled. Participants were recruited from the community and were eligible if they were 15-24 years old, from the clinic's catchment area, and willing to provide locator information. Sexually-active AGYW were purposively recruited. Participants were followed for one year. This is a cross-sectional analysis of baseline self-reported behavioral survey data.
Data Collection and Management
We performed a baseline survey in the full Malawian cohort assessing socioeconomic, individual behavioral and biomedical, and partnership characteristics; self-reported HIV status; and HIV risk perception and worry. Trained young female research officers administered surveys on electronic tablets using Open Data Kit software.28 Interviews were conducted in Chichewa and lasted 1-1.5 hours.
Outcomes of Interest
Baseline HIV status was elicited by asking participants if they had ever been tested for HIV and, if so, the most recent result. A dichotomous variable indicated those who reported being HIV-infected versus either HIV-uninfected or HIV status unknown. This specification was selected because all HIV-uninfected and HIV-unknown persons could perceive themselves to be at risk. HIV “risk perception” was elicited from those who did not report being HIV-infected. These participants were asked their perceived lifetime chance of acquiring HIV, with possible responses: “no chance,” “small chance,” or “high chance”. This variable was dichotomized into those participants who perceived a “high chance” versus all other responses. A second question asked participants how much they agreed or disagreed with the statement, “I worry a lot about getting infected with HIV,” with possible responses: “strongly disagree,” “disagree,” “neutral,” “agree,” and “strongly agree.” This variable (HIV worry) was dichotomized as those who agreed or strongly agreed versus other responses.
Exposures of Interest
Exposures of interest were obtained from questions in four domains: socio-economic, individual behavioral, individual biomedical, and sexual partnership factors. Each exposure variable associated with HIV infection and not already dichotomous was then dichotomized to allow for an analysis of association between having multiple risk factors and HIV prevalence, HIV risk perception, and HIV worry.
Socio-economic factors
Participant age was dichotomized into 15-19 years old versus 20-24 years old.
Educational attainment was dichotomized into those who had completed primary school versus those who had not.
Type of flooring in the participant's house was indicated as either earth or sand versus cement, tile or other types.
Running water and electricity were each dichotomous variables indicating whether participants had access in the home.
Asset score was the sum of household assets from a list of 14 items.13 This was dichotomized into ≤2 versus >2.
Marital status was dichotomized into single and not living with a partner versus married and/or living with a partner.
Orphan status was dichotomized into either one or both parents still alive versus both parents deceased (double orphan).
Participant travel was defined as having slept away from home on ≥3 nights during the past year.
Individual behavioral factors
Age at first sexual intercourse was elicited from each participant and dichotomized into <15 versus ≥15 years.
Forced first sex was a dichotomous variable indicating whether the participants' first sexual encounter was perceived as forced or consensual.
Consistent condom use was a dichotomous variable indicating whether or not the participant reported “always” using male or female condoms.
Number of sexual partners in the last year was dichotomized into either <3 versus ≥3 different sexual partners in the last year based on self-report.
Participants were asked about any drinking, frequency of drinking in a typical week, and quantity per occasion. Heavy drinking was defined as consuming ≥8 drinks per week or ≥4 drinks per occasion.29
Individual biomedical factors
STI symptoms were defined as reporting abnormal vaginal discharge and/or genital sores or ulcers within the past 6 months.
Pregnancy history indicated whether or not the participant had ever been or was currently pregnant.
Sexual partnership factors
Participants were asked about their three most recent sexual partners in a partner grid.
Partner travel was defined as the cohabiting partner having slept away from home ≥3 times during the past year.
Partner financial or material support indicated whether any partner had given the participant money or items like groceries, clothes or phone minutes to help her get by.
Transactional sex was elicited by asking participants if they felt like they had to have sex with any partner in exchange for receiving money or other items.
An uncircumcised partner was defined as any partner believed not to be circumcised.
Partner concurrency was defined as the participant believing a sexual partner had one or more other partners during the time they were sexually active.
An older partner was any partner the participant reported to be ≥10 years older than herself.
Perceived HIV status of each sexual partner was elicited from the participants. An HIV-positive partner was one who the participant knew to be HIV-infected.
The partner violence score was based on a validated 17-item scale that was adapted to this setting.13 Affirmative answers to yes and no questions were summed to produce a continuous variable that was subsequently categorized into three levels: 0–3; 4–7; and ≥8.
Data Analysis
We examined frequencies and percentages of each categorical variable and assessed their relationship to HIV status using Pearson's chi-squared tests. Variables associated with HIV status (p≤0.15) were considered risk factors. We fit individual models of each of these risk factors with self-reported HIV infection to estimate adjusted prevalence ratios (aPR) and 95% confidence intervals (CI), adjusting each model for age as a continuous variable, a known confounder. We then assessed whether having more risk factors was associated with a higher probability of HIV infection.
Among participants who reported a negative or unknown HIV status, we performed bivariable tests of association between risk factors and HIV risk perception and HIV worry. We then fit models of each risk factor separately for HIV risk perception and HIV worry to estimate aPRs and 95% CIs, adjusting each model for age. Analyses were performed using Stata statistical software (release 14, College Station, TX).
Ethical approvals
The University of North Carolina Institutional Review Board and Malawi's National Health Sciences Research Committee granted approval to conduct this study. Voluntary written informed consent was obtained from persons 18-24 years old. Assent and permission by a parent, guardian, or authorized representative were obtained from adolescents 15-17 years old. All consent procedures were read aloud and, in cases of limited literacy, an impartial witness was present.
Results
One thousand AGYW participated in the survey (Table 1). Median age was 19 years old (IQR 17–21). Thirty-three participants reported being HIV-infected; of the remaining 967, 69% reported an HIV-negative test within the past 6 months, 17% reported an HIV-negative test >6 months prior, and 14% had never tested.
Table 1. Baseline characteristics of adolescent girls and young women in Lilongwe, Malawi by self-reported HIV status, N=1000.
| Characteristic | Reported HIV- or Unknown (N=967) | Reported HIV+ (N=33) | p† | ||
|---|---|---|---|---|---|
|
| |||||
| N | %* | N | %* | ||
|
| |||||
| Socio-economic factors | |||||
|
| |||||
| Age, years | |||||
| 15–19 | 566 | 58% | 11 | 33% | |
| 20–24 | 401 | 42% | 22 | 67% | 0.004 |
|
| |||||
| Educational attainment | |||||
| No | 273 | 29% | 14 | 42% | |
| Yes | 684 | 71% | 19 | 58% | |
| Missing | 10 | 0 | 0.08 | ||
|
| |||||
| Type of flooring in house | |||||
| Earth / Sand | 291 | 30% | 12 | 36% | |
| Cement / Tile / Other | 675 | 70% | 21 | 64% | |
| Missing | 1 | 0 | 0.4 | ||
|
| |||||
| Running water in house | |||||
| No | 542 | 56% | 27 | 82% | |
| Yes | 425 | 44% | 6 | 18% | 0.003 |
|
| |||||
| Electricity in house | |||||
| No | 603 | 62% | 20 | 61% | |
| Yes | 362 | 38% | 13 | 39% | |
| Missing | 2 | 0 | 0.8 | ||
|
| |||||
| Household asset score | |||||
| ≤2 | 376 | 39% | 19 | 58% | |
| >2 | 591 | 61% | 14 | 42% | 0.03 |
|
| |||||
| Marital status | |||||
| Single and/or not living with partner | 755 | 78% | 24 | 72% | |
| Married and/or living with partner | 212 | 22% | 9 | 27% | 0.5 |
|
| |||||
| Orphan status | |||||
| One or both parents alive | 888 | 92% | 26 | 79% | |
| Both parents deceased | 76 | 8% | 7 | 21% | |
| Missing | 3 | 0 | 0.006 | ||
|
| |||||
| Participant travel in last year | |||||
| <3 times | 737 | 76% | 23 | 70% | |
| ≥3 times | 229 | 24% | 10 | 30% | |
| Missing | 1 | 0 | 0.4 | ||
|
| |||||
| Individual behavioral factors | |||||
|
| |||||
| Age at first sexual intercourse | |||||
| <15 | 170 | 18% | 8 | 24% | |
| ≥15 | 791 | 82% | 25 | 76% | |
| Missing | 6 | 0 | 0.3 | ||
|
| |||||
| Forced first sex | |||||
| No | 525 | 45% | 17 | 52% | |
| Yes | 432 | 55% | 16 | 48% | |
| Missing | 6 | 0.5 | |||
|
| |||||
| Consistent condom use | |||||
| No | 666 | 69% | 22 | 67% | |
| Yes | 296 | 31% | 11 | 33% | |
| Missing | 5 | 0.7 | |||
|
| |||||
| Number of sexual partners in last year | |||||
| <3 | 915 | 95% | 27 | 82% | |
| ≥3 | 52 | 5% | 6 | 18% | 0.002 |
|
| |||||
| Heavy alcohol use | |||||
| No | 925 | 96% | 25 | 76% | |
| Yes | 42 | 4% | 8 | 24% | <0.001 |
|
| |||||
| Individual biomedical factors | |||||
|
| |||||
| Symptoms of STI | |||||
| No | 725 | 75% | 21 | 64% | |
| Yes | 240 | 25% | 12 | 36% | |
| Missing | 2 | 0 | 0.1 | ||
|
| |||||
| Pregnancy history | |||||
| No | 563 | 58% | 7 | 22% | |
| Yes | 401 | 42% | 25 | 78% | |
| Missing | 3 | 1 | <0.001 | ||
|
| |||||
| Sexual partnership factors | |||||
|
| |||||
| Partners' travel in last year | |||||
| <3 times | 129 | 65% | 2 | 22% | |
| ≥3 times | 70 | 35% | 7 | 78% | |
| Missing/No partner | 768 | 24 | 0.01 | ||
|
| |||||
| Partners' financial or material support | |||||
| No | 42 | 4% | 1 | 3% | |
| Yes | 919 | 95% | 32 | 97% | |
| Missing | 6 | 0 | 0.7 | ||
|
| |||||
| Transactional sex | |||||
| No | 763 | 79% | 20 | 61% | |
| Yes | 201 | 21% | 13 | 39% | |
| Missing | 3 | 0 | 0.01 | ||
|
| |||||
| Uncircumcised partner | |||||
| No | 417 | 43% | 5 | 15% | |
| Yes | 475 | 49% | 23 | 70% | |
| Don't know | 68 | 7% | 5 | 15% | |
| Missing/No partner | 7 | 0 | 0.003 | ||
|
| |||||
| Partner concurrency | |||||
| No | 358 | 37% | 2 | 6% | |
| Yes | 468 | 49% | 30 | 91% | |
| Don't know | 134 | 14% | 1 | 3% | |
| Missing/No partner | 7 | 0 | <0.001 | ||
|
| |||||
| Older partner | |||||
| No | 906 | 94% | 27 | 82% | |
| Yes | 58 | 6% | 6 | 18% | |
| Missing/No partner | 3 | 0 | 0.005 | ||
|
| |||||
| HIV-positive partner | |||||
| No | 960 | >99% | 28 | 85% | |
| Yes | 3 | <1% | 5 | 15% | |
| Missing/No partner | 4 | 0 | <0.001 | ||
|
| |||||
| Partner violence score | |||||
| 0-3 | 417 | 43% | 10 | 30% | |
| 4-7 | 335 | 35% | 14 | 42% | |
| 8+ | 215 | 22% | 9 | 27% | 0.3 |
STI = sexually transmitted infection
Percents may not equal 100 because of rounding
Categorical correlations and p-values based on Pearson's chi-square.
Socio-economic factors and HIV infection
Self-reported HIV infection was higher among older participants: 2% of participants <20 years reported HIV infection compared to 5% of those ≥20 years old. Most participants (71%) finished primary school. A majority (57%) reported not having running water in their home; 61% reported having ≤2 household assets. Two-thirds reported both parents being alive; 26% were single orphans and 8% were double orphans. Not having running water, having ≤2 household assets, and being a double orphan were associated with HIV infection in bivariable analysis. After adjusting for age, not having running water (aPR 3.10, 95% CI 1.26–7.63) remained associated with prevalent HIV.
Individual behavioral factors and HIV infection
First sexual intercourse <15 years old was reported by 18% of participants. Forced first sexual intercourse was reported by 45%. HIV status was not associated with either of these variables. Most participants either never used condoms (19%) or used them inconsistently (50%). HIV status was not associated with consistent condom use. In the past year, most (78%) respondents had ≤1 sexual partner, 16% had 2, and 6% had ≥3 partners. Respondents with ≥3 sexual partners in the past year had higher prevalence of HIV infection (10%) than those who had <3 partners in the past year (3%). Alcohol use was reported by 37% of respondents; 14% of those reported heavy drinking. HIV infection was more prevalent among those participants who reported heavy drinking (16%) than those who did not (3%). After adjusting for age, ≥3 sexual partners in past year (aPR 3.49, 95% CI 1.35–9.02) and heavy alcohol use (aPR 5.80, 95% CI 2.40–14.02) were associated with HIV infection.
Individual biomedical factors and HIV infection
One-quarter of participants reported STI symptoms in the past 6 months. HIV prevalence was higher among women who reported STI symptoms (5%) than those who did not (3%). Participants who had ever been pregnant had higher HIV prevalence (6%) than those who had never been pregnant (1%). Pregnancy history remained associated with HIV infection after adjusting for age (aPR 2.97, 95% CI 1.16–7.61).
Sexual partnership factors and HIV infection
Approximately one-third of the 221 participants with a cohabiting partner reported that their partner slept away from home ≥3 times over the past year. HIV prevalence was higher among those reporting partner travel (9%) than those not (2%). Half reported that ≥1 partner was uncircumcised. Respondents who reported having an uncircumcised partner had higher HIV prevalence (5%) than those reporting partner circumcision (2%).
Eight participants reported having a partner with known HIV infection. HIV infection was more common among participants with an HIV-positive partner (15%) than among those with unknown or HIV-negative partners (0.3%). Half of participants reported that their partner had a concurrent partner, 14% reported not knowing, and 36% reported that their partners did not have another partner. Participants reporting partner concurrency had higher prevalence of HIV infection (6%) compared to those who did not (0.6%). Most participants (94%) had partners who were <10 years older. Those who reported a partner ≥10 years older had higher HIV prevalence (9%) than those who did not (3%). Transactional sex was reported by 21%. HIV infection was more common among those reporting transactional sex (6%) than among those denying transactional sex (3%). Partner violence was prevalent—82% reported emotional violence, 36% reported physical violence, and 46% reported sexual violence—but no type of violence was associated with HIV infection.
After adjusting for age, partner travel (aPR 7.00, 95% CI 1.37–35.78), transactional sex (aPR 2.26, 95% CI 1.10–4.67), uncircumcised partner (aPR 2.40, 95% CI 1.00–5.80), partner concurrency (aPR 8.27, 95% CI 2.48–27.60), and an HIV-positive partner (aPR 51.70, 95% CI 10.78–248.01), were each associated with reported HIV.
Multiple risk factors and HIV infection
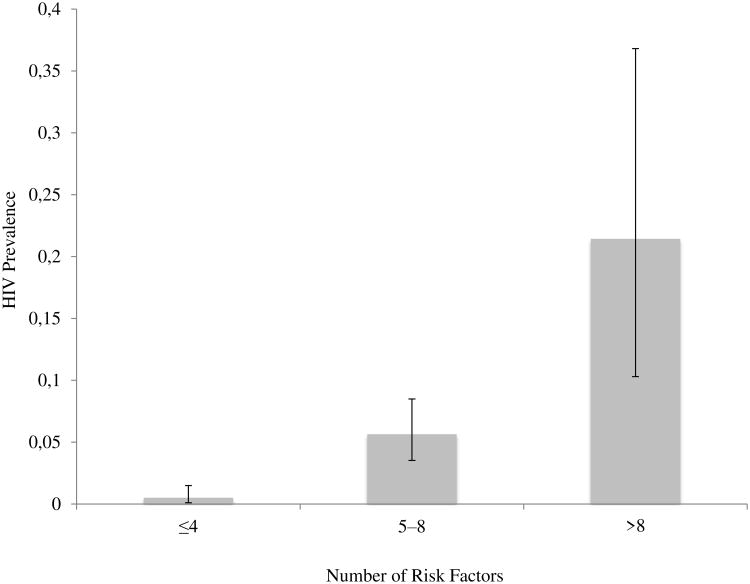
In total, 15 risk factors were associated with HIV infection. Among all respondents, the median number of risk factors was 4 (IQR 2–6). Fifty-nine percent had ≤4, 37% had 5–8, and 4% had >8 risk factors. Having more risk factors was associated with a higher prevalence of HIV infection: 0.5% among those with ≤4 risk factors; 6% among those with 5–8 risk factors; and 21% among those with >8 risk factors (p<0.001) (Figure 1).
Figure 1.
Prevalence of HIV infection increases with number of risk factors among adolescent girls and young women in Lilongwe, Malawi, N=1000.
HIV risk perception
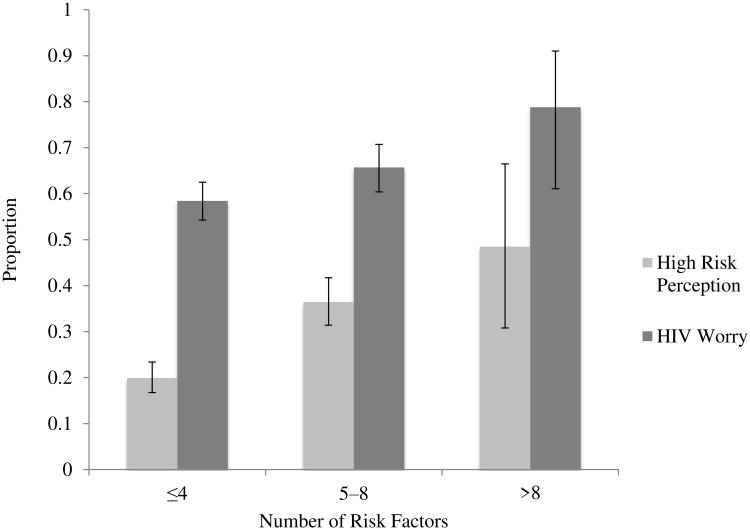
Of the participants who reported being HIV-uninfected or of unknown HIV status, 27% (n=831) and 26% (n=135), reported high HIV risk perception, respectively. Ten of the 15 risk factors associated with HIV infection were individually associated with high HIV risk perception (Table 2). The presence of more HIV risk factors was associated with higher HIV risk perception (p<0.001) (Figure 2). However, 63% of 384 women with ≥5 risk factors did not perceive themselves to be at high risk of HIV; and 52% of 33 women with >8 risk factors did not perceive themselves to be at high risk.
Table 2. Bivariable analysis of HIV risk perception (N=966) and worry (N=944) by risk factors associated with HIV infection among at-risk adolescent girls and young women in Lilongwe, Malawi.
| Risk Factor | HIV Risk Perception* | HIV Worry* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No/Small Risk % (N=706)† | High Risk % (N=260)† | p‡ | No Worry % (N=361)† | Worry % (N=583)† | p‡ | |
|
| ||||||
| Age 15–19 | 61 | 51 | .005 | 66 | 55 | |
| Age 20–24 | 39 | 49 | 34 | 45 | .001 | |
|
| ||||||
| Completed primary school | 74 | 64 | .002 | 71 | 71 | |
| Did not complete primary school | 26 | 36 | 29 | 29 | .9 | |
|
| ||||||
| Running water | 48 | 33 | <.001 | 44 | 43 | |
| No running water | 52 | 67 | 56 | 57 | .8 | |
|
| ||||||
| >2 household assets | 64 | 53 | .001 | 61 | 61 | |
| ≤2 household assets | 36 | 47 | 38 | 39 | .8 | |
|
| ||||||
| One or both parents alive | 92 | 92 | .9 | 90 | 94 | |
| Both parents deceased | 8 | 8 | 10 | 6 | .02 | |
|
| ||||||
| <3 sexual partners in past year | 95 | 93 | .1 | 97 | 94 | |
| ≥3 sexual partners in past year | 5 | 7 | 3 | 6 | .04 | |
|
| ||||||
| Not heavy alcohol | 96 | 95 | .8 | 97 | 95 | |
| Heavy alcohol use | 4 | 5 | 3 | 5 | .2 | |
|
| ||||||
| No STI symptoms | 75 | 76 | .8 | 79 | 73 | |
| STI symptoms | 25 | 24 | 21 | 27 | .06 | |
|
| ||||||
| Never pregnant | 63 | 47 | <.001 | 64 | 55 | |
| Ever pregnant | 37 | 53 | 36 | 45 | .004 | |
|
| ||||||
| Partner slept <3 nights away | 72 | 51 | .003 | 80 | 58 | |
| Partner slept ≥3 nights away§ | 28 | 49 | 20 | 42 | .002 | |
|
| ||||||
| No transactional sex | 82 | 71 | <.001 | 82 | 77 | |
| Transactional sex | 18 | 29 | 18 | 23 | .06 | |
|
| ||||||
| Partner circumcised | 45 | 40 | .2 | 45 | 42 | |
| Partner uncircumcised / Unknown | 55 | 60 | 55 | 58 | .2 | |
|
| ||||||
| Partner has no other partners | 42 | 23 | <.001 | 48 | 31 | |
| Partner has other partners / Unknown | 58 | 77 | 52 | 69 | <.001 | |
|
| ||||||
| Partner <10years older | 95 | 90 | .002 | 98 | 92 | |
| Partner ≥10 years older | 5 | 10 | 3 | 8 | <.001 | |
|
| ||||||
| No known HIV+ partner | 100 | 100 | .3 | 100 | 100 | |
| Partner HIV+ | 0 | 0 | 0 | 0 | .9 | |
STI = sexually transmitted infection
Missing answer to question on HIV risk perception N=1; missing answer to question on HIV worry N=23
Percents may not equal 100 because of rounding
p-values based on Pearson's chi-square
N=212, reflecting only cohabiting participants
Figure 2.
Prevalence of high HIV risk perception (N=966) and HIV worry (N=944) increases with number of HIV risk factors among adolescent girls and young women in Lilongwe, Malawi.
After adjusting for age, no running water (aPR 1.82, 95% CI 1.35–2.45), ≤2 household assets (aPR 1.55, 95% CI 1.16, 2.07), pregnancy history (aPR 1.77, 95% CI 1.27, 2.47), partner travel (aPR 2.48, 95% CI 1.34–4.61), partner concurrency (aPR 2.33, 95% CI 1.72–3.15), and transactional sex (aPR 1.92, 95% CI 1.37–2.67) were associated with high HIV risk perception (Table 3). These six variables remained significant predictors of risk perception when the analysis was restricted to participants who reported being HIV-uninfected.
Table 3. Adjusted prevalence ratios of factors associated with known HIV infection (N=1000), high HIV risk perception (N=966) and HIV infection worry (N=944) among adolescent girls and young women in Lilongwe, Malawi.
| Risk Factor | Reported HIV Infection | HIV Risk Perception* | HIV Worry* |
|---|---|---|---|
|
| |||
| Adjusted PR† (95% CI) | Adjusted PR† (95% CI) | Adjusted PR† (95% CI) | |
|
| |||
| Completed primary school | 1.00 | 1.00 | 1.00 |
| Did not complete primary school | 1.88 (0.92,3.83) | 1.68 (1.23,2.29) | 1.05 (0.78,1.41) |
|
| |||
| Running water | 1.00 | 1.00 | 1.00 |
| No running water | 3.10 (1.26,7.63) | 1.82 (1.35,2.45) | 1.01 (0.78,1.32) |
|
| |||
| >2 assets | 1.00 | 1.00 | 1.00 |
| ≤2 assets | 1.79 (0.88,3.67) | 1.55 (1.16,2.07) | 0.98 (0.75,1.29) |
|
| |||
| One or both parents alive | 1.00 | 1.00 | 1.00 |
| Both parents deceased | 2.21 (0.91,5.41) | 0.92 (0.54,1.57) | 0.49 (0.30,0.79) |
|
| |||
| <3 sexual partners in past year | 1.00 | 1.00 | 1.00 |
| ≥3 sexual partners in past year | 3.49 (1.35,9.02) | 1.58 (0.88,2.84) | 1.92 (0.98,3.75) |
|
| |||
| Not heavy alcohol use | 1.00 | 1.00 | 1.00 |
| Heavy alcohol use | 5.80 (2.40,14.02) | 1.06 (0.53,2.11) | 1.85 (0.89,3.84) |
|
| |||
| No STI symptoms in past 6 months | 1.00 | 1.00 | 1.00 |
| STI symptoms in past 6 months | 1.66 (0.80,3.46) | 0.94 (0.67,1.31) | 1.34 (0.98,1.83) |
|
| |||
| Never pregnant | 1.00 | 1.00 | 1.00 |
| Ever pregnant | 2.97 (1.16,7.61) | 1.77 (1.27,2.47) | 1.23 (0.90,1.68) |
|
| |||
| Partner slept <3 nights away | 1.00 | 1.00 | 1.00 |
| Partner slept ≥3 nights away‡ | 7.00 (1.37,35.78) | 2.48 (1.34,4.61) | 2.87 (1.43,5.77) |
|
| |||
| No transactional sex | 1.00 | 1.00 | 1.00 |
| Transactional sex | 2.26 (1.10,4.67) | 1.92 (1.37,2.67) | 1.36 (0.98,1.91) |
|
| |||
| Partner circumcised | 1.00 | 1.00 | 1.00 |
| Partner uncircumcised | 2.40 (1.00,5.80) | 1.13 (0.83,1.53) | 1.07 (0.81,1.42) |
|
| |||
| Partner has no other partners | 1.00 | 1.00 | 1.00 |
| Partner has ≥1 other partners/Unknown | 8.27 (2.48,27.60) | 2.33 (1.72,3.15) | 1.98 (1.50,2.60) |
|
| |||
| Partner <10 years older | 1.00 | 1.00 | 1.00 |
| Partner ≥10 years older | 2.20 (0.84,5.77) | 2.03 (1.17,3.52) | 2.96 (1.42,6.16) |
|
| |||
| No known HIV+ Partner | 1.00 | 1.00 | |
| Partner HIV+ | 51.70 (10.78,248.01) | --§ | 1.35 (0.12,15.14) |
PR = prevalence ratio; STI = sexually transmitted infection
Analyses only completed among the 967 respondents who did not report known HIV infection. Missing HIV risk perception responses N=1; missing HIV worry responses N=23
All PRs adjusted for age
N=221, reflecting only cohabiting participants. For PR calculations, this variable was transformed into a 3-level variable: (1) not cohabiting; (2) cohabiting partner slept <3 nights away in the past year; (3) cohabiting partner slept ≥3 nights away in the past year and the reported PR reflects the ratio of the outcome variable between the latter two categories
PR could not be calculated given zero observations in one category (zero responses of “high risk perception” among 3 women who reported having a HIV-positive partner)
HIV worry
Among participants who reported being HIV-negative or of unknown HIV status, 62% (n=813) and 60% (n=131) reported worrying about acquiring HIV, respectively. Nine of the 15 risk factors associated with HIV infection were associated with HIV worry (Table 2). Having more risk factors was associated with a higher probability of HIV worry (p<0.001) (Figure 2). However, 33% of those with ≥5 risk factors and 21% with >8 risk factors did not worry about acquiring HIV.
After adjusting for age, partner travel (aPR 2.87, 95% CI 1.43–5.77), partner concurrency (aPR 1.98, 95% CI 1.50–2.60), and an older partner (aPR 2.96, 95% CI 1.42–6.16) were each significant predictors of HIV worry (Table 3). These three variables remained significant predictors of worry after restricting the analysis to participants who reported being HIV-uninfected.
Discussion
In this cross-sectional analysis of HIV risk factors, risk perception, and worry among AGYW in Malawi, we found 15 socio-economic, behavioral, biomedical, and partnership factors associated with HIV infection. Having more of these factors predicted higher prevalence of HIV infection, higher lifetime HIV risk perception, and higher HIV infection worry. Despite these associations, most AGYW, including the majority of those at highest risk for HIV, did not report high risk perception.
Our findings suggest that the context of sexual behavior may predict HIV prevalence more than individual behaviors themselves. Although number of sexual partners in the past year was predictive of HIV infection, two other key female sexual behaviors—age of debut and consistent condom use—each a focus of HIV prevention programs among young women,30,31 were not associated with HIV status in our study. Causal associations are difficult to infer using cross-sectional data alone. Condom use may have a strong reciprocal relationship with both perceived risk and risky sexual behavior, and it is possible that other factors such as the circumstances of sexual initiation and partner factors may be more salient than age at debut. We noted a strong association between HIV status and heavy alcohol use, a contextual behavior that may result in disinhibited sexual activity with high-risk partners.32-34 Similarly, no running water was associated with HIV status, suggesting that socio-economic status may play a role in HIV risk. While particular socioeconomic risk factors may vary across settings, many are modifiable through social protection approaches.35
In our study, more sources of vulnerability were associated with higher HIV prevalence, consistent with risk scores demonstrating that more risk factors are associated with higher risk of HIV acquisition17,19 and with observations that more sources of social protection are associated with fewer risky behaviors, better utilization of preventive strategies, and lower probability of unintended pregnancy among AGYW.36,37 Social protection, behavioral, and biomedical interventions that aim to mitigate vulnerabilities in combination could produce greater impacts on HIV prevention than single-domain HIV prevention.35
Although the majority of HIV-uninfected participants had at least one risk factor, few had >8, the level associated with the highest HIV prevalence. This observation suggests that identifying a small subset of women at highest risk of HIV infection is possible. Such identification is important, as it would allow for PrEP targeting to those at highest risk. Replicating these findings in a larger cohort with an HIV incidence outcome is a critical next step.
HIV risk factors were moderately aligned with HIV risk perception and worry among uninfected AGYW. Overall, partnership factors (e.g. partner travel, partner concurrency, transactional sex, older partner) were more strongly correlated with HIV risk perception and worry than individual behavioral factors (e.g. heavy alcohol use, ≥3 sexual partners over the past year, older partner). Previous research has shown that Malawian women worry most about infection from partners; and primary individual behavioral strategies to prevent HIV often focus on modifying key partnership characteristics.25,38 Partner-specific risk factors are known to influence individual risk perception and can drive safer sex behavior and willingness to use biomedical prevention strategies.39 Even so, perception of partner concurrency was associated with an 8-fold increase in HIV prevalence, but only a 2-fold increase in risk perception or worry. Heavy alcohol use, strongly predictive of HIV infection in our study and the region,17,32,33,40,41 predicted neither high perceived risk nor worry. Similarly, although female HIV prevalence increases with number of sexual partners in our study and in the region,13,19 having ≥3 sexual partners in the past year did not predict HIV risk perception or worry. Discrepancies between the presence or magnitude of association between factors associated with HIV infection and factors predictive of risk perception or worry represent opportunities for education in vulnerable populations.
A large proportion of women with multiple HIV risk factors did not perceive themselves to be at high risk. This may be because risk is multi-factorial, with a number of the identified factors (e.g., no running water, household assets, orphanhood) being demographic and/or structural rather than more proximal behavioral factors. The successful application of prevention services depends on at-risk individuals first perceiving risk, accessing services, and adopting behavioral changes and biomedical interventions designed to mitigate that risk.24 Furthermore, long-term effectiveness of preventive strategies will depend on maintained risk perception among those who face ongoing risk. In PrEP trials among young women in SSA, risk perception was associated with pill adherence,42-45 the strongest predictor of PrEP efficacy.46-50 In the FEM-PrEP trial, half the participants who sero-converted had not perceived themselves to be at any risk of HIV infection, despite participation in an HIV prevention trial targeted at a high-risk population.43
More participants reported HIV acquisition worry than high lifetime risk perception. By including one cognitive measure (perceived likelihood of infection) and one intuitive measure (worry of becoming infected), we aimed to explore whether one metric was more sensitive in measuring participants' understanding of individual risk.51 Although worry was more common than high risk perception, some factors associated with worry were not associated with risk perception (e.g., older partner), while others predicted high risk perception but not worry (e.g., transactional sex). Given the discrepancies between worry and perceived risk, future studies could determine the optimal metric or set of metrics that best aligns risk perception with likelihood of HIV acquisition.
Our study design presents several limitations. First, due to the cross-sectional nature of the data, we cannot infer causality between participant characteristics and HIV prevalence. Whereas in young women many infections are likely to be recent, a few of those infected may have been perinatally infected, such that the identified risk factors would not be relevant. However, based on staff interactions with participants, perinatal infection was uncommon in our study and most participants with HIV reported that their most recent diagnostic HIV test was within the last 2 years. Second, these results are based on self-report, and AGYW may have been HIV-infected, but not known it or reported it. Although a cross-sectional survey based on self-report may have limited our risk factor analysis, it had less effect on our analyses of risk perception and worry, as persons with unknown HIV status could still perceive themselves as at-risk. Third, interviewer-administered surveys can cause misreporting of HIV status, sexual behaviors, and socioeconomic markers because of social desirability or memory challenges. Finally, while single-item HIV risk perception assessments are common,52-55 multidimensional tools may elicit perceived risk more accurately.56,57
It has long been recognized that HIV incidence is disproportionately high among AGYW in SSA, but less clear which women are at highest risk and whether these women accurately perceive this risk. Our work shows that it is indeed possible to identify these women, but additional work is needed to help them appreciate their own risk and then seek and adhere to appropriate prevention strategies. Identifying the most vulnerable and developing strategies to enhance risk perception will bring us one step closer to reducing HIV incidence.
Acknowledgments
We thank the staff of Girl Power for their hard work and dedication to this project. We also thank each participant for her invaluable contribution to this research.
Source of Funding: The study presented in this manuscript is funded by Evidence for HIV Prevention in Southern Africa (EHPSA), a DFID programme managed by Mott MacDonald. N.E.R. is supported by funding from the National Institutes of Health (K99 MH104154). Trainee support has been provided through the National Institutes of Health for J.T.P (T32 HD075731) and N.L.B. (R25 TW009340). The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Joint United Nations Programme on HIV/AIDS. [Accessed March 14, 2016];The Gap Report. 2014 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 2.Pettifor AE, van der Straten A, Dunbar MS, Shiboski SC, Padian NS. Early age of first sex: a risk factor for HIV infection among women in Zimbabwe. AIDS. 2004;18(10):1435–1442. doi: 10.1097/01.aids.0000131338.61042.b8. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Bruce J, Dude A. Protecting young women from HIV/AIDS: the case against child and adolescent marriage. Int Fam Plan Perspect. 2006;32(2):79–88. doi: 10.1363/3207906. [DOI] [PubMed] [Google Scholar]
- 4.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet. 2010;376(9734):41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Jha P, Stirling B, et al. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One. 2007;2(10):e1001. doi: 10.1371/journal.pone.0001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramjee G, Wand H. Geographical clustering of high risk sexual behaviors in “hot-spots” for HIV and sexually transmitted infections in Kwazulu-Natal, South Africa. AIDS and behavior. 2014;18(2):317–322. doi: 10.1007/s10461-013-0578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polis CB, Curtis KM, Hannaford PC, et al. Update on hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence, 2016. AIDS. 2016 doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettifor AE, Measham DM, Rees HV, Padian NS. Sexual power and HIV risk, South Africa. Emerg Infect Dis. 2004;10(11):1996–2004. doi: 10.3201/eid1011.040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(2):S144–153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 11.Joint United Nations Programme on HIV/AIDS. HIV prevention among adolescent girls and young women. [Accessed March 14, 2016];2016 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_HIV_prevention_among_adolescent_girls_and_young_women.pdf.
- 12.Abdool Karim Q, Kharsany AB, Frohlich JA, et al. HIV incidence in young girls in KwaZulu-Natal, South Africa--public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 2012;16(7):1870–1876. doi: 10.1007/s10461-012-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Statistical Office (NSO) and ICF Macro. Malawi Demographic and Health Survey 2015-2016. Zomba, Malawi, and Rockville, Maryland, USA: 2017. [Google Scholar]
- 14.National Statistical Office (NSO) and ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi, and Calverton, Maryland, USA: 2011. [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Accessed January 25, 2016];2015 http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed]
- 16.Shisana O, Rehle T, Simbayi L, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- 17.Balkus JE, Brown E, Palanee T, et al. An Empiric HIV Risk Scoring Tool to Predict HIV-1 Acquisition in African Women. J Acquir Immune Defic Syndr. 2016;72(3):333–343. doi: 10.1097/QAI.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pintye J, Drake AL, Kinuthia J, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis. 2017;64(6):751–758. doi: 10.1093/cid/ciw850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wand H, Reddy T, Naidoo S, et al. A Simple Risk Prediction Algorithm for HIV Transmission: Results from HIV Prevention Trials in KwaZulu Natal, South Africa (2002-2012) AIDS Behav. 2017 doi: 10.1007/s10461-017-1785-7. [DOI] [PubMed] [Google Scholar]
- 20.Baeten JM, Heffron R, Kidoguchi L, et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1-Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. PLoS medicine. 2016;13(8):e1002099. doi: 10.1371/journal.pmed.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekker LG, Grant R, Hughes J, Roux S. HPTN 067/ADAPT Cape Town: a comparison of daily and nondaily PrEP dosing in African women. Conference on Retroviruses and Opportunistic Infections; 23-26 February, 2015; Seattle, WA. [Google Scholar]
- 23.Celum CL, Delany-Moretlwe S, McConnell M, et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc. 2015;18(4 Suppl 3):20227. doi: 10.7448/IAS.18.4.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargreaves JR, Delany-Moretlwe S, Hallett TB, et al. The HIV prevention cascade: integrating theories of epidemiological, behavioural, and social science into programme design and monitoring. Lancet HIV. 2016;3(7):e318–322. doi: 10.1016/S2352-3018(16)30063-7. [DOI] [PubMed] [Google Scholar]
- 25.Smith KP, Watkins SC. Perceptions of risk and strategies for prevention: responses to HIV/AIDS in rural Malawi. Soc Sci Med. 2005;60(3):649–660. doi: 10.1016/j.socscimed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Kaler A. AIDS-talk in everyday life: the presence of HIV/AIDS in men's informal conversation in Southern Malawi. Soc Sci Med. 2004;59(2):285–297. doi: 10.1016/j.socscimed.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Angotti N, Frye M, Kaler A, Poulin M, Watkins SC, Yeatman S. Popular Moralities and Institutional Rationalities in Malawi's Struggle Against AIDS. Popul Dev Rev. 2014;40(3):447–473. doi: 10.1111/j.1728-4457.2014.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunette W, Sundt M, Dell N, Chaudhri R, Breit N, Borriello G. Open Data Kit 2.0: Expanding and Refining Information Services for Developing Regions. 2013 [Google Scholar]
- 29.Alcoholism NIoAAa. Rethinking Drinking: Alcohol and Your Health. Bethesda, MD: 2010. NIH Publication No. 15-3770. [Google Scholar]
- 30.Harrison A, Colvin CJ, Kuo C, Swartz A, Lurie M. Sustained High HIV Incidence in Young Women in Southern Africa: Social, Behavioral, and Structural Factors and Emerging Intervention Approaches. Curr HIV/AIDS Rep. 2015;12(2):207–215. doi: 10.1007/s11904-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mavedzenge SN, Luecke E, Ross DA. Effective approaches for programming to reduce adolescent vulnerability to HIV infection, HIV risk, and HIV-related morbidity and mortality: a systematic review of systematic reviews. J Acquir Immune Defic Syndr. 2014;66(2):S154–169. doi: 10.1097/QAI.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg M, Pettifor A, Van Rie A, et al. The Relationship between Alcohol Outlets, HIV Risk Behavior, and HSV-2 Infection among South African Young Women: A Cross-Sectional Study. PLoS One. 2015;10(5):e0125510. doi: 10.1371/journal.pone.0125510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34(11):856–863. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 34.Scott-Sheldon LA, Carey KB, Cunningham K, Johnson BT, Carey MP, Team MR. Alcohol Use Predicts Sexual Decision-Making: A Systematic Review and Meta-Analysis of the Experimental Literature. AIDS Behav. 2016;20(1):S19–39. doi: 10.1007/s10461-015-1108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cluver LD, Orkin FM, Yakubovich AR, Sherr L. Combination Social Protection for Reducing HIV-Risk Behavior Among Adolescents in South Africa. J Acquir Immune Defic Syndr. 2016;72(1):96–104. doi: 10.1097/QAI.0000000000000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cluver L, Boyes M, Orkin M, Pantelic M, Molwena T, Sherr L. Child-focused state cash transfers and adolescent risk of HIV infection in South Africa: a propensity-score-matched case-control study. Lancet Glob Health. 2013;1(6):e362–370. doi: 10.1016/S2214-109X(13)70115-3. [DOI] [PubMed] [Google Scholar]
- 37.Cluver LD, Orkin FM, Boyes ME, Sherr L. Cash plus care: social protection cumulatively mitigates HIV-risk behaviour among adolescents in South Africa. AIDS. 2014;28(3):S389–397. doi: 10.1097/QAD.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 38.Schatz E. ‘Take your mat and go!’: rural Malawian women's strategies in the HIV/AIDS era. Cult Health Sex. 2005;7(5):479–492. doi: 10.1080/13691050500151255. [DOI] [PubMed] [Google Scholar]
- 39.Morrow KM, Fava JL, Rosen RK, Christensen AL, Vargas S, Barroso C. Willingness to use microbicides varies by race/ethnicity, experience with prevention products, and partner type. Health Psychol. 2007;26(6):777–786. doi: 10.1037/0278-6133.26.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson-Jones D, Weiss HA, Rusizoka M, et al. Risk factors for herpes simplex virus type 2 and HIV among women at high risk in northwestern Tanzania: preparing for an HSV-2 intervention trial. J Acquir Immune Defic Syndr. 2007;46(5):631–642. doi: 10.1097/QAI.0b013e31815b2d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ao TT, Sam NE, Masenga EJ, Seage GR, 3rd, Kapiga SH. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis. 2006;33(3):163–169. doi: 10.1097/01.olq.0000187204.57006.b3. [DOI] [PubMed] [Google Scholar]
- 42.Corneli A, Wang M, Agot K, et al. Perception of HIV risk and adherence to a daily, investigational pill for HIV prevention in FEM-PrEP. J Acquir Immune Defic Syndr. 2014;67(5):555–563. doi: 10.1097/QAI.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 43.Corneli AL, McKenna K, Headley J, et al. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17(3 Suppl 2):19152. doi: 10.7448/IAS.17.3.19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59(5):463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 49.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolcini MM, Catania JA, Choi KH, Fullilove MT, Coates TJ. Cognitive and emotional assessments of perceived risk for HIV among unmarried heterosexuals. AIDS Educ Prev. 1996;8(4):294–307. [PubMed] [Google Scholar]
- 52.Stringer EM, Sinkala M, Kumwenda R, et al. Personal risk perception, HIV knowledge and risk avoidance behavior, and their relationships to actual HIV serostatus in an urban African obstetric population. J Acquir Immune Defic Syndr. 2004;35(1):60–66. doi: 10.1097/00126334-200401010-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenkorang EY, Rajulton F, Maticka-Tyndale E. Perceived risks of HIV/AIDS and first sexual intercourse among youth in Cape Town, South Africa. AIDS Behav. 2009;13(2):234–245. doi: 10.1007/s10461-008-9470-5. [DOI] [PubMed] [Google Scholar]
- 54.Johnston L, O'Bra H, Chopra M, et al. The associations of voluntary counseling and testing acceptance and the perceived likelihood of being HIV-infected among men with multiple sex partners in a South African township. AIDS Behav. 2010;14(4):922–931. doi: 10.1007/s10461-008-9362-8. [DOI] [PubMed] [Google Scholar]
- 55.Garfinkel DB, Alexander KA, McDonald-Mosley R, Willie TC, Decker MR. Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care. 2016:1–8. doi: 10.1080/09540121.2016.1234679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley H, Tsui A, Hindin M, Kidanu A, Gillespie D. Developing scales to measure perceived HIV risk and vulnerability among Ethiopian women testing for HIV. AIDS Care. 2011;23(8):1043–1052. doi: 10.1080/09540121.2010.543880. [DOI] [PubMed] [Google Scholar]
- 57.Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS Behav. 2012;16(4):1075–1083. doi: 10.1007/s10461-011-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]